Abstract
Objective
A long-hypothesized pathway through which low socioeconomic status (SES) harms health is through dysregulation of the physiologic stress response systems. No previous studies have tested this hypothesis by investigating cortisol reactivity and recovery to acute stress in relation to SES at different times in the life course in adults. Alteration of the cortisol response to an acute stressor could signal dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and has been associated with chronic illness.
Methods
We used data on 997 adults aged ≥ 54 years from a multi-ethnic, multi-site United States study to examine associations between life course SES and cortisol response to a laboratory stress challenge. Informed by life course theory, we hypothesized that lower child and adult SES would be associated with lower reactivity (i.e., smaller increase in cortisol) and a slower recovery rate (i.e., slower rate of decline in cortisol following the challenge).
Results
In demographics-adjusted multilevel piecewise linear regression models, low child and adult SES were associated with a 19% (95% CI: 4%–50%) and 27% (7%–55%) slower recovery rate compared to high child and adult SES, respectively. Compared to participants with stable high SES, those with stable low SES had a 48% (16%–70%) slower recovery rate. Differences in reactivity by SES were small.
Conclusions
Our results support the hypothesis that low SES throughout life affects the HPA axis, and in turn the ability to recover from exposure to acute stressors. This mechanism can help explain how socioeconomic disparities contribute to disparities in chronic disease.
Keywords: socioeconomic status, life course, cortisol, physiological stress response
Introduction
Low adult socioeconomic status (SES) is robustly related to poorer health and higher mortality compared to high adult SES (1, 2). There is increasing evidence that child SES and patterns of SES throughout the life course also have profound influences on later health (3-5). One pathway through which low SES may contribute to chronic illnesses and mortality is through psychological and physiological responses to stressors. Social stress theory was developed to explain why individuals with disadvantaged social status, including low SES, tend to have worse health than individuals with higher status (6, 7). A key component of social stress theory is the differential exposure hypothesis, which states that members of socially disadvantaged groups are exposed to more stressful life events and chronic stressors (6, 8). Indeed, a substantial body of evidence has shown that adults with low SES are exposed to more stressors than their higher status peers (see 9 for a review), and a smaller but growing body of evidence suggests that children with low SES have greater stress exposure than children from more advantaged families (see 10 for a review). At the same time, individuals with low SES have less access to social and material resources that would help mitigate the effects of these stressors (4, 11, 12). This chronic exposure to stressors, coupled with a lack of resources for dealing with them, can result in dysregulation of the body’s ability to respond to new chronic or acute stressors. This alteration of the physiologic stress response systems contributes in turn to chronic illnesses such as cardiovascular disease (13).
Using measures of the hormone cortisol as a biomarker can allow us to measure dysregulation of the stress response associated with low SES. Cortisol is produced by the hypothalamic-pituitary-adrenal (HPA) axis, a neuroendocrine system that contributes to the physiologic stress response (14). Cortisol typically follows a diurnal cycle in which it rises quickly after wakeup, peaks within approximately 30–45 minutes, and declines gradually throughout the day, reaching the lowest level in the evening. In response to an acute stressor (generally tested in a laboratory setting), salivary cortisol levels typically increase sharply and then return to baseline levels after the threat has passed (15). Variation in the degree of increase or the ability to successfully return to baseline levels can be a marker of dysregulation of the HPA axis (13, 16). This dysregulation can then potentially lead to increased cardiovascular risk through increased insulin resistance, visceral adiposity, and/or hypertension (17).
Allostatic load theory, which posits that repeated or prolonged exposure to stressors results in “wear and tear” that interferes with the body’s ability to maintain stability in the face of environmental changes, suggests that exposure to stressful environments will initially lead to hyperreactivity of the HPA system, as indicated by greater cortisol reactivity to a stress challenge (13). However, prolonged exposure may ultimately result in hyporeactivity of the stress response systems, as indicated by a blunted cortisol response to a stress challenge (13). Both exaggerated and blunted cortisol responses to acute stressors in laboratory settings have been related to past exposure to stressful life events (18, 19). These contrasting results may reflect differences in the timing of the life events and subsequent process of stress response dysregulation (as suggested by allostatic load theory) and highlight the fact that meaningful deviations from the typical response may vary in direction.
Research on how cortisol responses to acute laboratory stressors relate to SES is lacking. Alterations in diurnal cortisol patterns have been related to low SES in several study populations, although the literature is quite mixed (20, 21). A handful of studies have examined how lifetime SES exposure relates to diurnal cortisol patterns, relying on single-gender or racially/ethnically homogenous cohorts (22-25). To our knowledge, no previous studies have examined acute cortisol reactivity or recovery to a laboratory stressor in relation to life course SES. This gap in the literature limits our ability to understand how the acute stress response, a major theorized mechanism to explain well documented associations of life course SES with cardiovascular disease and other chronic illnesses, gets “under the skin” to create socioeconomic health disparities. While there is some evidence that some aspects of the diurnal cortisol profile are related to the cortisol response to an acute stressor, they are distinct processes that may relate differently to overall health and the development of chronic disease (26).
We addressed this gap by using data from a multi-site, multi-ethnic study of older adults in the United States (US) to investigate how SES in childhood and adulthood is associated with cortisol reactivity in response to a cognitive stress challenge in a laboratory setting. Cortisol levels rise sharply and then gradually return to the basal level in response to an acute stressor. Because there is still uncertainty about which characteristics of this pattern vary by SES, as well as which are most relevant for future health, we used measures of both reactivity and recovery (described below) to characterize variations in the cortisol response by child and adult SES.
Drawing on life course epidemiology theory, we also used several measures of SES that represent three different mechanisms through which SES may affect the acute stress response over time (3, 27). First, using parent education reported by study participants as a proxy for child SES and participant education, income, and wealth as measures of adult SES, we first examined independent associations of child and adult SES with the cortisol outcomes. We used these associations as a broad test of the “latent effect” mechanism through which low SES during one or the other life period may be particularly salient for the cortisol stress response (3). We hypothesized that in this older population, compared to higher SES, lower SES in childhood and adulthood would each be independently associated with a blunted cortisol reaction in response to a stress challenge (i.e., a smaller increase in cortisol) but a slower recovery (i.e., return to basal cortisol level). Second, we investigated cumulative lifetime exposure to low SES by combining the child and adult SES measures; we hypothesized that, compared to higher cumulative SES, lower cumulative SES (regardless of the timing) would be associated with the same pattern of blunted response and slow recovery. Third, we tested specific trajectories of SES that reflect experiences of upward and downward social mobility (27). We hypothesized that a pattern of downward social mobility would be associated with blunted response and slow recovery.
Methods
Study Population
Data came from the Multi-Ethnic Study of Atherosclerosis (MESA) Stress II Study, an ancillary study to the MESA cohort study. MESA is a six-site cohort study of older adults designed to study subclinical and clinical cardiovascular disease. There were five waves of data collection, with the baseline wave in 2000–2002 and wave 5 in 2010–2012. Study design details are available elsewhere (28). The MESA Stress II Study was conducted in 2010–2012 in three of the MESA sites (New York, Los Angeles, and Baltimore) and included non-Hispanic white, non-Hispanic black, and Hispanic participants aged 54–93 (29). All participants gave written consent for participation. There were 1,040 participants in the stress challenge portion of the study. We excluded participants with missing time of cortisol collection (n = 1), or who did not complete the entire stress challenge or were colorblind (n = 42; one of the tests in the challenge required color recognition), giving an analysis sample of 997.
Procedure
Participants completed a cognitive stress challenge consisting of two 6-minute tasks (a math test and a Stroop test) separated by a 6-minute rest period (30). The order of the tests was automatically and randomly selected at the time of data collection for each participant. Participants then underwent an orthostatic stressor (i.e., physiologic challenge) by standing and remained standing quietly, supported by a wall if necessary. Stress challenge start times ranged from 10:35 AM to 7:17 PM, with the large majority 11:00 AM – 6:00 PM.
Cortisol levels were measured using a standardized protocol during the lab stress challenge using saliva samples collected using Salivette collection tubes. Salivary cortisol was measured four times, as shown in Figure 1: at baseline (Y1: 0 minutes), after the second stress challenge task (Y2; ~ 38 minutes after baseline), 6 minutes after the orthostatic stressor (Y3; ~ 55 minutes after baseline), and 30 minutes after the third measurement (Y4; ~ 85 minutes after baseline).
Figure 1.
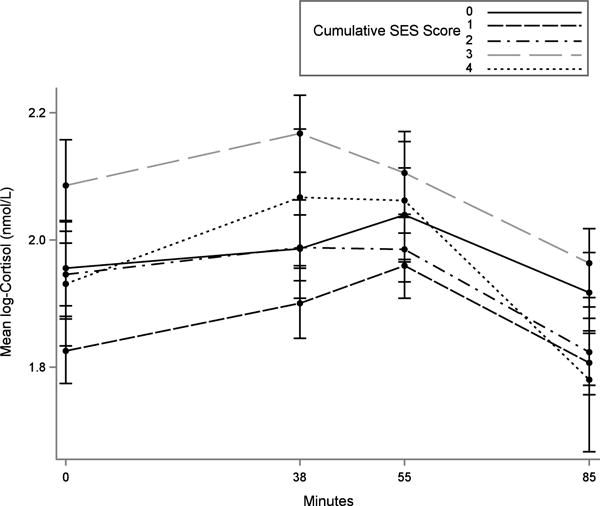
Timeline and mean log-transformed salivary cortisol measurements for lab stress challenge, by cumulative socioeconomic status (SES), MESA Stress Study. Salivary cortisol was measured four times: at baseline (Y1), after the second stress challenge task (Y2), 6 minutes after the orthostatic stressor (Y3), and 30 minutes after the third measurement (Y4). Stress reactivity was defined as Y2 – Y1. Stress recovery rate was defined as (Y4 – Y2)/(time in hours from Y2 to Y4). Vertical bars show standard error of the mean. Higher cumulative SES score denotes higher SES.
Measures
Cortisol
Our outcome variable was the log-transformed cortisol sample concentration (nmol/L), with each cortisol sample treated as a separate observation (i.e., four observations per person). Samples were stored at –20° C until analysis. They were then thawed and centrifuged at 3000 rpm for 3 minutes to obtain clear saliva with low viscosity. A commercially available chemi-luminescence assay (CLIA) with sensitivity of 0.16 ng/mL was used to determine cortisol levels, measured in nmol/L. Intra- and inter-assay coefficients of variation were below 8% (29).
Socioeconomic status
Our choices of measures of SES were guided by previous research on life course SES and health in this cohort (33) and other studies (31, 32, 34-37), as well as by the availability of SES indicators in this study. Child SES was measured using a single item of father’s or caregiver’s education level reported by participants during the second MESA exam. Participants were asked to think about their father or caregiver when they were a child up to age 5, and to report their highest educational level completed. We combined the six response options into three categories: 0 = less than high school, 1 = high school degree, or 2 = high school degree or higher. Adult SES, for which additional indicators were available, was measured using a composite index incorporating information on participant education, income, and wealth (33). Education was coded 0 = high school or less, 1 = some college but no degree, 2 = associate or bachelor’s degree, or 3 = graduate/professional degree. Annual household income was coded as 0 = < $25,000; 1 = $25,000–39,999; 2 = $40,000–74,999; or 3 = ≥ $75,000. Wealth was coded as the sum of the following four indicators: (1) participant owned own home, (2) participant or family had investments such as stocks, bonds, mutual funds, or retirement investments, (3) participant or family owned any land, business property, apartments or houses other than their residence, and (4) participant or family owned a car. The three adult SES measures were then summed to create an index ranging 0–10, then categorized as low (0–2), medium (3–6), or high (7–10).
The measure of cumulative SES was the sum of the 3-category child SES (coded 0–2) and 3-category adult SES (coded 0–2) measures, giving a total range 0–4. To measure SES trajectories, we created the following trajectories based on cross-classifications of the 3-category child and adult SES measures: stable low (Low-Low, i.e., low child SES and low adult SES), upward mobility (Low-High, Low-Medium, or Medium-High), downward mobility (High-Low, High-Medium, or Medium-Low), stable medium (Medium-Medium), and stable high (High-High).
Covariates
Models were adjusted for age in years, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic), and nativity (US- or foreign-born).
Analysis
We addressed missing data (see Table 1) using multiple imputation with 25 imputations using a chained equations approach in IVEware software (38, 39). All other analyses were conducted using SAS version 9.3 software.
Table 1.
Demographic characteristics of sample, by cumulative lifetime socioeconomic status (N = 997)
| Original sample
|
Imputed sample
|
||||
|---|---|---|---|---|---|
| Total | Total | Low cumulative lifetime SESf | High cumulative lifetime SESf | ||
|
|
|
||||
| Characteristic | n or mean | % or SD | % or mean | % or mean | % or mean |
| Age (mean, SD) | 69.3 | 9.0 | 69.3 | 70.3 | 68.4 |
| Female (n, %) | 552 | 55 | 55 | 60.6 | 50.4 |
| Race/ethnicity (n, %) | |||||
| Hispanic | 406 | 41 | 41 | 61 | 22 |
| Non-Hispanic black | 315 | 32 | 32 | 28 | 35 |
| Non-Hispanic white | 276 | 28 | 28 | 11 | 43 |
| Foreign-borna (n, %) | 402 | 30 | 40 | 58 | 24 |
| Missing | 1 | 0 | — | — | — |
| Study site (n, %) | |||||
| Baltimore, MD | 254 | 25 | 25 | 15 | 35 |
| Los Angeles, CA | 431 | 43 | 43 | 37 | 26 |
| New York, NY | 312 | 31 | 32 | 48 | 39 |
| Education (n, %) | |||||
| ≤ High school/GED | 394 | 40 | 39 | 65 | 15 |
| Some college, technical or associate’s degree | 299 | 30 | 30 | 26 | 34 |
| ≥ Bachelor’s degree | 304 | 30 | 31 | 9 | 52 |
| Household annual income (n, %) | |||||
| < $25,000 | 315 | 32 | 32 | 52 | 13 |
| $25,000-39,999 | 205 | 21 | 21 | 28 | 14 |
| $40,000-74,999 | 248 | 25 | 25 | 18 | 32 |
| ≥ $75,000 | 210 | 21 | 22 | 2 | 41 |
| Missing | 19 | 2 | — | — | — |
| Wealth indicators (n, %) | |||||
| Owns own home | 286 | 30 | 29 | 25 | 33 |
| Missing | 37 | 4 | — | — | — |
| Investmentsb | 527 | 55 | 55 | 33 | 77 |
| Missing | 35 | 4 | — | — | — |
| Owns a car | 757 | 79 | 79 | 72 | 85 |
| Missing | 34 | 3 | — | — | — |
| Owns propertyc | 332 | 34 | 34 | 23 | 44 |
| Missing | 34 | 3 | — | — | — |
| Child SESd (n, %) | |||||
| Low | 568 | 60 | 60 | 90 | 31 |
| Medium | 198 | 21 | 21 | 10 | 32 |
| High | 177 | 19 | 19 | 0 | 37 |
| Missing | 54 | 5 | — | — | — |
| Adult SESe (n, %) | |||||
| Low | 257 | 27 | 26 | 49 | 5 |
| Medium | 385 | 41 | 39 | 51 | 28 |
| High | 298 | 32 | 34 | 0 | 67 |
| Missing | 57 | 6 | — | — | — |
| Cumulative lifetime SESf (n, %) | |||||
| 0 | 175 | 20 | 19 | 39 | 0 |
| 1 | 275 | 31 | 30 | 61 | 0 |
| 2 | 231 | 26 | 27 | 0 | 53 |
| 3 | 115 | 13 | 13 | 0 | 26 |
| 4 | 98 | 11 | 11 | 0 | 21 |
| Missing | 103 | 10 | — | — | — |
| Lifetime SES trajectoryg (n, %) | |||||
| Stable low | 175 | 20 | 19 | 39 | 0 |
| Stable medium | 82 | 9 | 9 | 0 | 9 |
| Stable high | 98 | 11 | 11 | 0 | 21 |
| Upwardly mobile | 425 | 48 | 49 | 51 | 46 |
| Downwardly mobile | 114 | 13 | 13 | 10 | 16 |
| Missing | 103 | 10 | — | — | — |
Includes Puerto Rico
Includes stocks, bonds, mutual funds, retirement investments, or other investments
Includes any land, business property, apartments or houses other than own residence
Measured using father’s education: Low= < High school, Medium = High school, High = > High school
Sum of education (in categories shown, scored 0-2), household income (in categories shown, scored 0-3), and wealth indicators: Low = 0-2, Medium = 3-6, High = 7-10
Sum of child SES (scored 0-2) and adult SES (scored 0-2). For columns, Low is defined as 0–1 and High is defined as 2–4.
Stable low = low-low (i.e., low child SES, low adult SES); stable medium = medium-medium; stable high = high-high; upwardly mobile = low-medium, low-high, or medium-high; downwardly mobile = high-medium, high-low, or medium-low.
We used piecewise linear mixed effects models to examine unadjusted and adjusted associations between the SES measures and log-transformed cortisol concentration. The piecewise models included three time splines (see Figure 1: baseline to 2nd sample, 2nd sample to 3rd sample, and 3rd sample to 4th sample) and included the individual sociodemographic variables (age, gender, race, nativity, and SES) and their interactions with each of the time splines. In addition, the models included participant-level random intercepts and random slopes for each time spline to account for within-person correlations between cortisol samples. We estimated robust standard errors. Using these models, we tested our hypotheses by examining two features of the cortisol reactivity curve, the stress reactivity response to the cognitive challenge (2nd sample – baseline sample) and the recovery rate ([4th sample – 2nd sample]/hour). We also used likelihood-ratio tests to test overall improvement in model fit by inclusion of the SES measures.
We ran three types of models corresponding to the three hypothesized life course mechanisms. Latent effect models included 3-level child and adult SES as separate measures, with high SES as the referent group for both; we tested models with and without adjustment for SES during the other time period. The models adjusting for the other time period (i.e., including both child and adult SES) allowed us to test whether child and adult SES were each associated with the outcomes independent of SES for the other time period. In particular, associations of child SES with the outcomes after adjustment for adult SES would give evidence suggesting a latent effect of child SES on the cortisol stress response that was independent of adult SES. Cumulative SES models included indicators for the 5 levels of cumulative SES, with the highest as the referent group. These models served as a test of a “dose-response” mechanism through which exposure to lower SES is associated with the stress response irrespective of the timing of the exposure. Trajectory models included indicators for the trajectory groups, with stable high as the referent group. These models allowed us to test how specific trajectories of SES (e.g., upward and downward mobility) were associated with the cortisol response.
Sensitivity analyses
We tested the robustness of our results to additional adjustment for study site, time of day, and use of steroids and hormone replacement therapy. We also tested models excluding cortisol observations < 1st or > 99th percentile of the sample distribution. We also conducted a sensitivity analysis to assess the extent to which the fact that our child SES measure (based solely on father’s education) was less comprehensive than our adult SES measure (including participant education, income, and wealth) might have affected our results. To do this, we repeated analyses using participant education as the sole measure of adult SES, categorized the same way as the child SES measure, to increase comparability between the measures.
Results
The sample had mean age 69.3 years (SD: 9.0) and was 55% female and racially/ethnically diverse: 41% Hispanic, 32% non-Hispanic black, and 28% non-Hispanic white (Table 1). Largely because of the high proportion of Hispanic participants, 40% of the sample was foreign-born. Median salivary cortisol concentrations at the four measurements were 7.63 nmol/L, 8.25 nmol/L, 8.15 nmol/L, and 7.09 nmol/L. Variable distributions in the original and imputed samples were nearly identical (Table 1). Over half (60%) of the sample had low child SES (i.e., father with less than a high school degree) while 61% had themselves attained education beyond high school. Prevalence of wealth indicators ranged from 29% who owned their own home outright (in the imputed sample) to 79% who owned a car. The most prevalent SES trajectory was upward mobility (49%), followed by stable low (19%), downward mobility (13%), stable high (11%), and finally stable medium (9%). Participants with low cumulative SES were more likely to be Hispanic or foreign-born, and to live in Los Angeles or New York.
Latent Effect Model
Table 2 shows results from models using separate measures of child and adult SES to test the hypothesis that child and adult SES are associated with the cortisol stress response independent of each other. Results are presented as percent differences in the outcome relative to the high SES referent group. There was little evidence that lower child or adult SES was associated with stress reactivity: point estimates were small and not statistically significant at α = .05. Point estimates for child SES were negative, suggesting lower stress reactivity among participants with lower SES (consistent with our hypothesis), but the opposite was observed for adult SES.
Table 2.
Percent differences in cortisol reactivity and cortisol recovery rate by child and adult SES (latent effect model)
| Cortisol Measure | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
|
| ||||||
| % Diff | 95% CI | % Diff | 95% CI | % Diff | 95% CI | |
| Child SES (vs. High) | ||||||
| Stress Reactivity | ||||||
| Medium | −2 | (−12, 40) | 1 | (−10, 41) | 0 | (−10, 41) |
| Low | −5 | (−14, 38) | −4 | (−12, 39) | −4 | (−13, 39) |
| Recovery Rate (per hour) | ||||||
| Medium | 4 | (−11, 45) | 5 | (−11, 45) | 3 | (−12, 45) |
| Low | 16 | (3, 49) | 19 | (4, 50) | 18 | (3, 49) |
| Adult SES (vs. High) | ||||||
| Stress Reactivity | ||||||
| Medium | −2 | (−9, 39) | 2 | (−7, 41) | 2 | (−6, 41) |
| Low | 2 | (−7, 41) | 4 | (−6, 43) | 4 | (−6, 43) |
| Recovery Rate (per hour) | ||||||
| Medium | 12 | (0, 46) | 14 | (1, 47) | 13 | (0, 47) |
| Low | 21 | (5, 51) | 28 | (8, 56) | 27 | (8, 55) |
For reactivity, a positive point estimate indicates higher reactivity (i.e., a larger increase in cortisol). For recovery rate, a positive point estimate indicates slower recovery (i.e., a less negative hourly change in cortisol).
Bold indicates p < .05 compared to high SES.
Unadjusted. For global test of model fit compared to model excluding SES, mean p-value across imputations = .43 (range .21-.63) for child SES and mean p = .08 (range .03-.27) for adult SES.
Adjusted for age, gender, race/ethnicity, and nativity. For global test of model fit compared to model excluding SES, mean p-value across imputations = .36 (range .16-.56) for child SES and mean p = .02 (range .004-.08) for adult SES.
Adjusted for age, gender, race/ethnicity, nativity, and SES during the other time period. For global test of model fit compared to model excluding SES, mean p-value across imputations = .41 (range .19-.66) for child SES and mean p = .02 (range .005-.09) for adult SES.
There was more consistent evidence that lower child and adult SES were associated with slower recovery (the positive associations indicate less-negative hourly changes—i.e., slower declines—in cortisol concentrations after the stress challenge). Associations were slightly larger in magnitude after adjustment for covariates (Table 2, Model 2) and remained similar after adjustment for SES during the other time period (Table 2, Model 3). Compared to high child SES, medium child SES was associated with a 3% ([95% confidence interval] −12%, 45%) slower hourly decline in cortisol concentration during the recovery period and low child SES was associated with a 18% (3%, 49%) slower hourly decline, independent of adult SES. Compared to high adult SES, medium and low SES were associated with 13% slower (0%, 47%) and 27% slower (8%, 55%) hourly declines, respectively, independent of child SES. Estimates were somewhat larger for adult than child SES. In addition, model fit was more improved by the addition of adult SES than by the addition of child SES: Model 3 mean p = .41 (range .19-.66) across imputations for child SES and mean p = .02 (range .005-.09) for adult SES. Therefore, although low child SES was related to slower recovery compared to high child SES, there was weak evidence that the inclusion of the child SES measures significantly improved overall model fit while there was more robust evidence for adult SES.
Cumulative SES Model
Results for cumulative SES were consistent with those for the latent effect model (Table 3). Stress reactivity was not related to cumulative SES. Lower cumulative SES was consistently related to slower declines in cortisol during recovery in a dose-response manner. In the adjusted model (Table 3, Model 2), hourly declines in cortisol compared to the highest cumulative SES group (score = 4) ranged from 16% (−6%, 52%) slower for a score of 3 to 52% (20%, 71%) slower for a score of 0.
Table 3.
Percent differences in cortisol reactivity and cortisol recovery rate by cumulative lifetime SESa
| Cortisol Measure | Model 1b | Model 2c | ||
|---|---|---|---|---|
|
| ||||
| % Diff | 95% CI | % Diff | 95% CI | |
| Stress Reactivity (vs. 4 = Highest) | ||||
| 3 | −5 | (−18, 40) | −2 | (−15, 42) |
| 2 | −7 | (−18, 39) | −1 | (−13, 41) |
| 1 | −4 | (−15, 40) | 1 | (−12, 43) |
| 0 = Lowest | −6 | (−19, 40) | −3 | (−17, 42) |
| Recovery Rate (per hour; vs.4 = Highest) | ||||
| 3 | 14 | (−6, 51) | 16 | (−6, 52) |
| 2 | 19 | (0, 53) | 26 | (4, 56) |
| 1 | 30 | (10, 57) | 41 | (16, 63) |
| 0 = Lowest | 35 | (9, 61) | 52 | (20, 71) |
For reactivity, a positive point estimate indicates higher reactivity (i.e., a larger increase in cortisol). For recovery rate, a positive point estimate indicates slower recovery (i.e., a less negative hourly change in cortisol).
Bold indicates p < .05 compared to highest cumulative lifetime SES group.
Cumulative lifetime SES is sum of 3-category child SES and 3-category adult SES. Higher score corresponds to higher SES.
Unadjusted. For global test of model fit compared to model excluding SES, mean p-value across imputations = .19 (range .05-.46).
Adjusted for age, gender, race/ethnicity, and nativity. For global test of model fit compared to model excluding SES, mean p-value across imputations = .04 (range .01-.17).
SES Trajectory Model
Results for SES trajectories were also generally consistent with the other models (Table 4). SES trajectories were weakly and inconsistently associated with stress reactivity. For stress recovery all trajectories had positive point estimates, suggesting slower recovery compared to the stable high trajectory. Compared to stable high SES, stable medium SES was associated with a 14% (−12%, 54%) slower hourly decline during recovery and stable low SES was associated with a 46% (15%, 68%) slower hourly decline. Estimates for downward and upward mobility were similar to each other: 35% (9%, 61%) and 27% (7%, 56%) slower than stable high SES, respectively.
Table 4.
Percent differences in cortisol reactivity and cortisol recovery rate by SES trajectory
| Cortisol Measure | Model 1a | Model 2b | ||
|---|---|---|---|---|
|
| ||||
| % Diff | 95% CI | % Diff | 95% CI | |
| Stress Reactivity (vs. Stable high) | ||||
| Downward mobility | −2 | (−15, 42) | 3 | (−11, 44) |
| Upward mobility | −6 | (−16, 39) | −1 | (−13, 41) |
| Stable medium | −8 | (−23, 41) | −1 | (−17, 44) |
| Stable low | −6 | (−19, 40) | −4 | (−17, 41) |
| Recovery Rate (per hour; vs. Stable high) | ||||
| Downward mobility | 30 | (7, 58) | 35 | (9, 61) |
| Upward mobility | 23 | (4, 53) | 27 | (7, 56) |
| Stable medium | 13 | (−12,53) | 14 | (−12, 54) |
| Stable low | 35 | (9, 61) | 46 | (15, 68) |
For reactivity, a positive point estimate indicates higher reactivity (i.e., a larger increase in cortisol). For recovery rate, a positive point estimate indicates slower recovery (i.e., a less negative hourly change in cortisol).
Bold indicates p < .05 compared to stable high SES.
Unadjusted.For global test of model fit compared to model excluding lifetime SES trajectory indicators, mean p-value across imputations = .14 (range .04-.29).
Adjusted for age, gender, race/ethnicity, and nativity. For global test of model fit compared to model excluding lifetime SES trajectory indicators, mean p-value across imputations = .03 (range .003-.10).
Sensitivity Analyses
Additional adjustment for study site, study start time, or steroid or hormone replacement therapy use yielded nearly identical results (data available upon request). Exclusion of observations below the 1st or above the 99th percentile of the log-cortisol distribution also produced similar estimates; point estimates tended to be slightly attenuated (although patterns of statistical significance at α = .05 were unchanged), as would be expected given the lesser variability in the restricted sample (data available upon request). Results from the sensitivity analysis using only participant education as the measure of adult SES followed the same patterns as those in the primary analysis but were attenuated (see Appendix, Supplemental Digital Content 1). Attenuation was most evident in the latent-effect models (Appendix, Table A2), where associations between low adult SES and the recovery rate were minimal and in fact smaller than associations with low child SES.
Discussion
In a multi-ethnic sample of older adults living in the US, lower child and adult SES was inconsistently related to cortisol reactivity in response to a cognitive stress challenge but was related to slower cortisol recovery following the challenge. These results were supported across three types of models reflecting three hypothesized life course mechanisms: latent effect, cumulative, and trajectory. Taken together, results from the three types of models give clearest support for a cumulative effect of SES over time on the body’s ability to recover after exposure to an acute stressor. This is most readily evident in the cumulative SES models, which showed that when the two time periods were combined there was a clear dose-response relationship between lower lifetime SES and slower cortisol recovery. Trajectory models also showed the strongest associations for the contrast of stable low vs. stable high SES. Estimates for upward and downward mobility were intermediwate and were similar to each other, suggesting that the “dose,” rather than the specific pattern, of SES may be of importance. Similarly, in the latent effect models, low child and adult SES were both associated with slower recovery compared to high SES in the same time period while associations for medium vs. high SES were weaker. The weak associations for medium SES may be consistent with a threshold effect of SES within a specific time period.
Socioeconomic status is a complex, multifactorial construct and there is no consensus on how best to measure it or to combine multiple SES indicators in studies of health (4, 31, 32, 40). As an indicator of SES, education has the benefit of being easily measurable and applicable across the socioeconomic spectrum, relatively stable over time, and less susceptible to reverse causation than measures such as income or wealth (32); it has also been called the most “basic” measure of SES because of its influence on later occupation, income, wealth, and social standing (2). However, education may still represent only some aspects of SES. Therefore, comparison of our results for child vs. adult SES requires caution because the measure of adult SES used in our primary analyses was more comprehensive than our measure of child SES (a single measure of father’s/caregiver’s education) and may therefore have better captured any influence of SES on the stress response. This is consistent with the attenuated results for adult SES we observed in the sensitivity analysis using participant education as the sole measure of adult SES. It is possible that the weaker results for child SES compared to adult SES are driven by limitations in the childhood SES measure we used (use of education as the single indicator plus reduced ability to capture variability in childhood SES). There may also have been more misclassification of the child SES measure because of inaccurate recall by participants of their father’s education, or if the father/caregiver was not living with or contributing financially to the family. However, there is evidence that adults tend to recall their parents’ SES accurately (37, 41).
Although the multiple cortisol samples in our study were a strength, as in previous lab challenge studies (15) we depended on point-in-time cortisol measurements and therefore could not fully capture the cortisol response and recovery curve for each individual. In our study, there was some indication of higher cortisol levels at the third measurement (Y3) than the second measurement (Y2) among lower SES participants (see Figure 1). Through our modeling strategy we assumed that these Y3 values resulted from the physiologic stressor (i.e., the orthostatic challenge) and did not include them in our measure of the cortisol response, but it is possible that they also reflect the cognitive stressor, our exposure of interest, to some degree. It is not possible with these data to parse out the relative contributions of the two stressors to the third sample. In a post hoc descriptive analysis in which cortisol reactivity for each individual was defined more flexibly as the higher of the second or third measurement, differences between cumulative SES groups in mean reactivity did not vary in a graded manner and were not statistically significant. More generally, measured cortisol responses have differed across studies depending on the time of measurement following the stressor (15).
Our analysis was also subject to other limitations, many of which are shared with other studies of life course SES (3). We were limited to measures of SES corresponding to two time periods—age 0–5 and the time of the interview—which limited our ability to characterize patterns of SES throughout the life course. The strong graded result between cumulative SES and the outcome may largely reflect strong effects of adult SES, as cumulative SES models do not distinguish between SES at different times. In addition, the latent effect models do not capture the fact that effects of child SES may be mediated by adult SES. Statistical power, particularly in the trajectory models, may have been limited by the rarity in the population of some patterns of SES; some studies have addressed this by explicitly recruiting participants with different socioeconomic backgrounds (42, 43). Although we observed consistent differences in the cortisol response between groups, the overall response elicited in the lab challenge was modest; prior research has found that group differences may vary depending on the type of stressor (44). The stressor we used may not have been the best one to elicit the types of stress responsivity differences most likely to be affected by life course SES. Finally, while the multi-ethnic nature of our sample was a strength, we did not explicitly examine differences between racial/ethnic groups in associations between SES and the cortisol stress response. Future research doing so is warranted, as the salience and effects of various aspects of SES for health may vary across groups (45-47). Despite these limitations, strengths of our study were the relatively large, multi-ethnic study sample and our ability to incorporate multiple measures of adult SES and control for important sociodemographic confounders.
While most empirical studies on chronic stress and responses to acute laboratory stressors have not included an extended recovery period, one study using data from the Whitehall II cohort found that occupational role uncertainty was related to slower recovery following a laboratory stress challenge (48). Out of the handful of studies examining lifetime SES and diurnal cortisol patterns, DeSantis et al. (25) found in a Philippines-based cohort that lower lifetime SES was associated with a flatter diurnal cortisol decline (25) while Franz et al. (23) did not find an association and two other studies did not specifically examine the slope (22, 24).
The inconsistent results we observed for cortisol reactivity mirror the mixed findings of previous research examining chronic stress and stressful life events (18, 19, 44, 49). This literature has tended to support associations of stressful life events and chronic stress with heightened cortisol reactivity in children but blunted reactivity in adults (44). This is consistent with allostatic load theory, which would predict hyperreactivity initially but hyporeactivity as either more time passes between the experienced stressors and the reactivity measurement or as the stressors are experienced over a longer period of time.
The stronger associations we observed for recovery compared to reactivity are analogous to animal and human models of aging and HPA axis function, which show little effect of age on cortisol reactivity but consistent evidence that aging is related to slower recovery following acute stress (50). One hypothesized mechanism is that continued exposure to glucocorticoids such as cortisol—which occurs over time with aging but could also occur through repeated or sustained exposure to stressors—causes declines in concentrations of glucocorticoid receptors in the hippocampus, compromising negative feedback mechanisms of the HPA axis (50). In this way, cumulative exposure to stressors associated with low SES may interfere with the physiological ability to maintain allostasis.
Our hypothesis that life course SES is related to stress reactivity relied on prior evidence that low-SES individuals experience higher exposure to stressful events and conditions while also having lower access to resources for dealing with them (4, 6, 9). Future research may complete the puzzle by measuring the specific nature and timing of stressors experienced by individuals with low SES at various life stages, and determining how and when these different stressors contribute to HPA axis dysregulation. For example, a chronic stressor such as living in overcrowded or decrepit housing may exact a different toll than repeated exposure to discrete traumatic events. A meta-analysis of relations between chronic stressors and diurnal cortisol found that associations varied depending on the timing, nature, and controllability of the stressor (51). It also remains to distinguish between the roles of SES in increasing exposure to stressors, decreasing access to coping resources, and changing the contexts in which stressors are encountered and interpreted (52). Finally, alterations in cortisol levels and changes have been related to cardiovascular risk factors such as obesity, inflammation, and diabetes, and to measures of subclinical cardiovascular disease including coronary artery calcification and ankle-brachial index (19, 44, 53-55). But research is still needed to fully characterize the mechanisms through which HPA axis dysregulation contributes to cardiovascular and other chronic diseases.
We found across models representing three different hypothesized life course mechanisms that among older adults, recovery to baseline cortisol levels following exposure to an acute lab stressor was slower among those who had experienced lower SES in childhood and adulthood. These associations followed a graded pattern, so that differences in recovery rate were most pronounced between those who had experienced the highest and lowest SES across the two time periods. Our findings support alteration of the body’s response to acute stressors as one possible mechanism through which socioeconomic circumstances become incorporated into the biological processes underlying disease progression. Identifying and further elucidating such mechanisms is challenging (31), but is ultimately necessary in order to move beyond simply documenting socioeconomic health disparities to taking action to reduce the translation of socioeconomic inequality into population health inequities.
Supplementary Material
Supplemental Digital Content.
Supplemental Digital Content 1. docx.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Source of funding:
MESA was supported by contracts NO1-HC-95159 through NO1-HC-95165 and NO1-HC-95169 from the National Heart, Lung, and Blood Institute. The MESA Stress Study was supported by RO1 HL10161-01A1 and R21 DA024273 (PI: Dr.Diez Roux). This work was also supported in part by the Michigan Center for Integrative Approaches to Health Disparities (P60 MD002249) funded by the National Institute on Minority Health and Health Disparities.
Acronyms
- HPA
hypothalamic-pituitary-adrenal
- MESA
Multi-Ethnic Study of Atherosclerosis
- SES
socioeconomic status
- US
United States of America
Footnotes
Conflicts of interest: We have no conflicts of interest to report.
References
- 1.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4 Pt 1):1973–98. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 2.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Affairs. 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 3.Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- 5.Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. Milbank Q. 2002;80(3):433–79. iii. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearlin LI. The sociological study of stress. Journal of Health & Social Behavior. 1989;30(3):241–256. [PubMed] [Google Scholar]
- 7.Aneshensel CS, Rutter CM, Lachenbruch PA. Social structure, stress, and mental health: Competing conceptual and analytic models. American Sociological Review. 1991;56:166–178. [Google Scholar]
- 8.Thoits PA. Stress, coping, and social support processes: Where are we? What next? Journal of Health & Social Behavior. 1995;35:53–79. Extra Issue. [PubMed] [Google Scholar]
- 9.Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: a review of the research. Am J Community Psychol. 2007;40(3-4):313–32. doi: 10.1007/s10464-007-9134-z. [DOI] [PubMed] [Google Scholar]
- 10.Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future. Ann N Y Acad Sci. 2010;1186:146–73. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- 11.Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22(4):337–56. [PubMed] [Google Scholar]
- 12.Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: some conceptual perspectives. J Health Soc Behav. 2005;46(2):205–19. doi: 10.1177/002214650504600206. [DOI] [PubMed] [Google Scholar]
- 13.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 15.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 17.Steptoe A, Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–54. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- 18.Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. Int J Psychophysiol. 2013;90(1):8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter AL, Minnis H, Wilson P. Altered stress responses in children exposed to early adversity: a systematic review of salivary cortisol studies. Stress. 2011;14(6):614–26. doi: 10.3109/10253890.2011.577848. [DOI] [PubMed] [Google Scholar]
- 20.Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol. 2009;38(5):1297–309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, et al. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35(6):932–43. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafsson PE, Janlert U, Theorell T, Hammarstrom A. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology. 2010;35(4):613–23. doi: 10.1016/j.psyneuen.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Franz CE, Spoon K, Thompson W, Hauger RL, Hellhammer DH, Jacobson KC, et al. Adult cognitive ability and socioeconomic status as mediators of the effects of childhood disadvantage on salivary cortisol in aging adults. Psychoneuroendocrinology. 2013;38(10):2127–39. doi: 10.1016/j.psyneuen.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32(7):824–33. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Desantis AS, Kuzawa CW, Adam EK. Developmental origins of flatter cortisol rhythms: socioeconomic status and adult cortisol activity. Am J Hum Biol. 2015 doi: 10.1002/ajhb.22668. [DOI] [PubMed] [Google Scholar]
- 26.Kidd T, Carvalho LA, Steptoe A. The relationship between cortisol responses to laboratory stress and cortisol profiles in daily life. Biol Psychol. 2014;99:34–40. doi: 10.1016/j.biopsycho.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health. 2003;57(10):778–83. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Sanchez BN, Golden SH, Shrager S, Kirschbaum C, Karlamangla AS, et al. Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology. 2014;49:310–20. doi: 10.1016/j.psyneuen.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroop JR. Studies of interference in serial verbal reactions [Ph D] Nashville, Tenn.: George Peabody College for Teachers; 1935. [Google Scholar]
- 31.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 32.Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull. 2007:81–82. 21–37. doi: 10.1093/bmb/ldm001. [DOI] [PubMed] [Google Scholar]
- 33.Lemelin ET, Diez Roux AV, Franklin TG, Carnethon M, Lutsey PL, Ni H, et al. Life-course socioeconomic positions and subclinical atherosclerosis in the multi-ethnic study of atherosclerosis. Soc Sci Med. 2009;68(3):444–51. doi: 10.1016/j.socscimed.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16(2):91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 35.Harper S, Lynch J, Hsu WL, Everson SA, Hillemeier MM, Raghunathan TE, et al. Life course socioeconomic conditions and adult psychosocial functioning. Int J Epidemiol. 2002;31(2):395–403. [PubMed] [Google Scholar]
- 36.Best LE, Hayward MD, Hidajat MM. Life course pathways to adult-onset diabetes. Soc Biol. 2005;52(3-4):94–111. [PubMed] [Google Scholar]
- 37.McKenzie SK, Carter KN. Are retrospective measures of childhood socioeconomic position in prospective adult health surveys useful? Australasian Epidemiologist. 2009;16(3):22–24. [Google Scholar]
- 38.Raghunathan TE. What do we do with missing data? Some options for analysis of incomplete data. Annu Rev Public Health. 2004;25:99–117. doi: 10.1146/annurev.publhealth.25.102802.124410. [DOI] [PubMed] [Google Scholar]
- 39.Raghunathan TE, Lepkowski J, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27(1):85–95. [Google Scholar]
- 40.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 41.Krieger N, Okamoto A, Selby JV. Adult female twins’ recall of childhood social class and father’s education: a validation study for public health research. Am J Epidemiol. 1998;147(7):704–8. doi: 10.1093/oxfordjournals.aje.a009512. [DOI] [PubMed] [Google Scholar]
- 42.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106(34):14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20(3):267–75. [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips AC, Ginty AT, Hughes BM. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int J Psychophysiol. 2013;90(1):1–7. doi: 10.1016/j.ijpsycho.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Williams DR. Race/ethnicity and socioeconomic status: measurement and methodological issues. Int J Health Serv. 1996;26(3):483–505. doi: 10.2190/U9QT-7B7Y-HQ15-JT14. [DOI] [PubMed] [Google Scholar]
- 46.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99(9):1013–23. [PMC free article] [PubMed] [Google Scholar]
- 47.Le-Scherban F, Diez Roux AV, Li Y, Morgenstern H. Associations of Grandparental Schooling With Adult Grandchildren’s Health Status, Smoking, and Obesity. Am J Epidemiol. 2014 doi: 10.1093/aje/kwu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wirtz PH, Ehlert U, Kottwitz MU, La Marca R, Semmer NK. Occupational role stress is associated with higher cortisol reactivity to acute stress. J Occup Health Psychol. 2013;18(2):121–31. doi: 10.1037/a0031802. [DOI] [PubMed] [Google Scholar]
- 49.Armbruster D, Mueller A, Strobel A, Lesch KP, Brocke B, Kirschbaum C. Predicting cortisol stress responses in older individuals: influence of serotonin receptor 1A gene (HTR1A) and stressful life events. Horm Behav. 2011;60(1):105–11. doi: 10.1016/j.yhbeh.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Seeman TE, Robbins RJ. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocr Rev. 1994;15(2):233–60. doi: 10.1210/edrv-15-2-233. [DOI] [PubMed] [Google Scholar]
- 51.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 52.Wheaton B. The domains and boundaries of stress concepts. In: Kaplan HB, editor. Psychosocial Stress: Perspectives on Structure, Theory, Life-course, and Methods. San Diego, CA: Academic Press; 1996. pp. 29–70. [Google Scholar]
- 53.DeSantis AS, DiezRoux AV, Hajat A, Aiello AE, Golden SH, Jenny NS, et al. Associations of salivary cortisol levels with inflammatory markers: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2012;37(7):1009–18. doi: 10.1016/j.psyneuen.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajat A, Diez-Roux AV, Sanchez BN, Holvoet P, Lima JA, Merkin SS, et al. Examining the association between salivary cortisol levels and subclinical measures of atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2013;38(7):1036–46. doi: 10.1016/j.psyneuen.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matthews KA, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68(5):657–61. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content.
Supplemental Digital Content 1. docx.


