Abstract
Aims
Calcium-sensing receptor (CaSR) is expressed on neurons of both submucosal and myenteric plexuses of the enteric nervous system (ENS) and the CaSR agonist R568 inhibited Cl− secretion in intestine. The purpose of this study was to localize the primary site of action of R568 in the ENS and to explore how CaSR regulates secretion through the ENS.
Materials and Methods
Two preparations of rat proximal and distal colon were used. The full-thickness preparation contained both the submucosal and myenteric plexuses, whereas for the “stripped” preparation the myenteric plexus with the muscle layers was removed. Both preparations were mounted onto Ussing chambers and Cl− secretory responses were compared by measuring changes in short circuit current (Isc). Two tissue-specific CaSR knockouts (i.e., neuron-specific vs. enterocyte-specific) were generated to compare the effect of R568 on expression of c-fos protein in myenteric neurons by immunocytochemistry.
Key findings
In full-thickness colons, tetrodotoxin (TTX) inhibited Isc, both in proximal and distal colons. A nearly identical inhibition was produced by R568. However, in stripped preparations, while the effect of TTX on Isc largely remained, the effect of R568 was nearly completely eliminated. In keeping with this, R568 reduced c-fos protein expression only in myenteric neurons of wild type mice and mutant mice that contained CaSR in neurons (i.e., villinCre/Casrflox/flox mice), but not in myenteric neurons of nestinCre/Casrflox/flox mice in which neuronal cell CaSR was eliminated.
Significance
These results indicate that R568 exerts its anti-secretory effects predominantly via CaSR-mediated inhibition of neuronal activity in the myenteric plexus.
Keywords: Calcium sensing receptor, myenteric neurons, c-fos, short-circuit current, colon, calcimimetic, Ussing chamber
Introduction
The function of the gut is controlled by the central nervous system (CNS) as well as the enteric nerve system (ENS), the brain of the gut. The ENS is composed of two inter-connected neural plexuses, the Meissner’s plexus located in the submucosa and the myenteric Auerbach’s plexus located between the circular and the longitudinal smooth muscle layers. Both plexuses comprise afferent sensory neurons, interneurons, and efferent motor neurons to accomplish secretion and motility reflexes. Given their locations, it is generally thought that the submucosal plexus supplies the mucosal epithelium and controls secretion whereas the myenteric plexus innervates the circular and longitudinal muscles and regulates motility [1–4]. However, there is evidence that the myenteric plexus also plays a role in coordinating these two fundamental aspects of gastrointestinal function, as myenteric neurons have projections to the submucosal plexus [5,6]. These myenteric neurons respond to mechanical and chemical stimuli and elicit neuronal reflexes during food digestion [7]. However, the mechanism(s) that govern the activity of myenteric neurons and their reflexes upon completion of digestion and extraction of nutrients remains unclear.
The extracellular calcium-sensing receptor (CaSR) is a G protein-coupled receptor that uses nutrients including calcium, polyamines, certain amino acids, and peptides as its activating ligands. As such, CaSR provides a key mechanism for tissue function regulation and feedback by these nutrients [8]. In addition to its expression in parathyroid gland, kidney and bone to regulate systemic calcium homeostasis [9], CaSR is also expressed in intestinal mucosal epithelium along the entire small and large intestines [10–12] and regulates intestinal fluid movement. For example, CaSR activation in colonic crypts was shown to inhibit net fluid secretion [10,11,13] and cyclic nucleotide accumulation induced by synthetic and natural secretagogues such as forskolin and guanylin [13]. Recently, studies also demonstrated that CaSR is highly expressed in ENS [14]. The ex vivo study using rat colon mounted in Ussing chambers showed that both basal and forskolin-induced secretory current responses were inhibited 60–70% by serosally applied R568, a small-molecule CaSR agonist or calcimimetic, in the absence, but not presence of tetrodotoxin (TTX) [14]. While the results suggested requirement of the activity of the ENS for CaSR agonist-mediated inhibition of electrolyte secretion, the previous study neither addressed the location of R568 action in the ENS nor the mechanism used.
Thus, the present study examined the R568 effect on short-circuit current (Isc) responses in the full-thickness preparation that contain both the submucosal and myenteric plexuses as well as the “stripped” preparation in which the myenteric plexus with the muscle layers was removed, and examined the effect of R568 on expression of c-fos protein in neurons of myenteric plexus in two tissue-specific CaSR knockouts (i.e., neuron-specific and enterocyte-specific). Our results indicate the inhibitory effect of R568 on ENS-mediated Cl− secretory response is largely mediated by neurons in the myenteric plexus through a direct CaSR-mediated process that leads to inhibition of neuronal excitability.
Materials and Methods
Animals
Ussing chamber experiments were performed using non-fasting male Sprague-Dawley rats, whereas immunolocalization experiments were performed on C57BL/6 mice. Rats weighting 100–400 g were obtained from Charles River Laboratories. Mice lacking CaSR expression in intestinal epithelial cells (villinCre/Casrflox/flox mice or villin mice) and mice lacking CaSR expression in intestinal neurons (nestinCre/Casrflox/flox mice or nestin mice) and their wild type littermates were bred and maintained in-house at the University of Florida Communicore Animal Facility in accordance with the Animal Welfare Act and the Public Health Policy on Humane Care. CaSR KO mice were generated as previously described [15]. Briefly, CaSR flox/flox mice were bred with transgenic mice expressing Cre Recombinase under the control of the villin 1 or nestin promoter and genotyped prior to all experiments after an approximate 10–12 generations. Animals were fed and maintained on regular chow (Harlan) with free access to water before sacrifice. Animals were sacrificed with standard CO2 inhalation and by cervical dislocation before removing the colon. The use of animals as well as the protocol for isolating colon tissues (see below) was approved by the Institutional Animal Care and Use Committee (IACUC# 201307567) at University of Florida.
Ussing chamber-short-circuit current measurements from colonic segments
After animals were euthanized and segments of colons were quickly isolated. The colons were cut open along the mesenteric border into a flat sheet, and flushed with ice-cold Ringer solutions. The intact full-thickness segments containing all the layers of the proximal and distal colons and the colons stripped off the muscle layers comprising the myenteric plexus were used. A pair of adjacent colonic segments were incised and mounted between two halves of a modified Ussing chamber (Physiologic Instruments, San Diego, CA) and short circuited by a voltage clamp (VCC MC6, Physiologic Instruments) with correction for solution resistance. The exposure area was 0.3 cm2. Unless otherwise indicated, the mucosal and serosal surfaces of the tissue were bathed in reservoirs with 3 ml HEPES-Ringer solution containing 110 mM NaCl, 1.25 mM CaCl2, 1.2 mM MgCl2, 5 mM KCl, 10 mM glucose, and 22 mM HEPES, pH 7.4, maintained at 37°C and continuously bubbled with 100% O2. On average, an interval of 10 min elapsed between euthanizing the animal and mounting the tissue in the chamber. Tissues were allowed a minimum of 15 min stabilization and basal recording period before R568 (10 μM, Tocris Bioscience, Ellisville, MI), TTX (2 μM, Enzo Life Science, Plymouth Meeting, PA) or vehicles were added to the serosal side of the intestine. Short-circuit current, Isc, was monitored continuously and data were acquired via DATAQ instruments and were stored in a PC and processed using the program Acquire & Analyze. The Isc is defined as the current flow through the tissue when the tissue is short-circuited (i.e., when the voltage across the tissue is zero). Magnitudes (μA/cm2) of change in Isc were determined before and after additions of test reagents or vehicle. In a previous study, we have determined that the Isc measured under current experimental conditions reflects primarily the secretion of Cl− [14].
Immunolocalization of c-fos protein within the ENS
A piece of tissue (about 1.5 cm each) from proximal (1 cm from cecum) or distal (1 cm from anal end) colon of mice was isolated, rinsed with 37°C pre-warmed Krebs solution and then transferred into Ussing chamber superfused with 5ml 37°C Krebs solution containing nifedipine (10 μM) to avoid muscle contraction and gassed with mixture of 5% CO2/95% O2. The composition of Krebs solution was 120.9 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 14.4 mM NaHCO3, 11.5 mM Glucose and 1.25 mM CaCl2. Afterward, R568 (100 μM) or vehicle (DMSO) was added to stimulate CaSR. Three hours later, the colon segments were opened longitudinally along the mesenteric border and pinned flat on a Sylgard-coated Petri dish (Sylgard 184, Dow Corning Midland, MI). The whole-mount preparations were fixed in freshly prepared 4% paraformaldehyde solution for 2 h in room temperature followed by washing in 0.1 M phosphate-buffered saline (PBS) three times. The mucosa, sub-mucosa and circle muscle layer of colon were removed by gentle stroking with fine forceps and the longitudinal muscle/myenteric plexus (LMMP) was obtained. The LMMP was then permeabilized and nonspecific binding sites were blocked by incubation for 1 h in 0.1 M PBS with 0.5% Triton X-100 and 4% goat serum at room temperature. Then tissues were incubated with rabbit anti-c-fos antibody (9F6, 1:400, Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C. After three 10 min washes in 0.1 M PBS, tissues were incubated with Alexa Fluor 488 conjugated goat anti-rabbit IgG (H+L) F(ab′)2 fragment (1:800, Cell Signaling Technology, Beverly, MA, USA) for 1 h, washed 10 min in 0.1 M PBS ×3, and the sections were mounted with DAPI Fluoromount-GTM (Citifluor, UK). All fluorophores were visualized under a Nikon microscope equipped with a fluorescence unit and the appropriate filter sets. Photographs were taken under the same exposure conditions with an Olympus C-3040 zoom (Tokyo, Japan).
For quantification of c-fos immunoreactive signals, digital images captured from the fluorescence microscope were transferred to Adobe Photoshop. Neurons were defined as c-fos positive cells when nuclear staining was evident and a clear nuclear border was formed above the background. For comparison of c-fos signals, ten largest ganglia in each preparation were selected, and the total number of c-fos positive neurons in these 10 ganglia was calculated. Two preparations (proximal colon and distal colon) per animal and 10 animals per treatment group were assessed.
Statistical analysis
Data were expressed as means ± SE. Statistical comparisons between two means were performed by Student’s t-test using either Microsoft Excel 10 for Windows or Graph Pad Prism version 6 for Windows (Graph Pad Software, San Diego, CA). P < 0.05 was considered significant.
Results
Inhibitory effects of TTX on Isc in full-thickness and muscle stripped colons
Active Cl− secretion by the intestinal epithelium is modulated by the TTX-sensitive neurons in the ENS, including those comprising the submucosal plexus (i.e., Meissner’s plexus) and myenteric plexus (i.e., Auerbach’s plexus) [16]. Our previous study has shown expression of the extracellular calcium-sensing receptor (CaSR) on neurons of both plexuses and the inhibition of Cl− secretion by CaSR activator, R568, in the absence, but not presence of the neurotoxin, TTX [14]. However, whether the effect of R568 is mediated through the submucosal or myenteric plexus or both is unknown. Thus, our 1st set of experiments was performed to assess the relative contribution of neurons from these two plexuses to active anion secretion by the intestine. This was achieved by comparing the short circuit current (Isc ) responses to TTX in two preparations of both proximal and distal colons: 1) full-thickness preparation, which contained both the submucosal and myenteric plexuses, and 2) “stripped” preparation, which contained the submucosal plexus only without the myenteric plexus. In these experiments, the neurotransmission inhibitor TTX (2 μM) was added to the serosal side to inactivate the activity of neurons. Our preliminary studies showed that when added apically, the same dose of TTX produced no significant effect on Isc in either of colon preparations.
Under present experimental conditions, a relatively high basal Isc was observed in full-thickness (Figure 1, A and C) or non-stripped preparations (Figure 1, B and D) of proximal and distal colon. This basal Isc was stable for at least 60 min. TTX inhibited Isc in both proximal and distal colon. Thus, in non-stripped preparation, TTX decreased Isc from 53.6±9.6 to 16.7±3.1 μA/cm2 (P<0.01) in proximal colon and from 47.6±8.1 to 12.3±2.1 μA/cm2 (P<0.01) in distal colon (Figure 1, C). In stripped preparation, TTX decreased Isc from 38.0±4.1 to 19.6±3.1 μA/cm2 (P=0.01) in proximal colon and decreased Isc from 41.8±4.2 to 24.9±3.0 μA/cm2 (P<0.01) in distal colon (Figure 1, D).
Figure 1. Inhibitory effects of TTX on Isc in full-thickness and myenteric plexus stripped colon.
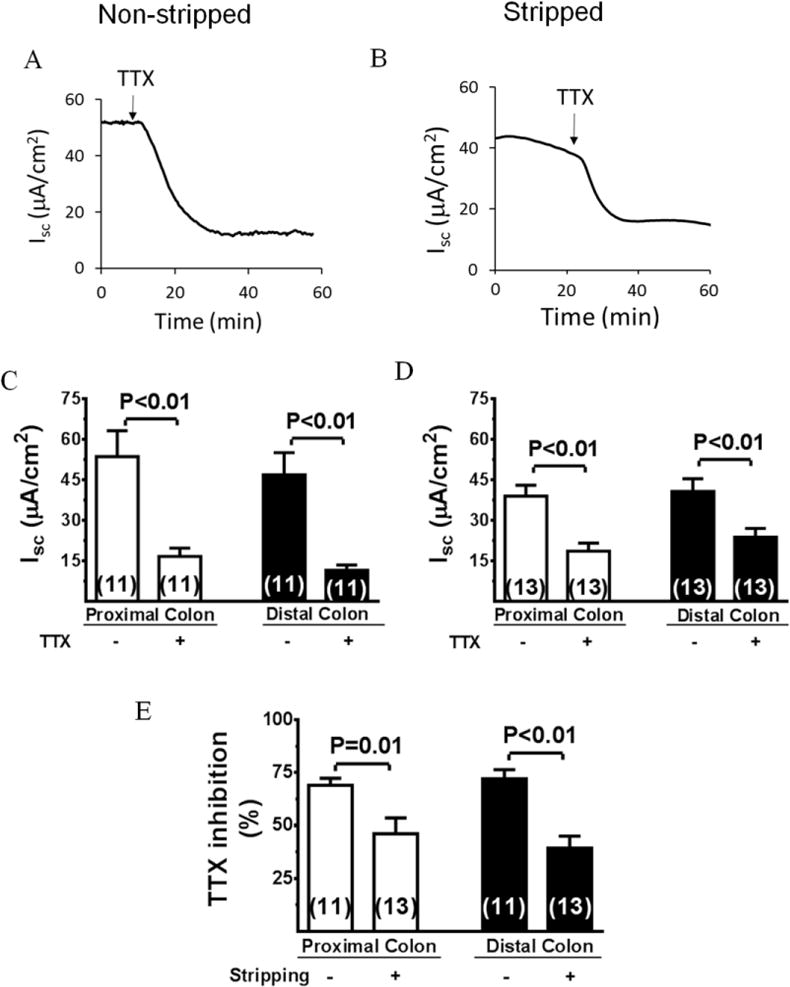
A and B, Representative recordings of Isc response to TTX in non-stripped and stripped distal colons. C and D, Summary of effects of TTX on Isc. E, Summary of effect of stripping on Isc inhibition by TTX. Data are means ± SE. The number of experiments is shown individually in each data bar.
The percent inhibition by TTX was shown in Figure 1, E. In non-stripped colon, Isc decreased 68.8±3.6% in proximal colon and 74.1±3.8% in distal colon. However, in stripped colon, such decreases were 48.4±9.6% and 40.4±5.6%. Thus, stripping off the myenteric plexus only reduced the TTX effect by 29.7 ±8.6% and 45.5 ±5.1%, respectively. This result is consistent with the literature [16] and our previous observations made in rat colon [14], suggesting that, in rodent colon, the secretory Isc response is controlled, to a large extent, by secretomotor neurons in the submucosal plexus. This comparable result also indicates that, in conjunction with the Ussing chamber, the two freshly isolated perfused stripped and non-stripped colon preparations are excellent ex vivo models to study regulation of ENS-mediated neural regulation of ion transport.
Inhibitory effects of R568 on Isc in full-thickness and stripped colons
To assess the role of CaSR in the ENS, we next examined the effect of R568, a specific agonist of CaSR, on Isc in intact non-stripped and stripped colon preparations. As shown in Figure 2, activation of CaSR by addition of 10 μM R568 to serosal solution significantly reduced Isc in the proximal and distal colon of both intact (Figure 2, A and C) and stripped preparations (Figure 2, B and D). In non-stripped colon, R568 decreased Isc from 30.2±5.1 to 10.6±2.1 μA/cm2 (P<0.01) in proximal colon and from 33.6±2.6 to 8.6±1.4 μA/cm2 (P<0.01) in distal colon. On the other hand, in stripped colon, R568 decreased Isc from 44.8±5.2 to 37.0±4.9 μA/cm2 (P<0.05) in proximal colon and from 37.6±3.3 (μA/cm2) to 29.6±4.6 μA/cm2 (P<0.05) in distal colon. Figure 2, E, summarizes the percent inhibition induced by R568. In non-stripped colon, Isc decreased 64.9±4.3% by R568 in proximal colon and 74.4±3.9% in distal colon. In stripped colon, however, R568 only decreased Isc 17.4±2.6% in proximal colon and 21.3±4.6% in distal colon. Thus, stripping eliminated the R568 effect in proximal and distal colon by 73.2±3.9% and 71.4±4.2%, respectively. Furthermore, the R568 effects are neutrally-mediated, as in both preparations, prior treatment with TTX abolished the R568 effect (data not shown). These results suggest that R568 produces its anti-secretory effect in proximal and distal colon primarily through inhibition of neurons in the myenteric plexus.
Figure 2. Inhibitory effects of R568 on Isc in full-thickness and myenteric plexus-stripped colon.
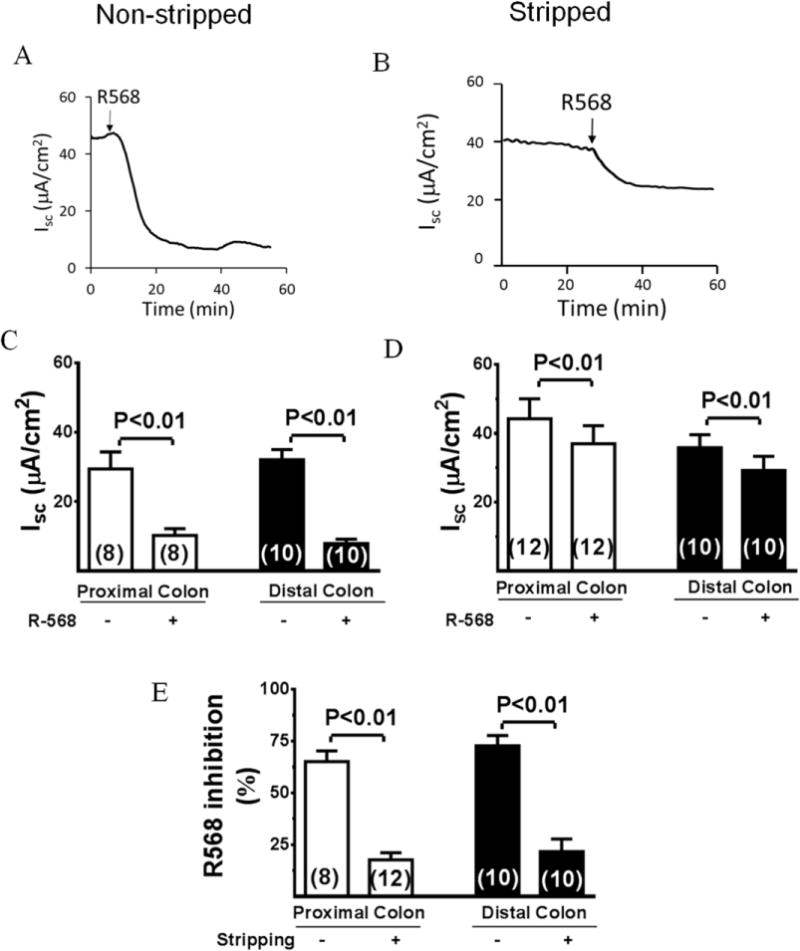
A and B, Representative recordings of Isc response to R568 in non-stripped and stripped distal colon. C and D, Summary of effects of R568 on Isc. E, Summary of effect of stripping on Isc inhibition by R568. Data are means ± SE. The number of experiments is shown individually in each data bar.
Effects of R568 on the c-fos expression in myenteric plexus
To confirm the inhibitory effect of R568 on neuronal activity in myenteric plexus, we performed immunofluorescent labeling study to examine the effect of R568 on the expression of c-fos protein in neurons of the myenteric plexus of proximal and distal colon of mice. In the brain as well as in the ENS, the expression of the immediate early gene, c-fos, is dynamically changed in response to stimulations and has therefore been widely used as a molecular marker for neuronal activity and excitability [17,18]. In these studies, the c-fos positive nuclei demonstrated a circular or oval profile (Figure 3, B; green fluorescence). In a pilot study, we had verified the c-fos immunoreactivity in neurons of the myenteric ganglia using the neuron-specific marker β-tubulin staining (Figure 3, C; red fluorescence) and observed co-localization of c-fos and β-tubulin signals in DAPI stained nuclei (Figure 3, A; blue fluorescence) of myenteric ganglia (Figure 3, D). In subsequent studies, only the data of c-fos immunoreactivity are shown.
Figure 3. Photomicrographs showing c-fos expression in myenteric plexus of colon.
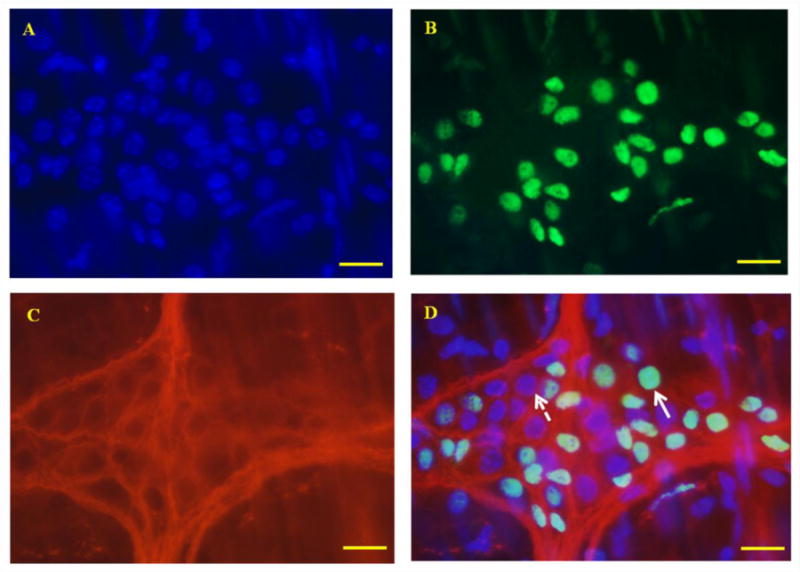
A: DAPI staining of the nuclei. B: c-fos staining. C: β-tubulin staining. D: merge of A, B and C. All c-fos protein co-expressed with DAPI and was restricted within myenteric ganglion areas. Arrow with dotted line indicates lack of expression of c-fos in a DAPI-stained nucleus. Arrow with solid line indicates co-expression of c-fos and DAPI in a nucleus of neuron. Scale Bars = 10μm
Figure 4 shows the changes in number of c-fos positive neurons in wild type mice. Figure 4, A–D, show representative images of c-fos fluorescence (green)-positive neurons. The boundaries of ganglions were identified and marked by yellow dotted line. The changes in in number of c-fos positive neurons are summarized in Figure 4, E. The c-fos positive neurons were noticed in both control and R568-treated preparations that were kept in 37°C pre-warmed Krebs solution for 3h. R568 treatment significantly reduced the number of c-fos positive neurons in myenteric plexus, both in the proximal colon [means±SE (n) in 10 ganglia in the absence vs. presence of R568: 132 ± 12 (10) vs. 85 ± 6 (10), P<0.01]and in distal colon [150 ± 14 (10) vs. 107 ± 10 (10), P<0.05]. The average decreases were 34.2 ± 6.5% and 26.4 ± 9.4%, respectively.
Figure 4. Effects of R568 on distribution of c-fos immunoreactive nuclei (in Green) in proximal and distal colons in wild type mice.
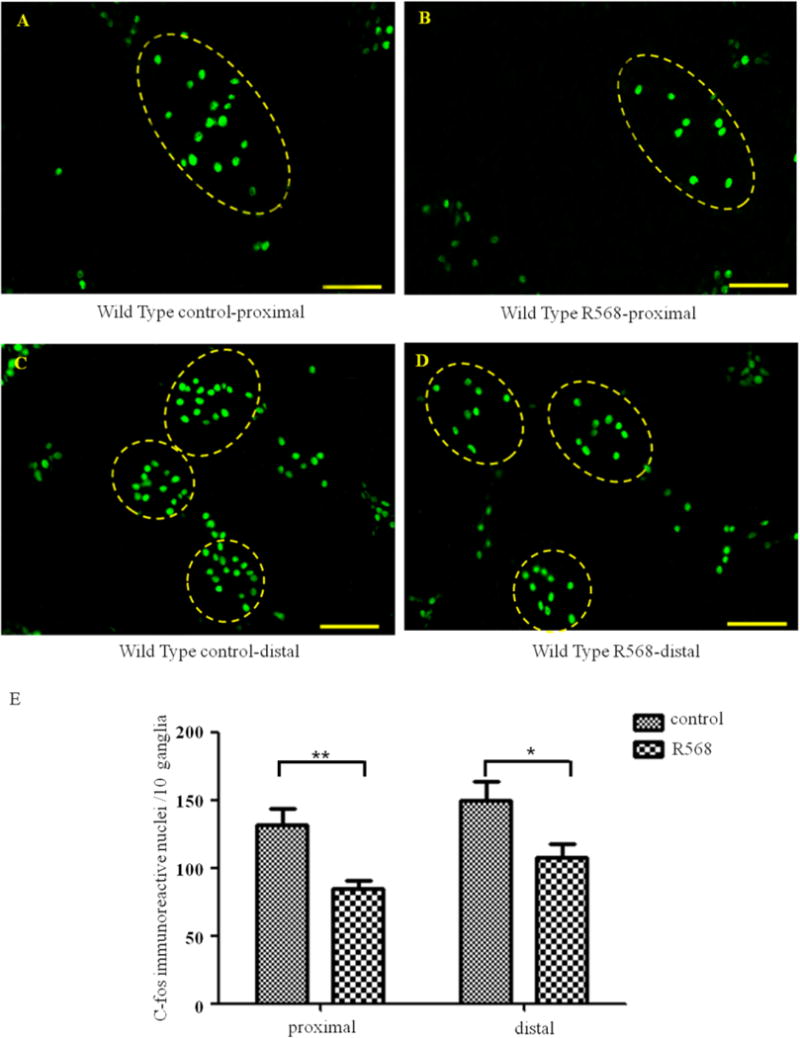
A–D show representative photomicrographs and E shows summary of expression of c-fos positive neurons in proximal (A–B) and distal (C–D) colons in the absence (A and C) and presence (B and D) of R568. Data are shown as mean ± SEM (n=10 independent experiments). Scale Bars = 50μm, *, P<0.05, **, P<0.01
To verify that CaSR mediated the effect of R568, additional studies were performed in both enterocyte-specific CaSR knockout mice (villin mice; Figure 5) and neuron-specific CaSR knockout mice (nestin mice; Figure 6). In villin mice where CaSR in enteric neurons remains unaltered, the R568 inhibitory effect remained, both in proximal colon [means±SE (n) in 10 ganglia in the absence vs. presence of R568: 140 ± 13(10) vs. 90 ± 9(10), average decrease of 33.4±8.0%, P<0.05, Figure 5] and in distal colon [145 ± 16(10) vs. 84 ± 8(10), average decrease of 41.2 ± 5.6%, P<0.01, Figure 5). These changes are comparable to those in wild type mice. However, in nestin mice where CaSR in enteric neurons was genetically ablated, such an inhibitory effect of R568 on the activity of myenteric neurons was not observed, neither in the proximal colon [124 ± 8(10) vs. 117 ± 8(10), average change of 5.4 ± 5.2%, P>0.05, Figure 6] nor in the distal colon [149 ± 12(10) vs. 155 ± 12(10), average change of 6.7 ± 11.6%, P>0.05, Figure 6]. These data suggest that R568 inhibits the activity of neurons via activation of CaSR in the myenteric neurons and that this inhibition is direct without requiring the presence of CaSR signaling from enterocyte.
Figure 5. Effects of R568 on distribution of c-fos immunoreactive nuclei in distal and proximal colons in villin mice.
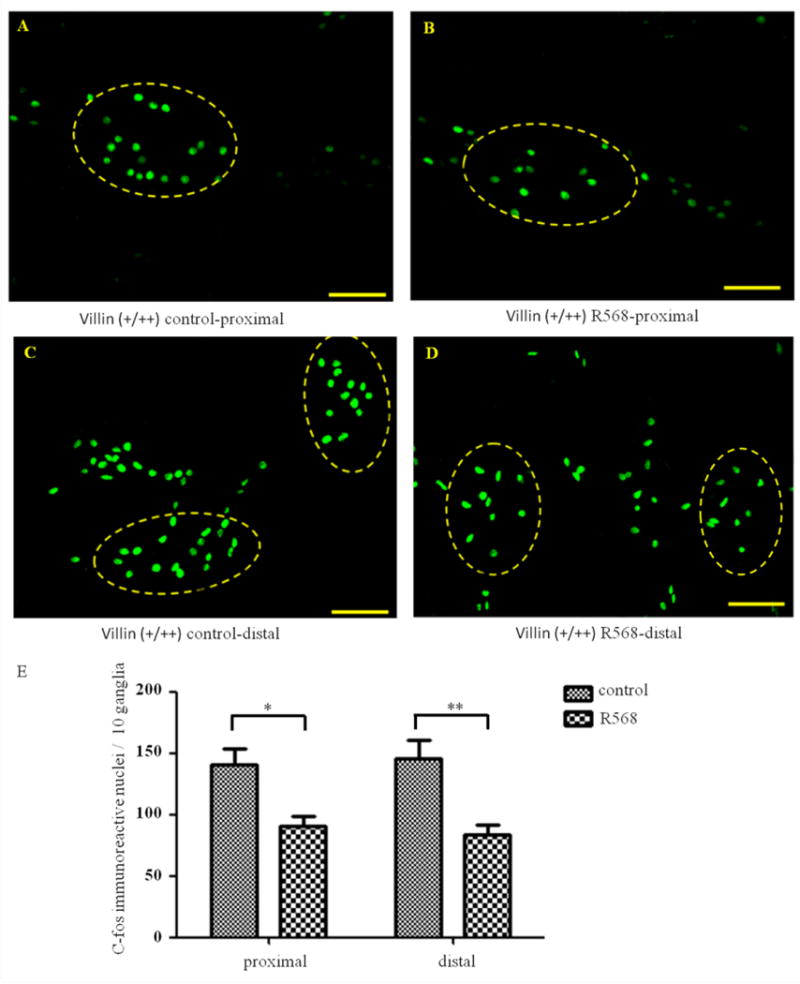
A–D show representatives and E shows summary of expression of c-fos positive neurons in proximal (A–B) and distal (C–D) colons in the absence (A and C) and presence (B and D) of R568. Data are shown as mean ± SEM (n=10 independent experiments). Scale Bars = 50μm, *, P<0.05, **, P<0.01
Figure 6. Effects of R568 on distribution of c-fos immunoreactive nuclei in distal and proximal colons in nestin mice.
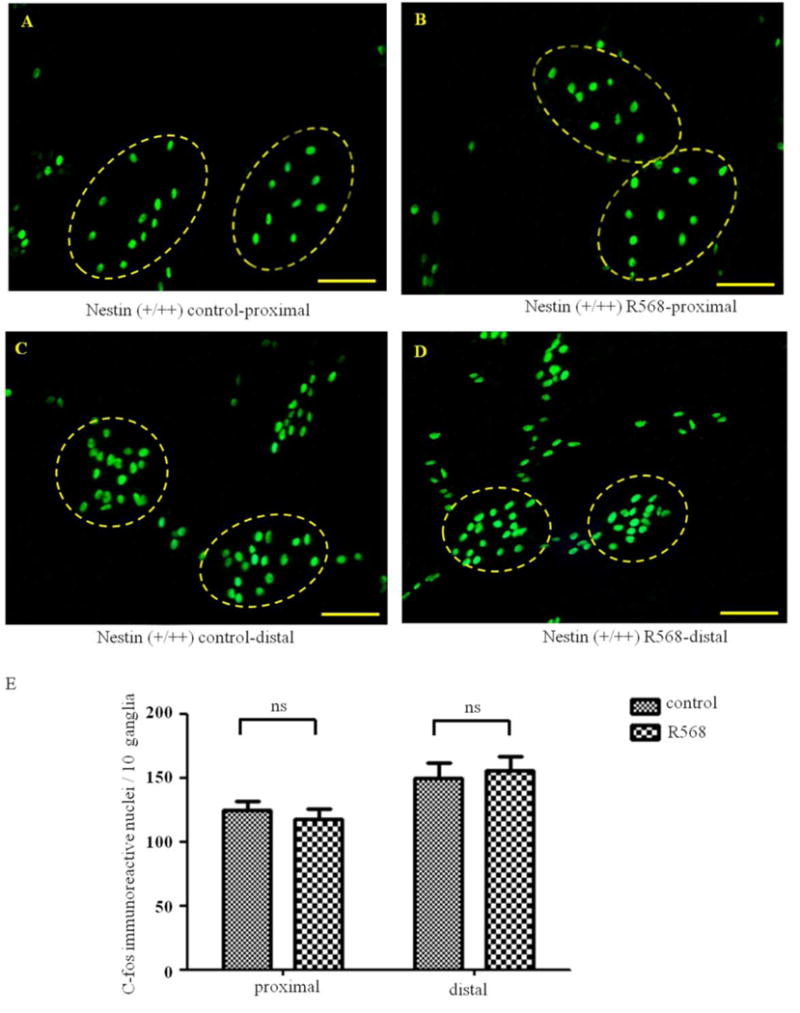
A–D show representatives and E shows summary of expression of c-fos positive neurons in proximal (A-B) and distal (C–D) colons in the absence (A and C) and presence (B and D) of R568. Data are shown as mean ± SEM (n=10 independent experiments). Scale Bars = 50μm, ns, not significant
Discussion
The present study employed the Ussing chamber ex vivo perfusion model and further investigated the role and function of CaSR in the control of electrolyte secretion by the colon. We used short-circuit current (Isc) to reflect Cl− secretion, TTX sensitivity to separate neuronally-mediated from non neuronally-mediated secretion, and physical stripping to separate the secretion mediated by the myenteric plexus from the submucosal plexus. Our results demonstrate that: 1) neurons in the submucosal and the myenteric plexus could both regulate the secretion of colon; 2) Neurons in submucosal plexus may take a more important part in stimulating secretion; 3) activation of CaSR has an anti-secretory effect on colon and this effect is mainly mediated by neurons in myenteric plexus. We then studied how activation of CaSR in the myenteric neurons leads to an inhibition of intestinal secretion. These include the use of neurotoxin to prior block neuronal activity to show the abolition of the R568 effect on Isc (which implies that R568 acts to inhibit neuronal activity), the use of immunostaining of expression of c-fos protein in nuclei of neurons, a biochemical measure of neuronal excitability, to confirm that R568 indeed alters neuronal activity, and the use of two tissue-specific CaSR knockouts, that is, neuron-vs. enterocyte-specific CaSR KO, to determine the specificity of the R568 action on CaSR in neurons, not indirectly via the receptor in enterocytes. Our results provide the first direct evidence demonstrating that CaSR in myenteric neurons is functioning and, when activated by R568, inhibiting the ENS activity and ENS-mediated Cl− secretory response.
In the present study, we showed that TTX inhibited Isc and that removal of the myenteric plexus only slightly reduced this TTX-sensitive Isc. This observation is consistent with the previous literatures [19–21], suggesting that the majority of the intestinal anion secretion is controlled by neurons in submucosal plexus of intestine. In the past, it was generally believed that, in gastrointestinal tract, neurons in submucosal plexus mainly modulate the function of secretion and blood flow [3,4], whereas neurons in myenteric plexus primarily regulate gastrointestinal motility [1,2]. However, subsequent immunohistochemical studies have identified several classes of neurons within the myenteric plexus that send out projections that terminate within the submucosal plexus [22–24]. Several in vivo studies further strengthen the concept that important connections exist between the myenteric and submucosal plexus and suggest that there is coordination of motility and secretion in the intestine [25,26]. In addition, secretory responses evoked by cholera toxin in the intestinal lumen appear to be mediated by neural pathways that involve the myenteric plexus [27,28]. In the present study, we noted that stripping off of the myenteric plexus reduced the TTX-sensitive neuronally-mediated Isc by ~30%. This effect, albeit small, does support the concept that myenteric plexus takes active part in this process possibly via modulating neuronal activity in submucosal plexus.
The expression of CaSR in the colon has previously been documented by RT-PCR, Northern blotting and immunohistochemical studies [11,12,29]. These studies show that the receptor is localized not only at basal and apical cell membranes of colonic crypt cells [11,29] but also in the serosa, submucosa, and intramuscular layers, in which the submucosal and myenteric plexus of the ENS are located [29]. Previous study in our lab has shown that the inhibitory effect of R-568 on Isc of colon mounted in Ussing chamber setup is mediated primarily through the ENS, and, to a lesser extent, via the epithelial cells [14]. However, the previous study did not address which part of the ENS is the target of R-568. So, in the present experiments, we examined and compared the effects of R-568 on Isc in colon preparations with and without the presence of the myenteric plexus. We found that removing myenteric neurons largely eliminated the R568 effect. Thus, in stripped colons only 20–30% of the R568 effect remained. This finding suggests that this agonist exerts its anti-secretory effect mainly through the myenteric neurons.
To further confirm that myenteric neurons are implicated in CaSR regulation, the effect of R568 on the expression of c-fos immunoreactivity in neurons of myenteric plexus was studied. Our results showed that the expression of c-fos protein in myenteric neurons was significantly inhibited by R568 in both distal and proximal colons of wild type mice; such an inhibitory effect was absent in colon of mice in which CaSR in the ENS was genetically deleted, but still remained in the mice that CaSR in the intestinal epithelium was genetically deleted. These results establish that R568 can activate CaSR in myenteric neurons and inhibit their excitability. Interestingly, a significant number of c-fos positive neurons were detected even under basal non-stimulated conditions, not only in WT mice, but also in nestin mice and villin mice. The reason might be related to the ex vivo tissues used. Normally, the enteric neurons receive a tonic inhibitory action by the sympathetic outflows [30,31]. These inhibitory signals become unavailable when the colon is isolated from the animal and incubated in Krebs solution ex vivo [31]. In our previous study, CaSR is expressed in virtually every single neuron in myenteric plexus [14] and, when activated, blocks virtually all TTX-sensitive Isc. However, in the present study, activation of CaSR by the same agonist R568 only reduced a fraction of c-fos positive neurons. One possibility is the relative insensitivity of the latter method, because it takes time for neurons to alter c-fos protein expression. Alternatively, while the results from both methods indicate R568 inhibition of neuronal activity, the former assays the short-term minute-to-minute effect, whereas the latter detects the long-term effect that requires nuclear transmission of CaSR cell membrane signal and c-fos gene transcription and translation – steps determined also by other receptors, neurotransmitters and activity-modifiers [32].
The mechanism how CaSR activation in neurons of myenteric plexus leads to inhibition of colonic secretion by intestinal epithelium remains unknown. In the ENS system, two types of excitatory postsynaptic potentials (EPSP) exist: the fast EPSP, mediated through the activation of postsynaptic nicotinic receptors by acetylcholine released from nerve terminals whose cell bodies lie mainly in the myenteric plexus [33], and the slow EPSP, which is also of enteric origin [33,34] but is mediated by a peptide, such as substance P or vasoactive intestinal polypeptide (VIP) [35]. Most submucosal neurons receive fast and slow EPSPs from myenteric neurons [36] and the majority of these submucosal neurons activate secretion [37]. There is evidence that EPSP plays an important role in controlling cellular excitability [38,39]. In this regard, it is tempting to postulate that activation of CaSR may reduce myenteric neuronal activity and then affect secretion through regulating the activity of submucosal neurons via modulation of EPSPs (see summary in Figure 7). Future work is needed to verify this hypothesis and define the type of neurons and neurotransmitters involved. Furthermore, since the main function of myenteric neurons is to regulate intestinal motility, we need to define if R568 modulates the motility of the intestine. In view of the roles the myenteric plexus plays in the coordination of motility and secretion, an even broader question is raised of whether CaSR on myenteric neurons function as a ‘motility sensor’ via sensing the local Ca2+ released as a result of muscle contraction, facilitating the coupling of contractile behavior with secretion.
Figure 7. Model showing how CaSR regulates epithelial Cl− secretion through the ENS.
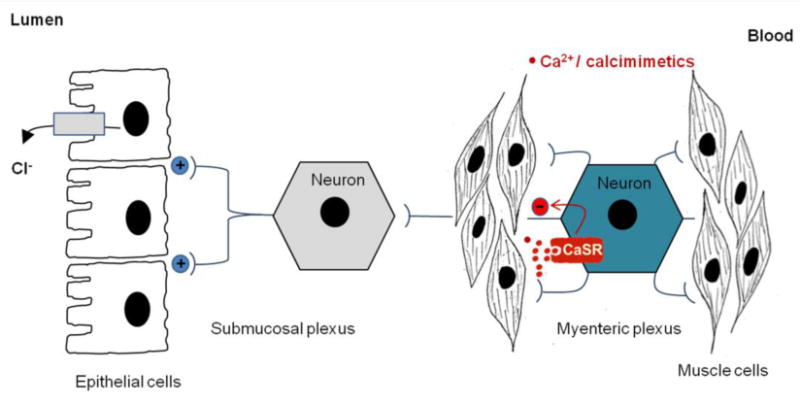
The ENS is composed of two neural plexuses and their connecting fiber. While the submucosal plexus controls epithelial secretion and the myenteric plexus controls muscle contraction, the connecting nerve fiber, projected from secretomotor neuron of myenteric plexus, functions to coordinate them. CaSR is expressed in this myenteric secretomotor neuron, and when activated by calcimimetics/Ca2+, released either as a result of food digestion or from muscle contraction, inhibits myenteric neuronal excitability/activity required for submucosal neuron-mediated fluid secretion, thus coupling contractile behavior with secretion. +, stimulation; −, inhibition.
The finding that CaSR agonist inhibits secretion through receptor-mediated modulation of neuronal activity in myenteric plexus may have both physiological and pathophysiological significance. Since in the intestine the ENS network goes along with the nutrient-carrying blood and lymph vessel networks, the interstitial Ca2+ and other nutrients undergo dynamic changes [40], and CaSR may provide a mechanism for the ENS to sense these changes and fine-tune its activity/function in accordance with the status of digestion and absorption[41]. Upon food ingestion, the mechanical signals activate the ENS, thereby promoting secretion and motility to facilitate digestion. Following the completion of digestion, the chemical signals (e.g., absorbed nutrients) carried in the parallel blood or lymph vessels may activate CaSR on neurons in the ENS to downregulate neuronal activity, thereby slowing down the secretion and motility to facilitate absorption. Pathologically, CaSR may play a role in the pathophysiology of irritable bowel syndrome (IBS), a common clinical condition characterized by chronic diarrhea and/or constipation in addition to abdominal pain and cramping. Given CaSR expression and function in neurons of the myenteric plexus, it is tempting to speculate that over activity of CaSR may result in under activation of the ENS, leading to constipation-dominate form of IBS (IBS-C), whereas under activation of this receptor may result in over activity of the ENS, leading to diarrhea-dominate form of IBS (IBS-D).
Conclusions
In summary, we show that while both neurons in submucosal plexus and myenteric plexus regulate the secretion of colon, neurons in submucosal plexus play a more important role in stimulating secretion. In contrast, while CaSR is expressed in both plexuses, CaSR inhibits neural-mediated secretion predominantly via neurons in the myenteric plexus (Figure 7). In view of the unique property of this receptor in sensing Ca2+ and the essential role that the myenteric plexus plays in the coordination of secretion and motility, the data raise a question of whether CaSR on myenteric neurons function as a ‘motility sensor’ via sensing the Ca2+ released as a result of contraction, coupling motility to secretion. The ability of myenteric CaSR to reduce intestinal secretion might provide a new insight into the mechanism by which the gut coordinates secretion with motility and fine-tunes secretion in accordance with the state of digestion and absorption.
Acknowledgments
This work was supported by National Institutes of Health HD079674 (SXC); R01AR067291 (WC); Xiangrong Sun was a visiting scholar funded by the China Scholar Council. We would like to extend our gratitude to Shi Jin for participating in the discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors report no conflicts of interest. No writing assistance is provided for this manuscript.
Author contribution to study
Xiangrong Sun wrote the first draft of the manuscript. Xiangrong Sun, Lieqi Tang and Steven Winesett acquired the data. Wenhan Chang provide the materials (KO mice) and revised the manuscript. Sam Xianjun Cheng supervised the experiments and revised and finalized the manuscript. All the authors have read and approved the final manuscript
References
- 1.Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience. 1982;7:341–349. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- 2.Song ZM, Brookes SJ, Costa M. Projections of specific morphological types of neurons within the myenteric plexus of the small intestine of the guinea-pig. Cell Tissue Res. 1996;285:149–156. doi: 10.1007/s004410050630. [DOI] [PubMed] [Google Scholar]
- 3.Vanner S, Jiang MM, Surprenant A. Mucosal stimulation evokes vasodilation in submucosal arterioles by neuronal and nonneuronal mechanisms. Am J Physiol. 1993;264:G202–212. doi: 10.1152/ajpgi.1993.264.2.G202. [DOI] [PubMed] [Google Scholar]
- 4.Vanner S, Macnaughton WK. Submucosal secretomotor and vasodilator reflexes. Neurogastroenterol Motil. 2004;16(Suppl 1):39–43. doi: 10.1111/j.1743-3150.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith TK, Koh SD. A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am J Physiol Gastrointest Liver Physiol. 2017;312:G1–G14. doi: 10.1152/ajpgi.00337.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed DE, Vanner S. Mucosal stimulation activates secretomotor neurons via long myenteric pathways in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G608–614. doi: 10.1152/ajpgi.00364.2006. [DOI] [PubMed] [Google Scholar]
- 7.Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil. 2007;19:869–878. doi: 10.1111/j.1365-2982.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 8.Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci. 2005;42:35–70. doi: 10.1080/10408360590886606. [DOI] [PubMed] [Google Scholar]
- 9.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology. 2004;126:148–158. doi: 10.1053/j.gastro.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 11.Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol. 2002;283:G240–250. doi: 10.1152/ajpgi.00500.2001. [DOI] [PubMed] [Google Scholar]
- 12.Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2+-sensing receptors in intestinal epithelium. Am J Physiol. 1997;273:C1168–1175. doi: 10.1152/ajpcell.1997.273.4.C1168. [DOI] [PubMed] [Google Scholar]
- 13.Geibel J, Sritharan K, Geibel R, Geibel P, Persing JS, Seeger A, Roepke TK, Deichstetter M, Prinz C, Cheng SX, et al. Calcium-sensing receptor abrogates secretagogue-induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci U S A. 2006;103:9390–9397. doi: 10.1073/pnas.0602996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng SX. Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2012;303:G60–70. doi: 10.1152/ajpgi.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rey O, Chang W, Bikle D, Rozengurt N, Young SH, Rozengurt E. Negative crosstalk between calcium-sensing receptor and beta-catenin signaling systems in colonic epithelium. J Biol Chem. 2012;287:1158–1167. doi: 10.1074/jbc.M111.274589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 18.Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- 19.Baldassano S, Liu S, Qu MH, Mule F, Wood JD. Glucagon-like peptide-2 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro. Am J Physiol Gastrointest Liver Physiol. 2009;297:G800–805. doi: 10.1152/ajpgi.00170.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LS, Zheng LF, Xu JD, Ji T, Guo H, Li XF, Li Y, Zhang Y, Zhu JX. Entacapone promotes cAMP-dependent colonic Cl(-) secretion in rats. Neurogastroenterol Motil. 2011;23:657–e277. doi: 10.1111/j.1365-2982.2011.01715.x. [DOI] [PubMed] [Google Scholar]
- 21.Nishikitani M, Kokubo A, Takemura H, Saigenji K, Kawahara K. Serosal application of Ba(2+) induces oscillatory chloride secretion via activation of submucosal cholinergic neurones in guinea-pig distal colon. Acta Physiol Scand. 2002;174:257–264. doi: 10.1046/j.1365-201x.2002.00938.x. [DOI] [PubMed] [Google Scholar]
- 22.Costa M, Furness JB. The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983;8:665–676. doi: 10.1016/0306-4522(83)90002-7. [DOI] [PubMed] [Google Scholar]
- 23.Furness JB, Costa M, Miller RJ. Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983;8:653–664. doi: 10.1016/0306-4522(83)90001-5. [DOI] [PubMed] [Google Scholar]
- 24.Steele PA, Brookes SJ, Costa M. Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience. 1991;45:227–239. doi: 10.1016/0306-4522(91)90119-9. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood B, Davison JS. Role of extrinsic and intrinsic nerves in the relationship between intestinal motility and transmural potential difference in the anesthetized ferret. Gastroenterology. 1985;89:1286–1292. doi: 10.1016/0016-5085(85)90644-4. [DOI] [PubMed] [Google Scholar]
- 26.Read NW, Smallwood RH, Levin RJ, Holdsworth CD, Brown BH. Relationship between changes in intraluminal pressure and transmural potential difference in the human and canine jejunum in vivo. Gut. 1977;18:141–151. doi: 10.1136/gut.18.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. 1993;104:1285–1292. doi: 10.1016/0016-5085(93)90336-b. [DOI] [PubMed] [Google Scholar]
- 28.Nocerino A, Iafusco M, Guandalini S. Cholera toxin-induced small intestinal secretion has a secretory effect on the colon of the rat. Gastroenterology. 1995;108:34–39. doi: 10.1016/0016-5085(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca(2+)-sensing receptor in rat intestine. Am J Physiol. 1998;274:G122–130. doi: 10.1152/ajpgi.1998.274.1.G122. [DOI] [PubMed] [Google Scholar]
- 30.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 31.Yuyama N, Mizuno J, Tsuzuki H, Wada-Takahashi S, Takahashi O, Tamura K. Effects of extrinsic autonomic inputs on expression of c-Fos immunoreactivity in myenteric neurons of the guinea pig distal colon. Brain Res. 2002;948:8–16. doi: 10.1016/s0006-8993(02)02943-8. [DOI] [PubMed] [Google Scholar]
- 32.Lim HD, Kim MH, Lee CY, Namgung U. Anti-Inflammatory Effects of Acupuncture Stimulation via the Vagus Nerve. PLoS One. 2016;11:e0151882. doi: 10.1371/journal.pone.0151882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bornstein JC, Costa M, Furness JB. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol. 1988;398:371–390. doi: 10.1113/jphysiol.1988.sp017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bornstein JC, Furness JB, Costa M. Sources of excitatory synaptic inputs to neurochemically identified submucous neurons of guinea-pig small intestine. J Auton Nerv Syst. 1987;18:83–91. doi: 10.1016/0165-1838(87)90137-8. [DOI] [PubMed] [Google Scholar]
- 35.Mihara S. Intracellular recordings from neurones of the submucous plexus. Prog Neurobiol. 1993;40:529–572. doi: 10.1016/0301-0082(93)90034-p. [DOI] [PubMed] [Google Scholar]
- 36.Moore BA, Vanner S. Properties of synaptic inputs from myenteric neurons innervating submucosal S neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 2000;278:G273–280. doi: 10.1152/ajpgi.2000.278.2.G273. [DOI] [PubMed] [Google Scholar]
- 37.Evans RJ, Jiang MM, Surprenant A. Morphological properties and projections of electrophysiologically characterized neurons in the guinea-pig submucosal plexus. Neuroscience. 1994;59:1093–1110. doi: 10.1016/0306-4522(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 38.Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973;231:471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood JD, Mayer CJ. Intracellular study of tonic-type enteric neurons in guinea pig small intestine. J Neurophysiol. 1979;42:569–581. doi: 10.1152/jn.1979.42.2.569. [DOI] [PubMed] [Google Scholar]
- 40.Mupanomunda MM, Ishioka N, Bukoski RD. Interstitial Ca2+ undergoes dynamic changes sufficient to stimulate nerve-dependent Ca2+-induced relaxation. Am J Physiol. 1999;276:H1035–1042. doi: 10.1152/ajpheart.1999.276.3.H1035. [DOI] [PubMed] [Google Scholar]
- 41.Tang L, Cheng CY, Sun X, Pedicone AJ, Mohamadzadeh M, Cheng SX. The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity. Front Physiol. 2016;7:245. doi: 10.3389/fphys.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]


