Abstract
Objective
To test the novel hypothesis that, among Black Americans who used John Henryism coping, those from low socioeconomic status backgrounds would be more likely to develop metabolic syndrome than would those from higher socioeconomic backgrounds.
Methods
This is an ancillary analysis of SHAPE, a longitudinal cohort of 391 Black youths and their caregivers. From ages 11 to 18 years, family socioeconomic status was assessed. At age 25, John Henryism was assessed, blood samples were drawn, and measurements were taken of blood pressure and waist circumference. Metabolic syndrome status was based on International Diabetes Federation guidelines.
Results
A significant interaction emerged between family socioeconomic disadvantage and John Henryism coping in predicting metabolic syndrome diagnosis (OR = 1.047, 95% CI [1.004, 1.091]). Participants who were high in John Henryism coping were more likely to display metabolic syndrome if they were from disadvantaged backgrounds (predicted prevalence of 26.7%) than if they were from more privileged backgrounds (predicted prevalence of 9.6%).
Conclusions
These patterns illustrate for the first time that John Henryism coping can undermine cardiometabolic health among Black youths from disadvantaged backgrounds.
Keywords: Black, health disparities, John Henryism, metabolic syndrome, socioeconomic status, young adulthood
INTRODUCTION
The well-documented prevalence of the chronic diseases of aging among Black adults does not originate in adulthood; rather, it results from changes in biological processes beginning at earlier stages of development (1). This may be particularly true for Black children in the rural American South. Risk factors include chronic poverty, frequent housing adjustments in response to economic pressures, substandard housing, interpersonal and institutional racism, difficulty in accessing health care, poor-quality schools, and a lack of access to nutritious food (2). Many Black families in the rural South thus live under conditions of severe, ongoing economic stress that have the potential to take a toll on health and well-being among children and adolescents (3, 4).
Despite the economic hardships and contextual risks that Black youths face, a significant number of them overcome these difficulties. They perform well in school, avoid drug use, and go on to attend college. Black youths do this, in part, through unwavering determination and persistence. James (5) called this single-minded determination to succeed that is accompanied by an uncompromising work ethic, even when encountering tremendous challenges, John Henryism (JH) after the legend of a Black railroad worker who, in the 19th century, was said to have defeated a steam-powered drill in a steel-driving contest only to collapse, dead from exhaustion. For James (5), the fabled actions of John Henry serve to illuminate associations among high-effort coping, chronic sympathetic nervous system arousal, and health problems. James (5) hypothesized that Black Americans from low socioeconomic status (SES) backgrounds who used a high-effort coping style in pursuit of upward mobility would incur outwardly undetectable wear and tear on their bodies that could be detected through indicators of shortened life expectancies and cardiometabolic health impairments.
Epidemiological investigations of the associations between JH and health among Black Americans have focused primarily on blood pressure among middle-aged and elderly participants. Reviews of this research present mixed findings, but more studies support a link between JH and blood pressure than do not support it, particularly for hypertension in community samples (6). Recently, a 15-year longitudinal study involving Finnish men expanded the research focus beyond blood pressure by demonstrating that a JH × low-SES interaction predicted differential risk for fatal and nonfatal heart attacks (7). It is not known whether the JH pattern for Black Americans from disadvantaged backgrounds appears earlier in the life course, during young adulthood. It is also not known whether the contributions of JH to health among Black adults may extend to significant clinical outcomes beyond blood pressure.
In this study, we examined the JH coping pattern to determine whether it would forecast a chronic condition that is highly prevalent among young Black adults, metabolic syndrome (MetS) (8). MetS is a cluster of cardiometabolic risk factors that includes central adiposity, hypertension, impaired glucose control, and lipid dysregulation. It is a precursor to several diseases, including diabetes, heart disease, and stroke. The prevalence of MetS varies substantially across demographic, racial, and ethnic groups. The highest rates occur among Black adults and adults of low socioeconomic status (8). Black women have a higher prevalence than do Black men, and the prevalence rates of MetS increase with age for all racial and ethnic groups (9). We gathered data to test the hypothesis that the prevalence of MetS would be greatest among young Black adults who use the JH coping style and grew up in disadvantaged circumstances.
We investigated the study hypotheses with a cohort of Black youths who were 11 years of age at the first, and 25 years of age at the last, annual data collection. Data on family socioeconomic disadvantage was gathered from ages 11 to 13 years and from ages 16 to 18 years. At age 25 years, participants completed the JH coping questionnaire and provided blood samples from which fasting blood glucose and triglyceride levels were assayed; blood pressure and waist circumference also were recorded.
METHODS
Participants
Data were gathered from the Strong African American Families Healthy Adult Project panel study. Starting in 2001, 667 Black 5th-grade children (M age = 11.2 years) and their primary caregivers were enrolled, to track the youths’ socioeconomic backgrounds. The families resided in nine rural counties in which poverty rates are among the highest in the nation (10). In 2009–2010, when the youths were 19–20 years of age, a subgroup of 500 young adults was randomly selected for a study of stress hormones and blood pressure. This cohort was reassessed in 2015–2016, when the participants had reached a mean age of 25 years; MetS was the focus of this assessment. The age 25 data collection included 408 participants from the age 19–20 subsample. Of this subsample, 391 agreed to have their blood drawn to detect MetS; they constituted the sample in the present study. T tests were executed to evaluate the equivalence of the demographic and study variables for the age 11 and age 25 samples. Compared with the original cohort, the cohort in the present study had a higher percentage of female participants (59.8% vs. 52.8%) and their families reported more indicators of socioeconomic disadvantage, as described in the Measures section (M = 2.33, SD = 1.20 vs. M = 2.25, SD = 1.25), but were otherwise similar demographically. The University of Georgia’s Institutional Review Board approved all study procedures, and all participants provided written informed consent at each wave of assessment. Primary caregivers consented to minor youths’ participation in the study, and the minor youths assented to their own participation. Youths 18 years of age or older consented to their own participation.
Measures
Family Socioeconomic Disadvantage
When participants were 11 to 13 and 16 to 18 years of age, caregivers provided data on their families’ socioeconomic disadvantage. Six dichotomous variables formed a socioeconomic disadvantage index. A score of 1 was assigned to each of the following: family poverty based on federal guidelines, primary caregiver unemployment, receipt of Temporary Assistance for Needy Families, primary caregiver single parenthood, primary caregiver education level less than high school graduation, and caregiver-reported inadequacy of family income. The scores were summed and averaged across waves to form the index, which ranged from 0 to 6 (M = 2.33, SD = 1.20).
John Henryism
At age 25, young adults reported their high-active coping strategies on the 12-item John Henryism Active Coping Scale (11); Cronbach’s α = .83. The items emphasized mental and physical vigor, commitment to hard work, and a single-minded determination to succeed (11). Example items included, “Once I make up my mind to do something, I stay with it until the job is completely done,” “When things do not go the way I want them to, that makes me work even harder,” and “In the past, even when things got really tough, I never lost sight of my goals.”
Metabolic Syndrome Diagnostic Status
At the age 25 assessment, a phlebotomist visited each participant’s home in the morning hours to draw an overnight fasting blood sample. Blood was drawn into serum separator tubes (Becton-Dickinson, Franklin Lakes, NJ, USA). Samples were centrifuged and assayed at the Foundations of Health Research Center Laboratory at Northwestern University. The serum was harvested, divided into aliquots, and immediately frozen on dry ice. At the end of the study, glucose was measured photometrically on a Roche/Hitachi cobas c502 analyzer. The average intra- and inter-assay coefficients of variation were 0.7% and 1.8%, respectively. This assay has a dynamic range of 2–750 mg/dL. High-density lipoproteins (HDL) and triglycerides were measured on a Roche/Hitachi cobas c701 analyzer. The average intra- and inter-assay coefficients of variation for these assays were below 1.6% and 2.4%, respectively. The assay’s detection ranges are 8.85–885 mg/dL (triglycerides) and 3–120 mg/dL (HDL). Resting blood pressure was monitored with a Critikon Dinamap Pro 100 (Critikon; Tampa, FL, USA) while youths sat reading quietly. Three readings were taken every 2 minutes, and the average of the last two readings was used as the resting index. Field researchers measured participants’ waist circumferences at the midpoint of the upper iliac crest and lower costal margin, at the midaxillary line.
MetS was diagnosed according to International Diabetes Federation (IDF) guidelines (12). To qualify, an individual must show central adiposity, defined by ethnic and sex-specific cutoffs for waist circumference (for individuals of African descent, cutoffs are ≥ 94 cm and ≥ 80 cm for men and women, respectively). At least two of four additional components must also be present. They include (a) systolic blood pressure ≥ 130 or diastolic blood pressure ≥ 85, (b) triglyceride levels ≥ 150 mg/dL, (c) fasting glucose levels ≥ 100 mg/dL, and (d) high-density lipoprotein levels < 40 mg/dL in men and < 50 mg/dL in women. A dichotomous classification indicating whether a participant met the IDF definition of MetS was the primary outcome of interest. We note that the applicability of the IDF MetS criteria to Black persons has been questioned (13). We chose, however, to use in this investigation established criteria for the diagnosis of MetS. Of the 391 25-year-old participants, 67 (17.1%) met IDF criteria for MetS. For comparison purposes, the best available epidemiological data for young adults indicated a prevalence of 18% among 20 to 39-year-old participants in the National Health and Nutrition Examination Survey (9).
Self-Control
Self-control was assessed when youths were 11 to 13 and 16 to 17 years of age using parents’ reports on the 12-item Self-Control Inventory (14). Each item was rated on a scale ranging from 0 (never) to 4 (almost always). Example items included, “sticks to what he/she is doing even during long, unpleasant tasks until finished,” “works toward a goal,” and “pays attention to what he/she is doing.” Responses were summed to yield a self-control score; alphas across waves ranged from .86 to .89. Self-control was operationalized as the average of the parents’ ratings across the five assessments during adolescence.
Self-Regulation
At ages 19 to 21 years, youths completed the 23-item Self-Regulation Questionnaire (15). Each item was rated on a scale ranging from 1 (strongly disagree) to 4 (strongly agree). Example items included, “Once I have a goal, I can usually plan how to reach it,” “I set goals for myself and keep track of my progress,” and “If I make a resolution to change something, I pay a lot of attention to how I am doing.” Responses were summed to yield a self-regulation score; alphas across waves ranged from .91 to .92. Self-regulation was operationalized as the average of the youths’ ratings across the three assessments during young adulthood.
Covariates
The SHAPE cohort was initially recruited for a randomized controlled trial of a family-centered intervention designed to prevent behavior problems and substance abuse (16). Participation in the intervention was not associated with MetS diagnosis (p = .24). To minimize any residual confounding, however, we included a dichotomous covariate reflecting intervention assignment (treatment vs. control group) in all models. Sex was dummy coded (male participants were coded 1 and female participants were coded 0) and controlled in the analyses. At age 25, participants reported monthly income (M = 1098, SD = 924) and educational achievement (1 = less than high school graduate; 2 = high school graduate or GED; 3 = some college or trade school; 4 = bachelor’s degree; 5 = some graduate school or more; M = 2.67, SD = 0.87). Unhealthful behavior at age 25 was indexed using items from the Youth Risk Behavior Survey (17). This scale has been used in several national, ethnically diverse surveys and has shown good validity and reliability. Participants reported the number of days during the past 7 days on which they consumed fruit, vegetables, 100% fruit juices, and milk. Exercise was measured with the single item, “During the past 7 days, on how many days were you physically active for a total of at least 60 minutes per day?” The nutrition and exercise items were reverse coded so that higher numbers indicated less healthful behaviors.
Statistical Analysis
The study hypothesis was tested using a logistic regression equation with three sequentially entered blocks of variables: (a) the covariates of sex, intervention status, monthly income at age 25, educational achievement, and unhealthful behaviors; (b) the main effects of family socioeconomic disadvantage and JH coping; and (c) a two-way interaction term of family socioeconomic disadvantage × JH coping. The analysis was executed based on the conventions that Aiken and West (18) prescribed, whereby the variables are first mean centered and interactions are calculated as the product of the centered variables.
RESULTS
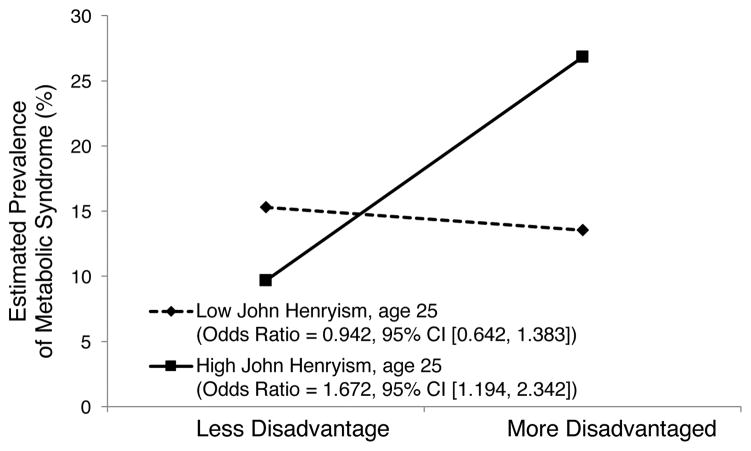
Table 1 presents descriptive statistics for the sample, along with bivariate correlations. Family socioeconomic disadvantage, assessed at ages 11 to 18 years, was associated positively with MetS diagnosis at age 25. The regression analysis revealed a significant interaction between family socioeconomic disadvantage and JH coping (see Table 2). To interpret this interaction, we plotted the estimated prevalence of a diagnosis of MetS for low (1 SD below the mean) and high (1 SD above the mean) levels of family socioeconomic disadvantage and JH coping. These results, depicted in Figure 1, show that the findings for MetS were consistent with the predicted JH pattern. For young adults whose families were the most disadvantaged throughout their adolescent years, higher JH coping was associated with a greater likelihood of MetS (predicted prevalence of 26.8%). For young adults in the most privileged families, higher JH coping conferred greater protection from MetS (predicted prevalence of 9.7%). Additional comparisons showed that young adults with high JH coping from the most disadvantaged families were 3.4 times more likely to be diagnosed with MetS than were young adults with similar levels of JH coping living in the most privileged families. Additional analyses were performed to determine whether participant sex conditioned any of these findings; no interaction was detected. Together, these results support the JH pattern in which the prevalence of MetS during young adulthood is predicted to be greatest among those using JH coping who grew up in disadvantaged circumstances.
TABLE 1.
Descriptive Statistics and Correlations among Family Socioeconomic Disadvantage, John Henryism Coping, and Metabolic Syndrome Status.
| Variable | Mean (SD) | Correlations
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| 1. Sex (male = 1, female = 0) | 0.402 (0.491) | — | |||||||
| 2. Intervention status | 0.583 (0.494) | −.027 | — | ||||||
| 3. Youth educational achievement (age 25) | 2.670 (0.866) | −.170** | −.101* | — | |||||
| 4. Youth monthly income (age 25) | 1098.578 (923.882) | .131** | .046 | .186*** | — | ||||
| 5. Youth unhealthful behaviors (age 25) | 0.052 (3.500) | .057 | −.003 | −.100* | −.056 | — | |||
| 6. Family socioeconomic disadvantage (ages 11–18) | 2.335 (1.196) | −.020 | .084 | −.338*** | −.172** | −.020 | — | ||
| 7. John Henryism (age 25) | 51.706 (6.244) | −.140** | −.010 | .097 | .049 | −.144** | −.042 | — | |
| 8. Metabolic syndrome diagnostic status (age 25) | 0.171 (0.377) | −.123* | .054 | −.038 | −.085 | .056 | .128* | .056 | — |
Note: N = 391.
p < .05.
p < .01.
p < .001.
TABLE 2.
Family Socioeconomic Disadvantage at Ages 11–18 and John Henryism at Age 25 as Predictors of Metabolic Syndrome Diagnostic Status at Age 25.
| Predictors | Metabolic Syndrome Diagnostic Status
|
|
|---|---|---|
| Odds Ratio | 95% CI | |
| 1. Sex (male = 1, female = 0) | 0.506* | 0.274, 0.937 |
| 2. Intervention status | 1.245 | 0.706, 2.196 |
| 3. Youth educational achievement (age 25) | 1.031 | 0.727, 1.462 |
| 4. Youth monthly income (age 25) | 1.000 | 0.999, 1.000 |
| 5. Youth unhealthful behaviors (age 25) | 1.056 | 0.974, 1.145 |
| 6. Family socioeconomic disadvantage (ages 11–18) | 1.255 | 0.976, 1.614 |
| 7. John Henryism (age 25) | 1.013 | 0.965, 1.064 |
| 8. Socioeconomic disadvantage × John Henryism | 1.047* | 1.004, 1.091 |
Note: CI = confidence interval.
p < .05
FIGURE 1.
Metabolic syndrome diagnostic status at age 25 as a function of family socioeconomic disadvantage at ages 11–18 and John Henryism coping at age 25: Georgia, 2001–2016. The lines represent the regression lines for different levels of John Henryism coping (low: 1 SD below the mean; high: 1 SD above the mean). Numbers in parentheses refer to simple slopes.
We also sought to determine whether the reported results would emerge when the individual indicators of MetS were disaggregated and analyzed separately. The results of these analyses are presented in Tables 3 and 4. When the multisystem, individual indicators of MetS were disaggregated, none of them evinced an interaction between family socioeconomic disadvantage and JH.
TABLE 3.
Family Socioeconomic Disadvantage at Ages 11–18 and John Henryism at Age 25 as Predictors of Blood Pressure and Glucose at Age 25.
| Predictors | Systolic Blood Pressure | Diastolic Blood Pressure | Glucose | |||
|---|---|---|---|---|---|---|
|
| ||||||
| B | 95% CI | B | 95% CI | B | 95% CI | |
| 1. Sex (male = 1, female = 0) | 10.687*** | 8.108, 13.266 | 1.620 | −0.605, 3.845 | −1.469 | −5.741, 2.803 |
| 2. Intervention status | 0.365 | −2.139, 2.868 | 0.644 | −1.516, 2.804 | 2.533 | −1.595, 6.661 |
| 3. Target educational achievement (age 25) | −0.926 | −2.492, 0.639 | 0.868 | −0.482, 2.219 | 0.057 | −2.520, 2.634 |
| 4. Target monthly income (age 25) | −0.001 | −0.002, 0 | −0.001* | −0.002, 0 | 0 | −0.002, 0.002 |
| 5. Target unhealthy behaviors (age 25) | −0.048 | −0.404, 0.308 | 0.065 | −0.242, 0.372 | −0.095 | −0.681, 0.492 |
| 6. Family socioeconomic disadvantage (ages 11–18) | 1.677** | 0.587, 2.768 | 1.347** | 0.406, 2.288 | 1.267 | −0.552, 3.086 |
| 7. John Henryism (age 25) | 0.139 | −0.065, 0.343 | 0.079 | −0.097, 0.255 | 0.039 | −0.296, 0.373 |
| 8. Socioeconomic disadvantage × John Henryism | 0.056 | −0.118, 0.231 | 0.040 | −0.190, 0.111 | 0.250 | −0.038, 0.538 |
Note: CI = confidence interval.
p < .001,
p < .01,
p < .05
TABLE 4.
Family Socioeconomic Disadvantage at Ages 11–18 and John Henryism at Age 25 as Predictors of Triglyceride Levels and High Density Lipoprotein at Age 25.
| Predictors | Triglyceride Levels | High Density Lipoprotein | ||
|---|---|---|---|---|
|
| ||||
| B | 95% CI | B | 95% CI | |
| 1. Sex (male = 1, female = 0) | 12.570* | 2.567, 22.572 | 0.203 | −2.719, 3.125 |
| 2. Intervention status | 3.001 | −6.664, 12.667 | 0.429 | −2.395, 3.252 |
| 3. Target educational achievement (age 25) | −2.610 | −8.644, 3.423 | 1.914* | 0.152, 3.677 |
| 4. Target monthly income (age 25) | −0.003 | −0.008, 0.003 | 0 | −0.002, 0.001 |
| 5. Target unhealthy behaviors (age 25) | −0.840 | −2.213, 0.533 | −0.148 | −0.549, 0.253 |
| 6. Family socioeconomic disadvantage (ages 11–18) | 0.784 | −3.475, 5.042 | −0.457 | −1.701, 0.787 |
| 7. John Henryism (age 25) | 0.230 | −0.552, 1.013 | −0.026 | −0.255, 0.202 |
| 8. Socioeconomic disadvantage × John Henryism | 0.287 | −0.387, 0.962 | −0.122 | −0.319, 0.075 |
Note: CI = confidence interval.
p < .05.
Finally, to explore the possibility of circumstantial evidence indicating that JH may have preceded the emergence of MetS, we correlated parent reports of youth self-control averaged across ages 16 to 18 years, and youth reports of self-regulation averaged across ages 19 to 21 years, with JH at age 25. The self-control and self-regulation assessments tap some dimensions of JH, most notably the key characteristic of persistence in meeting goals. Both self-control during adolescence (r = .124, p = .013) and self-regulation during young adulthood (r = .407, p = .001) were significantly associated with JH. These findings are consistent with the proposition that a propensity toward JH preceded rather than followed the emergence of MetS in this particular sample of young Black adults.
DISCUSSION
In a sample of Black youths from rural Georgia, a region with some of the highest rates of MetS in the nation, the JH pattern emerged among young adults from disadvantaged backgrounds. If, from ages 11 to 18 years, youths lived in more disadvantaged circumstances and, at age 25 years, used high levels of JH coping, their likelihood of having MetS at age 25 was elevated. This is the first demonstration that the JH pattern is associated with a heightened risk among young Black adults of developing a chronic disease. MetS can inhibit quality of life and is a precursor of two public health problems, diabetes and cardiovascular disease. Hence, the three-fold elevation in MetS among young adults from disadvantaged backgrounds who are high in JH is likely to have both individual and public health ramifications.
Several possible explanations exist for this study’s findings. First, the presence of MetS that we observed could predate the development of JH and be secondary to other confounders associated with socioeconomic risk. Though plausible, we consider this explanation to be unlikely, particularly given the results of models adjusted for young adults’ educational achievement, health behaviors, contemporaneous income, and the correlations of self-control across ages 16 to 18 years and self-regulation across ages 19 to 21 years with JH at age 25 years. These correlations suggest that it is more likely that JH preceded MetS than vice versa. Nevertheless, follow-up studies in which the measurement of JH precedes by a year or more the assessment of MetS should be conducted, along with follow-up studies that include more detailed assessments of potential third-variable effects. A second possibility involves the recognition that young adulthood can be a stressful developmental period during which young people are trying to establish independence, careers, and families. During this challenging time, an unrelenting determination to succeed despite interpersonal, community, and institutional stressors is likely to occasion frequent activation of the hypothalamic-pituitary-adrenal axis and sympathetic nervous system stress responses. The hormonal end products of these systems, glucocorticoids and catecholamines, have been found to be elevated in Black youths who grow up in disadvantaged circumstances (19, 20). Sustained exposure to high doses of these hormones can promote weight gain, elevate blood pressure, dysregulate lipids, and promote inflammation, all of which could hasten the development of MetS (3, 21). Accordingly, future research should examine the roles of sympathetic and adrenocortical activity in persons who adopt the JH coping style to navigate interpersonal and institutional impediments to success.
Limitations
Limitations of the present study include the nature of the sample, which was composed solely of rural Black families; thus, it is not known whether the findings would generalize to urban Black families or to members of other racial or ethnic groups. In addition, the research reported here was designed to test the JH hypothesis; it was beyond the scope of the study to examine other important and interesting questions such as the following. Why are women, both in this study and others (9), more likely than men to develop MetS? Does MetS prevalence vary between young adults exposed to episodic versus chronic SES risk across childhood and adolescence? These questions need to be addressed in future research. These limitations notwithstanding, this study’s findings extend knowledge of the JH coping phenomenon, documenting for the first time its association with a chronic medical risk, MetS. The results also serve as a reminder that determined efforts among young Black adults to “beat the odds” may exact an unanticipated toll on health.
Acknowledgments
Source of Funding: This work was supported by the National Institutes of Health through the National Institute of Child Health and Human Development (grant R01 HD030588 to G. H. Brody) and the National Institute on Drug Abuse (grant P30 DA027827 to G. H. Brody).
Acronyms
- IDF
International Diabetes Federation
- JH
John Henryism
- MetS
metabolic syndrome
Footnotes
Conflicts of Interest: None of the authors declare any conflicts of interest.
Author Contributions: Gene H. Brody conceptualized the research and drafted the original manuscript. Tianyi Yu contributed to the conceptualization of the research and executed the data analyses. Gregory E. Miller, Edith Chen, and Katherine B. Ehrlich contributed to the conceptualization of the research and the drafting of the manuscript.
Contributor Information
Gene H. Brody, Center for Family Research, University of Georgia, Athens.
Tianyi Yu, Center for Family Research, University of Georgia, Athens.
Gregory E. Miller, Department of Psychology and Institute for Policy Research, Northwestern University, Evanston, Illinois.
Katherine B. Ehrlich, Department of Psychology and Center for Family Research, University of Georgia, Athens.
Edith Chen, Department of Psychology and Institute for Policy Research, Northwestern University, Evanston, Illinois.
References
- 1.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. Am J Public Health. 2006;96:826–33. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody GH, Yu T, Beach SRH. Resilience to adversity and the early origins of disease. Dev Psychopathol. 2016;28:1347–65. doi: 10.1017/S0954579416000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–9. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 5.James SA. John Henryism and the health of African-Americans. Cult Med Psychiatry. 1994;18:163–82. doi: 10.1007/BF01379448. [DOI] [PubMed] [Google Scholar]
- 6.Kiecolt KJ, Hughes M, Keith VM. Can a high sense of control and John Henryism be bad for mental health? Sociol Q. 2009;50:693–714. [Google Scholar]
- 7.Mujahid MS, James SA, Kaplan GA, Salonen JT. Socioeconomic position, John Henryism, and incidence of acute myocardial infarction in Finnish men. Soc Sci Med. 2017;173:54–62. doi: 10.1016/j.socscimed.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 9.Aguilar M, Bhuket T, Torres S, Liu B, Wong RM. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–4. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 10.DeNavas-Walt C, Proctor BD. Income and poverty in the United States: 2013 (Current Population Reports P60-249) Washington, DC: U.S. Census Bureau; 2014. [Google Scholar]
- 11.James SA. John Henryism and the health of African-Americans. In: LaVeist TA, editor. Race, ethnicity, and health: A public health reader. 1. San Francisco, CA: Jossey-Bass; 2002. pp. 350–68. [Google Scholar]
- 12.Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard TR, Schuster DP, Osei K. Metabolic syndrome in Black people of the African diaspora: the paradox of current classification, definition and criteria. Ethn Dis. 2009;19:S2-1–S2-7. [PubMed] [Google Scholar]
- 14.Humphrey LL. Children’s and teachers’ perspectives on children’s self-control: The development of two rating scales. J Consult Clin Psychol. 1982;50:624–33. doi: 10.1037//0022-006x.50.5.624. [DOI] [PubMed] [Google Scholar]
- 15.Brown JM, Miller WR, Lawendowski LA. The Self-Regulation Questionnaire. In: VandeCreek L, Jackson TL, editors. Innovations in clinical practice: A source book. Sarasota, FL: Professional Resource Press; 1999. pp. 281–9. [Google Scholar]
- 16.Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair LD, Brown AC, Wills TA, Spoth RL, Luo Z, Chen Y-f, Neubaum-Carlan E. The Strong African American Families program: Translating research into prevention programming. Child Dev. 2004;75:900–17. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 17.Youth Risk Behavior Surveillance System. Youth Risk Behavior Survey. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2009. [Google Scholar]
- 18.Brody GH, Yu T, Chen Y-f, Kogan SM, Evans GW, Beach SRH, Windle M, Simons RL, Gerrard M, Gibbons FX, Philibert RA. Cumulative socioeconomic status risk, allostatic load, and adjustment: A prospective latent profile analysis with contextual and genetic protective factors. Dev Psychol. 2013;49:913–27. doi: 10.1037/a0028847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen E, Miller GE, Brody GH, Lei MK. Neighborhood poverty, college attendance, and diverging profiles of substance use and allostatic load in rural African American youth. Clin Psychol Sci. 2015;3:675–85. doi: 10.1177/2167702614546639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charmandari E, Tsigos C, Chrousos GP. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]