Abstract
Patients who suffer from lymphedema have impaired immunity and, as a result, are at an increased risk for infections. Furthermore, previous studies have shown that lymphadenectomy impairs acquisition of adaptive immune responses and antibody production in response to foreign antigens. Although it is clear that antigen presentation in lymph nodes plays a key role in adaptive immunity, the cellular mechanisms that regulate impaired immune responses in patients with lymphedema or following lymphatic injury remain unknown. We have previously found that axillary lymph node dissection (ALND), both clinically and in a mouse model, results in a marked increase in the number of T-regulatory cells (Tregs) in the ipsilateral limb. In this study, we focus on the role of Tregs in immunosuppression and show that Treg proliferation in tissues distal to site of lymphatic injury contributes to impaired innate and adaptive immune responses. More importantly, using Foxp3-diptheria toxin receptor (Foxp3-DTR) transgenic mice, we show that depletion of Tregs in the setting of lymphatic injury restores these critical immune-mediated responses. These findings provide additional evidence that immune responses following lymphatic injury play a key role in mediating the pathology of lymphedema.
Keywords: Treg, lymphedema, immunosuppression
INTRODUCTION
Lymphedema is a morbid disease that commonly occurs following lymphadenectomy in the course of oncologic treatment (Barry et al., 2012). The primary symptoms of swelling and progressive fibroadipose deposition result in disability and decreased quality of life. It is also associated with a high risk of severe, recurrent soft tissue infections that may lead to sepsis and even death (Mehrara et al., 2011, Wilke et al., 2006). Patients with lymphedema-related infections often require intravenous antibiotics and, in some cases, lifelong prophylactic antibiotics. The lack of an understanding of the cellular mechanisms regulating these infectious complications has prevented the development of novel therapies. Thus, the elucidation of the means by which lymphatic injury impairs immune responses is an important clinical goal.
The disproportionately high risk of infections suggests the presence of persistent localized immunologic dysfunction, a hypothesis supported by animal and clinical studies demonstrating that lymphatic defects correspond to impaired humoral immunity, dysregulated autoimmune responses, and abnormal trafficking of dendritic cells (DCs) to regional lymph nodes (Baird et al., 2002, El Saghir et al., 2005, Simon and Cody, 1992, Sugaya et al., 2012). Thomas et al., for example, showed that the absence of dermal lymphatic capillaries in K14-VEGFR3-Ig mice hinders the development of tolerance to skin-encountered antigens (Thomas et al., 2012). Similarly, others have reported that depletion or inhibition of lymphangiogenesis promotes immune tolerance and decreases graft rejection following transplantation (Chen et al., 2004, Dohlman et al., 2015, Yin et al., 2011). Clinical studies have also found that vaccination in patients’ lymphedematous extremities is associated with decreased antibody titers (Nookala et al., 2004, Sugaya et al., 2012). Taken together, these results suggest that the lymphatic system is a key regulator of immune function. However, the cellular mechanisms resulting in such immune deficits in lymphedema remain unknown.
We have previously shown that CD4+ cells play a key role in lymphedema pathophysiology by regulating tissue fibrosis and inhibiting lymphangiogenesis (Avraham et al., 2010, Avraham et al., 2013, Savetsky et al., 2014). Further characterization revealed that a substantial portion of these cells are CD4+CD25+Foxp3+ T-regulatory cells (Tregs) (Zampell et al., 2012). Interestingly, we found that depletion of CD25+ cells did not induce changes in lymphatic function or fibroadipose deposition. Consistent with our initial findings, Gousopoulos et al. also noted that lymphedema is associated with upregulation of Foxp3 expression and Treg accumulation (Gousopoulos et al., 2016). In contrast, however, these authors found that Treg depletion led to increased edema and inflammation and that adoptive transfer of Tregs reduced fibrosis and swelling. This discrepancy may be related to the fact that systemic CD25+ depletion likely also depletes other activated CD4+ cells in addition to Tregs. Collectively, these data indicate that Treg infiltration into lymphedematous tissues is increased and that these cells may regulate the chronic inflammatory reaction that characterizes this disease.
Tregs are immunoregulatory cells known to play an important role in tolerance and autoimmunity. Both natural (i.e., thymic-derived; nTregs) and adaptive (i.e., induced; iTregs) types suppress immune responses through various mechanisms, including cytolysis and cytokine-mediated signaling (Josefowicz et al., 2012, Kretschmer et al., 2005). Activated Tregs also passively bind interleukin 2 (IL-2), thereby depriving other T cells of an important signal of cellular proliferation and activation (Josefowicz et al., 2012). Based on this knowledge, we tested the hypothesis that Tregs in lymphedematous tissues play a critical role in impaired immunity following lymphatic injury. We first show that Tregs accumulate in both clinical and animal models of lymphedema. Then, using a mouse model of axillary lymph node dissection (ALND) with Treg reporter and Foxp3-diphtheria toxin receptor (Foxp3-DTR) mice, we demonstrate that proliferating nTregs in lymphedematous tissues suppress infiltration of inflammatory cells distal to the zone of lymphatic injury. Treg depletion resulted not only in marked inflammation, but also increased clearance of bacterial particles and restored adaptive immune responses initiated in the affected forelimb.
RESULTS
Treg infiltration is increased in lymphedematous tissues
Comparison of matched biopsies from affected and contralateral upper extremities of patients with unilateral breast cancer-related lymphedema revealed a 5.5-fold increase in Tregs in the lymphedematous extremity (Fig. 1a; P=0.036). Importantly, however, previous studies have presented evidence that the seemingly normal contralateral limbs also have lymphatic abnormalities even though the dysfunction is not overtly manifested clinically (Aldrich et al., 2012, Burnand et al., 2012, Pain et al., 2004). These lymphatic abnormalities may be secondary to the formation of collateral vessels to the contralateral limb that bypass the zone of injury, a phenomenon shown by Suami et al. in a canine model of lymphedema (Suami et al., 2013). Based on this knowledge, we chose to also examine the ipsilateral ALND-treated forelimbs in comparison to forelimbs of mice that underwent sham surgery (axillary incision without lymphadenectomy) only.
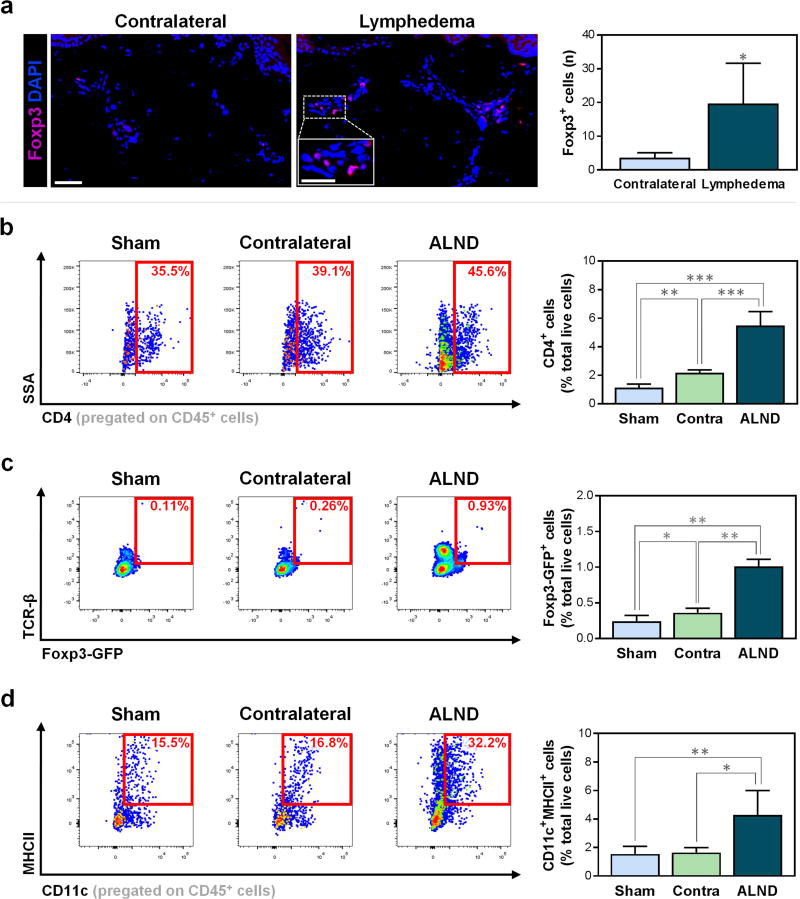
Figure 1. Treg infiltration is increased in lymphedema.
a) Representative immunofluorescent images (left; scale bar 50 µm) and quantification of Foxp3+ Tregs per 0.25 mm2 (right) in matched human biopsies from contralateral and lymphedematous limbs (n=4/group, 3–4 HPF/patient). Inset represents magnified view; scale bar 25 µm.
b) Representative FACS plots (left) and quantification (right) of CD45+CD4+ cells (n=4–5/group).
c) Representative FACS plots (left) and quantification (right) of TCR-β+Foxp3-GFP+ Tregs (n=5/group).
d) Representative FACS plots (left) and quantification (right) of CD45+CD11c+MHCII+ DCs (n=4–5/group).
Mice harvested six weeks after surgery. Data expressed as mean ± SD. Statistically significant differences represented by *P<0.05, **P<0.01, and ***P<0.001. Tregs, Tregulatory cells; HPF, high-powered field; sham, forelimbs ipsilateral to sham surgery; contra/contralateral, forelimbs contralateral to axillary lymph node dissection (ALND); ALND, forelimbs ipsilateral to ALND; DCs, dendritic cells.
To confirm our human findings, we examined the infiltration of immune cells in distal forelimbs ipsilateral and contralateral to the area where ALND was performed using flow cytometry on single-cell preparations. As previously mentioned, we also evaluated forelimb tissues harvested from sham-operated mice as an additional control. Consistent with our clinical results, we found that, compared to contralateral and sham-operated controls, the ipsilateral ALND-treated forelimbs had significantly increased CD4+ cells in general (Fig. 1b; P=0.0001 and P<0.0001, respectively) and Foxp3+ Tregs in particular (Fig. 1c; P<0.0001 for both). Interestingly, the infiltration of CD4+ cells and Tregs in tissues harvested from the forelimb contralateral to the ALND site was modestly, but significantly, increased as compared to that collected from sham-operated mice (Fig. 1b–c; P=0.0042 and P=0.032, respectively). These findings suggest that structural changes noted in prior studies may also correlate with mild inflammatory changes in the contralateral limb and that comparison to immune responses in sham-operated animals may be more meaningful when attempting to understand the changes that result from ALND.
To test our hypothesis that differences in CD4+ cell and Treg infiltration in the contralateral forelimbs are due to altered inflammatory cell trafficking through collateral vessels rather than innate changes, we evaluated forelimb skin CD11c+MHCII+ DCs, which are potent antigen-presenting cells (APCs) known to activate CD4+ T cells (Bousso, 2008). We found that the proportion of DCs was significantly increased only in the limb ipsilateral to the ALND as compared with contralateral and sham-operated groups (Fig. 1d; P=0.0245 and P=0.0093, respectively); the difference between the contralateral and sham groups was not significant (P=0.8). This finding supports the hypothesis that DCs are activated in the tissues of the ipsilateral limb rather than systemically.
Lymphatic injury increases proliferation and activation of natural Tregs
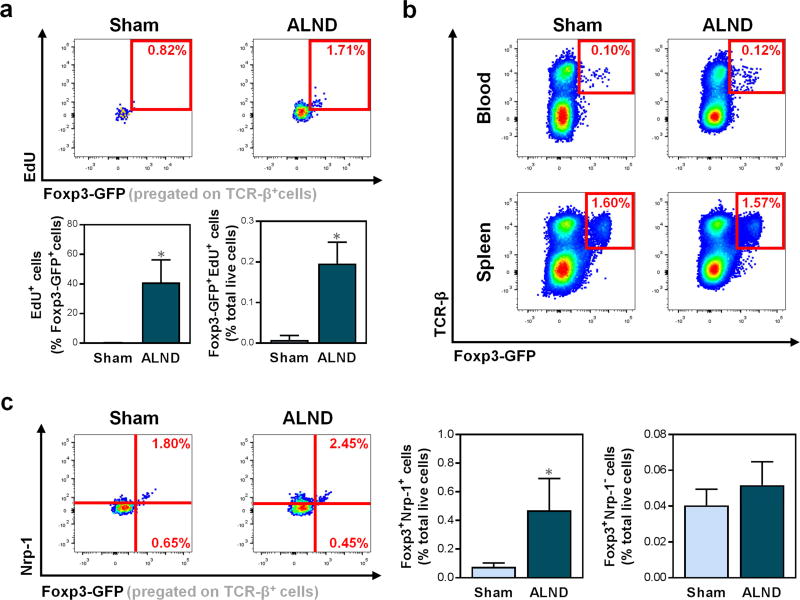
Based on studies demonstrating the potential for Tregs to proliferate in response to antigens (Walker et al., 2003), we analyzed the number of proliferating cells as indicated by the expression of 5-ethynl-2’-deoxyuridine (EdU) in the ipsilateral forelimbs of sham-operated and ALND-treated mice. We found that ALND resulted in an increase in proliferating Tregs in the ipsilateral forelimbs compared to sham surgery (Fig. 2a; P<0.0001). In contrast, there were no differences in Tregs in the blood or spleens of ALND-treated and sham-operated mice, thus suggesting that Treg proliferation was localized to the tissues distal to the zone of lymphatic injury resulting from ALND (Fig. 2b).
Figure 2. Lymphatic injury increases proliferation and activation of natural Tregs.
a) Representative FACS plots (upper) and quantification (lower) of proliferating (EdU+) TCR-β+Foxp3-GFP+ Tregs in mouse forelimbs (n=6/group).
b) Representative FACS plots of TCR-β+Foxp3-GFP+ Tregs in mouse blood (upper) and spleens (lower).
c) Representative FACS plots (left) and quantification (right) of TCR-β+Foxp3-GFP+Nrp-1+ nTregs and TCR-β+Foxp3-GFP+Nrp-1− iTregs in mouse forelimbs (n=5/group).
Mice harvested six weeks after surgery. Data expressed as mean ± SD. Statistically significant differences represented by *P<0.05, **P<0.01, and ***P<0.001. nTregs, natural Tregs; iTregs, induced Tregs.
Knowing that both nTregs and iTregs have been noted to contribute to immune tolerance in asthma and colitis (Curotto de Lafaille et al., 2008, Haribhai et al., 2011), we also sought to determine the predominant Treg type in lymphedema by analyzing the presence of nTreg marker neuropilin-1 (Nrp-1). We noted that ipsilateral forelimbs of ALND-treated mice had a sixfold increase in Foxp3+Nrp-1+ nTregs compared to sham-operated controls (Fig. 2c; P=0.0044), but no difference in Foxp3+ Nrp-1− iTregs (P=0.17).
Tregs downregulate local tissue inflammation after lymphatic injury
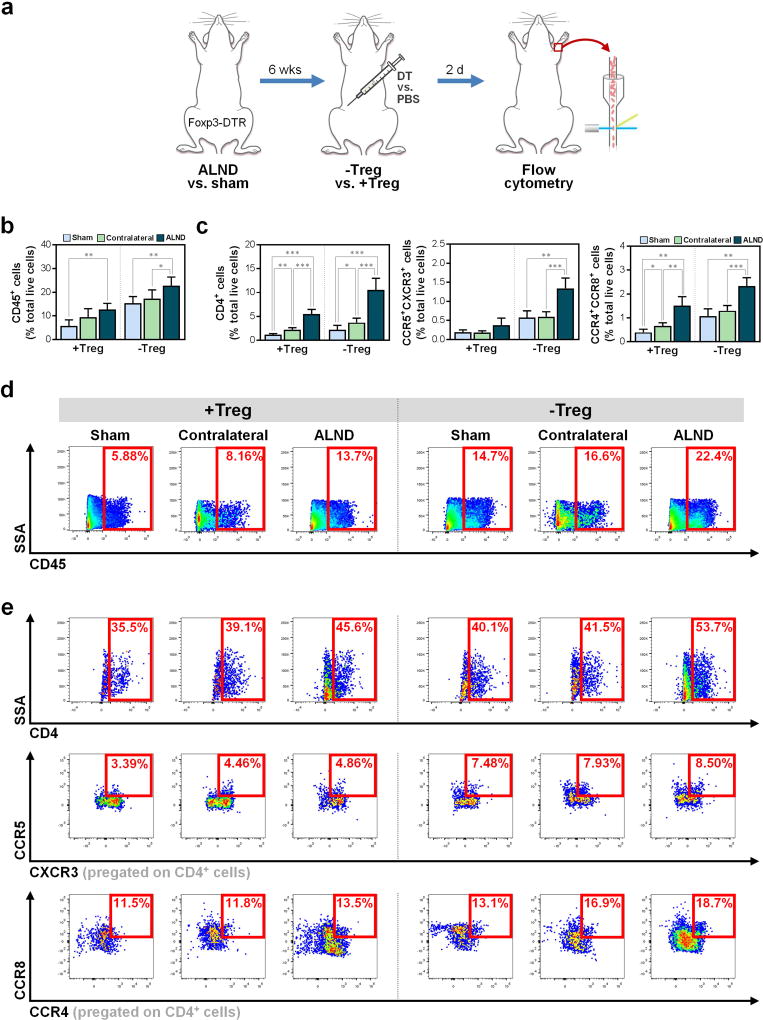
Knowing that Tregs proliferate in local tissues after lymphatic injury, we then evaluated the physiologic role of these cells in lymphedema using Foxp3-DTR mice, in which Treg depletion can be achieved with systemic diphtheria toxin (DT) injection (Kim et al., 2007). After the dose and timing of DT were optimized, we analyzed the effects of Treg depletion (>80% reduction; Fig. S1; P<0.0001) following ALND versus sham surgery (Fig. 3a).
Figure 3. Treg depletion following results in increased local tissue inflammation.
a) Schematic depicting Treg depletion after sham or ALND surgery in Foxp3-DTR mice.
b) FACS quantification of CD45+ cells (n=5–6/group).
c) FACS quantification of CD4+ cells (left), CD4+CCR5+CXCR3+ Th1 cells (middle), and CD4+CCR4+CCR8+ Th2 cells (right) (n=4–5/group).
d) Representative FACS plots of CD45+ cells.
e) Representative FACS plots of CD4+ (top), CD4+CCR5+CXCR3+ Th1 (middle), and CD4+CCR4+CCR8+ Th2 (bottom) cells.
Mice harvested six weeks after surgery. Data expressed as mean ± SD. Statistically significant differences represented by *P<0.05, **P<0.01, and ***P<0.001. Th1, T helper 1; Th2, T helper 2.
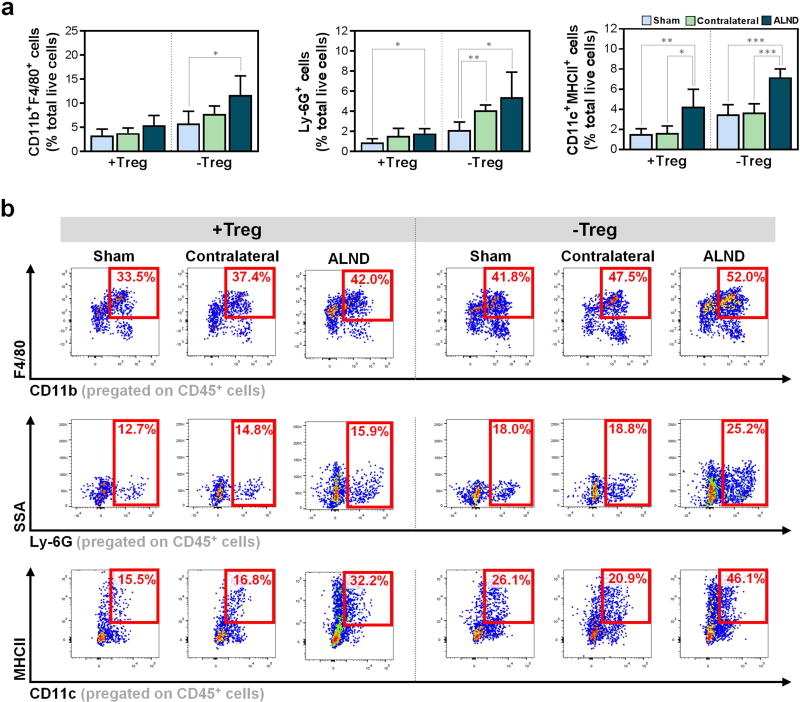
Consistent with our prior studies (Avraham et al., 2013, Zampell et al., 2012), we found that ALND results in a distinct local inflammatory infiltrate comprised largely of CD4+ cells (Fig. 3b–e). This response was amplified with Treg depletion, which resulted in increases in not only CD45+ leukocytes (Fig. 3b, d) and CD4+ cells, including CCR5+CXCR3+ T helper 1 (Th1) and CCR4+CCR8+ T helper 2 (Th2) cells (Fig. 3c, e), but also in CD11b+F4/80+ macrophages, Ly-6G+ neutrophils, and CD11c+MHCII+ DCs (Fig. 4a–b). Importantly, Treg depletion resulted in significant differences between sham-operated and ALND-treated mice among all studied immune cell types (Fig. 3b–c, 4b; P<0.05 for all), thus suggesting that Tregs regulate leukocyte infiltration to a much greater extent in ALND. Of note, similar to our initial findings, the Treg-depleted contralateral ALND-treated forelimbs had inflammation that was generally greater than Treg-depleted ipsilateral sham-operated forelimbs but less than the ipsilateral ALND-treated forelimbs (Fig. 3b–e, 4a–b).
Figure 4. Tregs regulate leukocyte infiltration following lymphatic injury.
a) FACS quantification of CD11b+F4/80+ macrophages (left), Ly-6G+ neutrophils (middle), and CD11c+MHCII+ DCs (right) (n=4–6/group).
b) Representative FACS plots of CD45+CD11b+F4/80+ macrophages (top), CD45+Ly-6G+ neutrophils (middle), and CD45+CD11c+MHCII+ DCs (bottom).
Data expressed as mean ± SD. Statistically significant differences represented by *P<0.05, **P<0.01, and ***P<0.001.
Tregs contribute to impaired T cell-mediated inflammation after lymphatic injury
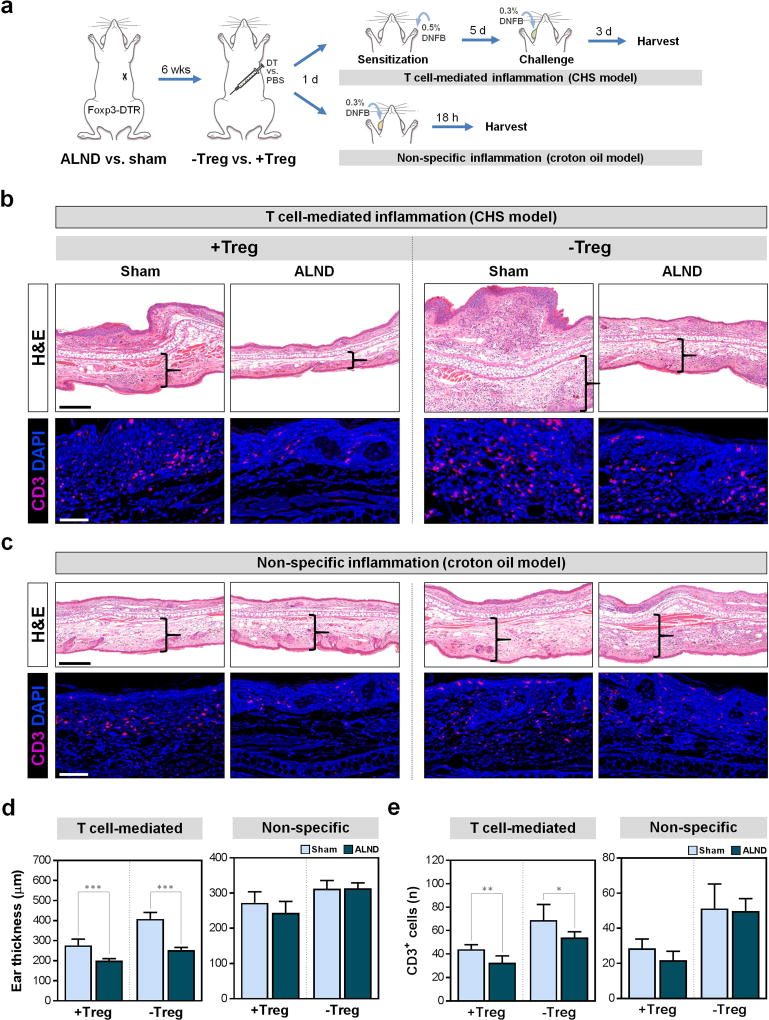
We next sought to determine how Treg accumulation after ALND affects immune responses in the ipsilateral limb by using contact hypersensitivity (CHS) experiments to elicit T cell-mediated inflammation (Fig. 5a, upper right) (Sugaya et al., 2012). We also analyzed the responses to a non-antigen specific irritant applied directly to a distant part of the body to test systemic immunity for comparison (Fig. 5a, lower right). In both experiments, animals underwent ALND or sham incision followed by DT or control injection six weeks later. In the CHS experiment, animals were sensitized with 1-fluoro-2,4-dinitrobenzene (DNFB) applied to the ipsilateral forepaw one day post-injection and then challenged with DNFB applied to the contralateral ear five days later. In the other experiment, croton oil was applied to the ear (without exposure to the ipsilateral forelimb) to induce non-specific inflammation.
Figure 5. Lymphatic injury decreases T cell-mediated immune responses.
a) Schematic depicting CHS (top right) and croton oil models (bottom right) of inflammation.
b) Representative H&E images with brackets indicating ear dermal thickness (upper; scale bar 250 µm) and immunofluorescent images localizing ear CD3+ cells (lower; scale bar 50 µm) in CHS model.
c) Representative H&E images with brackets indicating ear dermal thickness (upper; scale bar 250 µm) and immunofluorescent images localizing ear CD3+ cells (lower; scale bar 50 µm) in croton oil model.
d) Quantification of ear thickness (n=5/group, 3 HPF/mouse).
e) Quantification of ear CD3+ cells per 0.25 mm2 (n=5/group, 3–4 HPF/mouse).
Data expressed as mean ± SD. Statistically significant differences represented by *P<0.05, **P<0.01, and ***P<0.001. CHS, contact hypersensitivity; DNFB, 1-fluoro-2,4-dinitrobenzene; H&E, hematoxylin and eosin.
Compared to sensitization in the sham-operated forelimb, sensitization of mice in the forelimb ipsilateral to the area where ALND was performed resulted in decreased inflammatory responses as indicated by less ear edema (Fig. 5b, d; P=0.0008) and fewer T cells (Fig. 5b, e; P=0.0067). Consistent with our previous observation that skin harvested from the forelimb contralateral to the ALND site had more Tregs as compared with sham-operated controls, we found that mice sensitized in their contralateral forelimbs had mildly diminished CHS responses as compared with sham-operated controls (Fig. S2). Furthermore, although the contralateral forelimbs had better CHS responses than the ipsilateral ALND-treated forelimbs, the differences approached, but did not achieve, significance. Not surprisingly, Treg depletion resulted in markedly increased inflammatory responses in all groups (Fig. 5b, d, e and S2). However, the increase in T cell-mediated responses in mice sensitized in ipsilateral ALND-treated forelimb was not as great as that in sham-operated- or contralateral limb-sensitized mice, likely because these controls have an intact draining lymphatic system to facilitate a more robust immune response after Treg depletion. Taken together, these results suggest that Tregs in tissues ipsilateral to the area where ALND was performed inhibit acquisition of T cell-mediated immune responses and that these responses are diminished to a greater extent by the loss of draining lymph nodes.
To test the hypothesis that ALND results in local immunosuppressive changes (i.e., in the ipsilateral forelimb) rather than systemic defects in immune responses, we analyzed inflammation in response to croton oil application to the ear since this non-specific irritant does not require T cell sensitization. Consistent with our hypothesis, we found no differences in ear inflammation between the groups (Fig. 5c–e). This finding indicates that Treg infiltration in the limb ipsilateral to the ALND site combined with lymphatic injury due to ALND hinders the acquisition of T cell-mediated immune responses but does not cause systemic immune defects.
Tregs impair bacterial phagocytosis after lymphatic injury
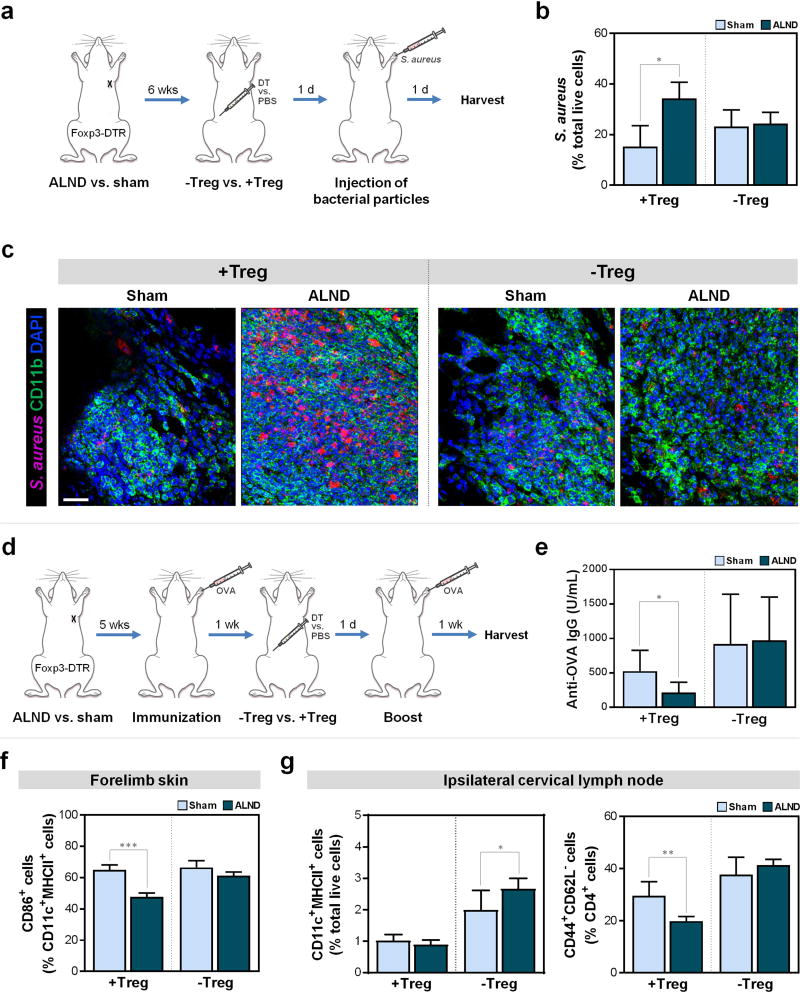
Given that lymphedema predisposes to local infections by bacteria commonly found in the skin flora, we next investigated the role of Tregs in regulating bacterial phagocytosis. To accomplish this, we injected heat-inactivated staphylococcal particles into the forelimbs of Treg-intact and Treg-depleted sham-operated and ALND-treated mice (Fig. 6a). We found that Treg-intact ALND-treated mice had more than double the number of bacterial particles remaining at the inoculation site compared to sham-operated controls, suggesting that ALND impairs bacterial clearance (Fig. 6b–c, S3; P=0.011). Interestingly, Treg depletion had no significant effect on bacterial clearance in sham-operated controls (Fig. 6b–c, S3; P=0.1846), but resulted in a significant decrease in residual particles in ALND-treated mice (to levels resembling that in sham-operated animals), suggesting that Treg infiltration after ALND contributes to defects in bacterial clearance after ALND.
Figure 6. Treg infiltration after lymphatic injury decreases phagocytosis and humoral responses by impairing DCs.
a) Schematic of the bacterial clearance model.
b) FACS quantification of S. aureus bacterial particles at the dermal injection site (n=4/group).
c) Representative immunofluorescent images co-localizing S. aureus and CD11b+ cells at dermal injection sites; scale bar 500 µm.
d) Schematic of the OVA immunization model.
e) Quantification of anti-OVA IgG in serum (n=8/group).
f) FACS quantification of CD11c+MHCII+CD86+ DCs in mouse forelimb skin harvested six weeks post-surgery and two days after DT or PBS injection (n=6/group).
g) FACS quantification of CD45+CD11c+MHCII+ DCs (left) and CD45+CD4+CD44+CD62L− cells (right) in mouse ipsilateral cervical lymph nodes harvested six weeks post-surgery and two days after DT or PBS injection (n=6/group).
Data expressed as mean ± SD. Statistically significant differences represented by *P<0.05, **P<0.01, and ***P<0.001.
Tregs regulate humoral responses after lymphatic injury
Prior studies have shown that mice with impaired lymphatics are unable to mount appropriate antibody responses (Thomas et al., 2012). To explore the role of Tregs in this phenomenon, we immunized Foxp3-DTR mice that underwent ALND or sham surgery with or without subsequent Treg depletion with OVA, a T cell-dependent antigen, in the ipsilateral forelimb (Fig. 6d).
Consistent with reports showing that patients are anergic in their lymphedematous extremities (Mehrara et al., 2011, Nookala et al., 2004), we found that Treg-intact ALND-treated mice had a more than twofold decrease in anti-OVA antibodies compared to shamoperated counterparts (Fig. 6e; P=0.032). Treg depletion led to a fourfold increase in ALND-treated mice (Fig. 6e; P=0.0089), but no significant change in sham-operated mice (P=0.2). Interestingly, Treg-depleted ALND-treated mice had titers comparable to that in sham-operated mice. These findings therefore indicate that Tregs contribute to the mitigation of antibody responses in lymphedema.
Tregs impair DC activation after lymphatic injury
Knowing that Tregs play a role in T cell-mediated inflammatory responses, bacterial phagocytosis, and humoral responses, we then sought to delineate the mechanism(s) by which Tregs mediate these effects. Previous studies have noted the contribution of Tregs to the maturation of APCs (Schmetterer et al., 2012), so we first examined the activation status of CD11c+MHCII+ DCs as indicated by CD86 in the ipsilateral forelimb skin of sham-operated and ALND-treated mice. We found that, although Treg-intact ALND-treated mice had more DCs (Fig. 4a, right; P=0.0093 for Treg-intact sham-operated vs. ALND-treated), these mice had a smaller percentage of activated DCs compared to sham-operated controls (Fig. 6f, S4a; P<0.0001). Importantly, Treg depletion led to an increase in the proportion of DCs that are activated in ALND-treated mice such that they more resembled that in sham-operated mice (Fig. 6f, S4a). Taken together, this suggests that Tregs impair DC activation in the setting of lymphatic injury. Consistent with this, Treg depletion also led to significantly more DCs in the ipsilateral cervical lymph nodes (the closest draining lymph node basin after ALND) of ALND-treated mice compared to sham-operated controls (Fig. 6g, left and S4; P=0.04), indicating that more forelimb skin DCs were migrating to lymph nodes, where they can interact with T cells (Bousso, 2008). Correspondingly, the percentage of activated CD44+CD62L− T cells in the lymph nodes increased to a greater extent in ALND-treated mice compared to control mice after Treg depletion (2.1 times greater compared to 1.2 times; Fig. 6g, right and S4).
DISCUSSION
The pathophysiology of lymphedema remains poorly understood, but studies of postsurgical lymphedema and lymphatic filariasis have suggested that Tregs play a significant role (Gousopoulos et al., 2016, Wammes et al., 2012, Zampell et al., 2012). Consistent with these reports, we have shown that lymphatic injury secondary to ALND results in increased infiltration and proliferation of Foxp3+ Tregs in the lymphedematous tissues of both humans and mice (Fig. 1a, c). Of note, Foxp3 may also be expressed by human effector T cells (Roncarolo and Gregori, 2008). However, given the transient nature of Foxp3 by non-Tregs, combined with our results in the mouse models and support from prior studies, it is more likely that the increase of Foxp3+ cells in the biopsies is reflective of an increase in Tregs.
Consistent with previous reports demonstrating abnormal lymphatic function in the seemingly normal contralateral limbs of patients with unilateral upper extremity lymphedema (Aldrich et al., 2012, Burnand et al., 2012, Pain et al., 2004), we found that ALND resulted in an increase in CD4+ cells in general and Tregs specifically in both the ipsilateral and contralateral forelimbs as compared to sham-operated forelimbs (Fig. 1b, c). In our study, however, we also noted that there were no significant differences in DCs between the contralateral and sham-operated forelimbs (Fig. 1d; P=0.8). Taken together with our finding that changes in Treg populations are not noted in the blood or spleens following ALND (Fig. 2b), we hypothesize that DCs are activated in the ipsilateral ALND-treated forelimb with subsequent activation of CD4+ cells in regional lymph nodes, which may include those in the contralateral forelimb as a result of collateral vessels that form to bypass to the area of injury (Suami et al., 2013). Alternatively, it is also possible that CD4+ cells activated in the ipsilateral lymph nodes may travel to the contralateral limb through the collateral vessels. Further studies are necessary to delineate the exact mechanisms underlying this altered immune cell trafficking. However, while it is necessary to recognize that there are changes in the contralateral limb, especially in the identification of the true effect of ALND, it is unlikely that these changes are significant enough to be clinically relevant.
Our findings of the importance of Tregs in the modulation of the inflammatory response in lymphedema are supported by a study showing that adoptive transfer of Tregs leads to decreased inflammation (Gousopoulos et al., 2016). Our results are additive, as we further demonstrate that, as the local proliferation of nTregs work to dampen chronic inflammation, they also cause local impairment of adaptive immunity. This is clinically relevant, as patients with lymphedema have an increased risk of cellulitis and secondary malignancies in the ipsilateral extremity (Ahmed et al., 2008).
Consistent with a study by Kuan et al. demonstrating that antigen-bearing DCs in skin are recruited to lymph nodes via collecting lymphatic vessels (Kuan et al., 2015), we showed that impaired lymphatic function secondary to ALND results in recruitment and activation of DCs (Fig. 1d). We found that Treg depletion significantly increases DC activation in tissues distal to the zone of lymphatic injury (Fig. 6f) with corresponding increases of DCs and activated T cells in the closest draining lymph nodes (Fig. 6g), thus suggesting that Tregs mediate immune responses by modulating APCs such as DCs. This hypothesis is supported by prior studies showing that Tregs indirectly inhibit DC maturation, down-modulate CD80/86 in DCs, and induce immunoregulatory enzyme indoleamine-2,3-desoxygenase (IDO) via CTLA-4 (Schmetterer et al., 2012). While the exact relative contribution of antigen transport versus impaired DC priming remains unresolved, it appears that the latter may be more important, as prior studies have shown that FLT4Chy/FLT4+ mice, which have abnormal lymphatic vasculature due to heterozygous mutations of the VEGFR3 gene, are capable of mounting normal adaptive immune responses despite impaired global transport (Platt et al., 2013).
Importantly, Treg depletion did not result in complete resolution of impaired immune responses. This was most clearly demonstrated by the CHS experiment, in which Treg depletion in ALND-treated mice resulted in inflammation that only approximated that of Treg-intact sham-operated mice. Treg depletion likely did not lead to an even greater immune response because the issues of lymphatic injury (thus hindering antigen transport, among other phenomena) and inflammation remain with their own resultant effect on immunosuppression. The greater improvement in humoral immunity following Treg depletion compared to cell-mediated immunity is expected, as B cells acquire antigen more efficiently than DCs, thus suggesting that only a small amount of OVA needs to transported to produce a strong IgG response (Pape et al., 2007). Nevertheless, our findings demonstrate that while Tregs play an important role, they do not comprise the sole mechanism in the orchestration of the tolerogenic response. Consistent with our prior findings of increased Th2 infiltration, immune dysfunction in lymphedema is likely multimodal and further studies are required to identify the means by which these important factors interact.
In conclusion, we have shown that Treg proliferation in tissues distal to the zone of lymphatic injury contributes to impaired innate and adaptive immunity. More importantly, we have shown that Treg depletion in the setting of lymphatic injury restores these critical responses. Selective targeting of this pathway may be a novel means to treat lymphedema-related local immunosuppression and its consequent morbidities.
MATERIALS AND METHODS
Human lymphedema tissues and staining
Matched tissue biopsies of the lymphedematous and contralateral upper extremities from six patients were obtained from the Stanford Center for Lymphatic and Venous Disorders to test the hypothesis that lymphedema is associated with increased Treg infiltration. Patients gave their written informed consent and this was approved by the Institutional Review Boards of both Stanford University and Memorial Sloan Kettering Cancer Center (MSKCC).
Animals
Experimental protocols were approved by the MSKCC Institutional Animal Care and Use Committee (IACUC), which operates under the Animal Welfare Act (AWA) and Health Research Extension Act of 1985. Adult female C57/BL6 mice and transgenic Foxp3-GFP reporter mice (10–12 weeks old) were purchased (Jackson Laboratories; Bar Harbor, Maine), while Foxp3-DTR mice were gifted by A. Rudensky (MSKCC). Further information regarding these mice can be found in supplemental text.
Axillary lymph node dissection (ALND)
The ALND model of lymphedema was utilized as previously described (Zampell et al., 2012). Further details can be found in supplemental text.
Histology
Histologic and immunohistochemical analysis was performed on tissues fixed in 4% ice-cold paraformaldehyde (Sigma-Aldrich), paraffin-embedded, and cut into 5 µm sections. Hematoxylin and eosin sections were prepared using standard techniques. Sections were scanned using a Mirax slide scanner (Carl Zeiss; Jena, Germany). Subcutaneous tissue thickness analysis was performed by two blinded reviewers and analyzg/ed using Pannoramic Viewer (3D HISTECH, Budapest, Hungary). Immunohistochemical staining was performed according to our published techniques (Avraham et al., 2010). Cell counts were performed on 3–4 high-powered fields per section by two blinded reviewers. Further details can be found in supplemental text.
Flow cytometry
Flow cytometry was performed on forelimb tissues 1 cm distal to axillary incisions using previously published techniques (Avraham et al., 2010). Further details can be found in supplemental text.
Contact hypersensitivity and non-specific inflammatory responses
The CHS model used to test T cell-mediated responses was performed with a modification of previously reported methods (Martin-Romero et al., 2000). In contrast, croton oil was used to assess non-specific inflammation. Details of both can be found in supplemental text.
Bacterial clearance
Staphylococcus aureus bacterial particles were into the ipsilateral forelimbs of mice to evaluate the role of Tregs in infectious complications of lymphedema. Details can be found in supplemental text.
Humoral responses
Patients have found to be anergic in their lymphedematous extremities, so we sought to evaluate antibody production in the mouse model using chicken ovalbumin (OVA). Details can be found in supplemental text.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, Inc.; San Diego, CA). Student’s T-test and ANOVA with post-hoc tests were used to compare differences between two or multiple groups, respectively. Descriptive analysis and graphical methods were used to analyze and summarize results. Data presented as mean ± standard deviation (SD), with P<0.05 considered significant.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Alexander Rudensky at the Memorial Sloan Kettering Cancer Center for his generous gift of the Foxp3-DTR mice. The authors are also grateful to Mesruh Turkekul, Sho Fujisawa, Yevgeniy Romin, Elvin Feng, and Evan Darling of the Molecular Cytology Core at Memorial Sloan Kettering Cancer Center for assistance with histology and tissue imaging (Core Grant P30 CA008748).
Funding sources: NIH R01 HL111130-01 and R21-CA194882 awarded to B.J.M. NIH T32 CA009501-27 grant to G.G.N.; NIH T32 CA9501-29 grant to C.L.L.; 2013 Plastic Surgery Foundation Pilot Research Grant 274165 awarded to S.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women's Health Study. J Clin Oncol. 2008;26(35):5689–96. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich MB, Guilliod R, Fife CE, Maus EA, Smith L, Rasmussen JC, et al. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomedical optics express. 2012;3(6):1256–65. doi: 10.1364/BOE.3.001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, et al. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010;177(6):3202–14. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27(3):1114–26. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JB, Charles JL, Streit TG, Roberts JM, Addiss DG, Lammie PJ. Reactivity to bacterial, fungal, and parasite antigens in patients with lymphedema and elephantiasis. Am J Trop Med Hyg. 2002;66(2):163–9. doi: 10.4269/ajtmh.2002.66.163. [DOI] [PubMed] [Google Scholar]
- Barry JM, Weber WP, Sacchini V. The evolving role of axillary lymph node dissection in the modern era of breast cancer management. Surgical oncology. 2012;21(2):143–5. doi: 10.1016/j.suronc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol. 2008;8(9):675–84. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- Burnand KM, Glass DM, Mortimer PS, Peters AM. Lymphatic dysfunction in the apparently clinically normal contralateral limbs of patients with unilateral lower limb swelling. Clinical nuclear medicine. 2012;37(1):9–13. doi: 10.1097/RLU.0b013e31823931f5. [DOI] [PubMed] [Google Scholar]
- Chen L, Hamrah P, Cursiefen C, Zhang Q, Pytowski B, Streilein JW, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10(8):813–5. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29(1):114–26. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Dohlman TH, Omoto M, Hua J, Stevenson W, Lee SM, Chauhan SK, et al. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 2015;99(4):678–86. doi: 10.1097/TP.0000000000000512. [DOI] [PubMed] [Google Scholar]
- El Saghir NS, Otrock ZK, Bizri AR, Uwaydah MM, Oghlakian GO. Erysipelas of the upper extremity following locoregional therapy for breast cancer. Breast. 2005;14(5):347–51. doi: 10.1016/j.breast.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Gousopoulos E, Proulx ST, Bachmann SB, Scholl J, Dionyssiou D, Demiri E, et al. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI insight. 2016;1(16):e89081. doi: 10.1172/jci.insight.89081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35(1):109–22. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8(2):191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nature immunology. 2005;6(12):1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol. 2015;194(11):5200–10. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199(1):15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- Mehrara BJ, Zampell JC, Suami H, Chang DW. Surgical management of lymphedema: past, present, and future. Lymphat Res Biol. 2011;9(3):159–67. doi: 10.1089/lrb.2011.0011. [DOI] [PubMed] [Google Scholar]
- Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infection and immunity. 2004;72(5):2598–604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain SJ, Barber RW, Ballinger JR, Solanki CK, Mortimer PS, Purushotham AD, et al. Local vascular access of radioprotein injected subcutaneously in healthy subjects and patients with breast cancer-related lymphedema. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2004;45(5):789–96. [PubMed] [Google Scholar]
- Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26(4):491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Platt AM, Rutkowski JM, Martel C, Kuan EL, Ivanov S, Swartz MA, et al. Normal dendritic cell mobilization to lymph nodes under conditions of severe lymphatic hypoplasia. J Immunol. 2013;190(9):4608–20. doi: 10.4049/jimmunol.1202600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? European journal of immunology. 2008;38(4):925–7. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, et al. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. American journal of physiology Heart and circulatory physiology. 2014;307(2):H165–72. doi: 10.1152/ajpheart.00244.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 2012;26(6):2253–76. doi: 10.1096/fj.11-193672. [DOI] [PubMed] [Google Scholar]
- Simon MS, Cody RL. Cellulitis after axillary lymph node dissection for carcinoma of the breast. The American journal of medicine. 1992;93(5):543–8. doi: 10.1016/0002-9343(92)90583-w. [DOI] [PubMed] [Google Scholar]
- Suami H, Yamashita S, Soto-Miranda MA, Chang DW. Lymphatic territories (lymphosomes) in a canine: an animal model for investigation of postoperative lymphatic alterations. PloS one. 2013;8(7):e69222. doi: 10.1371/journal.pone.0069222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya M, Kuwano Y, Suga H, Miyagaki T, Ohmatsu H, Kadono T, et al. Lymphatic dysfunction impairs antigen-specific immunization, but augments tissue swelling following contact with allergens. J Invest Dermatol. 2012;132(3 Pt 1):667–76. doi: 10.1038/jid.2011.349. [DOI] [PubMed] [Google Scholar]
- Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189(5):2181–90. doi: 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. The Journal of experimental medicine. 2003;198(2):249–58. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wammes LJ, Hamid F, Wiria AE, Wibowo H, Sartono E, Maizels RM, et al. Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLoS Negl Trop Dis. 2012;6(5):e1655. doi: 10.1371/journal.pntd.0001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS, Leitch AM, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Annals of surgical oncology. 2006;13(4):491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Yin N, Zhang N, Xu J, Shi Q, Ding Y, Bromberg JS. Targeting lymphangiogenesis after islet transplantation prolongs islet allograft survival. Transplantation. 2011;92(1):25–30. doi: 10.1097/TP.0b013e31821d2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PloS one. 2012;7(11):e49940. doi: 10.1371/journal.pone.0049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.