Abstract
Background & Aims
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States, affecting 75–100 million Americans. However, the disease burden may not be equally distributed among races or ethnicities. We conducted a systematic review and meta-analysis to characterize racial and ethnic disparities in NAFLD prevalence, severity, and prognosis.
Methods
We searched MEDLINE, EMBASE, and Cochrane databases through August 2016 for studies that reported NAFLD prevalence in population-based or high-risk cohorts, NAFLD severity including presence of nonalcoholic steatohepatitis (NASH) and significant fibrosis, and NAFLD prognosis including development of cirrhosis complications and mortality. Pooled relative risks, according to race and ethnicity, were calculated for each outcome using the DerSimonian and Laird method for a random-effects model.
Results
We identified 34 studies comprising 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis. NAFLD prevalence was highest in Hispanics, intermediate in Whites, and lowest in Blacks, although differences between groups were smaller in high-risk cohorts (range 47.6%–55.5%) than population-based cohorts (range, 13.0%–22.9%). Among patients with NAFLD, risk of NASH was higher in Hispanics (relative risk, 1.09; 95% CI, 0.98–1.21) and lower in Blacks (relative risk, 0.72; 95% CI, 0.60–0.87) than Whites. However, the proportion of patients with significant fibrosis did not significantly differ among racial or ethnic groups. Data were limited and discordant on racial or ethnic disparities in outcomes of patients with NAFLD.
Conclusion
In a systematic review and meta-analysis, we found significant racial and ethnic disparities in NAFLD prevalence and severity in the United States, with the highest burden in Hispanics and lowest burden in Blacks. However, data are discordant on racial or ethnic differences in outcomes of patients with NAFLD.
Keywords: fatty liver disease, disparities, nonalcoholic steatohepatitis, ethnicity
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of the metabolic syndrome, is the most common chronic liver disease in the U.S., affecting up to 100 million Americans1. NAFLD encompasses a spectrum of disease, including simple steatosis, nonalcoholic steatohepatitis (NASH), and NASH-related cirrhosis. Over one-third of children and adults in the U.S. are obese and over 20 million Americans are diabetic, resulting in a large at-risk population for NAFLD.2 Approximately 2–5% of patients with NAFLD will progress to NASH, among whom a subset will develop cirrhosis and cirrhosis-related complications including hepatocellular carcinoma (HCC).3
Prior studies have suggested Hispanics may have disproportionately higher and Blacks may have lower NAFLD prevalence and severity compared to non-Hispanic Whites4, 5. Additionally, NAFLD is increasingly being recognized in Asian patients, even at “normal” BMI values.6 However, the magnitude of these disparities is currently poorly characterized, as prior studies have been underpowered. Further, most studies evaluated NAFLD prevalence, severity or prognosis, with no studies evaluating disparities across the entire NAFLD spectrum. Racial/ethnic disparities in NAFLD prevalence and severity can be multifactorial, driven not only by genetic and environmental factors but also socioeconomic status and differential access to health care. Further these influences may differ in significance along the NAFLD spectrum, with some influences playing a central role in NAFLD development and others being more important for disease progression or NAFLD prognosis. Having a better understanding of what disparities exist and their magnitude is the first step to identifying contributing factors and reducing disparities through targeted interventions. Thus, the aim of this meta-analysis was to characterize racial/ethnic disparities in NAFLD prevalence, disease severity, and prognosis among patients in the U.S.
METHODS
Literature search strategy
We searched Ovid MEDLINE, Ovid MEDLINE In-Process, Ovid EMBASE, and the Cochrane Library from inception to August 2, 2016 using search terms described in Supplemental Methods. A manual search of references from relevant articles was performed to identify publications missed by search terms. A manual search of American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver, Digestive Diseases Week, and American College of Gastroenterology meeting abstracts from 2014–2016 was performed. The study was conducted in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines7.
Eligibility Criteria
Included studies met the following criteria: 1) cohort, cross-sectional, or case-control trials including original data characterizing NAFLD-related racial/ethnic disparities among adult patients in the U.S., 2) NAFLD diagnosed using biochemical, radiologic, or histologic criteria per AASLD guidelines, and 3) reported at least one of the following: NAFLD prevalence, severity, or prognosis. We excluded studies that: 1) did not stratify NAFLD prevalence, severity, or prognosis by race/ethnicity, 2) had insufficient data to determine necessary denominator for prevalence or severity, or 3) included patients with other causes of hepatic steatosis (e.g. alcoholic liver disease, medication-induced, or HIV infection). Additional exclusion criteria included: 1) lack of original data (e.g. commentaries, review articles), 2) non-human studies; 3) incomplete data, and 4) non-English language. For studies with overlapping cohorts, articles with the most contemporary cohort or complete data were selected.
Study Selection and Data Extraction
Studies were screened and reviewed in a collaborative, multi-step process. After removing duplicates, two investigators (N.R. and S.O.) independently reviewed publications identified by the search strategy. Articles were screened based on title and abstract for relevance, followed by full text review to assess for inclusion. Disagreements between authors were resolved by discussion with a third reviewer (A.S.). Using standardized forms, two authors (N.R. and S.O.) independently extracted data including patient demographics (including race/ethnicity), method of NAFLD diagnosis, NAFLD prevalence, NAFLD severity, and NAFLD prognosis outcomes (including liver-related and all-cause mortality). Study quality was assessed using a modified checklist based upon the NIH Quality Assessment Tool for Observational Cohort and Cross-sectional Studies, which rates observational studies on a 14-point scale based on study sample appropriateness, comparability of study groups, and adequacy of assessing exposure and outcomes.
Data Synthesis and Statistical Analysis
For each study, a risk ratio for each study outcome of interest was calculated according to race/ethnicity (White vs. Black and White vs. Hispanic). Data for other racial/ethnic groups, e.g. Asians, were limited by small sample sizes and not included in analyses. We first evaluated NAFLD prevalence in population-based and high-risk cohorts. Cohorts were deemed high-risk if they predominantly included patients with obesity (e.g., patients undergoing bariatric surgery), diabetes, or history of chronic liver disease. We next evaluated NAFLD severity, as assessed by two measures: 1) presence of NASH, the progressive form of NAFLD that can lead to cirrhosis and cirrhosis-related complications, and 2) presence of advanced (stage F3–F4) fibrosis. Although some studies reported components of NAFLD activity score (NAS) including degree of inflammation and steatosis, an insufficient number reported racial/ethnic differences in composite NAS to include as a severity measure. Finally, we evaluated NAFLD prognosis, including liver-related and all-cause mortality. Association between NAFLD and cardiovascular outcomes has been described elsewhere and not evaluated in this study8.
For each study outcome of interest, point estimates and 95% confidence intervals were calculated using the adjusted Wald method. We calculated pooled risk ratio estimates for NAFLD prevalence and severity using the DerSimonian and Laird method for a random effects model. Heterogeneity was evaluated graphically by examining forest plots and statistically using the inconsistency index (I2 statistic). I2 values >75% are consistent with high heterogeneity, and values between 50–75% are considered moderate heterogeneity. When there were concerns for heterogeneity, sensitivity analyses, in which one study was removed at a time, were performed to evaluate for undue influence of a single study. Publication bias was evaluated graphically by funnel plot analysis and statistically using Egger’s test. An asymmetric funnel plot suggests possibility of small studies not being published. All data analysis was performed using Stata 14.2 (StataCorp, College Station, TX, USA).
RESULTS
Literature Search
The search strategy yielded 7,921 potentially relevant citations. After removing 1,893 duplicate citations there were 6,028 unique citations. After initial review, 2,428 titles met inclusion criteria, and abstracts were reviewed. Of 351 publications that underwent full text review, 46 met inclusion criteria. Fourteen studies contained overlapping data from NHANES, so we selected the most inclusive studies representing patients from non-overlapping NHANES cohorts (n=2)9,10. We also excluded an additional study only reporting prevalence for racial/ethnic minority patients but not Whites, as risk ratios comparing minority populations to the index white population could not be calculated11. Searches of meeting abstracts yielded 3 abstracts with sufficient data for inclusion, and recursive literature searches revealed 1 additional article meeting inclusion criteria, for a total of 34 studies. Agreement between reviewers for final study inclusion exceeded 90%. Supplemental Figure 1 represents a flow diagram depicting study selection.
Study Characteristics
We identified 34 studies, with a total of 368,569 patients, characterizing racial/ethnic disparities in NAFLD prevalence, severity or prognosis. Studies had racial/ethnic diversity including 216,501 (58.7%) Whites, 57,412 (15.6%) Blacks, 43,737 (11.9%) Hispanics, 10, 100 (2.7%) Asians, and 40,819 (11.1%) “other” race/ethnicity. All included studies reported races/ethnicities as mutually exclusive categories. Of included studies, 17 (n=350,076) characterized disparities in NAFLD prevalence, 18 (n=16,083) in NAFLD severity, and 6 (n=15,187) reported disparities in NAFLD prognosis; seven studies reported disparities in more than one study outcome. Six studies focused on White-Black disparities and did not include data for Hispanics, while one study focused on White-Hispanic disparities and did not include data for Blacks12–18. Of NAFLD prevalence studies, 9 were conducted among population-based cohorts and 8 were conducted in high-risk cohorts. Of NAFLD severity studies, 10 evaluated presence of NASH and 11 characterized fibrosis staging. There were fewer studies of NAFLD prognosis including two full publications and 4 meeting abstracts, and reporting of outcomes was not standardized among available studies. We found no evidence of publication bias for NAFLD prevalence or severity by Egger’s test (p>0.05 for each) or funnel plot inspection.
NAFLD Prevalence in Population-Based Cohorts
Nine studies (n=343,393) assessed NAFLD prevalence in population-based cohorts (Table 1)9,10,12,13,16,19–22. NAFLD diagnosis was determined by presence of steatosis on ultrasound (n=3), steatosis per MR spectroscopy (n=2), steatosis on CT (n=2), elevated aminotransferases alone (n=1), and ICD-9 codes (n=1). Four studies required exclusion of other liver diseases to make a NAFLD diagnosis; however, only one required histologic confirmation.22
Table 1.
Studies characterizing racial/ethnic disparities in NAFLD prevalence among U.S. patients
| Prevalence (unselected cohort) | |||||||
|---|---|---|---|---|---|---|---|
| Author/Year | Study Design | Cohort | Method of NAFLD diagnosis | n | n (white/black/Hispanic) | n NAFLD [overall prevalence] | n NAFLD (white/black/Hispanic) |
| Birerdinc, 201217 | Cross-sectional | Retrospective, NHANES 2001–2008 | Aminotransferases | 18550 | 13422 / 2061 / 2242 | 1782 [9.6%] | 1247 / 131 / 314 |
| Browning, 200438 | Cross-sectional | Prospective, Dallas Heart Study | MR spectroscopy | 2240 | 734 / 1105 / 401 | 687 [30.7%] | 242 / 265 / 180 |
| Kim, 201332 | Cross-sectional | Prospective, Study of Women’s Health Across the Nation, Michigan site | Ultrasound | 331 | 130 / 201 / 0 | 94 [28.3%] | 47 / 47 / 0 |
| Loomba, 201531 | Cross-sectional | Prospective, Twins in Southern California | MR spectroscopy | 120 | 94 / 0 / 18 | 26 [21.7%] | 18 / 0 / 5 |
| North, 201235 | Cross-sectional | Retrospective, NHLBI family heart study | CT | 2,727 | 2221 / 506 / 0 | 181 [6.6%] | 159 / 22 / 0 |
| Reddy, 201339 | Cross-sectional | Retrospective, Nationwide Inpatient Sample | ICD-9 codes | 303,39 6 | 173,314 / 48,432 / 36,259 | 32,347 [10.7%] | 20536 / 2798 / 4135 |
| Tison, 201540 | Cross-sectional | Retrospective, MESA | CT | 4088 | 1503 / 1233 / 955 | 706 [17.3%] | 229 / 138 / 259 |
| Williams, 201141 | Cross-sectional | Prospective, Brooke Army Medical Center patients | Ultrasound +/− biopsy | 328 | 205 / 37 / 72 | 151 [46.0%] | 91 / 13 / 42 |
| Younossi, 201229 | Cross-sectional | Retrospective, NHANES 1988–1994 | Ultrasound +/− aminotransferases | 11,613 | 8887 / 1215 / 644 | 2492 [21.5%] | 1885 / 211 / 190 |
| Total | 343393 | 200,510 / 54,790 / 40,591 | 38466 [11.2%] | 24454 / 3625 / 5125 | |||
| Prevalence (high risk cohort) | |||||||
| Author | Study Design | Cohort | Method of NAFLD diagnosis | n | n (white/black/Hispanic) | n NAFLD [overall prevalence] | n NAFLD (white/black/Hispanic) |
| Bril, 201442 | Cross-sectional | Prospective, Diabetics/pre-diabetics | MR spectroscopy | 93 | 31 / 31 / 31 | 71 [76.3%] | 22 / 25 / 24 |
| Chen, 200943 | Cross-sectional | Prospective, Diabetics, patients with impaired glucose tolerance | MR spectroscopy | 76 | 22 / 5 / 49 | 59 [77.6%] | 13 / 2 / 44 |
| Corey, 201336 | Cross-sectional | Prospective, Obese undergoing weight loss surgery | Biopsy | 159 | 107 / 52 / 0 | 110 [69.2%] | 85 / 25 / 0 |
| Garcia, 201537 | Cross-sectional | Prospective, Obese women undergoing bariatric surgery | Biopsy | 51 | 41 / 10 / 0 | 33 [64.7%] | 28 / 5 / 0 |
| Kallwitz, 200944 | Cross-sectional | Prospective, Obese undergoing weight loss surgery | Biopsy | 238 | 110 / 92 / 36 | 212 [89.1%] | 105 / 73 / 34 |
| Setiawan, 201645 | Cross-sectional | Retrospective, Multiethnic cohort | ICD-9 codes | 5783 | 1148 / 609 / 1442 | 2990 [51.7%] | 467 / 239 / 658 |
| Stepanova, 201034 | Cross-sectional | Prospective, Obese undergoing weight loss surgery | Biopsy | 94 | 73 / 21 / 0 | 81 [86.2%] | 64 / 17 / 0 |
| Solga, 200533 | Cross-sectional | Prospective, Obese undergoing weight loss surgery | Biopsy | 189 | 163 / 26 / 0 | 166 [87.8%] | 149 / 17 / 0 |
| Total | 6683 | 1695 / 846 / 1558 | 3722 [55.7%] | 933 / 403 / 760 | |||
NAFLD prevalence ranged widely from 6.6% to 46.0% between studies, with a pooled prevalence of 11.2% (95%CI 11.1–11.3%). Compared to Whites, Hispanics had higher risk of NAFLD with a pooled RR of 1.36 (95% CI 1.08–1.73), and Blacks had lower risk of NAFLD with a pooled RR of 0.68 (95%CI 0.54–0.84). There was significant heterogeneity (I2 >90%) in both comparisons; on visual inspection of Forest plots, the study by Reddy was an outlier.20 This study was conducted among hospitalized patients and NAFLD diagnosis was based on ICD-9 codes, which have low accuracy, so it was excluded in sensitivity analyses. After removing this study, pooled NAFLD prevalence was 15.1% (95% CI 14.8–15.5%). NAFLD prevalence was highest in Hispanics (22.9%, 95% CI 21.6–24.1%), intermediate in Whites (14.4%, 95%CI 14.0–14.8%), and lowest in Blacks (13.0%, 95%CI 12.2–13.9%). Compared to Whites, the pooled RR of NAFLD in Hispanics was 1.47 (95% CI 1.35–1.61), and I2 decreased to 41% (Figure 1A); the pooled RR of NAFLD in Blacks compared to Whites was 0.74 (95% CI 0.69–0.80), and I2 decreased to 0% (Figure 1B).
Figure 1.
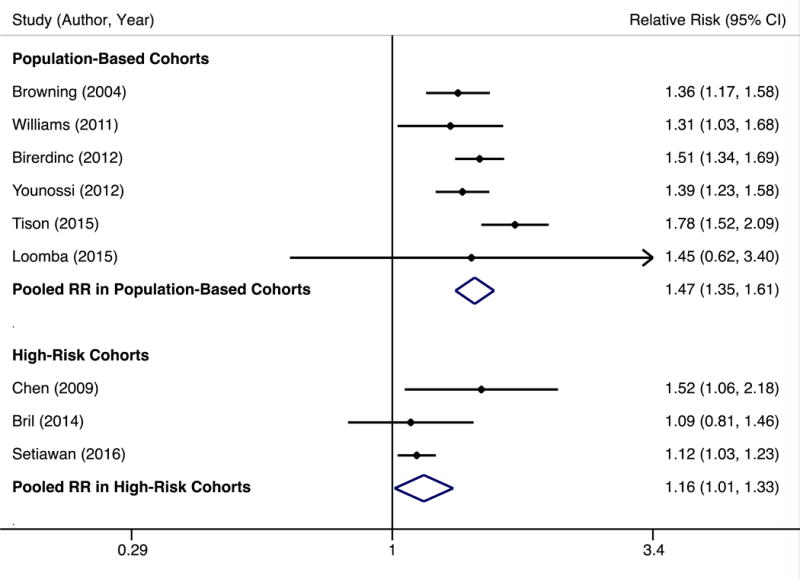

A. NAFLD prevalence among Hispanics vs. Whites in population-based and high-risk cohorts
B. NAFLD prevalence among Blacks vs. Whites in population-based and high-risk cohorts
NAFLD Prevalence in High-Risk Cohorts
Eight studies (n=6,683) assessed NAFLD prevalence in high-risk cohorts (Table 1)14,15,17,18,23–26. NAFLD diagnosis was ascertained using intraoperative liver biopsy (n=5), MR spectroscopy (n=2), and history/ICD-9 codes (n=1), with only one study requiring histologic confirmation.
NAFLD prevalence ranged from 51.7% to 89.1%, with a pooled prevalence of 55.7% (95% CI 54.5–56.9%). NAFLD prevalence was 55.5% (95%CI 52.6–57.4%) among Whites, 48.8% (95%CI 46.3–51.3%) among Hispanics, and 47.6% (95%CI 44.2–51.1%) among Blacks. NAFLD prevalence among other races/ethnicities, including Asians, was evaluated in few studies but appeared high at 62.9%. Risk of NAFLD in Hispanics among high-risk patients was not significantly different than Whites, with a pooled RR of 1.10 (95% CI 0.96–1.27), although there was moderate heterogeneity (I2 >70%). Studies by Chen and Kallwitz were outliers on visual inspection of Forest plots. There was not clinical heterogeneity justifying exclusion of Chen; however, Kallwitz was the only study to use intraoperative biopsy. On sensitivity analyses, we found a pooled RR of 1.26, 95%CI 1.09–1.46 (I2 = 94%) if the Chen study was excluded and a pooled RR of 1.16, 95%CI 1.03–1.33 (I2 = 25%) if the Kallwitz study was excluded (Figure 1A).
Blacks had lower risk of NAFLD than Whites in high-risk patients, with a pooled RR of 0.85 (95%CI 0.75–0.97). There was moderate heterogeneity (I2 >50%) and studies by Bril and Setiawan were outliers on visual inspection of Forest plots. Both studies used non-invasive means to define NAFLD – MR spectroscopy and ICD-9 codes, respectively – whereas remaining studies used liver biopsy. On sensitivity analysis removing all 3 studies defining NAFLD non-invasively, the pooled RR was 0.78 (95%CI 0.67–0.91), and I2 decreased to 47% (Figure 1B).
NAFLD Severity
Eighteen studies (n=16,083) addressed racial/ethnic differences in NAFLD severity (Table 2)10,14,18,22,23,27–39. Among 10 studies that evaluated presence of NASH among NAFLD patients, pooled NASH prevalence was 31.4% (95%CI 30.1–32.7%). NASH prevalence was highest in Hispanics (45.4%, 95% CI 40.7–50.2%), intermediate in Whites (32.2%, 95% CI 30.7–33.7%), and lowest in Blacks (20.3%, 95% CI 16.8–24.2%). The pooled RR of NASH in Hispanics compared to Whites was 1.24 (95%CI 1.02–1.52), with moderate heterogeneity (I2 >50%). The study by Bril was an outlier on visual inspection of Forest plots; however, there was not clinical heterogeneity justifying exclusion. The study by Younossi did not appear to be an outlier but was the only study to define NASH using liver enzymes instead of histology. On sensitivity analyses, we found a pooled RR of 1.30, 95%CI 1.05–1.63 (I2 = 63%) if the study by Bril was excluded and pooled RR of 1.09, 95%CI 0.98–1.21 (I2 = 4%) if the study by Younossi was excluded (Figure 2A). The pooled RR of NASH in Blacks compared to Whites was 0.72 (95%CI 0.60–0.87), with minimal heterogeneity (I2 <20%) (Figure 2B).
Table 2.
Studies characterizing racial/ethnic disparities in NAFLD severity among U.S. patients
| Author/Year | Study Design | Retrospective/Prospective | Cohort | Method of NAFLD/NASH diagnosis | n |
|---|---|---|---|---|---|
| Bambha, 201246 | Cross-sectional | Prospective | NASH CRN | Biopsy | 1026 |
| Bril, 201442 | Cross-sectional | Prospective | Diabetics/pre-diabetics | MR spectroscopy, biopsy | 93 |
| Campos, 200847 | Cross-sectional | Prospective | Obese undergoing weight loss surgery | Biopsy | 200 |
| Corbin, 201348 | Cross-sectional | Prospective | Single center NAFLD registry | Biopsy | 446 |
| Garcia, 201537 | Cross-sectional | Prospective | Obese, non-diabetic women | Biopsy | 51 |
| Ha, 201649 | Cohort | Retrospective | NAFLD patients at single center | Imaging, histology | 460 |
| Hossain, 200950 | Cross-sectional | Prospective | NAFLD patients at single center | Biopsy | 432 |
| Kallwitz, 200951 | Cross-sectional | Retrospective | Obese undergoing weight loss surgery | Biopsy | 238 |
| Lomonaco, 201152 | Cross-sectional | Prospective | Obese patients | Biopsy | 152 |
| Mohanty, 200953 | Cross-sectional | Retrospective | Pts with “steatosis” on pathology | Biopsy | 238 |
| Nelson, 200754 | Cross-sectional | Prospective | Multicenter cohort, NASH | Biopsy | 126 |
| Pimentel, 201655 | Cross-sectional | Prospective | Single center NAFLD registry | Biopsy | 183 |
| Rafiq, 200956 | Cohort | Retrospective | NAFLD registry (2 centers) | Biopsy | 173 |
| Solga, 200533 | Cross-sectional | Prospective | Obese undergoing weight loss surgery | Biopsy | 189 |
| Tabibian, 201157 | Cross-sectional | Retrospective | Single center NAFLD registry | Biopsy | 90 |
| Verna, 201558 | Cross-sectional | Prospective | Single center NAFLD registry | Biopsy | 45 |
| Williams, 201141 | Cross-sectional | Prospective | Single center NAFLD registry | Biopsy | 328 |
| Younossi, 201229 | Cross-sectional | Retrospective | NHANES 1988–1994 | Ultrasound +/− aminotransferases | 11613 |
Figure 2.

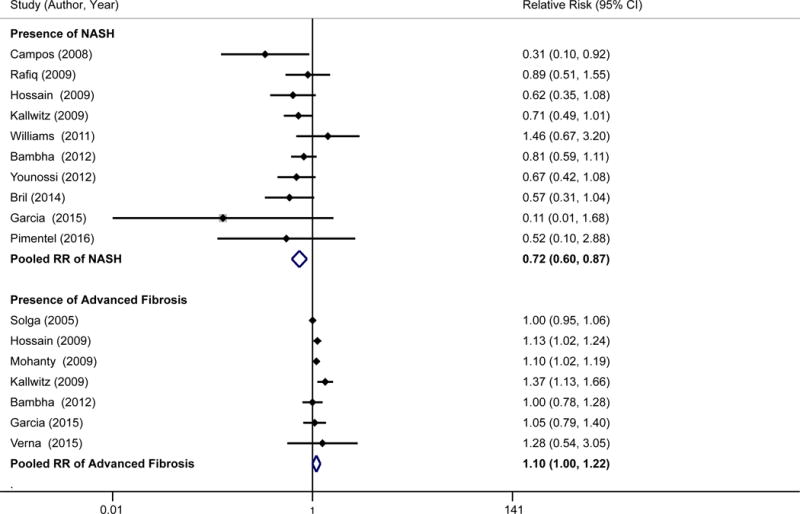
A. Presence of NASH and advanced fibrosis among patients with NAFLD for Hispanics vs. Whites
B. Presence of NASH and advanced fibrosis among patients with NAFLD for Blacks vs. Whites
Among 11 studies assessing stage of fibrosis, pooled proportion of NAFLD patients with significant fibrosis (stage F3–F4) was 19.5% (95%CI 18.1–20.9%). Significant fibrosis proportions were numerically highest in Whites (22.3%, 95% CI 20.5–24.2%) and Hispanics (19.6%, 95% CI 16.0–23.0%) and lowest among Blacks (13.1%, 95% CI 8.9–18.2%); however differences were not statistically significant (Whites vs. Blacks: RR of 1.10, 95% CI 1.00–1.22; Whites vs. Hispanics: RR 1.02, 95%CI 0.94–1.11).
NAFLD Prognosis
Six studies assessed racial/ethnic differences in prognosis among NAFLD patients (Table 3)30,40–44. All studies were retrospective and outcomes included progression to cirrhosis, development of HCC, liver-related mortality, and all-cause mortality. Heterogeneity of outcomes precluded pooling of data. One study reported Hispanics had higher odds of developing cirrhosis (OR 2.7, 95%CI 1.2–5.8) and HCC (OR 2.5, 95%CI 1.1–5.5) compared to Whites, although another study found no significant difference in cirrhosis proportions between Hispanics and Whites (6.7% vs. 5.6%, p=0.65). Studies on liver-related and all-cause mortality also reported discordant results. Younossi and colleagues reported significantly higher hazards of all-cause mortality among Blacks than Whites (HR 1.36, 95%CI 1.03–1.78) and lower hazards among other racial/ethnic minorities including Hispanics compared to Whites (HR 0.65, 95%CI 0.43–0.96). In contrast, another study reported non-significant higher hazards of liver-related and all-cause mortality among whites than racial/ethnic minorities and another reported no differences in all-cause mortality between racial/ethnic groups.
Table 3.
Studies characterizing racial/ethnic disparities in NAFLD prognosis among U.S. patients
| Author, Year | Study Design | Retrospective/Prospective | Cohort | n | Method of NAFLD/NASH diagnosis |
|---|---|---|---|---|---|
| De Martini, 201459 | Cohort | Retrospective | Single center registry | 626 | Clinical criteria, biopsy |
| Ditah, 201418 | Cohort | Retrospective | NHANES III + mortality-linked files to National Death Registry | 11,863 | Ultrasound |
| Ha, 201649 | Cohort | Retrospective | Single center NAFLD registry | 460 | Imaging, biopsy |
| Stepanova, 201360 | Cohort | Retrospective | NAFLD databases (2 centers) | 289 | Biopsy |
| Yip, 201561 | Cohort | Retrospective | Single center registry | 696 | Biopsy |
| Younossi, 201362 | Cohort | Retrospective | NHANES III + mortality-linked files to National Death Index | 6,709 | Ultrasound |
Quality Assessment
Quality assessment of studies is provided in Table 4. Four studies had quality scores <5, 17 had a score of 5–7, and 13 studies had quality scores >7. Three of the four studies with quality scores <5 were abstracts. Most studies had appropriate cohort selection, including representativeness of the at-risk cohort. The most common limitation observed was cross-sectional study design (27of 34 studies), which precluded the exposure being measured prior to outcome, sufficient time frame, repeated measurements of the exposure over time, and loss to follow-up reporting. Although outcomes were clearly defined and valid in most studies, ascertainment method for those outcomes varied. Some studies used imaging including ultrasound, CT, or MRI for NAFLD prevalence, whereas others used liver enzymes, which have lower sensitivity for NAFLD ascertainment. Similarly, most studies assessed NAFLD severity via histology although some studies used less reliable methods such as imaging and/or liver enzymes. Finally, several studies used large administrative databases, e.g. NHANES, which have inherent limitations, including missing or incomplete data on diagnosis of NAFLD thus increasing risk of ascertainment bias.
Table 4.
Quality assessment for all included studies
| Prevalence Studies | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Overall Score | Outcomes assessed |
| Bierdinc, 2012 | * | * | * | * | no | no | no | no | * | no | * | * | NA | * | 8 | NAFLD |
| Younossi, 2012 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | NAFLD/NASH | |
| Tison, 2015 | * | * | * | * | no | no | no | * | * | no | * | * | NA | * | 9 | NAFLD |
| Browning, 2004 | * | * | * | * | no | no | no | NA | * | no | * | NR | NA | NR | 6 | HCTG |
| Williams, 2011 | * | * | * | * | no | no | no | NA | * | no | * | NR | NA | * | 7 | NAFLD/NASH |
| Loomba, 2015 | * | * | NA | * | * | no | no | NA | * | no | * | NR | NA | * | 7 | NAFLD |
| Kim, 2013 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | Hepatic steatosis |
| Reddy, 2013 | * | * | NA | no | no | no | no | NA | no | no | no | NA | * | 3 | NAFLD | |
| North, 2012 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NAFLD |
| Bril, 2014 | * | no | NR | NR | no | no | no | NA | NR | * | NR | NA | * | * | 3 | NAFLD |
| Chen, 2009 | no | no | NR | NR | no | no | no | NA | * | no | * | NR | NA | NR | 2 | NAFLD |
| Corey, 2013 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NAFLD/NASH |
| Garcia, 2015 | * | * | NR | * | no | no | no | * | * | no | * | no | NA | NR | 6 | NAFLD/NASH |
| Kallwitz, 2009 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NAFLD/NASH |
| Setiawan, 2016 | * | * | NA | * | no | no | no | NA | * | no | * | NA | NA | no | 5 | NAFLD |
| Stepanova, 2010 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | NR | 7 | NAFLD/NASH |
| Solga, 2005 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NAFLD/NASH |
| Severity Studies | ||||||||||||||||
| Author, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Overall Score | Outcomes assessed |
| Younossi, 2012 | * | * | * | * | no | no | no | NA | * | no | no | * | NA | * | 7 | NAFLD/NASH |
| Williams, 2011 | * | * | * | * | no | no | no | NA | * | no | * | NR | NA | * | 7 | NAFLD/NASH |
| Bril, 2014 | no | NR | NR | no | no | no | NA | NR | no | * | NR | NA | * | 3 | NAFLD | |
| Garcia, 2015 | * | * | NR | * | no | no | no | * | * | no | * | no | NA | NR | 6 | NAFLD/NASH |
| Kallwitz, 2009 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NAFLD/NASH |
| Solga, 2005 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NAFLD/NASH |
| Bambha, 2012 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NAFLD/NASH |
| Campos, 2008 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NASH |
| Corbin, 2013 | * | * | NR | no | * | no | no | NA | * | no | * | NR | NA | * | 6 | Degree of steatosis |
| Ha, 2016 | * | * | NR | * | no | * | * | NA | * | no | * | NR | NR | NR | 7 | Progression to NASH, decompensation, HCC, all-cause mortality |
| Hossain, 2009 | * | * | NR | * | no | no | no | NA | * | no | * | * | NA | * | 7 | Degree of fibrosis |
| Lomonaco, 2011 | * | * | NR | * | no | no | no | NA | * | no | * | * | NA | no | 6 | NASH severity (histologic score) |
| Mohanty, 2009 | * | * | NA | * | no | no | no | NA | * | no | * | * | NA | * | 7 | NASH severity |
| Nelson, 2007 | * | * | NA | * | no | no | no | NA | * | no | * | no | NA | * | 6 | Degree of fibrosis |
| Pimentel, 2016 | * | * | NA | * | no | no | no | NA | * | no | * | * | NA | * | 7 | NASH/non-NASH |
| Rafiq, 2009 | * | * | NR | * | no | * | * | NA | * | no | * | NR | NR | NR | 7 | overall survival |
| Tabibian, 2011 | * | * | * | * | no | no | no | NA | * | no | * | * | NA | * | 8 | NASH severity |
| Verna, 2015 | * | * | NR | * | * | no | no | NA | * | no | * | * | NA | * | 8 | Fibrosis severity |
| Outcomes Studies | ||||||||||||||||
| Author, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Overall Score | Outcomes assessed |
| Ha, 2016‡ | * | * | NR | * | no | * | * | NA | * | no | * | NR | NR | NR | 7 | 15-year cumulative incidence of cirrhosis, HCC, all-cause mortality |
| De Martini, 2014‡ | * | * | NR | * | no | no | * | NA | NR | no | NR | NR | NA | * | 5 | Cirrhosis, HCC, liver transplantation |
| Ditah, 2014‡ | * | * | NA | * | no | * | * | NA | NR | NR | * | NR | NR | NR | 6 | All-cause mortality |
| Stepanova, 2013 | * | * | NR | * | no | * | * | NA | * | no | * | NR | NR | * | 8 | Liver-related and all-cause mortality |
| Yip, 2015‡ | * | no | NR | NR | no | NR | NR | NA | * | NR | NR | NR | NR | * | 3 | Cirrhosis, HCC, liver transplantation |
| Younossi, 2013 | * | * | NA | * | no | * | * | NA | * | no | * | no | NR | NR | 7 | Overall survival, Cardiac-specific mortality and Liver-specific mortality |
Key: An asterisk (*) indicates that the study met criteria NA = Not applicable; NR = Not reported.
abstract only
DISCUSSION
While NAFLD prevalence varied widely among studies, particularly in population-based cohorts, our systematic review highlights NAFLD is very common in the United States. We found nearly 1 in 6 of all Americans and 1 in 2 high-risk individuals have NAFLD. NAFLD prevalence appeared highest among Hispanics and lowest among Blacks, although differences were less marked in high-risk cohorts than population-based cohorts. Among patients with NAFLD, risk of NASH is greatest in Hispanics and lowest in Blacks; however, the proportion with advanced fibrosis did not significantly differ between racial/ethnic groups. Data are discordant regarding the presence of racial/ethnic disparities in NAFLD-related prognosis such as progression to cirrhosis and/or mortality. To the best of our knowledge, this is the first systematic review to quantify racial/ethnic differences in NAFLD prevalence, severity, and prognosis. These data provide insight into where in the NAFLD-to-NASH cirrhosis continuum disparities start to arise and suggest determinants of each step may differ.
Our findings reflect the wide variation in NAFLD prevalence among studies, particularly in population-based cohorts, ranging from 6.6% to 46.0%. Prevalence appeared to depend on method of NAFLD diagnosis, with highest prevalence reported in studies using ultrasound or MR spectroscopy. As expected, NAFLD prevalence was higher in high-risk cohorts than population-based cohorts, with approximately 50% of patients with obesity or diabetes having NAFLD, independent of race/ethnicity. Despite variation in reported prevalences, observed racial/ethnic disparities were fairly consistent across studies. The highest NAFLD burden was evident among Hispanics, the fastest growing demographic in the United States. Population-based studies suggest nearly 1 in 4 Hispanics have NAFLD compared to only approximately 1 in 10 blacks. However, differences in prevalence were less marked in high-risk cohorts, suggesting differences in prevalence may be, in part, driven by the differential distribution of underlying risk factors including obesity and diabetes. These data are important to consider when determining targets for NAFLD screening in the general population and for determining pre-test likelihood of NAFLD in the differential diagnosis for patients with aminotransferase elevations. Development and validation of accurate predictive models, including factors such as age, race/ethnicity, and metabolic syndrome features, may help target NAFLD screening to those at highest risk.
Among patients with NAFLD, nearly 30% had evidence of NASH and nearly 20% had evidence of advanced fibrosis (stage F3–F4), underscoring the high potential for NAFLD-related morbidity and need for effective treatment strategies. Currently, lifestyle modifications remain the cornerstone of NASH therapy and while there are no currently widely available pharmacologic therapies, a number of novel drugs are in development. Given the widespread burden of NAFLD in the U.S., it may not be feasible to identify all at-risk persons. It may be more cost-effective to concentrate efforts on identifying patients who are most likely to develop adverse consequences of NASH (including cirrhosis, portal hypertensive complications, and HCC) and those who would derive most benefit from early preventative and therapeutic interventions. These patients would also need to be closely monitored for development of cirrhosis, at which time measures such as HCC surveillance and varices screening should be implemented.
It is unclear from current literature if NAFLD-related severity and prognosis differs between racial/ethnic groups. Although Hispanic NAFLD patients were more likely to have NASH than their counterparts, presence of advanced fibrosis did not differ between racial/ethnic groups and data characterizing prognosis were discordant. These data highlight the need for further research in areas of NASH severity and prognosis, as current data are sparse and inconsistently reported.
Although several studies have described racial/ethnic and socioeconomic disparities, further studies are needed to characterize precisely why these disparities exist. While NAFLD disease burden is related to metabolic syndrome components such as obesity, diabetes, and dyslipidemia, NAFLD risk extends beyond environmental factors. For example, diabetes and obesity are both more common among Blacks compared to Whites; however, the latter group has significantly higher risk of NAFLD2,45. Cultural and socioeconomic factors are also likely implicated including dietary and exercise habits, access to health care, and allostatic load experienced by those living in poverty. Prior studies have demonstrated genetic risk factors play a large role in NAFLD. In particular, single nucleotide polymorphisms in PNPLA3, TM6SF2 and MBOAT have unequal distributions across races/ethnicities which contributes to the observed differences in NAFLD prevalence46–48. For instance, the I148M variant in PNPLA3 is strongly associated with hepatic fat content, and occurs most frequently in Hispanics (49%) compared to non-Hispanic Whites (23%) and Blacks (17%)46. Additionally, recent studies have demonstrated polymorphisms in PNPLA3 are frequent in Asian Indians with NAFLD, which is likely contributing to the increasing prevalence of NAFLD in this population.49 Though genetic factors are clearly implicated, further studies are needed to characterize the relative contribution of genetic and environmental factors toward NAFLD pathogenesis to help inform risk stratification and future prevention efforts. Additionally, it is possible mechanisms for NAFLD progression differ from mechanisms that lead to development of NAFLD. Our study supports this hypothesis, as racial/ethnic disparities are less apparent for NAFLD severity and prognosis than prevalence, though the paucity of data on disparities in NAFLD prognosis should be noted. Although some genetic factors such as polymorphisms in PNPLA3 are also associated with NAFLD severity and prognosis50, it is unclear if this is true for other genetic factors such as TM6SF2 or environmental factors.
Studies in our meta-analysis have several inherent limitations given the complexity of racial/ethnic health disparities. First, data on race and ethnicity is self-reported and may not be collected reliably. Second, differences between racial groups may be difficult to interpret, as there may be as much genetic heterogeneity within races as between races. One particular challenge becoming more common over time is the classification of multi-ethnic individuals, who are often forced into a single category. Finally, race and socioeconomic status are often highly correlated, complicating interpretation of observed disparities in health outcomes. Racial/ethnic differences in some health outcomes can be mitigated, or even disappear, if adequately adjusting for socioeconomic status; however, this can often be difficult, particularly for retrospective analyses. Additional limitations of the NAFLD-related literature are worth acknowledging. First, most included studies in our analysis were cross-sectional with limitations in study quality. There was also heterogeneity in method of NAFLD diagnosis; some studies used liver histology or MR spectroscopy, while others used ICD-9 codes and laboratory tests which may underestimate or overestimate NAFLD prevalence. Additionally, while the NAFLD Activity Score (NAS) is the most accepted measure of NASH severity, racial/ethnic differences in NAS scores were rarely reported. Finally, there were little data on other underrepresented minority groups such as Asian/Pacific Islanders and Native Americans.
While awaiting effective NAFLD chemoprevention and treatment, our meta-analysis provides important data characterizing NAFLD disparities. Our study highlights NAFLD is common in the U.S., with nearly 1 in 6 of all Americans and 1 in 2 high-risk individuals having NAFLD. There are notable racial and ethnic disparities in NAFLD prevalence and severity, with the highest burden in Hispanics and lowest in Blacks. Few studies have evaluated racial/ethnic differences in NAFLD prognosis, with discordant results, demonstrating the need for further research in this area. Studies are also needed to identify determinants of NAFLD disparities, which would be the first crucial step to identify appropriate intervention targets to reduce racial/ethnic disparities and improve NAFLD morbidity and mortality.
Supplementary Material
Supplemental Figure 1. Flow diagram for study selection
Acknowledgments
Financial Support: This work was conducted with support from the AHRQ Center for Patient-Centered Outcomes Research (R24 HS022418) and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001105). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AHRQ.
Abbreviations
- AASLD
American Association for the Study of Liver Disease
- ALT
Alanine Aminotransferase
- AST
Aspartate Aminotransferase
- BMI
Body Mass Index
- HCC
Hepatocellular carcinoma
- HIV
Human Immunodeficiency Virus
- HR
Hazard ratio
- MBOAT
Membrane-bound O-acyl transferase
- NAFLD
Nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
Nonalcoholic steatohepatitis
- NIH
National Institute of Health
- OR
Odds ratio
- PNPLA3
Patatin-like phospholipase domain-containing protein 3
- TM6SF2
Transmembrane 6 superfamily 2 human gene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None of the authors have relevant conflicts of interest.
Author Contributions:
Nicole E. Rich was involved in study concept and design, acquisition of data, interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Stefany Oji was involved in acquisition of data, interpretation of data, and critical revision of the manuscript for important intellectual content.
Arjmand R. Mufti was involved in interpretation of data and critical revision of the manuscript for important intellectual content.
Jeffrey D. Browning was involved in interpretation of data and critical revision of the manuscript for important intellectual content.
Neehar D. Parikh was involved in interpretation of data, and critical revision of the manuscript for important intellectual content.
Mobolaji Odewole was involved in interpretation of data, and critical revision of the manuscript for important intellectual content.
Helen Mayo was involved in acquisition of data and critical revision of the manuscript for important intellectual content.
Amit G. Singal was involved in study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript, critical revision of the manuscript for important intellectual content, and study supervision.
References
- 1.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Kumar KS, Saboorian MH, et al. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99:292–8. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell SH, Harris DM, Patrie JT, et al. Is NASH underdiagnosed among African Americans? The American journal of gastroenterology. 2002;97:1496–500. doi: 10.1111/j.1572-0241.2002.05795.x. [DOI] [PubMed] [Google Scholar]
- 6.Wei JL, Leung JC-F, Loong TC-W, et al. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. The American journal of gastroenterology. 2015;110:1306–1315. doi: 10.1038/ajg.2015.235. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Journal of Clinical Epidemiology. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Wu F, Ding Y, et al. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. doi: 10.1038/srep33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birerdinc A, Stepanova M, Pawloski L, et al. Caffeine is protective in patients with nonalcoholic fatty liver disease. Alimentary pharmacology & therapeutics. 2012;35:76–82. doi: 10.1111/j.1365-2036.2011.04916.x. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine. 2012;91:319–27. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 11.Wagenknecht LE, Palmer ND, Bowden DW, et al. Association of PNPLA3 with nonalcoholic fatty liver disease in a minority cohort: the Insulin Resistance Atherosclerosis Family Study. Liver international : official journal of the International Association for the Study of the Liver. 2011;31:412–6. doi: 10.1111/j.1478-3231.2010.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loomba R, Schork N, Chen CH, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology. 2015;149:1784–1793. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim C, Harlow SD, Karvonen-Gutierrez CA, et al. Racial/ethnic differences in hepatic steatosis in a population-based cohort of post-menopausal women: the Michigan Study of Women’s Health Across the Nation. Diabetic medicine : a journal of the British Diabetic Association. 2013;30:1433–41. doi: 10.1111/dme.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solga SF, Clark JM, Alkhuraishi AR, et al. Race and comorbid factors predict nonalcoholic fatty liver disease histopathology in severely obese patients. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2005;1:6–11. doi: 10.1016/j.soard.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Stepanova M, Hossain N, Afendy A, et al. Hepatic gene expression of Caucasian and African-American patients with obesity-related non-alcoholic fatty liver disease. Obesity surgery. 2010;20:640–50. doi: 10.1007/s11695-010-0078-2. [DOI] [PubMed] [Google Scholar]
- 16.North KE, Graff M, Franceschini N, et al. Sex and race differences in the prevalence of fatty liver disease as measured by computed tomography liver attenuation in European American and African American participants of the NHLBI family heart study. European journal of gastroenterology & hepatology. 2012;24:9–16. doi: 10.1097/MEG.0b013e32834a94fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corey KE, Misdraji J, Zheng H, et al. The absence of obstructive sleep apnea may protect against non-alcoholic fatty liver in patients undergoing bariatric surgery. PloS one. 2013;8:e62504. doi: 10.1371/journal.pone.0062504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia AE, Kasim N, Tamboli RA, et al. Lipoprotein Profiles in Class III Obese Caucasian and African American Women with Nonalcoholic Fatty Liver Disease. PloS one. 2015;10:e0142676. doi: 10.1371/journal.pone.0142676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 20.Reddy SK, Zhan M, Alexander HR, et al. Nonalcoholic fatty liver disease is associated with benign gastrointestinal disorders. World journal of gastroenterology. 2013;19:8301–11. doi: 10.3748/wjg.v19.i45.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tison GH, Blaha MJ, Nasir K, et al. Relation of Anthropometric Obesity and Computed Tomography Measured Nonalcoholic Fatty Liver Disease (from the Multiethnic Study of Atherosclerosis) Am J Cardiol. 2015;116:541–6. doi: 10.1016/j.amjcard.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Bril F, Subbarayan S, Maximos M, et al. Prevalence of nonalcoholic fatty liver disease (NAFLD) and disease severity among African Americans (AA) with prediabetes (PREDM) or type 2 diabetes (T2DM) Diabetes. 2014;63:A465. [Google Scholar]
- 24.Chen J, Mathew M, Finch J, et al. The prevalence of NAFLD in T2DM is highest among hispanics and is closely related to hepatic and adipose tissue insulin resistance. Diabetes. 2009;58 no pagination. [Google Scholar]
- 25.Kallwitz ER, Guzman G, TenCate V, et al. The histologic spectrum of liver disease in African-American, non-Hispanic white, and Hispanic obesity surgery patients. The American journal of gastroenterology. 2009;104:64–9. doi: 10.1038/ajg.2008.12. [DOI] [PubMed] [Google Scholar]
- 26.Setiawan VW, Porcel J, Lu SC, et al. Prevalence of chronic liver disease and dirrhosis by underlying cause in understudied ethnic groups in the United States: The multiethnic cohort (MEC) Gastroenterology. 2016;150:S1141. doi: 10.1002/hep.28677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bambha K, Belt P, Abraham M, et al. Ethnicity and Nonalcoholic Fatty Liver Disease. Hepatology (Baltimore, Md) 2012;55:769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos GM, Bambha K, Vittinghoff E, et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology (Baltimore, Md) 2008;47:1916–23. doi: 10.1002/hep.22241. [DOI] [PubMed] [Google Scholar]
- 29.Corbin KD, Abdelmalek MF, Spencer MD, et al. Genetic signatures in choline and 1-carbon metabolism are associated with the severity of hepatic steatosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:1674–89. doi: 10.1096/fj.12-219097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ha NB, Trinh S, Le RH, et al. Disease presentation and natural history of non-alcoholic fatty liver disease (NAFLD) in an ethnically diverse U.S. patient population: A long-term follow-up study. Gastroenterology. 2016;150:S1055. [Google Scholar]
- 31.Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7:1224–2. doi: 10.1016/j.cgh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Kallwitz ER, Guzman G, TenCate V, et al. The histologic spectrum of liver disease in African-American, non-Hispanic white, and Hispanic obesity surgery patients. Am J Gastroenterol. 2009;104:64–9. doi: 10.1038/ajg.2008.12. [DOI] [PubMed] [Google Scholar]
- 33.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology. 2011;54:837–45. doi: 10.1002/hep.24483. [DOI] [PubMed] [Google Scholar]
- 34.Mohanty SR, Troy TN, Huo D, et al. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50:797–804. doi: 10.1016/j.jhep.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Nelson JE, Bhattacharya R, Lindor KD, et al. HFE C282Y mutations are associated with advanced hepatic fibrosis in Caucasians with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md) 2007;46:723–9. doi: 10.1002/hep.21742. [DOI] [PubMed] [Google Scholar]
- 36.Pimentel CFMG, Jiang ZG, Otsubo T, et al. Poor Inter-test Reliability Between CK18 Kits as a Biomarker of NASH. Digestive diseases and sciences. 2016;61:905–12. doi: 10.1007/s10620-015-3916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7:234–8. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Tabibian JH, Lazo M, Durazo FA, et al. Nonalcoholic fatty liver disease across ethno-racial groups: do Asian-American adults represent a new at-risk population? Journal of gastroenterology and hepatology. 2011;26:501–9. doi: 10.1111/j.1440-1746.2010.06443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verna EC, Patel J, Bettencourt R, et al. Novel association between serum pentraxin-2 levels and advanced fibrosis in well-characterised patients with non-alcoholic fatty liver disease. Alimentary pharmacology & therapeutics. 2015;42:582–90. doi: 10.1111/apt.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Martini S, Wei E, Wakil AE, et al. Racial differences in cirrhosis, hepatocellular carcinoma and liver transplantation among patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:S-946. [Google Scholar]
- 41.Stepanova M, Rafiq N, Makhlouf H, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD) Digestive diseases and sciences. 2013;58:3017–23. doi: 10.1007/s10620-013-2743-5. [DOI] [PubMed] [Google Scholar]
- 42.Ditah IC, Ngwa TN, Ndzengue A, et al. Racial disparities in nonalcoholic fatty liver disease related mortality among US Adults: Results of the National Health and Nutrition Examination Survey 18-year mortality follow-up data. Hepatology. 2014;60:594A. [Google Scholar]
- 43.Yip B, Yee BE, Do A, et al. Characteristics and clinical outcomes of a diverse cohort of patients with biopsy-proven non-alcoholic fatty liver disease (NAFLD) Hepatology. 2015;62:1303A. [Google Scholar]
- 44.Younossi ZM, Otgonsuren M, Venkatesan C, et al. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism: clinical and experimental. 2013;62:352–60. doi: 10.1016/j.metabol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Menke A, Casagrande S, Geiss L, et al. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. Jama. 2015;314:1021–9. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 46.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature genetics. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozlitina J, Smagris E, Stender S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–6. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mancina RM, Dongiovanni P, Petta S, et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219–1230.e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatt SP, Nigam P, Misra A, et al. Genetic variation in the patatin-like phospholipase domain-containing protein-3 (PNPLA-3) gene in Asian Indians with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2013;11:329–35. doi: 10.1089/met.2012.0064. [DOI] [PubMed] [Google Scholar]
- 50.Rotman Y, Koh C, Zmuda JM, et al. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow diagram for study selection


