Abstract
Context
Risk profiling of oncology patients based on their symptom experience assists clinicians to provide more personalized symptom management interventions. Recent findings suggest that oncology patients with distinct symptom profiles can be identified using a variety of analytic methods.
Objectives
To evaluate the concordance between the number and types of subgroups of patients with distinct symptom profiles using latent class analysis (LCA) and K-modes analysis.
Methods
Using data on the occurrence of 25 symptoms from the Memorial Symptom Assessment Scale (MSAS), that 1329 patients completed prior to their next dose of chemotherapy (CTX), Cohen’s kappa coefficient was used to evaluate for concordance between the two analytic methods. For both LCA and K-modes, differences among the subgroups in demographic, clinical, and symptom characteristics, as well as quality of life outcomes were determined using parametric and nonparametric statistics.
Results
Using both analytic methods, four subgroups of patients with distinct symptom profiles were identified (i.e., All Low, Moderate Physical and Lower Psychological, Moderate Physical and Higher Psychological, All High). The percent agreement between the two methods was 75.32% which suggests a moderate level of agreement. In both analyses, patients in the All High group were significantly younger and had a higher comorbidity profile, worse MSAS subscale scores, and poorer QOL outcomes.
Conclusion
Both analytic methods can be used to identify subgroups of oncology patients with distinct symptom profiles. Additional research is needed to determine which analytic methods and which dimension of the symptom experience provides the most sensitive and specific risk profiles.
Keywords: symptom clusters, cancer, latent class analysis, machine learning, clustering, chemotherapy, k-modes analysis
INTRODUCTION
Both clinical experience and research findings suggest that oncology patients experience significant interindividual variability in their symptom experience.1,2 In the era of precision medicine,3 which focuses on the identification of patients who are at greater risk for chronic conditions like cancer, it is imperative that the optimal methods to risk profile patients based on their symptom burden is identified. In two reviews of the state of the science in symptom clusters research,4,5 it was noted that future studies need to focus on an evaluation of the concordance between the various analytic methods that can be used to identify patients who are at greatest risk for a higher symptom burden.
Recent findings from our group6–14 and others15–18 have identified subgroups of patients with distinct symptom experiences using approaches like hierarchical cluster analysis and latent class analysis (LCA). In the earliest of these studies,6,7,15,16 different clustering methods were used to create the patient subgroups. In the later studies,9–14,18 LCA was the preferred analytic approach. While across these thirteen studies, the number of subgroups ranged from two to five, a common finding across all of these studies was the identification of a group of patients who reported low levels of symptoms and a group of patients who reported high levels of symptoms. However, none of these studies determined whether the use of two different analytic approaches produces congruent results (e.g., the percentages of patients in the “all high” groups are equal and are the same patients).
As noted in a recent review,5 machine learning techniques may provide useful approaches to identify subgroups of patients with distinct symptom profiles. Some specific machine learning techniques that can be used for this purpose include: K-means,19 K-modes,20,21 spectral clustering,22 birch,23 or agglomerative hierarchical clustering (AHC).24,25 For binary variables (e.g., symptom occurrence), K-means and K-modes are two centroid based algorithms that calculate the distance between each pair of data points using Euclidean distance or a simple dissimilarity measure (e.g., Hamming distance), respectively. The clusters derived from K-means and K-modes analyses are described by the “centroid”, which is the multidimensional mean and mode, respectively, of the samples inside them.19,21 Spectral clustering is a graph distance based algorithm that performs a dimensionality reduction before clustering the lower-dimension dataset in a similar fashion to K-means. It is used when the clusters are not linearly separated in the original space, providing better results than algorithms such as K-means (which tends to find spherical clusters).26 Birch is a hierarchical clustering algorithm that can provide an advantage in datasets that are non-uniformly distributed and every data point is not equally important. It concentrates on densely occupied partitions and follows a hierarchical order of analysis that focuses on calculating and updating measurements that capture the natural closeness of data. Therefore, it is more robust to “noise” (i.e., data points that are not part of the underlying pattern).23 Finally, AHC is a decision tree, bottom-up clustering method that starts with every single data point in a single cluster. In each successive iteration, it agglomerates (merges) the closest pair of clusters by satisfying a similarity criterion, until all of the data are in one cluster. A matrix tree plot visually demonstrates the hierarchy within the final cluster, where each merger is represented by a binary tree. AHC can be both informative for data display and helpful for the discovery of smaller clusters.24
No studies were identified that evaluated for congruence between two methods of classifying oncology patients based on their distinct experiences with common symptoms associated with cancer treatment. Based on how well the machine learning methods described above performed during our initial analyses,27 for this paper, K-modes was selected as the method to compare with LCA. The purpose of this study, in a sample of patients (n=1329) who were undergoing chemotherapy (CTX) for breast, lung, gastrointestinal (GI), or gynecological (GYN) cancers was to evaluate the concordance between the number and types of subgroups of patients with distinct symptom experiences that were identified using LCA and K-modes analyses. We hypothesized that the number and types of subgroups would be similar using these two analytic methods.
METHODS
Patients and Settings
This study is part of a longitudinal study of the symptom experience of oncology outpatients receiving CTX. The methods for this study are described in detail elsewhere.13,28,29 According to the study’s eligibility criteria: patients were ≥18 years of age; had a diagnosis of breast, GI, GYN, or lung cancer; had received CTX within the preceding four weeks; were scheduled to receive at least two additional cycles of CTX; were able to read, write, and understand English; and gave written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs.
Instruments
A demographic questionnaire obtained information on age, gender, ethnicity, marital status, living arrangements, education, employment status, and income. The Karnofsky Performance Status (KPS) scale30 was used to evaluate patients’ functional status. The Self-administered Comorbidity Questionnaire (SCQ)31 evaluated the occurrence, treatment, and functional impact of thirteen common comorbid conditions (e.g., diabetes, arthritis).
A modified version of the Memorial Symptom Assessment Scale (MSAS) was used to evaluate the occurrence, severity, frequency, and distress of 38 symptoms commonly associated with cancer and its treatment. In this study, six symptoms were added to the original list of 32 MSAS symptoms (i.e., hot flashes, chest tightness, difficulty breathing, abdominal cramps, increased appetite, weight gain). The MSAS is a self-report questionnaire designed to measure the multidimensional experience of symptoms. Patients were asked to indicate whether or not they had experienced each symptom in the past week (i.e., symptom occurrence). If they had experienced the symptom, they were asked to rate its frequency of occurrence, severity, and distress. The reliability and validity of the MSAS is well established in oncology patients.32,33
Three subscale scores (i.e., physical [MSAS-PHYS], psychological [MSAS-PSYCH], global distress index [MSAS-GDI]) were calculated. The MSAS-PHYS is the average of the frequency, severity, and distress ratings for twelve physical symptoms (i.e., lack of energy, feeling drowsy, pain, nausea, vomiting, change in the way food tastes, lack of appetite, dry mouth, constipation, feeling bloated, dizziness, and weight loss). The MSAS-PSYCH is the average of the frequency, severity, and distress ratings for six psychological symptoms (i.e., worrying, feeling sad, feeling nervous, feeling irritable, difficulty in sleeping, difficulty concentrating). The MSAS-GDI is the average of the distress ratings for six physical symptoms (i.e., lack of energy, feeling drowsy, pain, lack of appetite, dry mouth, constipation) and the frequency ratings for four psychological symptoms (i.e., worrying, feeling sad, feeling nervous, feeling irritable).
Quality of life (QOL) was evaluated using disease-specific (i.e., Quality of Life Scale-Patient Version (QOL-PV))34–36 and generic (i.e., Medical Outcomes Study-Short Form-12 (SF-12))37 measures. The QOL-PV is a 41-item instrument that measures four dimensions of QOL (i.e., physical, psychological, social, and spiritual well-being) in oncology patients, as well as a total QOL score. Each item is rated on a 0 to 10 numeric rating scale (NRS) with higher scores indicating a better QOL. The QOL-PV has established validity and reliability.36,38–40
The SF-12 consists of 12 questions that evaluate physical, mental, and overall health status. Individual items on the SF-12 are evaluated. In addition, the instrument is scored into physical component summary (PCS) and mental component summary (MCS) scores. These scores can range from 0 to 100. Higher PCS and MCS scores indicate a better QOL. The SF-12 has well established validity and reliability.37
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Board at each of the study sites. Written informed consent was obtained from all patients. For this analysis, symptom occurrence data from the enrollment assessment, that asked patients to report on their symptom experience for the week prior to the administration of the next cycle of CTX, were analysed (i.e., recovery from previous CTX cycle).
Data Analyses
Symptom Occurrence Data
In order to have a sufficient number of patients who endorsed each symptom, the LCA and K-modes analyses were done with the 25 symptoms that occurred in ≥30% of the patients (i.e. difficulty concentrating, pain, lack of energy, cough, feeling nervous, hot flashes, dry mouth, nausea, numbness or tingling in hands or feet, feeling drowsy, difficulty sleeping, feeling bloated, diarrhea, feeling sad, sweats, problems with sexual interest or activity, worrying, lack of appetite, dizziness, feeling irritable, hair loss, constipation, change in the way food tastes, I do not look like myself, changes in skin).
Latent Class Analysis
LCA identifies latent classes based on an observed response pattern.41,42 It is a statistical method for finding subtypes of related cases (i.e., latent classes) from multivariate categorical data. The LCA was performed using Mplus™ Version 7.43 Estimation was carried out with robust Maximum-Likelihood (MLR) and the Expectation-Maximization (EM) algorithm.44 The optimal number of latent classes for this LCA was selected based on the Bayesian Information Criterion (BIC), the Vuong, Lo, Mendel, and Rubin (VLMR) likelihood ratio test, and entropy. Theoretically, the best fitting LCA model has the lowest BIC. Nevertheless, the BIC can be supplemented by an evaluation of the VLMR45 which tests whether a model with K classes fits the data better than a model with one fewer class (the K-1 class model). When this VLMR is significant, the K-class model is considered to be a better fit for the data. When models are evaluated sequentially, with each new model having one more class than the previous model, if a model is identified for which the VLMR is not significant, then too many classes were extracted and the K-1 class model is considered to fit the data better than the current K-class model. Furthermore, well-fitting models produce entropy values of ≥0.80.46 In addition, the optimal fitting model should “make sense” conceptually and its classes should differ as might be expected on variables not used in the generation of the model.
K-modes analysis
K-modes is a centroid method that is optimized for use with categorical variables.21 It defines clusters based on the number of matching categories between data points and not on their Euclidean distance (a common similarity index in agglomerative clustering methods). Although its performance is comparable to K-means,27 the K-modes distance measurement approach is theoretically a more appropriate approach to use to cluster the categorical variable of symptom occurrence.21,47 The K-modes analysis was implemented with PyCharm Professional Edition 4.5 and the Scikit-Learn library.48
The optimal number of clusters for the K-modes analysis was assessed using the Silhouette Coefficient (SC).49 The SC represents how well each case (i.e., patient) lies within its cluster and how appropriate each case’s assignment is inside a specific cluster. The average SC, called the Silhouette Index (SI), allows one to evaluate the overall quality of the separation between the clusters. The SC is calculated using its intra-cluster distance and its nearest-cluster distance.27 The SC is bounded between -1 for inappropriate clustering and +1 for highly compact clustering. A SC around zero indicates that a case is assigned inside overlapping clusters. In general, the average SI is high when clusters are dense and well separated.
Evaluation of Congruence
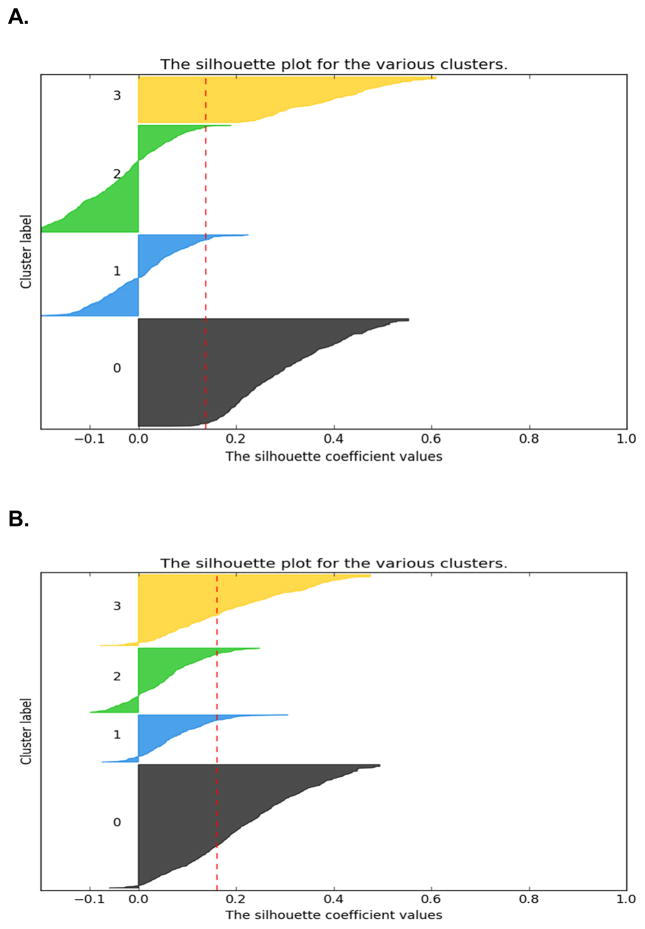
In order to evaluate the congruence between the LCA and K-modes solutions (i.e., number of subgroups identified), we compared the solutions using SCI diagrams (see Figures 1A and 1B, respectively).49 When the SC for a case is >0, its assignment to this cluster is considered appropriate. When the SC for a case is ≤0, this case may have equal similarities with cases in another, overlapping cluster and its assignment inside a specific cluster may not be an appropriate fit. In addition, Cohen’s kappa coefficient was used to evaluate the agreement between the two analytic approaches.
Figure 1.
Figure 1A. Silhouette coefficient diagram for the 4-class solution using latent class analysis. The sizes of the clusters in the diagram are proportional to their size inside the total sample of patients (n=1329). The labels represent the following clslusters: 0 (All Low (n=419, 31.5%)), 1 (Moderate Physical & Lower Psychological (n=316, 23.8%)), 2 (Moderate Physical & Higher Psychological (n=416, 31.3%)) and 3 (All High (n=178, 13.4%).
Figure 1B. Silhouette coefficient diagram for the 4-cluster solution using the K-modes analysis. The sizes of the clusters in the diagram are proportional to their size inside the total sample of patients (n=1329). The labels represent the following clusters: 0 (All Low (n=536, 40.3%)), 1 (Moderate Physical & Lower Psychological (n=205, 15.4%)), 2 (Moderate Physical & Higher Psychological (n=280, 21.1%)), and 3 (All High (n=308, 23.2%)).
Differences in Demographic, Clinical, and Symptom Characteristics and QOL Outcomes
Descriptive statistics and frequency distributions were calculated for demographic and clinical characteristics using SPSS version 23 (IBM, Armonk, NY). For each analytic approach, differences in demographic and clinical characteristics and QOL outcomes, among the groups, were evaluated using analyses of variance, Kruskal-Wallis, and Chi Square analyses. Post hoc contrasts were calculated using the Bonferroni corrected alpha of 0.008 (0.05/6 pairwise comparisons).
RESULTS
Number of Subgroups Identified Using LCA and K-modes Approaches
For the LCA, the fit indices for the candidate models are shown in Table 1. The four class solution was selected because its BIC was lower than for the 3- and 5-class solutions. In addition, the VLMR indicated that a 4-class solution was better than a 3-class solution. However, the VLMR for the 5-class solution was not better than the 4-class solution indicating that too many classes were extracted.
Table 1.
Latent Class Solutions and Fit Indices for Two- Through Five-Class Solutions
| Model | LL | AIC | BIC | Entropy | VLMR |
|---|---|---|---|---|---|
| 3 Class | −10998.00 | 22150.00 | 22505.64 | .85 | 413.57* |
| 4 Classa | −10835.22 | 21876.44 | 22352.17 | .82 | 325.55* |
| 5 Class | −10765.09 | 21788.17 | 22383.99 | .81 | 140.27NS |
The four-class solution was selected because the BIC for that solution was lower than the BIC for both the 3- and 5-class solutions. In addition, the VLMR for the 4-class solution indicates that it fits better than the 3-class solution and the VLMR for the 5-class solution does not fit better than the 4-class solution.
p < .001
Abbreviations: AIC = Akaike’s Information Criterion, BIC = Bayesian Information Criterion, LL = log-likelihood, NS = not significant, VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test for the K vs. K-1 model
Using K-modes, while the average SI for the 3-class solution was slightly larger than the average SI for the 4-class solution (Table 2), given this trivial difference and in order to compare the differences in demographic, clinical, and symptom characteristics and QOL outcomes between the two methods, we used the 4-class solution from the K-modes analysis.
Table 2.
K-modes Solutions and Silhouette Indices for Three- Through Five-Class Solutions
| Model | Silhouette Index |
|---|---|
| 3 Clustera | 0.159 |
| 4 Cluster | 0.156 |
| 5 Cluster | 0.129 |
Based on the Silhouette Index, the three-cluster solution performed higher than both the 4- and 5-cluster solutions.
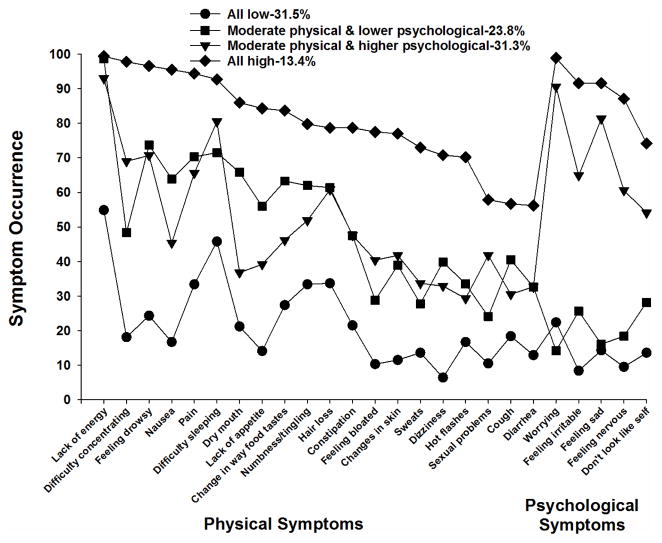
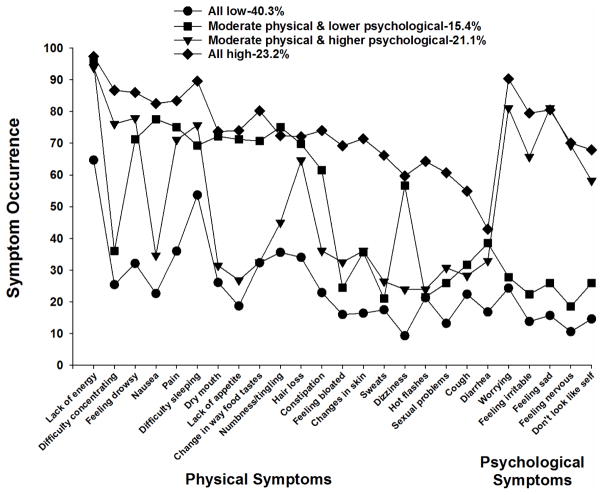
As shown in Figures 2 and 3, for the LCA and K-modes analyses, respectively, the four subgroups were named based on the probability of occurrence of the 25 MSAS symptoms that occurred in ≥30% of the patients. The All High and All Low groups included patients who reported relatively high and low occurrence rates for most of the 25 MSAS symptoms, respectively. The Moderate Physical and Higher Psychological and Moderate Physical and Lower Psychological groups included patients who reported relatively moderate occurrence rates for the majority of the physical symptoms and relatively higher or lower occurrence rates, respectively, for the five psychological symptoms (i.e., worrying, feeling irritable, feeling sad, feeling nervous, I don’t look like myself).
Figure 2.
Symptom occurrence for each of the subgroups identified using latent class analysis for the 25 symptoms on the Memorial Symptom Assessment Scale that occurred in ≥30% of the total sample (n=1329) at Time 1 (i.e., prior to next dose of chemotherapy).
Figure 3.
Symptom occurrence for each of the subgroups identified using K-modes analysis for the 25 symptoms on the Memorial Symptom Assessment Scale that occurred in ≥30% of the total sample (n=1329) at Time 1 (i.e., prior to next dose of chemotherapy).
The SC diagrams for all of the patient cases within each of the 4 clusters for the LCA and K-modes analyses (Figures 1A and 1B) showed that their inefficient assignments were mostly within two specific groups (i.e. Moderate Physical and Higher Psychological, Moderate Physical and Lower Psychological). Both well (SC >0) and inappropriately (SC ≤0) clustered cases were included within these clusters. As illustrated in the SC diagrams, K-modes assigned a larger proportion of cases to these two groups (SC >0). Of note, the two other groups (All Low, All High) were well defined and separated using both the LCA and K-modes approaches (SC >0.4).
Pairwise Agreement Between the LCA and K-modes Approaches
As shown in Table 3, the observed agreement among the four groups was 75.32% and the expected agreement was 26.08%. The two analyses separated patients into 4 distinct groups with substantial agreement beyond chance (range 0.6–0.7) as measured by the Cohen’s coefficient (kappa=0.666).(50) The biggest disagreements between the LCA and K-modes approaches were between: a) the Moderate Physical and Lower Psychological (LCA) and All Low (K-modes) and b) the Moderate Physical and Higher Psychological (LCA) and All High (K-modes) groups, with 92 and 101 divergent classifications, respectively.
Table 3.
Pairwise Agreement Among the Patient Groups Using Latent Class Analysis and K-modes Analysis
| Pairwise agreement among the patient groups | All Lowb | Moderate Physical & Lower Psychological | Moderate Physical & Higher Psychological | All High | Total n (%) |
|---|---|---|---|---|---|
| nc (%d) | nc (%d) | nc (%d) | nc (%d) | ||
| All Lowa | 406 (30.6) | 4 (0.3) | 9 (0.7) | 0 (0.0) | 419 (31.5) |
| Moderate Physical & Lower Psychological | 92 (6.9) | 171 (12.9) | 23 (1.7) | 30 (2.3) | 316 (23.8) |
| Moderate Physical & Higher Psychological | 38 (2.9) | 30 (2.3) | 247 (18.6) | 101 (7.6) | 416 (31.3) |
| All High | 0 (0.0) | 0 (0.0) | 1 (0.1) | 177 (13.3) | 178 (13.4) |
| Total | 536 (40.3) | 205 (15.4) | 280 (21.1) | 308 (23.2) | 1,329 (100.0) |
| Cohen’s kappa coefficient | |||||
| Agreement | Expected Agreement | Kappa | Standard Error | Z | p-value |
| 75.32% | 26.08% | 0.666 | 0.016 | 42.64 | <0.001 |
For LCA – All Low (n=419, 31.5%), Moderate Physical and Lower Psychological (n=316, 23.8%), Moderate Physical and Higher Psychological (n=416, 31.3%), and All High (n=178, 13.4%).
For K-modes analysis – All Low (n=536, 40.3%), Moderate Physical and Lower Psychological (n=205, 15.4%), Moderate Physical and Higher Psychological (n=280, 21.1%), and All High (n=308, 23.2%).
Number of the patients who were included in both classes
Percentage of patients from the total sample of 1329 patients
Group Characteristics Identified with LCA and K-modes Approaches
The All Low group consisted of 31.5% (n=419) of the sample using LCA and 40.3% (n=536) using K-modes. The probability of occurrence of the MSAS symptoms for this group ranged from 0.064 to 0.549 for LCA and 0.093 to 0.647 for K-modes.
The second largest group identified using LCA was named Moderate Physical and Higher Psychological and consisted of 31.3% (n=416) of the sample. Using K-modes, this group consisted of 21.1% (n=280) of the patients. The occurrence rates for the majority of the physical symptoms ranged from 0.293 to 0.930 for LCA and from 0.236 to 0.939 for K-modes. For the psychological symptoms, the occurrence rates were relatively high. They ranged from 0.541 to 0.906 for LCA and from 0.582 to 0.811 for K-modes.
The third largest group identified using LCA (23.8%, n=316) was named the Moderate Physical and Lower Psychological group. Using K-modes, this group was the smallest one identified (15.4%, n=205). The probability of occurrence for the physical symptoms ranged from 0.241 to 0.987 for LCA and from 0.210 to 0.956 for K-modes. For the psychological symptoms, the range was from 0.142 to 0.282 for LCA and from 0.185 to 0.278 for K-modes.
The All High group was the smallest one for LCA (13.4%, n=178) and the second largest for the K-modes analysis (23.2%, n=308). The probability of occurrence of the MSAS symptoms for this group ranged from 0.562 to 0.994 for LCA and from 0.429 to 0.974 for K-modes.
Differences in Patient Characteristics Among the Groups Identified with LCA and K-modes Approaches
Tables 4 and 5 summarize the differences in demographic and clinical characteristics among the four groups of patients identified using LCA and K-modes, respectively. For both analyses, compared to the “All Low” group, patients in the “Moderate Physical and Higher Psychological” and the “All High” groups were significantly younger, had a lower KPS score, had a higher SCQ score, were more likely to have breast cancer, and were more likely to report depression and back pain. In addition, for both analyses, compared to the “Moderate Physical and Lower Psychological” group and the “Moderate Physical and Higher Psychological” group, patients in the “All High” group had a lower KPS score and a higher SCQ score.
Table 4.
Differences in Demographic and Clinical Characteristics Among the Patient Subgroups Using Latent Class Analysis
| Characteristic | All Low n=419 (31.5%) (0) | Moderate Physical & Lower Psychological n=316 (23.8%) (1) | Moderate Physical & Higher Psychological n=416 (31.3%) (2) | All High n=178 (13.4%) (3) | Statistics |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| Age (years) | 60.0 (11.2) | 57.9 (12.3) | 55.3 (12.9) | 54.4 (12.0) | F(3,1325) = 14.66, p <.001 |
| 0 and 1 > 2 and 3 | |||||
|
| |||||
| Education (years) | 16.3 (3.1) | 16.2 (2.9) | 16.4 (3.1) | 15.5 (2.9) | F(3,1298) = 4.28, p = .005 |
| 0 and 2 > 3 | |||||
|
| |||||
| Body mass index (kg/m2) | 26.2 (5.5) | 26.2 (5.9) | 26.0 (5.4) | 26.9 (6.4) | F(3,1307) = 1.08, p = .358 |
|
| |||||
| Karnofsky Performance Status score | 85.8 (11.1) | 79.4 (12.2) | 78.0 (11.9) | 72.3 (11.2) | F(3,1271) = 62.75,Pp<.001 |
| 0 > 1, 2, and 3 | |||||
| 1 and 2 > 3 | |||||
|
| |||||
| Number of comorbidities | 2.1 (1.3) | 2.5 (1.4) | 2.5 (1.4) | 3.0 (1.6) | F(3,1325) = 19.32, p<.001 |
| 0 < 1, 2, and 3 | |||||
| 1 and 2 < 3 | |||||
|
| |||||
| SCQ score | 4.5 (2.6) | 5.6 (3.2) | 5.7 (3.1) | 7.1 (4.0) | F(3,1325) = 29.60, p<.001 |
| 0 < 1, 2, and 3 | |||||
| 1 and 2 < 3 | |||||
|
| |||||
| AUDIT score | 3.1 (2.4) | 2.5 (1.9) | 3.1 (2.7) | 3.1 (3.1) | F(3,856) = 2.61, p = .05 |
|
| |||||
| Time since cancer diagnosis (years) | 1.8 (3.1) | 2.1 (4.2) | 2.1 (4.4) | 1.9 (3.7) | KW = 2.64, p = .478 |
|
| |||||
| Time since cancer diagnosis (median) | 0.42 | 0.41 | 0.44 | 0.45 | |
|
| |||||
| Number of prior cancer treatments | 1.5 (1.5) | 1.5 (1.5) | 1.7 (1.5) | 1.8 (1.5) | F(3,1312) = 1.25, p = .290 |
|
| |||||
| Number of metastatic sites including lymph node involvement | 1.3 (1.2) | 1.3 (1.3) | 1.3 (1.3) | 1.0 (1.1) | F(3,1325) = 2.31, p = .075 |
|
| |||||
| Number of metastatic sites excluding lymph node involvement | 0.8 (1.0) | 0.8 (1.1) | 0.8 (1.1) | 0.6 (0.9) | F(3,1325) = 1.85, p = .136 |
|
| |||||
| % (n) | % (n) | % (n) | % (n) | ||
|
| |||||
| Gender | X2 = 48.63, p<.001 | ||||
| Female+ | 67.8 (284) | 76.3 (241) | 83.7 (348) | 89.9 (160) | 0 < 2 |
| Male | 32.2 (135) | 23.7 (75) | 16.1 (67) | 10.1 (18) | 1 < 3 |
| Transgender* | 0.0 (0) | 0.0 (0) | 0.2 (1) | 0.0 (0) | |
|
| |||||
| Ethnicity | X2 = 22.96, p = .006 | ||||
| White | 70.6 (291) | 66.6 (207) | 75.1 (310) | 61.9 (109) | 2 < 3 |
| Black | 13.1 (54) | 14.8 (46) | 8.0 (33) | 15.9 (28) | NS |
| Asian or Pacific Islander | 7.5 (31) | 9.3 (29) | 5.6 (23) | 6.8 (12) | 1 and 3 < 2 |
| Hispanic Mixed or Other | 8.7 (36) | 9.3 (29) | 11.4 (47) | 15.3 (27) | NS |
|
| |||||
| Married or partnered (% yes) | 67.7 (279) | 64.3 (202) | 64.0 (261) | 57.4 (101) | X2 = 5.78, p = .123 |
|
| |||||
| Lives alone (% yes) | 20.9 (86) | 20.4 (64) | 21.0 (86) | 26.6 (47) | X2 = 3.03, p = .387 |
|
| |||||
| Child care responsibilities (% yes) | 18.5 (76) | 21.3 (65) | 22.2 (91) | 31.0 (54) | X2 = 11.32, p = .010 |
| 0 < 3 | |||||
|
| |||||
| Care of adult responsibilities (% yes) | 5.2 (20) | 8.8 (25) | 9.6 (36) | 8.9 (14) | X2 = 5.97, p = .113 |
|
| |||||
| Currently employed (% yes) | 40.0 (165) | 34.4 (108) | 35.9 (148) | 23.3 (41) | X2 = 15.23, p = .002 |
| 0 and 2 > 3 | |||||
|
| |||||
| Income | |||||
| < $30,000+ | 14.4 (52) | 18.3 (52) | 15.9 (61) | 33.1 (54) | KW, p<.001 |
| $30,000 to <$70,000 | 19.7 (71) | 21.5 (61) | 23.2 (89) | 19.0 (31) | 0, 1, and 2 < 3 |
| $70,000 to < $100,000 | 18.9 (68) | 16.2 (46) | 15.4 (59) | 16.0 (26) | |
| ≥ $100,000 | 46.9 (169) | 44.0 (125) | 45.4 (174) | 31.9 (52) | |
|
| |||||
| Specific comorbidities (% yes) | |||||
|
| |||||
| Heart disease | 5.5 (23) | 7.6 (24) | 4.1 (17) | 7.3 (13) | X2 = 4.91, p = .178 |
|
| |||||
| High blood pressure | 30.5 (128) | 33.5 (106) | 26.9 (112) | 33.1 (59) | X2 = 4.48, p = .214 |
|
| |||||
| Lung disease | 9.8 (41) | 12.7 (40) | 10.6 (44) | 14.0 (25) | X2 = 3.10, p = .377 |
|
| |||||
| Diabetes | 9.5 (40) | 11.4 (36) | 6.7 (28) | 9.6 (17) | X2 = 4.97, p = .174 |
|
| |||||
| Ulcer or stomach disease | 2.9 (12) | 4.4 (14) | 5.3 (22) | 9.0 (16) | X2 = 10.55, p = .014 |
| 0 < 3 | |||||
|
| |||||
| Kidney disease | 0.7 (3) | 1.6 (5) | 2.2 (9) | 1.1 (2) | X2 = 3.27, p = .351 |
|
| |||||
| Liver disease | 7.2 (30) | 6.0 (19) | 5.8 (24) | 7.3 (13) | X2 = 0.98, p = .806 |
|
| |||||
| Anemia or blood disease | 7.2 (30) | 13.6 (43) | 14.4 (60) | 17.4 (31) | X2 = 16.77, p = .001 |
| 0 < 1, 2, and 3 | |||||
|
| |||||
| Depression | 7.2 (30) | 11.7 (37) | 28.4 (118) | 39.3 (70) | X2 = 119.64, p<.001 |
| 0 and 1 < 2 and 3 | |||||
|
| |||||
| Osteoarthritis | 10.5 (44) | 11.4 (36) | 13.0 (54) | 16.3 (29) | X2 = 4.32, p = .229 |
|
| |||||
| Back pain | 15.3 (64) | 26.6 (84) | 27.6 (115) | 44.9 (80) | X2 = 59.15, p<.001 |
| 0 < 1, 2, and 3 | |||||
| 1 and 2 < 3 | |||||
|
| |||||
| Rheumatoid arthritis | 2.6 (11) | 4.7 (15) | 2.6 (11) | 3.4 (6) | X2 = 3.28, p = .351 |
|
| |||||
| Exercise on a regular basis (% yes) | 73.2 (303) | 68.8 (212) | 74.9 (305) | 59.6 (102) | X2 = 15.41, p = .002 |
| 0 and 2 > 3 | |||||
|
| |||||
| Smoking, current or history of (% yes) | 34.2 (142) | 37.1 (114) | 36.3 (149) | 32.6 (57) | X2 = 1.40, p = .706 |
|
| |||||
| Cancer diagnosis | X2 = 34.25, p<.001 | ||||
| Breast | 32.9 (138) | 39.9 (126) | 45.7 (190) | 44.9 (80) | 0 < 2 and 3 |
| Gastrointestinal | 37.2 (156) | 33.5 (106) | 23.8 (99) | 25.8 (46) | 0 > 2 and 3; 1 > 2 |
| Gynecological | 16.7 (70) | 13.3 (42) | 21.2 (88) | 18.5 (33) | 1 < 2 |
| Lung | 13.1 (55) | 13.3 (42) | 9.4 (39) | 10.7 (19) | NS |
|
| |||||
| Type of prior cancer treatment | KW, p = .063 | ||||
| No prior treatment | 26.5 (108) | 29.0 (89) | 22.6 (91) | 19.9 (35) | |
| Only surgery, CTX, or RT | 41.0 (167) | 41.7 (128) | 42.5 (171) | 43.8 (77) | |
| Surgery & CTX, or Surgery & RT, or CTX & RT | 20.6 (84) | 17.3 (53) | 21.9 (88) | 18.2 (32) | |
| Surgery & CTX & RT | 11.8 (48) | 12.1 (37) | 12.9 (52) | 18.2 (31) | |
Abbreviations: AUDIT = Alcohol Use Disorders Identification Test, CTX = chemotherapy, kg = kilograms, KW = Kruskal Wallis; m2 = meter squared, NS = not significant, RT = radiation therapy, SCQ = Self-Administered Comorbidity Questionnaire, SD = standard deviation
Chi Square analysis and post hoc contrasts done without the transgender patient include in the analyses
Reference group for the post hoc comparisons
Table 5.
Differences in Demographic and Clinical Characteristics Among the Patient Subgroups Using K-Modes Analysis
| Characteristic | All Low n=536 (40.3%) (0) | Moderate Physical & Lower Psychological n=205 (15.4%) (1) | Moderate Physical & Higher Psychological n=280 (21.1%) (2) | All High n=308 (23.2%) (3) | Statistics |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| Age (years) | 59.6 (11.7) | 58.1 (12.1) | 55.3 (13.1) | 54.4 (12.1) | F(3,1325) = 15.10, p<.001 |
| 0 > 2 and 3 | |||||
| 1 > 3 | |||||
|
| |||||
| Education (years) | 16.3 (3.1) | 16.0 (2.9) | 16.7 (3.0) | 15.6 (2.9) | F(3,1298) = 6.44, p<.001 |
| 0 > 2 and 3 | |||||
|
| |||||
| Body mass index (kg/m2) | 26.2 (5.5) | 26.3 (5.8) | 25.8 (5.2) | 26.7 (6.3) | F(3,1307) = 1.26, p = .287 |
|
| |||||
| Karnofsky Performance Status score | 85.0 (11.3) | 77.8 (12.2) | 78.6 (11.9) | 74.2 (11.7) | F(3,1271) = 59.38, p<.001 |
| 0 >1, 2, and 3 | |||||
| 1 and 2 > 3 | |||||
|
| |||||
| Number of comorbidities | 2.1 (1.3) | 2.6 (1.4) | 2.4 (1.4) | 2.9 (1.6) | F(3,1325) = 20.27, p<.001 |
| 0 <1 and 3 | |||||
| 2 < 3 | |||||
|
| |||||
| SCQ score | 4.7 (2.7) | 5.9 (3.1) | 5.5 (3.0) | 6.6 (3.8) | F(3,1325) = 28.30, p<.001 |
| 0 <1, 2, and 3 | |||||
| 1 and 2 < 3 | |||||
|
| |||||
| AUDIT score | 3.1 (2.2) | 2.3 (1.9) | 3.1 (2.7) | 3.1 (2.9) | F(3,856) = 3.92, p = .009 |
| 1 < 0, 2 and 3 | |||||
|
| |||||
| Time since cancer diagnosis (years) | 2.0 (3.8) | 2.2 (4.0) | 2.1 (4.3) | 1.7 (3.6) | KW, p = .831 |
|
| |||||
| Time since cancer diagnosis (median) | 0.42 | 0.40 | 0.45 | 0.42 | |
|
| |||||
| Number of prior cancer treatments | 1.6 (1.5) | 1.6 (1.5) | 1.7 (1.5) | 1.6 (1.5) | F(3,1312) = 0.41, p = .748 |
|
| |||||
| Number of metastatic sites including lymph node involvement | 1.3 (1.2) | 1.4 (1.3) | 1.2 (1.2) | 1.1 (1.2) | F(3,1325) = 2.33, p = .073 |
|
| |||||
| Number of metastatic sites excluding lymph node involvement | 0.8 (1.0) | 0.9 (1.1) | 0.8 (1.1) | 0.7 (1.0) | F(3,1325) = 1.83, p = .140 |
|
| |||||
| % (n) | % (n) | % (n) | % (n) | ||
|
| |||||
| Gender | X2 = 50.10, p<.001 | ||||
| Female+ | 69.6 (373) | 74.1 (152) | 83.9 (235) | 88.6 (273) | 0 < 2 and 3 |
| Male | 30.4 (163) | 25.9 (53) | 15.7 (44) | 11.4 (35) | 1 < 3 |
| Transgender* | 0.0 (0) | 0.0 (0) | 0.4 (1) | 0.0 (0) | |
|
| |||||
| Ethnicity | X2 = 24.93, p = .003 | ||||
| White | 71.2 (375) | 60.7 (122) | 77.8 (217) | 66.6 (203) | 1 and 3 < 2 |
| Black | 12.7 (67) | 16.9 (34) | 8.6 (24) | 11.8 (36) | 1 > 2 |
| Asian or Pacific Islander | 7.2 (38) | 10.9 (22) | 4.3 (12) | 7.5 (23) | 1 > 2 |
| Hispanic Mixed or Other | 8.9 (47) | 11.4 (23) | 9.3 (26) | 14.1 (43) | NS |
|
| |||||
| Married or partnered (% yes) | 66.9 (354) | 64.4 (130) | 60.9 (167) | 63.0 (192) | X2 = 3.16, p = .367 |
|
| |||||
| Lives alone (% yes) | 20.7 (109) | 20.2 (41) | 23.3 (64) | 22.5 (69) | X2 = 1.12, p = .773 |
|
| |||||
| Child care responsibilities (% yes) | 19.4 (102) | 17.4 (34) | 20.4 (57) | 31.0 (93) | X2 = 19.01, p = .000 |
| 0, 1, and 2 < 3 | |||||
|
| |||||
| Care of adult responsibilities (% yes) | 6.1 (30) | 9.9 (18) | 8.3 (21) | 9.4 (26) | X2 = 4.15, p = .246 |
|
| |||||
| Currently employed (% yes) | 38.9 (206) | 36.0 (73) | 37.5 (104) | 25.9 (79) | X2 = 15.42, p = .001 |
| 0 and 2 > 3 | |||||
|
| |||||
| Income | KW, p = .001 | ||||
| < $30,000+ | 15.1 (70) | 20.4 (38) | 15.1 (39) | 25.6 (72) | 0 and 2 < 3 |
| $30,000 to <$70,000 | 19.8 (92) | 21.0 (39) | 22.8 (59) | 22.1 (62) | |
| $70,000 to < $100,000 | 18.8 (87) | 17.7 (33) | 13.1 (34) | 16.0 (45) | |
| ≥ $100,000 | 46.3 (215) | 40.9 (76) | 49.0 (127) | 36.3 (102) | |
|
| |||||
| Specific comorbidities (% yes) | |||||
|
| |||||
| Heart disease | 6.3 (34) | 7.3 (15) | 4.6 (13) | 4.9 (15) | X2 = 2.33, p = .507 |
|
| |||||
| High blood pressure | 30.4 (163) | 36.1 (74) | 25.7 (72) | 31.2 (96) | X2 = 6.13, p = .106 |
|
| |||||
| Lung disease | 11.2 (60) | 9.3 (19) | 12.1 (34) | 12.0 (37) | X2 = 1.21, p = .752 |
|
| |||||
| Diabetes | 8.8 (47) | 15.1 (31) | 5.7 (16) | 8.8 (27) | X2 = 12.97, p = .005 |
| 1 > 2 | |||||
|
| |||||
| Ulcer or stomach disease | 3.4 (18) | 4.9 (10) | 3.9 (11) | 8.1 (25) | X2 = 10.29, p = .016 |
| 0 < 3 | |||||
|
| |||||
| Kidney disease | 0.9 (5) | 1.5 (3) | 1.4 (4) | 2.3 (7) | X2 = 2.49, p = .476 |
|
| |||||
| Liver disease | 6.2 (33) | 8.3 (17) | 5.7 (16) | 6.5 (20) | X2 = 1.48, p = .688 |
|
| |||||
| Anemia or blood disease | 8.6 (46) | 15.1 (31) | 9.3 (26) | 19.8 (61) | X2 = 26.75, p<.001 |
| 0 and 2 < 3 | |||||
|
| |||||
| Depression | 7.5 (40) | 13.7 (28) | 28.6 (80) | 34.7 (107) | X2 = 115.51, p<.001 |
| 0 and 1 < 2 and 3 | |||||
|
| |||||
| Osteoarthritis | 9.9 (53) | 12.2 (25) | 13.2 (37) | 15.6 (48) | X2 = 6.20, p = .102 |
|
| |||||
| Back pain | 16.0 (86) | 29.3 (60) | 26.4 (74) | 39.9 (123) | X2 = 60.12, p<.001 |
| 0 <1, 2, and 3 | |||||
| 2 < 3 | |||||
|
| |||||
| Rheumatoid arthritis | 3.2 (17) | 3.9 (8) | 1.8 (5) | 4.2 (13) | X2 = 3.13, p = .372 |
|
| |||||
| Exercise on a regular basis (% yes) | 73.6 (388) | 69.0 (138) | 74.2 (204) | 64.4 (192) | X2 = 9.73, p = .021 |
| 0 > 3 | |||||
|
| |||||
| Smoking, current or history of (% yes) | 35.5 (188) | 34.0 (68) | 37.9 (105) | 33.6 (101) | X2 = 1.38, p = .710 |
|
| |||||
| Cancer diagnosis | X2 = 43.25, p<.001 | ||||
| Breast | 34.9 (187) | 37.1 (76) | 47.1 (132) | 45.1 (139) | 0 < 2 and 3 |
| Gastrointestinal | 34.9 (187) | 40.5 (83) | 20.4 (57) | 26.0 (80) | 0 and 1 > 2 and 3 |
| Gynecological | 16.8 (90) | 10.7 (22) | 22.1 (62) | 19.2 (59) | 1 < 2 |
| Lung | 13.4 (72) | 11.7 (24) | 10.4 (29) | 9.7 (30) | NS |
|
| |||||
| Type of prior cancer treatment | KW, p = .226 | ||||
| No prior treatment | 25.9 (135) | 30.8 (61) | 20.1 (55) | 24.0 (72) | |
| Only surgery, CTX, or RT | 41.7 (217) | 37.9 (75) | 44.3 (121) | 43.3 (130) | |
| Surgery & CTX, or Surgery & RT, or CTX & RT | 20.0 (104) | 19.7 (39) | 22.7 (62) | 17.3 (52) | |
| Surgery & CTX & RT | 12.5 (65) | 11.6 (23) | 12.8 (35) | 15.3 (46) | |
Abbreviations: AUDIT = Alcohol Use Disorders Identification Test, CTX = chemotherapy, kg = kilograms, KW = Kruskal Wallis; m2 = meter squared, NS = not significant, RT = radiation therapy, SCQ = Self-Administered Comorbidity Questionnaire, SD = standard deviation
Chi Square analysis and post hoc contrasts done without the transgender patient include in the analyses
Reference group for the post hoc comparisons
Differences in Symptom Occurrence Rates Among the Groups Identified with LCA and K-modes
Supplemental Table 1 summarizes differences in symptom occurrence rates among the four groups of patients identified using LCA and K-modes. Both analyses identified two groups of oncology patients who reported moderate levels of physical symptoms but differentiated on the occurrence of five psychological symptoms (i.e., worrying, feeling irritable, feeling sad, feeling nervous, I don’t look like myself). For patients in the Moderate Physical and Higher Psychological group, worrying (LCA: 0.906, K-modes: 0.811), feeling sad (LCA: 0.813, K-modes: 0.811), and feeling irritable (LCA: 0.649, K-modes: 0.657) were among the top symptoms. In contrast, in the Moderate Physical and Lower Psychological group, worrying (LCA: 0.142, K-modes: 0.278), feeling sad (LCA: 0.161, K-modes: 0.259), and feeling irritable (LCA: 0.256, K-modes: 0.224) were among the symptoms with the lowest probability of occurrences. The remaining psychological symptoms, namely: “feeling nervous” (Moderate Physical and Higher Psychological group: LCA: 0.606, K-modes: 0.693; Moderate Physical and Lower Psychological group: LCA: 0.184, K-modes: 0.185) and “I don’t look like myself” (Moderate Physical and Higher Psychological group: LCA: 0.541, K-modes: 0.582; Moderate Physical and Lower Psychological group: LCA: 0.282, K-modes: 0.259) had significant differences between the aforementioned groups for both analyses.
Across all four groups, lack of energy was the most common symptom. While the probability of its occurrence for the total sample was 0.832, values ranged from 0.549 to 0.994 for LCA and from 0.647 to 0.974 for K-modes. In addition, pain (LCA: 0.944-0.334, K-modes: 0.834-0.360), difficulty in sleeping (LCA: 0.927-0.458, K-modes: 0.896-0.537), numbness/tingling in hands/feet (LCA: 0.798-0.334, K-modes: 0.724-0.356), change in the way food tastes (LCA: 0.837-0.274, K-modes: 0.802-0.323), and feeling drowsy (LCA: 0.966-0.243, K-modes: 0.860-0.321) occurred in the top ten symptoms across all four groups for both analyses.
Differences in MSAS Summary Scores Among the Groups Identified with LCA and K-modes
Table 6 summarizes differences in the MSAS summary scores among the four groups of patients identified using LCA and K-modes. For the Physical subscale, the Psychological subscale, and the Global Distress index, the differences among the four groups followed the same pattern for both analyses. For the MSAS total score, as well as for the total number of MSAS symptoms, the pattern observed using the LCA was in the expected direction (i.e., All Low < Moderate Physical and Lower Psychological < Moderate Physical and Higher Psychological < All High). For the MSAS total score, as well as for the total number of MSAS symptoms, the pattern observed using K-modes was as follows: All Low < Moderate Physical and Lower Psychological, Moderate Physical and Higher Psychological and All High (i.e., 0 < 1, 2, and 3), as well as Moderate Physical and Lower Psychological and Moderate Physical and Higher Psychological < All High (i.e., 1 and 2 < 3).
Table 6.
Differences in Memorial Symptom Assessment Scale Scores Among the Patient Subgroups Using Latent Class Analysis or K-Modes Analysis
| MSAS scores | All Lowa,b (0) | Moderate Physical & Lower Psychological (1) | Moderate Physical & Higher Psychological (2) | All High (3) | Statistics |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| PATIENT SUBGROUPS USING LATENT CLASS ANALYSIS | |||||
|
| |||||
| Physical subscale | 0.3 (0.2) | 1.0 (0.4) | 0.8 (0.4) | 1.6 (0.5) | F(3,1325) = 578.78, p<.001 |
| 0 < 2 < 1 < 3 | |||||
|
| |||||
| Psychological subscale | 0.3 (0.3) | 0.6 (0.4) | 1.3 (0.5) | 1.9 (0.6) | F(3,1325) = 717.30, p<.001 |
| 0 < 1 < 2 < 3 | |||||
|
| |||||
| Global Distress Index | 0.4 (0.3) | 0.9 (0.4) | 1.3 (0.5) | 2.1 (0.6) | F(3,1305) = 770.22, p<.001 |
| 0 < 1 < 2 < 3 | |||||
|
| |||||
| Total Score | 0.3 (0.2) | 0.7 (0.3) | 0.8 (0.3) | 1.6 (0.4) | F(3,1325) = 11037.63, p<.001 |
| 0 < 1 < 2 < 3 | |||||
|
| |||||
| Total number of MSAS symptoms (out of 32) | 5.6 (2.5) | 12.9 (3.2) | 14.6 (3.0) | 23.0 (3.3) | F(3,1325) = 1601.27, P = 0.000, |
| 0 < 1 < 2 < 3 | |||||
|
| |||||
| Total number of MSAS symptoms (out of 38) | 6.3 (2.9) | 14.4 (3.5) | 16.1 (3.5) | 26.1 (4.4) | F(3,1325) = 1474.65, p<.001 |
| 0 < 1 < 2 < 3 | |||||
|
| |||||
| PATIENT SUBGROUPS USING K-MODES ANALYSIS | |||||
|
| |||||
| Physical subscale | 0.4 (0.3) | 1.1 (0.4) | 0.7 (0.4) | 1.4 (0.6) | F(3,1325) = 578.28, p<.001 |
| 0 < 2 < 1 < 3 | |||||
|
| |||||
| Psychological subscale | 0.4 (0.3) | 0.6 (0.4) | 1.3 (0.5) | 1.6 (0.7) | F(3,1325) = 553.73, p<.001 |
| 0 < 1 < 2 < 3 | |||||
|
| |||||
| Global Distress Index | 0.4 (0.3) | 1.0 (0.4) | 1.3 (0.5) | 1.8 (0.6) | F(3,1305) = 588.21, p<.001 |
| 0 < 1 < 2 < 3 | |||||
|
| |||||
| Total Score | 0.3 (0.2) | 0.8 (0.3) | 0.8 (0.3) | 1.3 (0.4) | F(3,1325) = 765.76, p<.001 |
| 0 < 1, 2, and 3 | |||||
| 1 and 2 < 3 | |||||
|
| |||||
| Total number of MSAS symptoms (out of 32) | 6.7 (3.2) | 13.9 (2.8) | 13.7 (2.8) | 20.6 (4.1) | F(3,1325) = 1187.40, p<.001 |
| 0 < 1, 2, and 3 | |||||
| 1 and 2 < 3 | |||||
|
| |||||
| Total number of MSAS symptoms (out of 38) | 7.6 (3.6) | 15.2 (3.1) | 15.0 (3.3) | 23.2 (5.1) | F(3,1325) = 1068.59, p<.001 |
| 0 < 1, 2, and 3 | |||||
| 1 and 2 < 3 | |||||
Abbreviations: MSAS = Memorial Symptom Assessment Scale, SD = standard deviation
For LCA – All Low (n=419, 31.5%), Moderate Physical and Lower Psychological (n=316, 23.8%), Moderate Physical and Higher Psychological (n=416, 31.3%) and All High (n=178, 13.4%).
For K-modes analysis – All Low (n=536, 40.3%), Moderate Physical and Lower Psychological (n=205, 15.4%), Moderate Physical and Higher Psychological (n=280, 21.13%) and All High (n=303, 23.24%).
Differences in QOL Scores Among the Groups Identified with LCA and K-modes
Table 7 summarizes differences in MQOLS-CA subscale and total scores among the four groups of patients identified using LCA and K-modes. For the MQOLS psychological and social well-being subscales, and total QOL scores, the differences among the four groups followed the same pattern for both analyses (i.e., All Low > Moderate Physical and Lower Psychological > Moderate Physical and Higher Psychological > All High). In addition, for the physical well-being subscale scores, the differences among the four groups followed the same pattern for both analyses (i.e., All Low > Moderate Physical and Lower Psychological, Moderate Physical and Higher Psychological, and All High (i.e., 0 > 1, 2, and 3) and Moderate Physical and Lower Psychological and Moderate Physical and Higher Psychological > All High (i.e., 1 and 2 > 3)).
Table 7.
Differences in Quality of Life Scores Among the Patient Subgroups Using Latent Class Analysis or K-Modes Analysis
| QOL scores | All Low (0) | Moderate Physical & Lower Psychological (1) | Moderate Physical & Higher Psychological (2) | All High (3) | Statistics |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| PATIENT SUBGROUPS USING LATENT CLASS ANALYSIS | |||||
|
| |||||
| MQOLS-PV - Physical well-being | 7.8 (1.4) | 6.5 (1.5) | 6.3 (1.5) | 4.7 (1.6) | F(3,1292) = 179.64, p<.001 |
| 0 > 1, 2, and 3 | |||||
| 1 and 2 > 3 | |||||
|
| |||||
| MQOLS-PV - Psychological well- being | 6.5 (1.6) | 6.0 (1.6) | 4.7 (1.6) | 4.0 (1.5) | F(3,1281) = 154.85, p<.001 |
| 0 > 1 > 2 > 3 | |||||
|
| |||||
| MQOLS-PV - Social well-being | 6.9 (1.7) | 6.0 (1.8) | 5.1 (1.8) | 4.1 (1.8) | F(3,1274) = 123.13, p<.001 |
| 0 > 1 > 2 > 3 | |||||
|
| |||||
| MQOLS-PV – Spiritual well-being | 5.5 (2.2) | 5.5 (2.1) | 5.3 (2.0) | 5.6 (2.0) | F(3,1286) = 0.61, p = .611 |
|
| |||||
| MQOLS-PV – Total QOL score | 6.7 (1.2) | 6.0 (1.2) | 5.2 (1.2) | 4.4 (1.2) | F(3,1276) = 177.88, p<.001 |
| 0 > 1 > 2 > 3 | |||||
|
| |||||
| SF12 – PCS score | 45.6 (9.6) | 39.0 (10.1) | 41.1 (10.5) | 35.7 (9.7) | F(3,1225) = 45.76, p<.001 |
| 0 > 2 > 1 > 3 | |||||
|
| |||||
| SF12 – MCS score | 54.0 (8.4) | 51.9 (8.5) | 45.4 (9.8) | 40.5 (11.1) | F(3,1225) = 113.49, p<.001 |
| 0 > 1 > 2 > 3 | |||||
|
| |||||
| PATIENT SUBGROUPS USING K-MODES ANALYSIS | |||||
|
| |||||
| MQOLS-PV - Physical well-being | 7.6 (1.5) | 6.3 (1.5) | 6.5 (1.5) | 5.2 (1.7) | F(3,1292) = 153.99, p<.001 |
| 0 > 1, 2, and 3 | |||||
| 1 and 2 > 3 | |||||
|
| |||||
| MQOLS-PV - Psychological well- being | 6.4 (1.6) | 5.9 (1.6) | 4.7 (1.6) | 4.3 (1.6) | F(3,1281) = 128.41, p<.001 |
| 0 > 1 > 2 > 3 | |||||
|
| |||||
| MQOLS-PV - Social well-being | 6.7 (1.8) | 5.9 (1.8) | 5.2 (1.8) | 4.4 (1.8) | F(3,1274) = 115.73, p<.001 |
| 0 > 1 > 2 > 3 | |||||
|
| |||||
| MQOLS-PV – Spiritual well-being | 5.5 (2.1) | 5.5 (2.1) | 5.3 (2.0) | 5.5 (2.0) | F(3,1286) = 0.71, p = .547 |
|
| |||||
| MQOLS-PV – Total QOL score | 6.5 (1.2) | 5.9 (1.3) | 5.3 (1.2) | 4.7 (1.3) | F(3,1276) = 152.38, p<.001 |
| 0 > 1 > 2 > 3 | |||||
|
| |||||
| SF12 – PCS score | 44.8 (9.9) | 38.1 (9.3) | 41.6 (10.3) | 37.0 (10.5) | F(3,1225) = 43.78, p<.001 |
| 0 > 1, 2, and 3 | |||||
| 2 > 1 and 3 | |||||
|
| |||||
| SF12 – MCS score | 53.7 (8.3) | 51.2 (9.0) | 45.3 (10.3) | 42.9 (10.5) | F(3,1225) = 98.06, p<.001 |
| 0 > 1 > 2 > 3 | |||||
Abbreviations: MCS = Mental Component Summary, MQOLS-PV = Multidimensional Quality of Life Scale – Patient Version, PCS = Physical Component Summary, SF12 – Medical Outcomes Study Short Form 12, SD = standard deviation
For LCA – All Low (n=419, 31.5%), Moderate Physical and Lower Psychological (n=316, 23.8%), Moderate Physical and Higher Psychological (n=416, 31.3%) and All High (n=178, 13.4%).
For K-modes analysis – All Low (n=536, 40.3%), Moderate Physical and Lower Psychological (n=205, 15.4%), Moderate Physical and Higher Psychological (n=280, 21.13%) and All High (n=303, 23.24%).
For the SF12, for both analyses, the MCS scores followed a similar pattern (i.e., All Low > Moderate Physical and Lower Psychological > Moderate Physical and Higher Psychological > All High). For the PCS scores, the post hoc contrasts were different depending on the method of analysis. For LCA, the pattern was All Low > Moderate Physical and Higher Psychological > Moderate Physical and Lower Psychological > All High. For the K-modes analysis, the pattern was as follows: All Low > Moderate Physical and Lower Psychological, Moderate Physical and Higher Psychological and All High (i.e., 0 > 1, 2, and 3), as well as Moderate Physical and Higher Psychological > Moderate Physical and Lower Psychological and All High (i.e., 2 > 1 and 3).
DISCUSSION
This study is the first to evaluate for congruence between the ability of two different analytic approaches to identifiy subgroups of oncology patients with distinct symptom profiles. Using both LCA and K-modes, four groups of patients with distinct symptom profiles were identified. The Cohen’s kappa coefficient of 0.666 represents a moderate level of agreement between the two approaches.51–53 Potential reasons for only a moderate level of agreement may be related to differences in the underlying assumptions of each of the methods. LCA is a model based approach where “clusters” (i.e. classes) are defined by parametric probability distributions that can be interpreted to generate homogenous points, while the whole data set is modelled by a mixture of such distributions.54 Its key assumption is the conditional independence of the observed variables given the latent class. Inside the same class, the presence or the absence of one symptom is viewed as unrelated to the presence or absence of all of the others. On the other hand, K-modes is a distance-based clustering method that separates clusters as data subsets that have small within-cluster distances and large separation from other clusters. K-modes tries to find clusters that bring similar observations together without making an assumption about their distribution or attempt to fit a mixture distribution. Our findings, as well as others,54–56 suggest that further research is needed, using both approaches, to determine the most sensitive and specific method(s) to risk profile oncology patients based on symptom occurrence rates.
While the absolute percentages of patients in the four groups differed depending on the analytic approach, the specific symptom profiles within each of the four groups were very similar. In addition, previous work in heterogeneous samples of oncology patients, using a different numbers of MSAS symptoms,9,57 found the same four phenotypic profiles identified in the current study. Across these three studies, the percentage of patients in the All Low group ranged from 28.0%9 to 40.3% (using K-modes in the current study) and the percentage of patients in the All High class ranged from 13.4% (using LCA in the current study) to 27.8%.57 Across these three studies, these relatively wide ranges may be related to differences in the number and types of symptoms evaluated, the timing of the symptom assessments in relationship to cancer diagnosis and treatments, and/or the specific cancer diagnoses of the patients in each of the studies. That said, these two extreme phenotypes were identified in previous studies that used only four symptoms6,7,10,11 or identified only two or three groups.15–17
Across the two previous studies9,57 and with the two analytic methods used in the current study, the consistent phenotypic characteristics associated with membership in the All High group were younger age and poorer functional status. The association between younger age and a higher symptom burden is consistent with previous studies.6,7 While younger patients may receive more aggressive cancer treatments,58 equally plausible hypotheses for this association include: that older adults experience a “response shift” in their perception of symptoms;59 that chronological age may not be an accurate representation of the biological age of oncology patients;60 and/or that accelerated aging occurs with cancer and its treatment.61–63
Similar to age, the association between a higher symptom burden and poorer functional status was reported previously.11,16,18 In the current study and in the one conducted in Norway,57 that both used the KPS scale, compared to patients in the All Low group who had KPS scores between 85 and 95, patients in the All High group reported KPS scores in the mid-70s. This difference represents a clinically meaningful change in functional status on this scale. Given that patients typically report lower KPS scores than their clinicians,64,65 patients should be interviewed not only about the number and severity of their symptoms but about changes in functional status during and following cancer treatment.
An equally important finding in this study and in the two previous studies9,57 is the identification of two groups of patients who differentiated based on the occurrence of psychological symptoms. While our phenotypic data suggest that these two groups have lower KPS scores and a higher comorbidity profile than the All Low group and better scores for both characteristics than the All High group, the demographic and clinical characteristics that distinguish between these two “Moderate” groups are not readily apparent. These findings are similar to previous reports9,57 and warrant investigation in future studies. An evaluation of additional psychosocial characteristics (e.g., coping styles, personality, social support) may improve the phenotypic characterization of these two “Moderate” groups.
In terms of the QOL outcomes, regardless of whether a generic (i.e., SF12) or disease-specific (i.e., MQOLS-PV) measure was used, the pattern of the differences in scores were in the expected direction, namely that as the symptom phenotype worsened, QOL decreased. The one interesting finding on Table 7, relates to the PCS scores from the SF12. While none of the groups had PCS scores of ≥50 (i.e., the normative value for the general population in the United States), patients in the Moderate Physical and Lower Psychological group had worse scores than patients in the Moderate Physical and Higher Psychological group. This finding is consistent with the report by Astrup and colleagues.57 Additional research is warranted to explain this finding and to determine the specific phenotypic characteristics that distinguish between these two Moderate groups.
In terms of study limitations, patients were recruited at various points in their CTX treatment. In addition, the types of CTX were not homogeneous. While we cannot rule out the potential contributions of clinical characteristics to patients’ symptom experiences, the relatively similar percentages of cancer diagnoses, reasons for current treatment, time since cancer diagnosis, and evidence of metastatic disease across the four groups, suggest that the patients were relatively similar in terms of disease and treatment characteristics. Although it is possible that patients in the “All Low” group were receiving more aggressive symptom management interventions, the occurrence rates for the five most common symptoms were relatively similar across the four classes for both analyses. It is possible that using ratings of frequency, severity or distress to create patients groups would provide additional information on inter-individual differences in the symptom experience of these patients.
Additional research is warranted using different analytic methods to optimize the identification of oncology patients with a higher symptom burden. Future studies can evaluate different machine learning approaches, as well as real time collection of different dimensions of a patient’s symptom experience (i.e., occurrence, severity, distress) to determine the most sensitive and specific methods to use to risk profile patients and design and test more effective symptom management interventions.
Supplementary Material
Acknowledgments
This study was funded by the National Cancer Institute (NCI, CA134900). Dr. Miaskowski is funded by grants from the American Cancer Society and NCI (CA168960). Dr. Wright is funded by a T32 grant from the National Institute of Nursing Research (NR008346). In addition, this project received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement number 602289.
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21:1525–1550. doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miaskowski C. Future directions in symptom cluster research. Semin Oncol Nurs. 2016;32:405–415. doi: 10.1016/j.soncn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. J Natl Cancer Inst. 2017:109. doi: 10.1093/jnci/djw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 7.Pud D, Ben Ami S, Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35:162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Illi J, Miaskowski C, Cooper B, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miaskowski C, Dunn L, Ritchie C, et al. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage. 2015;50:28–37. doi: 10.1016/j.jpainsymman.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford DJ, Paul SM, Cooper B, et al. Comparison of subgroups of breast cancer patients on pain and co-occurring symptoms following chemotherapy. Support Care Cancer. 2016;24:605–614. doi: 10.1007/s00520-015-2819-1. [DOI] [PubMed] [Google Scholar]
- 11.Dodd MJ, Cho MH, Cooper BA, et al. Identification of latent classes in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncol Nurs Forum. 2011;38:33–42. doi: 10.1188/11.ONF.33-42. [DOI] [PubMed] [Google Scholar]
- 12.Doong SH, Dhruva A, Dunn LB, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs. 2015;17:237–247. doi: 10.1177/1099800414550394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miaskowski C, Cooper BA, Aouizerat B, et al. The symptom phenotype of oncology outpatients remains relatively stable from prior to through 1 week following chemotherapy. Eur J Cancer Care (Engl) 2016 doi: 10.1111/ecc.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miaskowski C, Cooper BA, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer. 2014;120:2371–2378. doi: 10.1002/cncr.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira KA, Kimura M, Teixeira MJ, et al. Impact of cancer-related symptom synergisms on health-related quality of life and performance status. J Pain Symptom Manage. 2008;35:604–616. doi: 10.1016/j.jpainsymman.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Gwede CK, Small BJ, Munster PN, Andrykowski MA, Jacobsen PB. Exploring the differential experience of breast cancer treatment-related symptoms: a cluster analytic approach. Support Care Cancer. 2008;16:925–933. doi: 10.1007/s00520-007-0364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reese JB, Blackford A, Sussman J, et al. Cancer patients’ function, symptoms and supportive care needs: a latent class analysis across cultures. Qual Life Res. 2015;24:135–146. doi: 10.1007/s11136-014-0629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder CF, Garrett-Mayer E, Blackford AL, et al. Concordance of cancer patients’ function, symptoms, and supportive care needs. Qual Life Res. 2009;18:991–998. doi: 10.1007/s11136-009-9519-6. [DOI] [PubMed] [Google Scholar]
- 19.Arthur D, Vassilvitskii S. k-means++: The advantages of careful seeding. Proceedings of the eighteenth annual ACM-SIAM symposium on Discrete Algorithms; Society for Industrial and Applied Mathematics; 2007. pp. 1027–1035. [Google Scholar]
- 20.Cao F, Liang J, Bai L. A new initialization method for categorical data clustering. Expert Systems with Applications. 2009;36:10223–10228. [Google Scholar]
- 21.Huang Z. Extensions to the k-means algorithm for clustering large data sets with categorical values. Data Mining and Knowledge Discovery. 1998;2:283–304. [Google Scholar]
- 22.Ng AY, Jordan MI, Weiss Y. On spectral clustering: Analysis and an algorithm. Advances in Neural Information Processing Systems. 2002;2:849–856. [Google Scholar]
- 23.Zhang T, Ramakrishnan R, Livny M. BIRCH: an efficient data clustering method for very large databases. ACM SIGMOD Record. 1996;25:103–114. [Google Scholar]
- 24.Sasirekha K, Baby P. Agglomerative Hierarchical Clustering Algorithm – A review. International Journal of Scientific and Research Publications. 2013;3:1–3. [Google Scholar]
- 25.Zhao Y, Karypis G, Fayyad U. Hierarchical clustering algorithms for document datasets. Data Mining and Knowledge Discovery. 2005;10:141–168. [Google Scholar]
- 26.Dhillon IS, Guan Y, Kulis B. A unified view of kernel k-means, spectral clustering and graph cuts. UTCS Technical Report TR-04–25. 2005 [Google Scholar]
- 27.Papachristou N, Miaskowski C, Barnaghi P, et al. Comparing machine learning clustering with latent class analysis on cancer symptoms’ data. Proceedings of the IEEE Healthcare Innovation Point-of-Care Technologies Conference; 2016. [Google Scholar]
- 28.Wright F, Hammer M, Paul SM, et al. Inflammatory pathway genes associated with inter- individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine. 2017;91:187–210. doi: 10.1016/j.cyto.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kober KM, Cooper BA, Paul SM, et al. Subgroups of chemotherapy patients with distinct morning and evening fatigue trajectories. Support Care Cancer. 2016;24:1473–1485. doi: 10.1007/s00520-015-2895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustard in the palliative treatment of cancer. Cancer. 1948:634–656. [Google Scholar]
- 31.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 32.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 33.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale - an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30a:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 34.Ferrell BR, Wisdom C, Wenzl C. Quality of life as an outcome variable in the management of cancer pain. Cancer. 1989;63:2321–2327. doi: 10.1002/1097-0142(19890601)63:11<2321::aid-cncr2820631142>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 35.Padilla GV, Grant MM. Quality of life as a cancer nursing outcome variable. Adv Nurs Sci. 1985;8:45–60. doi: 10.1097/00012272-198510000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Padilla GV, Presant C, Grant MM, et al. Quality of life index for patients with cancer. Res Nurs Health. 1983;6:117–126. doi: 10.1002/nur.4770060305. [DOI] [PubMed] [Google Scholar]
- 37.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Padilla GV, Ferrell B, Grant MM, Rhiner M. Defining the content domain of quality of life for cancer patients with pain. Cancer Nurs. 1990;13:108–115. [PubMed] [Google Scholar]
- 39.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 40.Ferrell BR. The impact of pain on quality of life. A decade of research. Nurs Clin North Am. 1995;30:609–624. [PubMed] [Google Scholar]
- 41.Collins LM, Lanza ST. Latent class and latent transition analysis: with applications in the Social, Behavioral, and Health Science. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- 42.Nylund K, Bellmore A, Nishina A, Graham S. Subtypes, severity, and structural stability of peer victimization: what does latent class analysis say? Child Dev. 2007;78:1706–1722. doi: 10.1111/j.1467-8624.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 43.Muthen LK, Muthen BO. Mplus (Version 7.4) Los Angeles, CA: Muthen & Muthen; 2015. [Google Scholar]
- 44.Muthen B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 45.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling. 2007;14:535–569. [Google Scholar]
- 46.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13:195–212. [Google Scholar]
- 47.Ordonez C. Clustering binary data streams with K-means. Proceedings of the 8th ACM SIGMOD workshop on Research issues in data mining and knowledge discovery; 2003. pp. 12–19. [Google Scholar]
- 48.Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: Machine learning in Python. Journal of Machine Learning Research. 2011;12:2825–2830. [Google Scholar]
- 49.Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. Journal of Computational and Applied Mathematics. 1987;20:53–65. [Google Scholar]
- 50.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 51.Gisev N, Bell JS, Chen TF. Interrater agreement and interrater reliability: key concepts, approaches, and applications. Res Social Adm Pharm. 2013;9:330–338. doi: 10.1016/j.sapharm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 52.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 53.Steinijans VW, Diletti E, Bomches B, Greis C, Solleder P. Interobserver agreement: Cohen’s kappa coefficient does not necessarily reflect the percentage of patients with congruent classifications. Int J Clin Pharmacol Ther. 1997;35:93–95. [PubMed] [Google Scholar]
- 54.Anderlucci L, Hennig C. The clustering of categorical data: a comparison of a model- based and a distance-based approach. Communications in Statistics-Theory and Methods. 2014;43:704–721. [Google Scholar]
- 55.Hennig C, Liao TF. How to find an appropriate clustering for mixed-type variables with application to socio-economic stratification. Journal of the Royal Statistical Society: Series C. 2013;62:309–369. [Google Scholar]
- 56.Oberski DL. Beyond the number of classes: separating substantive from non-substantive dependence in latent class analysis. Advances in Data Analysis and Classification. 2016;10:171–182. [Google Scholar]
- 57.Astrup GL, Hofso K, Bjordal K, et al. Patient factors and quality of life outcomes differ among four subgroups of oncology patients based on symptom occurrence. Acta Oncol. 2017:1–9. doi: 10.1080/0284186X.2016.1273546. [DOI] [PubMed] [Google Scholar]
- 58.Klepin HD, Rodin M, Hurria A. Treating older adults with cancer: geriatric perspectives. Am Soc Clin Oncol Educ Book. 2015;35:e544–552. doi: 10.14694/EdBook_AM.2015.35.e544. [DOI] [PubMed] [Google Scholar]
- 59.Sprangers MA, Schwartz CE. The challenge of response shift for quality-of-life-based clinical oncology research. Ann Oncol. 1999;10:747–749. doi: 10.1023/a:1008305523548. [DOI] [PubMed] [Google Scholar]
- 60.Bae CY, Kang YG, Piao MH, et al. Models for estimating the biological age of five organs using clinical biomarkers that are commonly measured in clinical practice settings. Maturitas. 2013;75:253–260. doi: 10.1016/j.maturitas.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book. 2014:e423–430. doi: 10.14694/EdBook_AM.2014.34.e423. [DOI] [PubMed] [Google Scholar]
- 62.Hurria A, Jones L, Muss HB. Cancer treatment as an accelerated aging process: Assessment, biomarkers, and interventions. Am Soc Clin Oncol Educ Book. 2016;35:e516–522. doi: 10.1200/EDBK_156160. [DOI] [PubMed] [Google Scholar]
- 63.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schnadig ID, Fromme EK, Loprinzi CL, et al. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113:2205–2214. doi: 10.1002/cncr.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ando M, Ando Y, Hasegawa Y, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br J Cancer. 2001;85:1634–1639. doi: 10.1054/bjoc.2001.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.