Abstract
Rapidly fabricated, disposable sensor platforms hold tremendous promise for point-of-care detection. Here, we present an inexpensive (< $0.25) fully inkjet printed electrochemical sensor with integrated counter, reference, and working electrodes that is easily scalable for commercial fabrication. The electrochemical sensor platform featured an inkjet printed gold working 8-electrode array (WEA) and counter electrode (CE), along with an inkjet –printed silver electrode that was chlorinated with bleach to produce a Ag/AgCl quasi-reference electrode (RE). As proof of concept, the electrochemical sensor was successfully applied for detection of clinically relevant breast cancer biomarker Human Epidermal Growth Factor Receptor 2 (HER-2). Capture antibodies were bound to a chemically modified surface on the WEA and placed into a microfluidic device. A full sandwich immunoassay was constructed following a simultaneous injection of target protein, biotinylated antibody, and polymerized horseradish peroxide labels into the microfluidic device housing the WEA. With an ultra fast assay time, of only 15 mins a clinically relevant limit of detection of 12 pg mL−1 was achieved. Excellent reproducibility and sensitivity were observed through recovery assays preformed in human serum with recoveries ranging from 76–103%. These easily fabricated and scalable electrochemical sensor platforms can be readily adapted for multiplex detection following this rapid assay protocol for cancer diagnostics.
Keywords: Inkjet Printing, Printed Electronics, Microfluidic, Immunoassay, Human Epidermal Growth Factor Receptor 2 (HER-2), Breast Cancer
1. Introduction
Electrochemical detection has gained significant attention for point-of-care diagnostics due to ease of detection, low instrumentation cost, and ability to be miniaturized and automated (Wang, 2006)(Arduini et al., 2016). A typical electrochemical cell consists of a counter, working, and reference electrodes. Miniaturization and production cost of electrodes over the past few decades have been improved by patterning electrodes on a substrate surface. Common methods of production include chemical vapor deposition (CVD)(Chen et al., 2011), photolithography (Fourkas, 2010), screen-printing (Krebs et al., 2009)(Metters et al., 2011) and stencil printing (Siegel et al., 2010). Whereas each of these approaches permits for well-defined electrode construction and insulation, each also require the production of a template such as a patterned screen, mask, or stencil (Lessing et al., 2014). Manufacturing these templates is time consuming and costly, limiting rapid prototyping. In addition, methods such as CVD, and photolithography require expensive facilities and generate hazardous waste (Kamyshny et al., 2011). Inkjet printing has the ability to circumvent these limitations by offering the capability to rapidly produce patterns based on easily modifiable digital files with high resolution and low material waste (Kamyshny et al., 2011)(Khan et al., 2016)(Komuro et al., 2013)(Li et al., 2015).
Although inkjet printing of electrodes has been widely reported, the number of fully inkjet printed sensors has been limited and those with integrated counter and reference electrodes is even fewer (Moya et al., 2017). In this communication, we report a fully inkjet printed sensor array with onboard counter electrode and a bleach-activated reference electrode to enable for multiple detections of proteins on a single chip. Material cost of this electrochemical array is less than US $0.25 per chip, making it a viable option for a disposable point-of-care test strip that is attractive for mass-production.
Human Epidermal Growth Factor Receptor 2 (HER-2) is one of the few potential protein biomarkers for breast cancer that are officially approved by the Food and Drug Administration (FDA)(Sturgeon et al., 2008)(Li and Chan, 2014)(Goossens et al., 2015). The most common clinical methods for detection of HER-2 include immunohistochemistry (IHC) staining, and fluorescence in situ histochemistry (FISH), both of which require tumor tissues using invasive biopsies (Shah and Chen, 2010)(Tchou et al., 2015). However, HER-2 can also be found in patient serum. Recent reports indicated that serum concentrations above 7 ng mL−1 have lower rates of disease free survival. Therefore, sensitive assays that measure lower concentration levels in serum are needed for HER-2 detection (Tchou et al., 2015)(Banys-Paluchowski et al., 2017).
The gold standard for clinical protein serum biomarkers for nearly 50 years has been the enyzme-linked immunoasorbent assay (ELISA), which offers limits of detection in the low pg mL−1 range for some proteins (Kingsmore, 2006). ELISA however is limited by long assay times, cost, lack of multiplexing, and sample volume. Electrochemical immunosensors offer an alternative detection method to that of clinical analysis. They are inexpensive, technically simple, have short assay times, multiplexing capability, and low sample volumes (Rusling et al., 2010)(Kingsmore, 2006)(Munge et al., 2016). Reports of the electrochemical detection of HER-2 include an amplified bead –based assay approach on the surface of a screen printed carbon electrodes with limits of detection of 6 ng mL−1 in an 85 min assay (Al-Khafaji et al., 2012). Similarly, using a screen printed carbon electrochemical cell S.D. Tallapragada et al. with no surface modification recently detected HER-2 with a detection limit of 4 ng mL−1 in ~ 4 hr (Tallapragada et al., 2017). To the best of our knowledge, the lowest detection limit reported for HER-2 is 0.037 pg mL−1 demonstrated by Zhu et al. on a single glassy carbon electrode modified with a hydrazine –Aunanoparticle –aptamer bioconjugate with external reference and counter electrodes and assay time of 1 hr (Zhu et al., 2013). While all of these approaches reach the target limit (< 7 ng mL−1), they all require longer assay times.
For a point-of-care (POC) sensor to be clinically useful, it must be rapid, inexpensive, simple to use, sensitive and selective to the target analyte at normal and disease state levels. Here, we report for the first time, a fully inkjet printed electrochemical array platform with integrated counter and reference electrodes for determination of clinically relevant HER-2 in serum in 15 min with a limit of detection (LOD) of 12 pg mL−1 integrated into a simple microfluidic device.
2. Materials and Methods
Full list of materials and equipment can be found in supplementary material.
2.1 Preparation of the Inkjet Printed Electrochemical Array Chips
The electrochemical array chips were comprised of an inkjet printed gold working 8-electrode array (WEA), and counter electrode (CE) and of an inkjet –printed silver electrode that was chlorinated with bleach to produce a Ag/AgCl quasi-reference electrode (RE) (Refer to Fig. 1A for further details). The electrode Array with integrated reference and counter electrode design patterns were constructed using Microsoft Paint (Microsoft Inc. Redmond, WA). The patterns were imported to Dimatix materials printer software. Prior to printing silver and gold nanoparticle inks were filtered with a 0.2 μm cutoff PTFE filter. Both nanoparticle inks were characterized in terms of size and distribution by transmission electron microscopy (TEM) and dynamic light scattering (DLS) (refer to Fig. S1 and Fig. S2 in supplementary material). In addition, all new ink formulations were evaluated in terms of both their viscosity and surface tension to ensure they met the standards of jettable fluids for the piezo-driven inkjet printed nozzles namely viscosities between (10–12 cPs) and surface tensions between (2.8–3.3 N m−2). Each pattern was printed with a single nozzle and drop spacing of 15 μm using a custom waveform (see supplementary material, Fig. S3) to obtain optimal droplet formation and prevent clogs.
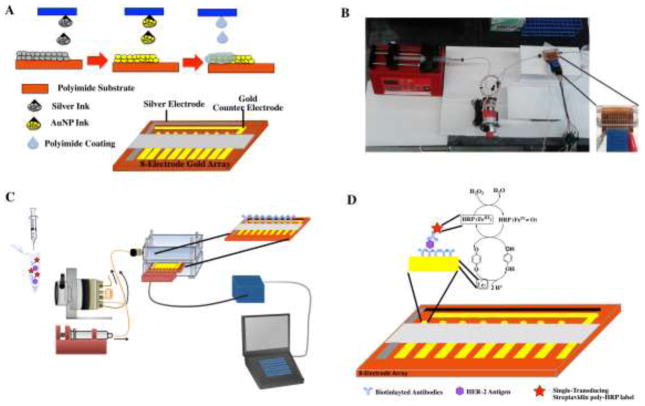
Fig. 1.
(A) Schematic representation of the fabrication of the fully inkjet printed electrochemical array platform with integrated counter, working and reference electrodes. (B) Picture of the microfluidic system. (C) Schematic representation of the system for electrochemical immunoarray detection of HER-2. (D) Fully developed sandwich immunoassay developed on electrochemical array platform and detection strategy generated by the injection of a hydroquinone and H2O2 mixture.
For printing Kapton sheets (22 × 28 cm) were cleaned with ethanol and water and dried with nitrogen prior to being placed in the Dimatix printer. The pattern files were set to allow for printing multiple electrodes or arrays in a single file. The silver nanoparticle ink for reference electrode patterns was printed first. Once printed the silver electrodes were sintered at 200 °C for 3 mins. The sheet was then returned to the printer to pattern the gold nanoparticle ink for construction of the 8-working electrode arrays and counter electrode. Again the electrodes and arrays were sintered for 3 mins at 200 °C. This allowed for the au cores to coalesce forming percolation paths (Jensen et al., 2011). The sheets were then returned to the printer for the last time to deposit the insulation layer of poly (amic) acid (PAA) ink. Five layers of PAA ink was applied over the leads of the 8-electrode arrays as well as reference and counter electrodes. Once insulation layer was deposited the sheets were sintered for 15 mins. A commercial bleach solution diluted to a concentration of 63 mg mL−1 was used to transform the silver inkjet printed electrode to obtain a pseudo-(Ag/AgCl) reference electrode. This was confirmed through energy-dispersive X-ray spectroscopy, EDX elemental analysis (Supplementary Material Fig S4 and S5). Optimized deposition time (60 s) and dilutions of commercial bleach are shown in supplementary material Fig. S6 along with storage stability (Fig. S7). Characterization of the inkjet printed electrode surface was carried out using tapping mode Atomic Force Microscopy details displayed in supplementary material Fig. S8.
Following this method of construction 75–100 electrochemical sensor chips with integrated counter and reference electrodes could be produced in a single batch. This could be further scaled up for commercial use using an industrial style inkjet printer.
2.2 Immunosensor Fabrication
Prior to immunosensor fabrication electrochemical array chips were cleaned in 0.18 M sulfuric acid, to remove any excess oxides. The electrochemical chips were then washed with water, then ethanol, and finally immersed in 4 mM 3-mercaptopropionic acid (MPA) in ethanol for 24 hours. The chips were then removed and washed with ethanol and water and dried with nitrogen. Carboxyl terminal ends of the MPA layer on the gold working electrodes arrays (WEA) were activated by placing 1 μL of 400 mM EDC and 100 mM NHSS on each of the 8 working electrodes for 10 mins. The electrodes were then rinsed with water and dried with nitrogen the HER-2 primary antibody solution was then added and the chips were then placed the fridge for overnight incubation. Once the primary antibodies were attached by amidization to the working electrode surface they were washed with 0.05% PBS T-20. To aid in blocking nonspecific binding on the surface of the working electrodes a 2% BSA solution was then added for 1 hour. Electrochemical Array chips were then washed with 0.05 % PBS-T-20 and inserted into the microfluidic device (Fig. 1B, and supplementary material Fig. S9). Arrays were flushed with 0.05% PBS-T-20 for 2 mins at a flow rate of 200 μL min−1. In a 1 mL centrifuge tube 100 μL of optimized secondary antibody-HER-2, 100 μL of optimized poly-HRP dilution of 1:250 in 0.1% BSA, and 100 μL of sample were offline captured mixed and immediately injected at a flow rate 200 μL min−1 into the microfluidic channel housing the electrochemical array chips (Fig. 1C). They were allowed to incubate for 10 mins. This was then followed by a 2 min wash with 0.05% PBS-T20. A solution of 1 mM hydroquinone was then passed through the microfluidic channel and amperometric detection was accomplished by injecting a mixture of 100 mM H2O2 and 1 mM HQ in PBS (Fig. 1D). A signal was then generated at −0.15 V vs pseudo-Ag/AgCl. For our assay procedures the total assay time was obtained by adding all times after antigen was added. This included incubation of the antigen (10 mins) wash step (2 mins) and detection (3 mins) for a total assay time of 15 mins.
3. Results and Discussion
3.1 Characterization and Electrochemical performance
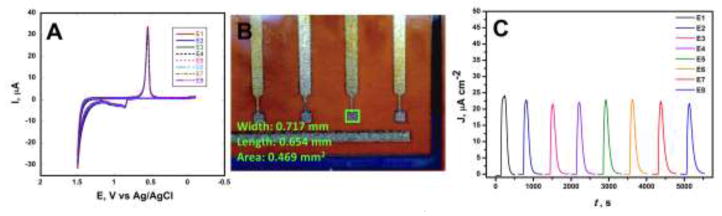
Electrochemical performance of the entire electrochemical array chip was evaluated by assessing the reproducibility of simultaneous cyclic voltammograms in 0.18 M sulfuric acid scanning between 1.5 V and – 0.1 V at 100 mV s−1. Fig. 2A displays similar peaks to that shown for bulk gold with formation of gold oxide at 0.9 V and reduction back to gold at 0.5 V vs a pseudo-Ag/AgCl reference. Following the protocol of Trasatti et al., the electrochemical surface are of the arrays was calculated to be 0.65 ± 0.02 mm2 (Trasatti and Petrii, 1991). This area is 135% of the geometric surface area calculated through Firefly software Fig. 2B. The overlay of the 8-working electrodes on a single array chip in sulfuric acid shows excellent reproducibility with RSD of 3%. However, similar to that reported by Krause et al (Krause et al., 2013), standard deviations were much higher when assessing multiple arrays. For 15 arrays, the average surface area was 0.63 ± 0.12 mm2 with an RSD of 19.8 %. Accordingly, each amperometric response was divided by the surface area calculated from CVs obtained from cleaning in 0.18 M H2SO4 prior to immunoassay assembly due to the larger impact on RSD in array chip to array chip reproducibility following Krause et al. (Krause et al., 2013). Reproducibility studies were further carried out in the microfluidic device using amperometry. Amperometric responses were measured on all eight electrodes in the microfluidic device following injection of 5 mM K3Fe(CN)6 in 0.1M KCl (Fig. 2C). All eight working electrodes exhibited minimum cross-talk between neighboring electrodes giving similar peak currents with an average of 22.9 ± 1.83 μA cm−2 (RSD < 8%). Additional characterization of the sustainability of the fully inkjet printed electrochemical platform was evaluated following several days of emersion in water with little to no etching observed (see supplementary material Fig. S10). Each electrochemical chip demonstrated high reproducibility and sustainability enabling them to serve as an excellent platform for immunosensor fabrication.
Fig. 2.
(A) Simultaneous CVs recorded at 100 mV s−1 on all working electrodes across the inkjet printed array in 0.18 M H2SO4. (B) A magnified photograph documented by FireflyPro digital microscope of the electrochemical array platform with a geometric surface area of a working electrode of 0.469 mm2. (C) Simultaneous amperometric responses at 0.1 V vs Ag/AgCl in the microfluidic device after a injection of 200 μL of 5 mM Fe(CN)6 3−/4− in 100 mM KCl at 200 μL min−1.
3.2 Immunosensor performance application for detection of clinically relevant HER-2 Biomarker
Sandwich immunoassays were constructed on the 8-working electrodes of the electrochemical array chips through covalent binding of an optimized concentration of primary anti-human HER-2 antibody (supplementary information Fig. S11B) to a self-assembled monolayer of 3-mercaptopropionic acid. Stability of this antibody-modified array was observed for over 14 days with minimal signal loss (supplementary information Fig. S12A). Electrochemical array chips were blocked with a solution of 2% BSA to address complications arising from non-specific binding to the working electrode surface. Once blocked and washed, arrays were positioned into the microfluidic device. Immediately, they were washed with PBS-T-20 followed by an injection of solution containing an optimized biotinylated secondary HER-2 antibody concentration of 3.125 μg mL−1 (Ab2) (supplementary material Fig. S11A), optimized dilution of poly-HRP at 1:250 in 0.1% BSA (poly-HRP) (supplementary material Fig. S11C), and a sample concentration ranging from (Ag) (2 pg mL−1 to 12.5 ng mL−1) in fivefold diluted calf serum. Controls contained the full immunoassay without HER-2. Amperometric responses were generated at a potential of −0.15 V vs Ag/AgCl, using a mixture of 1mM hydroquinone (HQ) mediator and 0.1 mM H2O2 injected into the microfluidic device through a 200 μL sample loop. H2O2 activates the iron heme protein on the sensor surface converting it to the ferryloxy form, the electron mediator HQ, then reduces the activated ferryloxy HRP, regenerating HRP refer to Fig. 1D for full schematic.
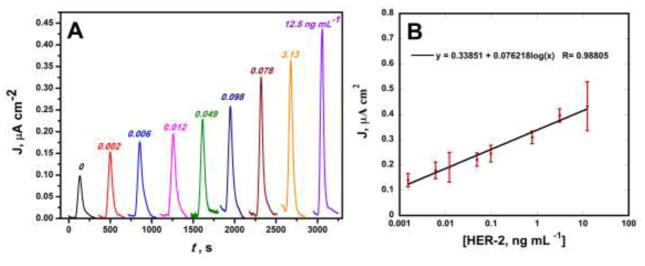
Immunoassay protocols featured streptavidin poly-HRP, consisting of polymers of multiple HRP molecules conjugated to streptavidin, binding to a biotinylated HER-2 secondary antibody. This enables for increased molar ratios of HRP per biotin binding event allowing for greater signal amplification as well as for shorter assay times. In contrast to previous reports of amplification strategies, using magnetic beads conjugated with hundreds of thousands of enzyme labels and thousands of antibodies, it enabled for a rapid amplification without the need for a conjugation of antibodies and target analytes to a beads surface further simplifying the immunoassay protocol (Krause et al., 2013)(Otieno et al., 2014)(Otieno et al., 2016)(Uliana et al., 2018). Following protocols outlined above, amperometric responses demonstrated high sensitivity over three decades of concentration from nanogram per milliliter to low picrogram per milliliter (Fig. 3A). Following the calibration curve in Fig. 3B, a linear relationship between amperometric signal and log protein concentration was determined with R2 value of 0.99 with small error bars indicating the excellent reproducibility of the electrochemical array chips. A detection limit of 12 pg mL−1, calculated as 3 standard deviation above the average control, was demonstrated for detection of HER-2 in 5-fold diluted calf serum, an excellent surrogate for human serum (Rusling et al., 2010) (Hanash et al., 2008). Additional selectivity studies of the biosensor were preformed in the presence of BSA with minimal signal response (see supporting information Fig. S12B).
Fig. 3.
(A) Amperometric responses for HER-2 in 5X diluted calf serum at −0.15 V vs Ag/AgCl following injection of 1 mM HQ and 0.1 mM H2O2. (B) Corresponding calibration curve y= 0.34 + 0.076 log (x); R= 0.99.
At the dynamic range observed in Fig. 3B, the assay lies in the clinically relevant range of HER-2 with ultrafast assay time of only 15 minutes. This rapid rate is well below the speed of ELISA (> 5 hrs) (Findlay et al., 2000) as well as other commercial assays. Sensitivities of the assay reported from the slope of the calibration curve was 0.076 μA cm−2 log [C]−1 comparable to that of bead based assays without the need for a conjugate preparation step (Krause et al., 2013)(Otieno et al., 2014)(Otieno et al., 2016).
3.3 Accuracy Validation of the Inkjet printed Electrochemical Sensor Chip
The accuracy of the fully inkjet printed sensor chip was validated by determining HER-2 in spiked pooled Human Serum though spiked recovery assays. These assays were carried out with the addition of 4 standard concentrations HER-2 to human serum, a fivefold dilution was required in order to achieve signals in the range of the analytical curve and compared to running spiked pooled human serum with a commercial ELISA Kit (Table S1). An average recovery of 91.3% was achieved with a range of 76.3–104%, demonstrating the ability to accurately and reliably measure HER-2 without matrix interference effects. At this recovery average and range, it is comparable to several commercially available ELISA kits for the detection of HER-2 in serum. (“ErbB2 Human ELISA Kit - Thermo Fisher Scientific,” n.d.), (“Human ErbB2/Her2 Quantikine ELISA Kit DHER20: R&D Systems,” n.d.).
Conclusions
Inkjet printing offers distinct advantages over conventional methods for low-cost fabrication of electrodes including rapid production, high resolution and minimal sample waste (Kamyshny, 2011)(Khan et al., 2016)(Komuro et al., 2013)(Li et al., 2015). The fully inkjet printed electrochemical platform featuring onboard counter and reference electrodes described here could be manufactured with total material cost of less than $0.25. This method of production could easily be scaled for commercial use with an industrial sized inkjet printer. Following this low-cost and simple method of fabrication a highly reproducible sensor platform was easily adapted for electrochemical immunosening of clinically relevant HER-2 in serum. A low detection limit of 12 pg mL1 was achieved in a total assay time of 15 minutes. In addition, the assay demonstrated high sensitivity in over three decades of concentration from nanogram per milliliter to low picogram per milliliter. At this dynamic range the assay remains within the clinically relevant range for detection of HER-2 in serum (Tchou et al., 2015) (Banys-Paluchowski et al., 2017). Moreover, the capability to detect HER-2 at this low detection limit with excellent sensitivity suggests a high selectivity as calf and human serums are complex medias containing hundreds to thousands of competing proteins (Rusling et al., 2010)(Hanash et al., 2008). This sensor platform could easily be modified for multiplex detection of a panel of proteins for breast cancer screening and large patient sample analysis. Efforts are currently underway to improve in the portability of the device for point –of –care use.
Supplementary Material
Highlights.
Commercially scalable fully inkjet printed electrochemical array platform with on-board reference and counter electrodes.
Inexpensive ($0.25) disposable sensor platform.
Easy to use and simple instrumentation for immunosensing.
Ultrafast assay (15 minutes) for detection of clinically relevant breast cancer biomarker in serum.
Acknowledgments
We like to thank Snehasis Bhakta for helping with TEM imaging and Esraa Elsanadidy for helping with DLS. Financial support from The University of Hartford startup funds and the Vincent B. Coffin Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Khafaji QAM, Harris M, Tombelli S, Laschi S, Turner APF, Mascini M, Marrazza G. An Electrochemical Immunoassay for HER2 Detection. Electroanalysis. 2012;24:735–742. [Google Scholar]
- Arduini F, Micheli L, Moscone D, Palleschi G, Piermarini S, Ricci F, Volpe G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal Chem. 2016;79:114–126. [Google Scholar]
- Banys-Paluchowski M, Witzel I, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer EF, Aktas B, Kasimir-Bauer S, Pantel K, Fehm T, Müller V. Clinical Relevance of Serum HER2 and Circulating Tumor Cell Detection in Metastatic Breast Cancer Patients. Anticancer Res. 2017;37:3117–3128. doi: 10.21873/anticanres.11669. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ren W, Gao L, Liu B, Pei S, Cheng HM. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat Mater. 2011;10:424–8. doi: 10.1038/nmat3001. [DOI] [PubMed] [Google Scholar]
- ErbB2 Human ELISA Kit - Thermo Fisher Scientific [WWW Document] n.d URL https://www.thermofisher.com/order/catalog/product/EHERBB2?gclid=CjwKCAiA3o7RBRBfEiwAZMtSCSBFOJd2DDpT2AzhiesvNYL_87NKxuPJ2EoHh0WhXri2ktbTL9DguBoCTOoQAvD_BwE&s_kwcid=AL!3652!3!231736819839!b!!g!!%2Bher2%2Belisa&ef_id=WSNOnAAAAF4Du3pm:20171204010829:s.
- Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, Khan MN, Bowsher RR. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal. 2000;21:1249–73. doi: 10.1016/s0731-7085(99)00244-7. [DOI] [PubMed] [Google Scholar]
- Fourkas JT. Nanoscale Photolithography with Visible Light. J Phys Chem Lett. 2010;1:1221–1227. [Google Scholar]
- Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res. 2015;4:256–269. doi: 10.3978/j.issn.2218-676X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–9. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- Human ErbB2/Her2 Quantikine ELISA Kit DHER20: R&D Systems [WWW Document] n.d URL https://www.rndsystems.com/products/human-erbb2-her2-quantikine-elisa-kit_dher20.
- Jensen GC, Krause CE, Sotzing Ga, Rusling JF. Inkjet-printed gold nanoparticle electrochemical arrays on plastic. Application to immunodetection of a cancer biomarker protein. Phys Chem Chem Phys. 2011;13:4888–94. doi: 10.1039/c0cp01755h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamyshny A. Metal-based Inkjet Inks for Printed Electronics. Open Appl Phys J. 2011;4:19–36. [Google Scholar]
- Kamyshny A, Steinke J, Magdassi S. Metal-based Inkjet Inks for Printed Electronics. Open Appl Phys J. 2011:4. [Google Scholar]
- Khan Y, Pavinatto FJ, Lin MC, Liao A, Swisher SL, Mann K, Subramanian V, Maharbiz MM, Arias AC. Inkjet-Printed Flexible Gold Electrode Arrays for Bioelectronic Interfaces. Adv Funct Mater. 2016;26:1004–1013. [Google Scholar]
- Kingsmore SF. Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat Rev Drug Discov. 2006;5:310–20. doi: 10.1038/nrd2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro N, Takaki S, Suzuki K, Citterio D. Inkjet printed (bio)chemical sensing devices. Anal Bioanal Chem. 2013;405:5785–5805. doi: 10.1007/s00216-013-7013-z. [DOI] [PubMed] [Google Scholar]
- Krause CE, Otieno BA, Latus A, Faria RC, Patel V, Gutkind JS, Rusling JF. Rapid Microfluidic Immunoassays of Cancer Biomarker Proteins Using Disposable Inkjet-Printed Gold Nanoparticle Arrays. ChemistryOpen. 2013;2:141–145. doi: 10.1002/open.201300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs FC, Jørgensen M, Norrman K, Hagemann O, Alstrup J, Nielsen TD, Fyenbo J, Larsen K, Kristensen J. A complete process for production of flexible large area polymer solar cells entirely using screen printing—First public demonstration. Sol Energy Mater Sol Cells. 2009;93:422–441. [Google Scholar]
- Lessing J, Glavan AC, Walker SB, Keplinger C, Lewis JA, Whitesides GM. Inkjet printing of conductive inks with high lateral resolution on omniphobic “R(F) paper” for paper-based electronics and MEMS. Adv Mater. 2014;26:4677–82. doi: 10.1002/adma.201401053. [DOI] [PubMed] [Google Scholar]
- Li D, Chan DW. Proteomic cancer biomarkers from discovery to approval: it’s worth the effort. Expert Rev Proteomics. 2014;11:135–6. doi: 10.1586/14789450.2014.897614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Rossignol F, Macdonald J. Inkjet printing for biosensor fabrication: combining chemistry and technology for advanced manufacturing. Lab Chip. 2015;15:2538–2558. doi: 10.1039/c5lc00235d. [DOI] [PubMed] [Google Scholar]
- Metters JP, Kadara RO, Banks CE. New directions in screen printed electroanalytical sensors: an overview of recent developments. Analyst. 2011;136:1067–76. doi: 10.1039/c0an00894j. [DOI] [PubMed] [Google Scholar]
- Moya A, Gabriel G, Villa R, Javier del Campo F. Inkjet-printed electrochemical sensors. Curr Opin Electrochem. 2017 doi: 10.1016/j.coelec.2017.05.003. [DOI] [Google Scholar]
- Munge BS, Stracensky T, Gamez K, DiBiase D, Rusling JF. Multiplex Immunosensor Arrays for Electrochemical Detection of Cancer Biomarker Proteins. Electroanalysis. 2016;28:2644–2658. doi: 10.1002/elan.201600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otieno BA, Krause CE, Latus A, Chikkaveeraiah BV, Faria RC, Rusling JF. On-line protein capture on magnetic beads for ultrasensitive microfluidic immunoassays of cancer biomarkers. Biosens Bioelectron. 2014;53:268–274. doi: 10.1016/j.bios.2013.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otieno BA, Krause CE, Rusling JF. Bioconjugation of Antibodies and Enzyme Labels onto Magnetic Beads. Methods Enzymol. 2016;571:135–50. doi: 10.1016/bs.mie.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Rusling JF, Kumar CV, Gutkind JS, Patel V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst. 2010;135:2496–511. doi: 10.1039/c0an00204f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Chen B. Testing for HER2 in Breast Cancer: A Continuing Evolution. Patholog Res Int. 2010;2011:903202. doi: 10.4061/2011/903202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AC, Phillips ST, Dickey MD, Lu N, Suo Z, Whitesides GM. Foldable Printed Circuit Boards on Paper Substrates. Adv Funct Mater. 2010;20:28–35. [Google Scholar]
- Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brünner N, Chan DW, Babaian R, Bast RC, Dowell B, Esteva FJ, Haglund C, Harbeck N, Hayes DF, Holten-Andersen M, Klee GG, Lamerz R, Looijenga LH, Molina R, Nielsen HJ, Rittenhouse H, Semjonow A, Shih IM, Sibley P, Sölétormos G, Stephan C, Sokoll L, Hoffman BR, Diamandis EP National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54:e11–79. doi: 10.1373/clinchem.2008.105601. [DOI] [PubMed] [Google Scholar]
- Tallapragada SD, Layek K, Mukherjee R, Mistry KK, Ghosh M. Development of screen-printed electrode based immunosensor for the detection of HER2 antigen in human serum samples. Bioelectrochemistry. 2017;118:25–30. doi: 10.1016/j.bioelechem.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Tchou J, Lam L, Li YR, Edwards C, Ky B, Zhang H. Monitoring serum HER2 levels in breast cancer patients. Springerplus. 2015;4:237. doi: 10.1186/s40064-015-1015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasatti S, Petrii OA. Real surface area measurements in electrochemistry. Pure Appl Chem. 1991;63:711–734. [Google Scholar]
- Uliana CV, Peverari CR, Afonso AS, Cominetti MR, Faria RC. Fully disposable microfluidic electrochemical device for detection of estrogen receptor alpha breast cancer biomarker. Biosens Bioelectron. 2018;99:156–162. doi: 10.1016/j.bios.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Wang J. Electrochemical biosensors: towards point-of-care cancer diagnostics. Biosens Bioelectron. 2006;21:1887–92. doi: 10.1016/j.bios.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Chandra P, Shim YB. Ultrasensitive and Selective Electrochemical Diagnosis of Breast Cancer Based on a Hydrazine–Au Nanoparticle–Aptamer Bioconjugate. Anal Chem. 2013;85:1058–1064. doi: 10.1021/ac302923k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.