Abstract
Objective
Suicide is a public health crisis with limited treatment options. We conducted a systematic review and individual participant data meta-analysis examining the effects of a single dose of ketamine on suicidal ideation.
Method
Individual participant data were obtained from 10 of 11 identified comparison intervention studies (using either saline or midazolam as control). The analysis included only participants with suicidal ideation at baseline (n=167). A one-stage, individual participant data, meta-analytic procedure was employed using a mixed-effects, multilevel, general linear model. The primary outcome measures were the suicide items from clinician-administered (Montgomery-Asberg Depression Rating Scale (MADRS) or Hamilton Depression Rating Scale (HAM-D)) and self-reported scales (Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) or Beck Depression Inventory (BDI)), obtained for up to one week post-ketamine administration.
Results
Ketamine rapidly reduced (one day) suicidal ideation on both the clinician-administered (p<0.001) and self-reported outcome measures (p<0.001). Effect sizes were moderate-to-large (Cohen’s d=0.51–0.85) at all time points post-dose. Sensitivity analysis demonstrated that, compared to controls, ketamine had significant benefits on the individual suicide items of the MADRS, HAM-D, and QIDS-SR (all p<0.001) but not on the BDI (p=0.080). Ketamine’s effect on suicidal ideation remained significant after adjusting for concurrent changes in severity of depressive symptoms.
Conclusions
Ketamine rapidly reduced suicidal thoughts within one day and for up to one week in depressed patients with suicidal ideation. Ketamine’s effects on suicidal ideation were partially independent of its effects on mood, though subsequent trials in transdiagnostic samples are required to confirm that ketamine exerts a specific effect on suicidal ideation. Additional research on ketamine’s long-term safety and its efficacy in reducing suicide risk is needed before clinical implementation.
Introduction
Suicide is a public health crisis and ranks among the top three causes of mortality worldwide for individuals ages 15–44 (1, 2). Unfortunately, the suicide rate has increased over the last two decades despite renewed efforts to address this crisis (3). Studies suggest that approximately 90% of individuals who commit suicide suffer from a treatable psychiatric disorder, most commonly a mood disorder (4). Nevertheless, current treatment options for patients at acute risk for suicide are limited and generally consist of hospitalization plus pharmacotherapy, psychotherapy, electroconvulsive therapy (ECT), or a combination thereof. The National Action Alliance for Suicide Prevention has highlighted the importance of identifying fast-acting interventions for suicidal individuals as a critical research goal to reduce the suicide rate (5). Treatment with lithium and clozapine, as well as dialectical behavioral therapy (DBT) and cognitive behavioral therapy (CBT), have been shown to reduce suicide deaths (2, 6, 7) and the rate of suicide attempts (8, 9). However, while these treatments and interventions function to reduce suicide risk long-term, they have not been shown to be effective in acute settings.
Since 2000, several small clinical trials have demonstrated that sub-anesthetic doses of ketamine have rapid-acting antidepressant properties (10–14) as well as potential anti-suicidal properties (15–19) in patients with mood disorders (both major depressive disorder (MDD) and bipolar depression). Given ketamine’s rapid antidepressant effects, considerable interest exists regarding its potential ability to stabilize patients suffering from mood disorders at imminent risk of suicide. Here, we performed a systematic review and meta-analysis using patient-level data of explicit measures of suicidal ideation to assess ketamine’s potential anti-suicidal effects.
Method
Study Identification and Selection
Two reviewers (STW, EDB) searched MEDLINE using the terms “ketamine”, “NMDA receptor antagonist”, “ketamine-like” or “rapid antidepressant” and “suicide”, “suicidality” or “suicidal ideation” (filter: clinical trials) between January 1, 2000 and November 15, 2016. The identified systematic reviews and meta-analyses were searched for relevant published and unpublished research. Additional studies were identified through cross-reference or communication with investigators.
This meta-analysis investigated studies of single-dose intravenous ketamine for the treatment of any psychiatric disorder; comparison intervention trials (using saline placebo or midazolam as a control, henceforth referred to as the “control”) were included. Studies that administered multiple ketamine doses were excluded. The corresponding authors of identified publications were contacted to provide individual subject data regarding how suicidal ideation was assessed in each potentially eligible trial. Authors also provided individual suicide assessment scores, individual depression severity scores, and baseline demographic and clinical information.
Statistical Analysis
Patient-level data were collected for several distinct variables, including: 1) suicidal ideation, assessed via two clinician-administered and two self-reported rating scales (Montgomery-Åsberg Depression Rating Scale (MADRS) item 10; 17-item Hamilton Depression Rating Scale (HAM-D) item 3; Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) item 12; and/or Beck Depression Inventory (BDI) item 9); 2) overall severity of depressive symptoms, assessed via MADRS, HAM-D, QIDS-SR, and/or BDI total scores); 3) treatment assignment (ketamine or control); and 4) potential moderators of treatment effect (age, gender, race, inpatient vs. outpatient status, use of concomitant medications). Whenever available, all data were collected from each investigator for baseline and Days 1, 2, 3, and 7 post-ketamine. Our method for handling missing data can be found in the Supplemental Information. Supplemental Table 1 lists which rating scales were used in each study.
Because this study sought to determine the effects of ketamine on suicidal ideation, subjects with no suicidal ideation at baseline were excluded from the analysis. Based on prior literature (15), we included active or passive suicidal ideation, which was operationalized a priori as a score ≥2 on MADRS item 10 (“weary of life/fleeting suicidal ideation”) or ≥1 on the HAM-D suicidal ideation item (“feels life is not worth living”) for the clinician-administered scales; for the self-report scales, suicidal ideation was defined as a score ≥1 on the QIDS-SR item 12 (“I feel that life is empty or wonder if it’s worth living”) or ≥1 on BDI item 9 (“I have thoughts of killing myself, but I would not carry them out”).
We used a standard one-stage, hierarchical modeling approach (20), with participants nested within studies. A general linear mixed-model (GLMM) was used; the dependent variable was suicidal ideation. The specific hypothesis tested using this model was that ketamine would resolve suicidal ideation more rapidly than the control condition. The following independent variables were used in the first model: baseline suicidal ideation held constant over time, group, time, and a group x time interaction. The following covariates were included in an additional model: age, gender, race, treatment setting (inpatient vs. outpatient), diagnosis, and whether the patient was taking concomitant psychotropic medications. In a final model, we dropped terms that had no main effect and adjusted for changes in severity of depressive symptoms over time in an attempt to assess whether ketamine’s effects on suicidal ideation were independent of its effects on other depressive symptoms. In these analyses, the suicide items were removed from the composite depression score.
Dichotomous outcomes (being free of suicidal ideation) among those with some level of baseline suicidal ideation were also analyzed in two separate models (using self-reported and clinician-administered rating scales; see Supplemental Information).
Effect sizes (Cohen’s d) were calculated using mean differences between baseline and each time point (Days 1, 2, 3, and 7 post-infusion). For all analyses, significance was set at p<0.05, two-tailed. Because several trials showed evidence of carryover effects associated with ketamine treatment, we only analyzed data from the first treatment (control or ketamine) in crossover trials.
The Supplemental Information describes how data from different scales were combined.
Correlations between change in suicidal ideation and overall severity of depressive symptoms were calculated using Pearson correlation coefficients. Changes were calculated between baseline and Days 1, 2, 3, and 7 post-ketamine infusion. For this analysis, suicide items were removed from the overall composite depression score.
Results
Included Studies
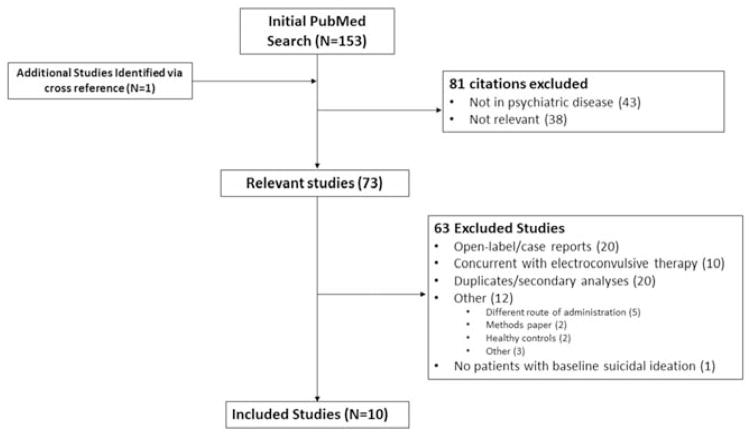
Eleven eligible trials were identified from 153 citations (Figure 1). Corresponding authors provided individual subject data for 10 of these 11 potentially eligible citations (10–14, 18, 21–24). Correspondence with authors of the remaining study (25) indicated that none of the subjects had baseline suicidal ideation and would thus not have been eligible for inclusion in the present analysis. It should be noted that three of these studies were conducted at the National Institutes of Health (NIH) (11–13) under a single protocol and were included in a review of ketamine’s ability to reduce suicidal ideation (15). Because these three studies were done under a single protocol, for purposes of statistical modeling, we considered this a single study (hence, k=8). In addition to participants from these published trials, 36 additional participants analyzed in the prior review were also included in this analysis (15).
Figure 1.
A flowchart depicting the procedure for selecting eligible trials from identified references.
Characteristics of the Included Sample
Individual patient-level data were obtained for 298 subjects who participated in the 10 included ketamine trials; 167 subjects met criteria for baseline suicidal ideation. Study characteristics appear in Table 1 and patient demographics appear in Supplemental Table 2. Subjects who were included for analysis did not differ from subjects who were excluded in terms of age (t=0.105, p=0.917) or gender (chi-square=0.27, p=0.604). Overall, included subjects had more severe depressive symptoms at baseline than excluded subjects as measured by the MADRS (33.4 vs. 25.9, t=9.08, p<0.001), HAM-D (20.5 vs. 16.3, t=4.8, p<0.001), QIDS-SR (17.7 vs. 14.2, t=4.81, p=<0.001), and BDI (29.2 vs. 21.1, t=5.62, p<0.001). Included subjects were also more likely than excluded subjects to be receiving concomitant psychotropic medications (47.9% vs. 27.5%, chi-square=12.9, p=<0.001).
Table 1.
Characteristics of studies included in the meta-analysis
| Reference | Total N | Included N | Setting | Control | Diagnosis | Patients with Concomitant Medications* | |
|---|---|---|---|---|---|---|---|
| N | % | ||||||
| Berman et al 2000 (10) | 8 | 5 | Outpatient | Saline | MDD | 0 | 0 |
| Valentine et al 2011 (25) | 11 | 4 | Outpatient | Saline | MDD | 0 | 0 |
| Sos et al 2013 (24) | 27 | 9 | Inpatient | Saline | MDD | 9 | 100 |
| Murrough et al 2013 (14) | 73 | 35 | Both** | Midazolam | MDD | 0 | 0 |
| Feder et al 2014 (22) | 41 | 5 | Both** | Midazolam | PTSD | 0 | 0 |
| Ballard et al 2014† (15) | 87 | 59 | Inpatient | Saline | MDD/BD | 26 | 44 |
| Hu et al 2015 (23) | 27 | 26 | Outpatient | Saline | MDD | 26 | 100 |
| Murrough et al 2015 (18) | 24 | 24 | Inpatient/Outpatient | Midazolam | Mixed†† | 19 | 79 |
| Total | 298 | 167 | 80 | 48 | |||
Abbreviations: MDD: major depressive disorder; BD: Bipolar Disorder; PTSD: posttraumatic stress disorder
Concomitant medications included antidepressants, antipsychotics, or mood stabilizers
Subjects were admitted as inpatients for ketamine infusion and then discharged as outpatients 24 hours following infusion
This was a review and secondary analysis of ketamine data from a research protocol evaluating the effects of ketamine on depressive symptoms (NCT00088699); publications from these results include Zarate CA et al., 2006 (11); Diazgranados et al., 2010 (13); and Zarate et al., 2012 (12).
The inclusion criterion for this investigation was suicidal ideation, not a specific DSM diagnosis. The most common diagnoses were MDD, BD, and PTSD.
Among subjects who were included, those who received ketamine were not different from those who received control in terms of age (t=-0.27, p=0.788), gender (chi-square=1.01, p=0.314), diagnosis (chi-square=0.40, p=0.527), inpatient v. outpatient status at time of drug exposure (chi-square=0.52, p=0.470), proportion receiving concomitant psychotropic medications (chi-square=1.23, p=0.268), or baseline MADRS score (t=0.867, p=0.388).
The Effects of Ketamine on Clinician-Administered Suicidal Ideation Item Scores
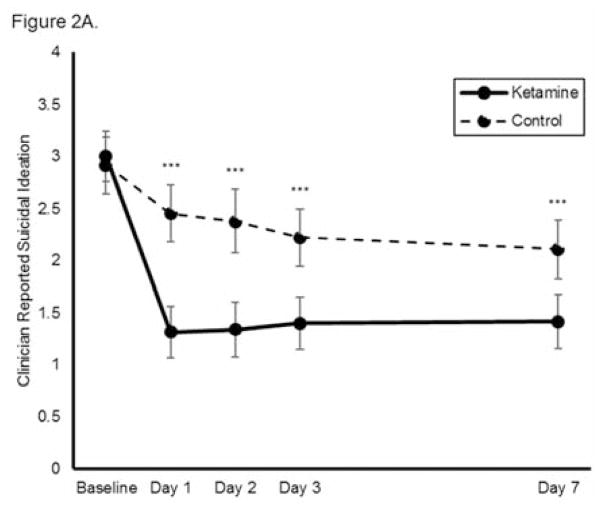
As assessed via clinician-administered rating scales, ketamine reduced suicidal ideation more rapidly than was observed in the control group, with significant benefits appearing as early as Day 1 and extending up to Day 7 (MADRS/HAM-D, total n=167, k=8, group x time interaction chi square=50.6, p<0.001, Figure 2A). The benefits of ketamine compared to control (either saline or midazolam, depending on the source study) remained significant after baseline covariates (age, gender, race, use of concomitant psychotropic medications, outpatient vs. inpatient status, diagnosis) were adjusted for in the model (group x time interaction chi square=50.5, p<0.001). None of the baseline variables significantly moderated ketamine’s effects on suicidal ideation. The mean (SD) MADRS scores for the group exposed to ketamine were 33.8 (6.8) at baseline, 19.5 (12.3) at Day 1, 19.4 (12.1) at Day 2, 20.1 (12.2) at Day 3, and 22.0 (11.5) at day 7.
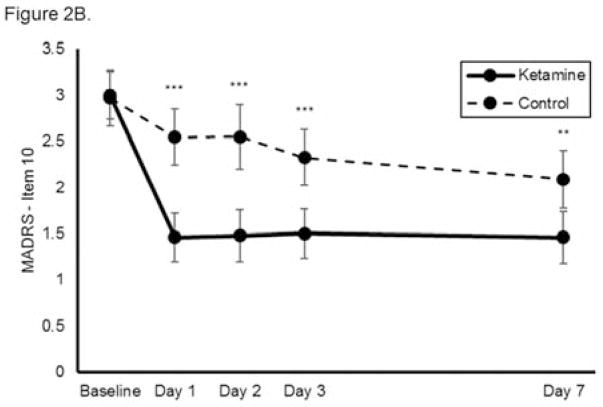
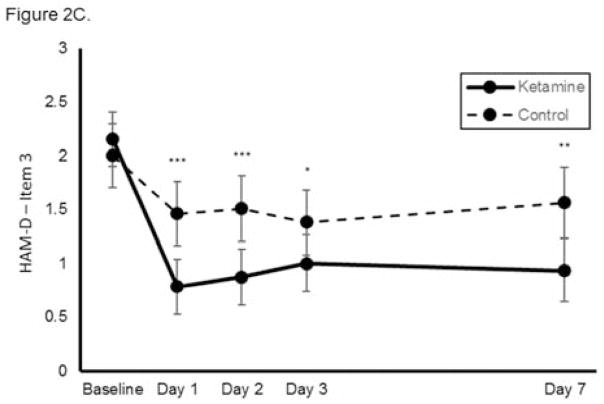
Figure 2.
Effect of a single dose of ketamine on suicidal ideation. Means from multilevel, mixed effects, general linear model (subjects nested within studies) adjusting for baseline suicidal ideation and between-study effects. Outcomes are: (A) combined clinician-reported, converted to Montgomery-Åsberg Depression Rating Scale (MADRS) units (N=167, k=8, chi-square=50.6, p<0.001 for overall time x treatment interaction), (B) MADRS item 10 (N=140, k=6, chi-square=35.0, p<0.001 for overall time x treatment interaction), and (C) Hamilton Depression Rating Scale (HAM-D) item 3 (N=89, k=4, chi-square=19.4; p<0.001 for overall time x treatment interaction); *p<0.05, **p<0.01, ***p<0.001. Error bars are 95% confidence intervals.
The mean (SD) MADRS scores for the group exposed to control were 32.9 (6.5) at baseline, 29.2 (9.3) at Day 1, 29.1 (10.5) at Day 2, 27.3 (9.8) at Day 3, and 27.5 (10.0) at Day 7.
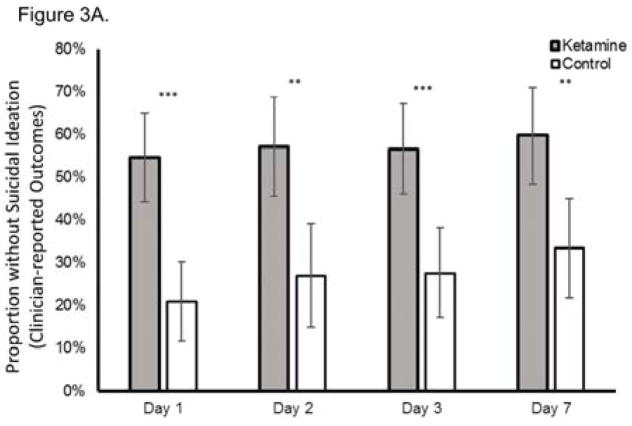
When each clinician-administered rating scale outcome was analyzed separately, ketamine continued to reduce suicidal ideation significantly more rapidly than control on both the MADRS (chi-square=35.0, p<0.001, group x time interaction; Figure 2B) and the HAM-D (chi-square=19.4; p<0.001, group x time interaction; Figure 2C). Meta-analysis demonstrated little heterogeneity between studies (F796=1.00, p=0.500). Effect sizes (group difference divided by pooled standard deviation) for ketamine on change in suicidal ideation were moderate-to-large for the clinician-administered rating scales at all time points (at Day 1, Cohen’s d=0.85, 95% CI 0.53–1.17; at Day 2, d=0.85, 95% CI 0.52–1.17; at Day 3, d=0.67, 95% CI 0.35–0.99; at Day 7, d=0.61, 95% CI 0.27–0.94). In addition, ketamine was associated with a significantly greater proportion of subjects being free from suicidal ideation, as assessed by clinician-administered ratings compared to the control group at Days 1, 2, 3, and 7 post-ketamine infusion; over half of participants reported no suicidal ideation across all time points (all t<−2.95, all p<0.005; Figure 3A). The Number Needed to Treat (NNT) for ketamine (compared to control) for being free of suicidal ideation was 3.1–4.0 for all time points one to seven days following ketamine infusion.
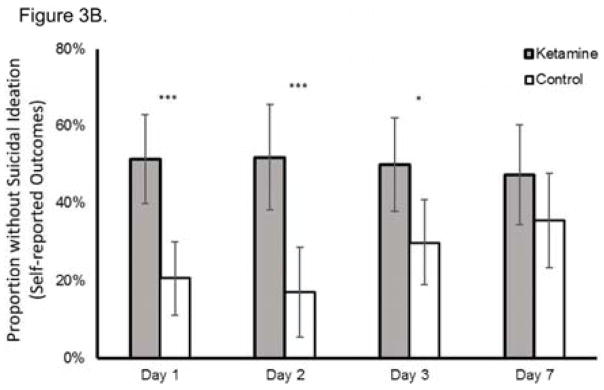
Figure 3.
Proportion of subjects without suicidal ideation at each time point post-dose using (A) clinician-reported outcome measures (Montgomery-Asberg Depression Rating Scale (MADRS) or Hamilton Depression Rating Scale (HAM-D)) and (B) self-reported outcome measures (Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR) or Beck Depressive Inventory (BDI)); *p<0.05, **p<0.01, ***p<0.001. Error bars are 95% confidence intervals.
The Effects of Ketamine on Self-Reported Suicidal Ideation
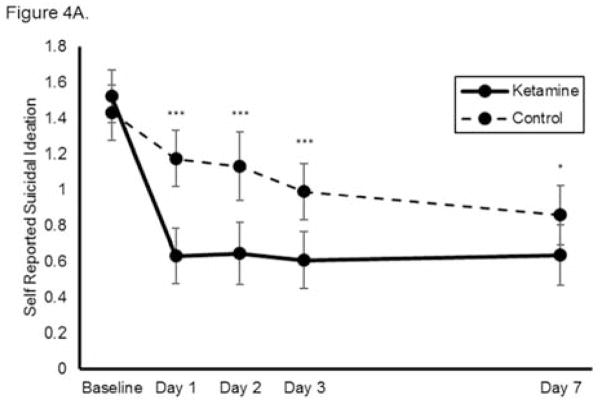
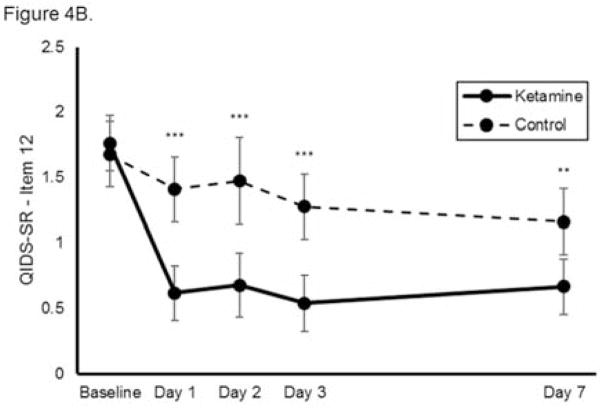
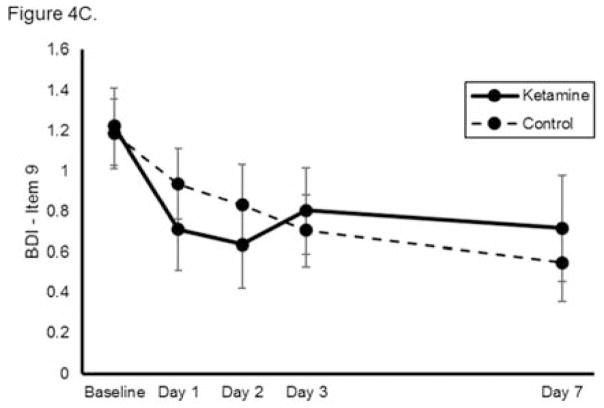
Using self-reported outcome measures, ketamine similarly reduced suicidal ideation significantly more rapidly than was observed in the control group, with significant benefits noted at each individual time point (n=144, k=8, group x time interaction chi-square=45.5, p<0.001, Figure 4A). The benefits of ketamine compared to control remained significant after baseline covariates were adjusted for in the model. None of the baseline variables significantly moderated ketamine’s effects on suicidal ideation. When each self-reported measure was analyzed separately, ketamine reduced suicidal ideation significantly more rapidly than was observed in the control group on the QIDS-SR (chi-square=32.5, p<0.001, group x time interaction; Figure 4B) but not the BDI (chi-square=8.34, p=0.080, group x time interaction; Figure 4C). Notably, these analyses had smaller sample sizes (n=77 and n=67, respectively) because not all studies used each measure. Meta-analysis demonstrated little heterogeneity between studies (F676=1.00, p=0.500).
Figure 4.
Effect of a single dose of ketamine on suicidal ideation. Means from multilevel, mixed effects, general linear model (subjects nested within studies), adjusting for baseline suicidal ideation and between-study effects. Outcomes are: (A) combined self-reported scale scores (N=144, k=8, chi-square=45.5, p<0.001 for overall group x time interaction); (B) Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) item 12 (N=77, k=4, chi-square=32.5, p<0.001 for overall group x time interaction); and (C) Beck Depression Inventory (BDI) item 9 (N=67, k=4, chi-square=8.34, p=0.080 for overall group x time interaction); *p<0.05, **p<0.01, ***p<0.001. Error bars are 95% confidence intervals.
Effect sizes (group difference divided by pooled standard deviation) of ketamine on change in suicidal ideation were moderate-to-large on self-reported outcomes (at Day 1, Cohen’s d=0.73, 95% CI 0.38–1.07; at Day 2: d=0.84, 95% CI 0.49–1.19; at Day 3, d=0.63, 95% CI 0.28–0.98; at Day 7, d=0.48, 95% CI 0.12–0.83). Ketamine was again associated with a significantly greater proportion of subjects being free from suicidal ideation compared to the control group at Days 1, 2, and 3 days post-ketamine infusion (all t<−2.30; all p<0.05) but not at Day 7 (t=−1.18, p=0.238) (Figure 3B). The NNT for ketamine (compared to control) for being free of suicidal ideation was 3.2–5.0 for all time points one to seven days following ketamine infusion, except at Day 7 (NNT=9.6).
Correlation Between Suicidal Ideation and Severity of Depressive Symptoms
Changes in suicidal ideation and overall severity of depressive symptoms were strongly correlated at all time points. As measured using clinician-administered rating scales, change in suicidal ideation and change in severity of depressive symptoms were significantly correlated at Day 1 (adjusted r2=0.411, t=10.73, p<0.001), Day 2 (adjusted r2=0.460, t=11.66, p<0.001), Day 3 (adjusted r2=0.370, t=9.60, p<0.001), and Day 7 (adjusted r2=0.414, t=10.08, p<0.001). Using self-reported outcome measures, change in suicidal ideation and change in severity of depressive symptoms were significantly correlated at Day 1 (adjusted r2=0.405, t=9.75, p<0.001), Day 2 (adjusted r2=0.212, t=5.46, p<0.001), Day 3 (adjusted r2=0.208, t=5.95, p<0.001), and Day 7 (adjusted r2=0.103, t=3.83, p<0.001).
Independence of Improvement Measures for Suicidal Ideation and Change in Severity of Depressive Symptoms
After adjusting for change in severity of depressive symptoms over time, the time x treatment interaction remained significant regardless of whether clinician-administered (chi square=10.84, p=0.028) or self-reported (chi square=13.19, p=0.010) outcome measures were used. Significant differences were observed between groups at all post-dose time points using clinician-administered outcome measures (all t<−2.30, all p<0.05). With self-reported outcome measures, significant differences were noted at Day 1 (t=−2.80, p=0.005), Day 2 (t=−2.62, p=0.009), and Day 3 (t=−1.99, p=0.047) but not Day 7 (t=−0.36, p=0.720).
Durability of effect
Among the group of subjects who achieved a resolution of suicidal ideation (as measured by clinician-reported measures) by 24 hours post-dose, the effect of ketamine on reduced suicidal ideation persisted for up to one week in 86.0% of subjects compared to 52.9% of subjects in the control condition (chi-square=7.98, p=0.005). Using self-reported outcomes, the effect of ketamine on suicidal ideation persisted for up to one week in 89.2% of subjects in the ketamine group compared to 42.9% in the control group (chi-square=12.1, p<0.001).
Midazolam versus saline as control
To assess the differences of midazolam versus saline as control, we calculated effect sizes (group difference divided by pooled standard deviation) separately for saline or midazolam as control condition. The outcome was the change in suicidal ideation from baseline (clinician-rated outcomes) at each time point post-dose. When only saline was used as a comparator (N=50 saline group, N=93 ketamine group), the effect sizes were as follows: at Day 1, d=0.90, 95% CI 0.54–1.26; at Day 2, d=0.90, 95% CI 0.53–1.26; at Day 3, d=0.82, 95% CI 0.45–1.19; at Day 7, d=0.69, 95% CI 0.31–1.07. When only midazolam was used as a comparator (N=24 midazolam group, N=93 ketamine group), the effect sizes were more modest: at Day 1, d=0.62, 95% CI 0.16–1.08; at Day 2, d=0.69, 95% CI 0.22–1.15; at Day 3, d=0.34, 95% CI −0.12–0.80; at Day 7, d=0.41, 95% CI −0.06–0.88. Comparing the effect of midazolam (n=24) versus saline (n=50) on suicidal ideation in the linear mixed model showed no group x time interaction nor main effect of group.
Discussion
This study is the first meta-analysis to use individual participant-level data to examine the effects of ketamine on suicidal ideation specifically in participants with some level of baseline suicidal ideation. We found that ketamine significantly reduced suicidal ideation, with moderate-to-large effect sizes observed within one day that extended one week post-ketamine administration. We also found that patients treated with ketamine were significantly more likely to be free of suicidal ideation at all time points post-ketamine (except Day 7 as assessed by self-reported outcome measures). Change in severity of depressive symptoms was strongly correlated with change in suicidal ideation and accounted for 10–46% of the variance in change in suicidal ideation. Notably, after controlling for improvement in severity of depressive symptoms, ketamine’s effects on suicidal ideation remained significant. This suggests that ketamine has a specific effect on suicidal ideation that depends only partly on change in overall severity of depressive symptoms. The study extends the existing literature by using an analytic sample comprising exclusively participants with some level of suicidal ideation—at an individual participant level—from all single-dose, comparison intervention trials of intravenous ketamine.
Taken together, these results suggest that ketamine’s salutary effects on suicidal ideation hold considerable promise, particularly given the lack of treatment options for patients who may be at risk of suicide. Indeed, in the present study, 54.9% of patients were free of suicidal ideation 24 hours after a single ketamine infusion, and 60.0% were free of suicidal ideation at one week post-ketamine. In comparison, in an open-label ECT study, 38.2% of 131 patients reporting significant suicidal ideation were free of suicidal ideation after three treatments (one week), 61.1% were free after six treatments (two weeks), and 80.9% were free of suicidal ideation at the end of the acute course (mean=7.5 treatments, SD=3.2) (26). To put this difference into context, it should be noted that ECT is standard care for patients with mood disorders and active suicidal ideation and generally leads to sustained resolution of suicidal ideation, while the effects of ketamine on suicidal ideation beyond one week have not yet been thoroughly investigated. Furthermore, clinical studies of ketamine may draw their subjects from a different patient population than clinical ECT studies.
Pseudospecificity
Considerable interest exists regarding whether ketamine’s anti-suicidal properties occur independently of its general antidepressant effects (pseudospecificity), particularly because this may impact the path towards US Food and Drug Administration (FDA) approval for ketamine or related compounds in treating suicidal ideation or behavior (27). A related example of pseudospecificity is vortioxetine, which underwent the FDA New Drug Application process for potential approval for cognitive dysfunction in MDD; the application was submitted based on the consideration that cognitive dysfunction may be phenomenologically distinct from other symptoms of depression and that it may have a course distinct from other depressive symptoms (28–30). Ultimately, the FDA did not grant vortioxetine approval for this pseudospecific indication. However, vortioxetine did receive an approval for a Type II Variation for the treatment of cognitive dysfunction in MDD by the European Medicines Agency, a process similar to that of the FDA for pseudospecificity.
The present analysis provides evidence drawn from the largest sample to date that ketamine reduces suicidal ideation partially independently of mood symptoms. However, the specificity of this effect requires further exploration. While we purposefully included patients across a range of psychiatric diagnoses, most of the patients had MDD or bipolar depression and were treatment-resistant per inclusion criteria. For ketamine to have a future as a potential anti-suicidal therapeutic in patients with a range of mood and anxiety disorders, studies specifically recruiting diverse populations must be conducted. Future studies should also consider whether ketamine may be a potential therapeutic for suicidal ideation in patients of varying levels of treatment resistance. This is particularly important because, in the emergency settings where anti-suicidal interventions might be most useful, clinical decisions must often be made quickly in the face of potential ambiguity of both diagnosis and treatment history. In one of the included studies, Murrough and colleagues (18) recruited a population considered at risk for suicide across diagnoses in an attempt to specifically measure ketamine’s effect on suicidal ideation; this differed from other trials included in the meta-analysis that recruited patients with specific diagnoses and excluded participants deemed at imminent risk of suicide. Notably, diagnoses such as substance use disorder or schizophrenia/schizoaffective disorder were uniformly exclusionary criteria in these studies. Given ketamine’s abuse liability (31) and concern for exacerbation of psychotic symptoms (32), additional research is clearly required before ketamine can be considered a treatment option in these areas.
Limitations
Despite the robust findings described above, several limitations require comment. First, the relatively small sample sizes of the included studies limited sensitivity analyses of the individual scales, which may account for our mixed findings in the BDI analysis; nevertheless, three of the four scales (both clinician-administered and self-reported) yielded consistent findings. Second, all of the studies included in this meta-analysis examined the effects of ketamine on suicidal ideation; whether ketamine’s effects on suicidal ideation translate to suicidal behavior has not been studied. Third, with one exception (18), the studies included in this meta-analysis did not specifically recruit patients deemed at imminent risk for suicide. As such, the assessment of suicidal ideation used in this analysis is limited to a single item from each scale. This limitation, however, is mitigated by our exclusion of patients with no suicidal ideation at baseline. Fourth, while our finding that ketamine exerts significant effects on suicidal ideation even when adjusting for the severity of depressive symptoms was replicated in a similar analysis of open-label ketamine (33), the bulk of the current ketamine studies have been conducted in patients with active mood disorders. Fifth, the short follow-up of studies precludes this analysis from providing further guidance on any sustained effects beyond seven days following treatment. Finally, given the psychoactive properties of ketamine, a limitation of many—though not all—of the included studies is the possibility of functional unblinding of trials using saline as the comparator.
Future Work
Despite these and other robust findings indicating that ketamine has significant anti-suicidal effects, a number of questions must be answered before ketamine can be regularly used in clinical settings to treat patients at risk of suicide. While ketamine’s anti-suicidal effects appeared to be maintained over one week, the possibility of rebound suicidal ideation remains, with accompanying potential negative outcomes within the weeks or months following exposure to ketamine (34). In addition, to date no study has demonstrated that ketamine specifically reduces the risk of suicidal behavior, only of suicidal ideation. Results from several ongoing clinical trials evaluating the efficacy of ketamine or esketamine (ketamine’s S-enantiomer) to stabilize outpatients with significant suicidal ideation (NCT02094898, NCT01700829) or patients hospitalized due to acute suicide risk (NCT02133001, NCT02299440) should provide longer-term follow-up data as well as protocols for repeated dosing. Future studies should also explore the effects of combining ketamine with established somatic/pharmacologic (i.e., ECT, lithium (NCT01880593)) or psychotherapeutic (i.e. DBT or CBT) modalities to extend beneficial response. One preliminary report of ketamine combined with CBT observed improved longer-term outcomes in mood disorders (35), though we know of no such protocols examining similar combinations specifically for the treatment of suicidal ideation. Future clinical studies should also assess details of suicidal ideation, including frequency, intensity, duration, and past suicidal behaviors. Differentiation between acute and chronic factors in the emergence of suicidal ideation may also help establish expectations regarding treatment outcomes. While using midazolam as a control may attenuate the effect size of ketamine to due improved integrity of blinding, our study did not find a significant difference between the two control conditions, which may have been due to the relatively small number of subjects who received midazolam. Also, no study has directly compared the effect of saline v. midazolam on suicidal ideation.
Conclusions
This study used individual participant-level data from a sample comprised exclusively of subjects with some level of active or passive suicidal ideation drawn from all single-dose, comparison intervention trials of intravenous ketamine. We found that across 10 controlled trials, a single ketamine infusion rapidly reduced the severity of suicidal thinking within 24 hours in more than half the patients, with benefits observed up to one week.
These results suggest that ketamine holds considerable promise as a potential rapid-acting treatment for patients at risk of suicide. Further research examining ketamine and similar compounds for the treatment of suicidal patients is urgently needed. In particular, questions remain regarding optimal patient selection, dosing frequency, clinical monitoring, and follow-up assessment. Although a great unmet need exists for novel and rapid-acting therapeutics for patients at risk of suicide, the evidence for using ketamine in this context remains preliminary. Any consideration of the clinical use of ketamine should balance the known risks of this treatment approach (32), the still limited evidence of its efficacy (36), and any possible delays it may cause in receiving more established therapies for reducing the risk of suicidal ideation, such as ECT or lithium (27).
Supplementary Material
Acknowledgments
We gratefully acknowledge Michael Kane, PhD, Assistant Professor of Biostatistics in the Yale School of Public Health, for his assistance in the statistical approach. We also thank Jessica A. Johnson (Child Study Center, Yale School of Medicine) for assistance in preparing the figures and Ioline Henter (NIMH) for invaluable editorial assistance.
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA-MH002857). Dr. Wilkinson acknowledges support from the National Institute of Mental Health (T32MH062994), the Brain and Behavioral Research Foundation (formerly NARSAD), and the Robert E. Leet and Clara Guthrie Patterson Trust. Dr. Sos acknowledges support from project No. LO1611/NPU I from the MEYS CR. Dr. Sanacora acknowledges the support of the State of Connecticut Department of Mental Health and Additive Services, the Brain and Behavior Research Foundation, the Pfeiffer Research Foundation, Yale New Haven Hospital, and the National Center for Posttraumatic Stress Disorder.
Footnotes
Presentations
The results of this specific meta-analysis will be presented in abstract form at the annual meeting of the Society of Biological Psychiatry in May 2017.
Disclosures
Dr. Bloch receives research support from Therapix Biosciences and Biohaven Pharmaceuticals. In the past three years, Dr. Murrough has provided consultation services to Novartis, Janssen Research and Development, and Genentech; he is named on patents pending for neuropeptide Y as a treatment for mood and anxiety disorders, a patent pending for the combination of ketamine and lithium for suicidal ideation, and a patent pending for ketamine plus lithium to extend the antidepressant response of ketamine. Dr. Feder and Icahn School of Medicine at Mount Sinai are named on a pending patent application of ketamine for the treatment of PTSD. Dr. Mathew reported receiving research funding from the Department of Veterans Affairs, National Institute of Mental Health, Janssen Research & Development, and Otsuka; receiving consulting fees from or serving on advisory boards for Acadia, Alkermes, Cerecor, Genentech, Naurex, Otsuka, Teva, Valeant, and Vistagen Therapeutics; and receiving support from the Johnson Family Chair for Research in Psychiatry at Baylor College of Medicine and resources and use of facilities at the Michael E Debakey Veterans Affairs Medical Center in Houston, Texas. Dr. Sanacora has received consulting fees form Allergan, Alkermes, BioHaven Pharmaceuticals Holding company, Janssen, Merck, Takeda, Taisho Pharmaceuticals, and Vistagen therapeutics over the last 24 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Johnson & Johnson, Hoffman La-Roche, Merck & Co., Naurex, and Servier over the last 24 months. Free medication was provided to Dr. Sanacora for an NIH-sponsored study by Sanofi-Aventis. In addition he holds shares in BioHaven Pharmaceuticals Holding Company and is a co-inventor on a patent “Glutamate agents in the treatment of mental disorders” (patent number: 8778979). Dr. Zarate is listed as a coinventor on a patent for the use of ketamine in major depression and suicidal ideation. Dr. Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorders. Dr. Zarate has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Drs. Wilkinson, Ballard, Sos, and Wang have no conflict of interest to disclose, financial or otherwise.
References
- 1.Aleman A, Denys D. Mental health: A road map for suicide research and prevention. Nature. 2014;509(7501):421–3. doi: 10.1038/509421a. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths JJ, Zarate CA, Jr, Rasimas JJ. Existing and novel biological therapeutics in suicide prevention. Am J Prev Med. 2014;47(3 Suppl 2):S195–203. doi: 10.1016/j.amepre.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtin SC, Warner M, Hedegaard H. Increase in Suicide in the United States, 1999–2014. NCHS Data Brief. 2016;(241):1–8. [PubMed] [Google Scholar]
- 4.Cavanagh JT, Carson AJ, Sharpe M, Lawrie SM. Psychological autopsy studies of suicide: a systematic review. Psychol Med. 2003;33(3):395–405. doi: 10.1017/s0033291702006943. [DOI] [PubMed] [Google Scholar]
- 5.National Action Alliance for Suicide Prevention: Research Prioritization Task Force. A prioritized research agenda for suicide prevention: An action plan to save lives. Rockville, MD: National Institute of Mental Health and the Research Prioritization Task Force; 2014. [Google Scholar]
- 6.Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- 7.Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819–34. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- 8.Brown GK, Ten Have T, Henriques GR, Xie SX, Hollander JE, Beck AT. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563–70. doi: 10.1001/jama.294.5.563. [DOI] [PubMed] [Google Scholar]
- 9.Linehan MM, Comtois KA, Murray AM, Brown MZ, Gallop RJ, Heard HL, et al. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs therapy by experts for suicidal behaviors and borderline personality disorder. Arch Gen Psychiatry. 2006;63(7):757–66. doi: 10.1001/archpsyc.63.7.757. [DOI] [PubMed] [Google Scholar]
- 10.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 11.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 12.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–46. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–6. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71(12):1605–11. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127–31. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 18.Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015:1–10. doi: 10.1017/S0033291715001506. [DOI] [PubMed] [Google Scholar]
- 19.Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31(4):335–43. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med. 2017;36(5):855–75. doi: 10.1002/sim.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(6):681–8. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 22.Hu YD, Xiang YT, Fang JX, Zu S, Sha S, Shi H, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med. 2015:1–13. doi: 10.1017/S0033291715002159. [DOI] [PubMed] [Google Scholar]
- 23.Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine's antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett. 2013;34(4):287–93. [PubMed] [Google Scholar]
- 24.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191(2):122–7. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–83. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellner CH, Fink M, Knapp R, Petrides G, Husain M, Rummans T, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977–82. doi: 10.1176/appi.ajp.162.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson ST, Sanacora G. Ketamine: a potential rapid-acting antisuicidal agent? Depress Anxiety. 2016;33(8):711–7. doi: 10.1002/da.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40(8):2025–37. doi: 10.1038/npp.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557–67. doi: 10.1017/S1461145714000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntyre RS, Xiao HX, Syeda K, Vinberg M, Carvalho AF, Mansur RB, et al. The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs. 2015;29(7):577–89. doi: 10.1007/s40263-015-0263-x. [DOI] [PubMed] [Google Scholar]
- 31.Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, et al. Potential Risks of Poorly Monitored Ketamine Use in Depression Treatment. Am J Psychiatry. 2016;173(3):215–8. doi: 10.1176/appi.ajp.2015.15081082. [DOI] [PubMed] [Google Scholar]
- 32.Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105(1):121–33. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 33.Ionescu DF, Swee MB, Pavone KJ, Taylor N, Akeju O, Baer L, et al. Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: secondary analysis of an open-label study. J Clin Psychiatry. 2016;77(6):e719–25. doi: 10.4088/JCP.15m10056. [DOI] [PubMed] [Google Scholar]
- 34.Vande Voort JL, Morgan RJ, Kung S, Rasmussen KG, Rico J, Palmer BA, et al. Continuation phase intravenous ketamine in adults with treatment-resistant depression. J Affect Disord. 2016;206:300–4. doi: 10.1016/j.jad.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson ST, Wright D, Fasula M, Fenton L, Griepp M, Ostroff RB, et al. Cognitive behavior therapy may sustain antidepressant effects of intravenous ketamine in treatment-resistant depression. Psychother Psychosom. 2017;56:352–4. doi: 10.1159/000457960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, et al. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry. 2017;74:399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.