Abstract
Objective
To evaluate the association between increased exposure to airborne fine particulate matter (PM2.5) during the periconception period with risk of congenital anomalies (CA).
Study Design
Using birth certificate data from the Ohio Department of Health (2006–2010) and PM2.5 data from the US Environmental Protection Agency’s 57 monitoring stations located throughout Ohio, geographic coordinates of the mother’s residence for each birth were linked to the nearest PM2.5 monitoring station and monthly exposure averages were calculated. The association between CA and increased PM2.5 levels was estimated, with adjustment for coexistent risk factors.
Results
After adjustment for coexisting risk factors, exposure to increased levels of PM2.5 in the air during the periconception period was modestly associated with risk of CA. Compared to other periconception exposure windows, increased exposure during the 1 month prior to conception was associated with the highest risk increase at smaller distances from monitor stations. The strongest influences of PM2.5 on individual malformations were found with abdominal wall defects, and hypospadias, especially during the 1 month preconception.
Conclusion(s)
Increased exposure to PM2.5 in the periconception period is associated with some modest risk increases for congenital malformations. The most susceptible time of exposure appears to be the one month prior to and after conception. Although the increased risk with PM2.5 exposure is modest, the potential impact on a population basis is noteworthy as all pregnant women have some degree of exposure.
Keywords: PM2.5, air pollution, birth defect, congenital anomaly, malformation
1. Introduction
Congenital malformations are among the most serious complications of pregnancy, affecting 3% of all births in the US.(1) The spectrum of birth defects is wide ranging from minor anomalies having no adverse health effects to severe major malformations that result in death. As a group, congenital anomalies are a leading cause of infant mortality in the United States.(1)
Although some specific malformations have a clear cause-effect relationship with periconception exposure such as poor glycemic control in diabetics and the caudal regression syndrome or thalidomide and limb reduction anomalies, most congenital anomalies have no known singular teratogenic etiology.(2) Considering that embryonic maldevelopment leading to congenital anomalies is a multifactorial disease process, investigators have become increasingly interested in the contribution of modifiable risk factors such as exposure to environmental pollutants. Prior studies that have indicated the possible association of particulate matter (PM) with birth defects, have been limited by inconsistency in definitions of high exposure levels, geographic measures of exposure, and timing of high exposure assignment.(3-12) Inconsistent definitions of high exposure levels not only lead to inconsistent findings, but also introduce bias into the estimates of odds ratios by improperly dichotomizing PM2.5 exposure.(13) The majority of prior studies on PM exposure examined coarse particles (aerodynamic diameter ≤ 10 µg/m3, PM10), which can be inhaled and accumulate in the respiratory system. Fine particles, PM2.5 (aerodynamic diameter < 2.5 µg/m3) are believed to be a more significant health hazard because they can deposit deep into lower airways and alveoli within the lungs and subsequently enter the systemic circulation. (14) However, the findings of studies examining PM2.5 on congenital anomaly risk have been inconsistent showing minor associations with a few individual anomalies or no effect.(3, 6, 7, 10-12, 15)
Because embryonic development occurs in the first trimester of pregnancy, the timing between an exposure and maldevelopment- assuming a true cause-effect relationship- must occur either before conception or in the early first trimester of pregnancy. Prior studies of PM2.5 have limited the exposure period studied to a small window during the early first trimester only, and did not investigate exposure in the months preceding conception.(3, 6, 7, 10, 11, 15) In this study, we aim to describe the association between exposure to airborne fine particle pollution, PM2.5, in a month-by-month fashion examining the time extending from two months prior to and through two months after conception with risk of congenital malformations. We also explore exposure-outcome associations by varying the cut-off values of geographic distance from monitor station.
2. Methods
2.1. Study Population
We developed a geo-spatial population-based cohort study using Ohio Department of Health live birth records. The Ohio Department of Health and Human Subjects Institutional Review Board approved a protocol for this study. This study was exempt from review by the Institutional Review Board at the University of Cincinnati, Cincinnati, Ohio. A data set generated from vital records of all live births that occurred in the state from 2006–2010 was provided for this analysis. We analyzed live births to women whose residential address was within a defined distance threshold of their nearest PM2.5 monitor. A detailed description of the study population is illustrated in Figure 1. Births with recorded Down syndrome or other suspected chromosomal disorder (pending or confirmed) (16, 17) were not included in the primary analysis; however, they were examined as an individual outcomes and also included in a separate sensitivity analysis.
Figure 1.
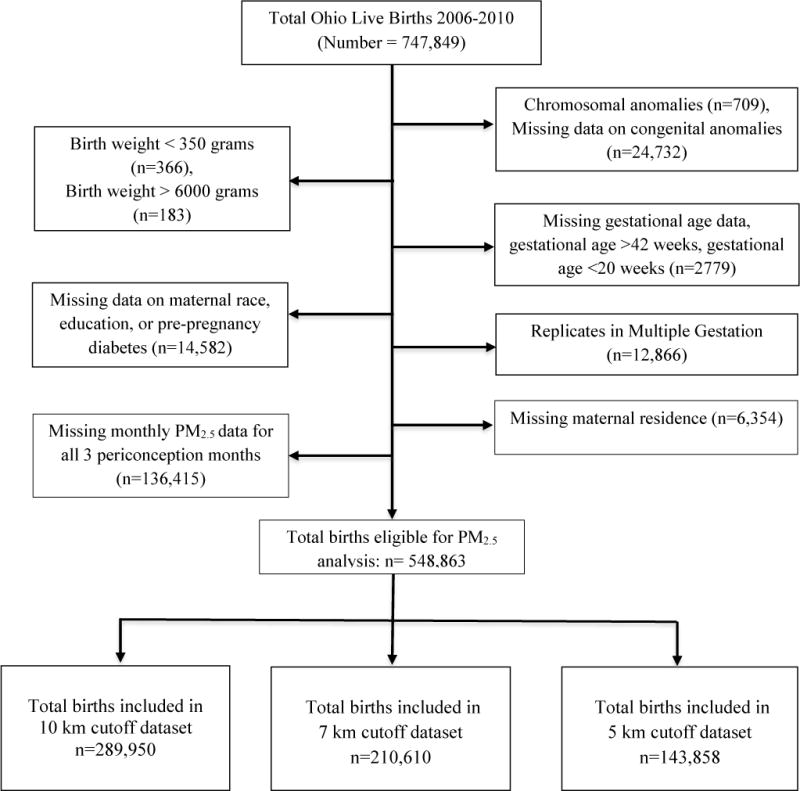
Flow diagram of the study population
Exposure values from central monitoring stations were used to estimate personal exposure levels of births during the study period. As there has been no clearly defined optimal distance cut-off to estimate exposure values related to a stationary monitor, we analyzed outcomes using multiple distances including residential address perimeters of 5, 7, and 10 km from monitor station. (6, 8, 16, 18, 19).
2.2. PM2.5 exposure assessment
PM2.5 levels were measured daily during the study period (2005–2010) by 57 US Environmental Protection Agency (EPA) stationary monitors across Ohio, and from this monthly averages were calculated. (20) The monthly averages were linked to Ohio birth records using the location of maternal residences. (21) We assigned the monthly average values of PM2.5 to each birth for five different monthly time periods: one and two months before/after conception, and the month of conception, linking data from the nearest monitoring stations using ArcGIS 10.1 (ESRI, Redlands, CA) software. These periconception time periods were calculated based on the gestational age at birth recorded in the birth certificate. There is no standard for the definition of “high exposure” in studies using stationary monitor exposure data for birth outcome.(16) Dichotomizing exposure values may be problematic and may introduce bias into the estimates of odds ratios.(13) Therefore, in our study, we treated PM2.5 exposure as a continuous variable, and reported odds ratios in both per IQR and per 10 µg/m3 increase.
2.3. Outcome measures
The primary outcome was major congenital anomaly at birth, as recorded on the 2003 version of the US birth certificate. Congenital anomaly of a newborn is recorded in the US standard certificate of live birth in a standardized manner. Strict criteria for the definition of each congenital anomaly are outlined in the Guide to Completing The Facility Worksheets for the Certificate of Live Birth and Report of Fetal Death (2003 revision), National Center for Health Statistics. (22) The presence of a major congenital anomaly in this study was defined as the presence of one or more of those reported anomalies. The frequency of the primary outcome of any congenital anomaly and the secondary outcomes of individual congenital anomalies were calculated.
2.4. Statistical Analysis
2.4.1. Descriptive analysis
The frequency of individual congenital anomalies recorded on the live birth certificate within the defined areas of study, stratified by distance cutoff from monitor (5 km, 7 km, and 10 km) were calculated. Baseline maternal and delivery characteristics were compared between the outcome group with congenital anomalies and the referent group of births with no anomaly, with p-value reported for χ2 test comparisons for - 10 km cutoff dataset (n=289,950). Rates of congenital anomalies among each baseline characteristic are reported as number of anomaly cases per 1000 live births. Summary statistics of PM2.5 levels were then calculated for births with congenital anomalies and those with no anomalies, stratified by month of exposure. No adjustment for multiple comparisons is preferable in this type of observational study as it leads to fewer errors of interpretation and allows for detection of natural observations of association.(23)
2.4.2. Outcome analysis
We used binomial regression with logistic link to analyze the association between congenital anomalies and PM2.5 exposures. Considering the spatial correlation of subjects sharing the same monitoring station, the use of a marginal model for this study is suitable. We utilized Generalized Estimating Equations (GEE) with exchangeable correlation structure to account for spatial correlation of subjects sharing the same PM2.5 monitor.(24) Estimates of association were adjusted for the confounding effects of maternal and newborn factors. Following model selection criteria described below, some of those individual covariates were removed from the final adjusted model.
To assess the influence of time of PM2.5 exposure on the primary outcome of any congenital anomaly, we stratified the exposure-outcome adjusted analyses by multiple time periods during the periconception period: one and two months before/after conception, the month of conception, and average of three periconception months. To provide a more comprehensive description of the influence of PM2.5 exposure on specific organ systems, analyses were repeated for the secondary outcomes of individual anomalies grouped by organ systems. Results are reported as adjusted odds ratio (adjOR) with 95% confidence interval (CI). Displaying the association as odds ratios with CI provides more information with regards to the direction of effect and effect size compared to just presenting association with p-values, and also avoids the need of complicated multiple testing adjustment methods for the p-values as they are highly correlated.(25)
2.4.3. Model selection
The following rules were considered in model selection:
Biological plausibility: Based on previously published data (8, 11, 26) as well as known factors associated with congenital anomalies, we included some covariates in our adjusted models based on their biological plausibility, rather than selection by small p-value or ‘best’ fit under a model selection rule. Such variables included maternal age, race, smoking status, season of conception and pre-pregnancy diabetes.
Quasi-Akaike information criterion (QIC): QIC is a model fitting criteria for generalized linear models using GEE.(27) Models with lower QIC values were favored as they represent better model fit.
Covariate set selection for consistent adjustment in multiple analyses. Due to varying quantity of missing data or observations in the multiple stratified analysis using different distance cutoffs and time periods of exposure, a single consistent set of covariates was chosen for all adjusted analyses rather than model-specific covariate sets selected for each individual analysis.
Final models were constructed incorporating a group of covariates selected based on biological plausibility and low QIC. The final models included the following covariates: maternal age (coded as categorical variables with 3 levels: less than or equal to 18, 19–34, and greater than 34 years of age(14), race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black and non-Hispanic others), pre-pregnancy diabetes, smoking status, marital status, educational level (coded as categorical variables with 3 levels: less than high school, high school graduate, and postsecondary education), season of conception and infant gender.
2.4.4. Sensitivity analyses
We performed sensitivity analyses modeling smoking in pregnancy as average number of cigarette smoked during immediate preconception period as a categorical variable composed of four groups: non-smokers, 1–9 cigarettes per day, 10–19 cigarettes per day and > 20 cigarettes smoked per day. In addition, we performed sensitivity analyses measuring the association between PM2.5 and congenital anomalies by including and excluding genetic abnormalities in the outcome analyses.
3. Results
3.1. Descriptive analyses
The locations of congenital anomaly cases, PM2.5 monitors, and the buffer regions representing 5 and 10 km circumferences around monitor stations in Ohio are demonstrated in Figure 2. The relationship of monitor stations, congenital anomaly cases and density by county in Ohio are further represented in Figure 3; online. Some of the most densely populated counties have the highest number of congenital anomalies cases (Figure 3a; online); however, they have relatively low frequency of congenital anomalies based on high birth density within the county (Figure 3b; online). Likewise, some counties with low birth density have higher rates of congenital anomalies.
Figure 2. Maternal residence of congenital anomalies cases, locations of monitor stations and their buffer regions.
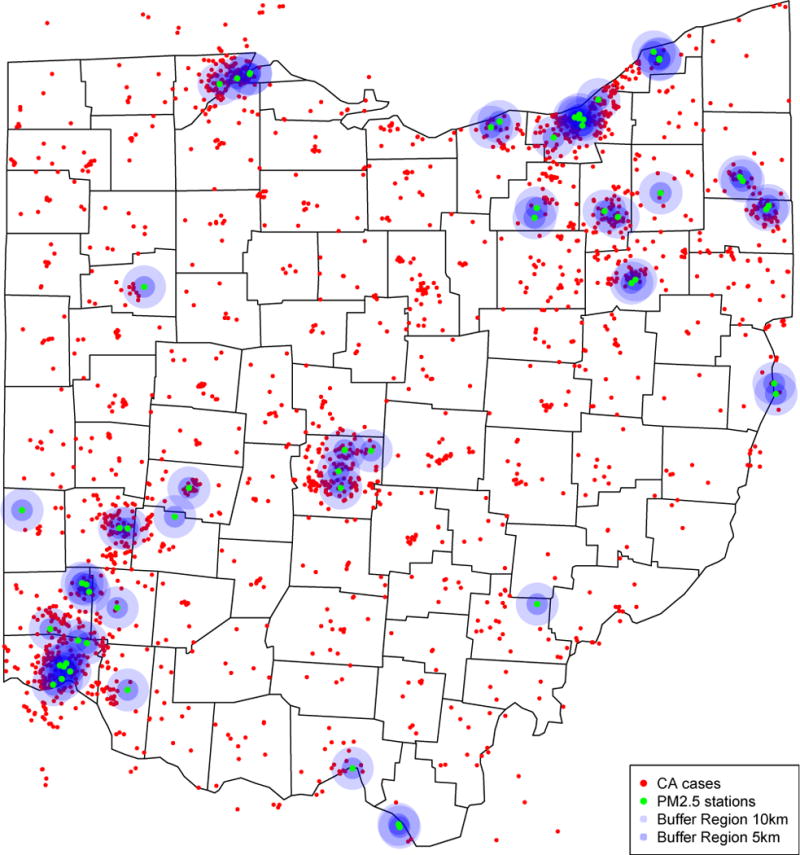
In this figure, red dots denote congenital anomalies cases, green dots denote the locations of monitor stations, 5km and 10km buffer regions were indicated by darker and lighter purple circles.
Figure 3.
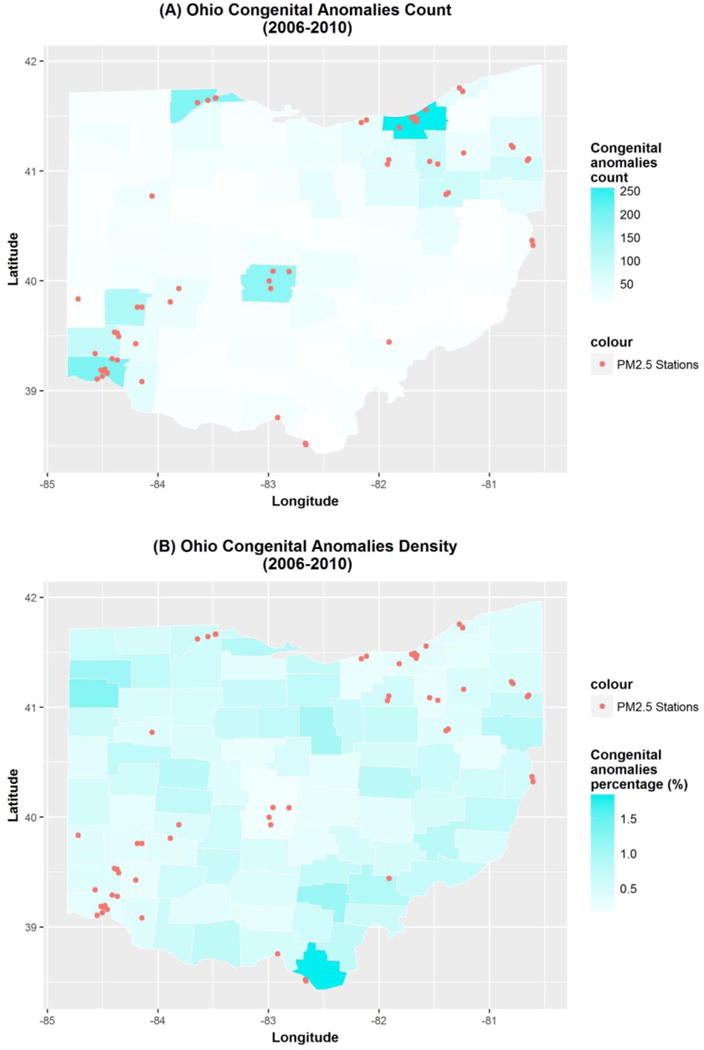
online. Congenital anomalies count and density by counties across Ohio from 2006 to 2010 (A) congenital anomalies count map by counties (B) congenital anomalies density map by counties
The frequency of congenital anomalies that occurred within the three specified distance cutoffs are presented in Table 1; online. The most common anomaly was cleft lip/palate, followed by abdominal wall defects. Differences in maternal demographic and pregnancy characteristics between the outcome group with congenital anomalies and the referent group of births with no anomaly are presented in Table 2; online. Births complicated by congenital anomalies occurred more commonly in young mothers ≤ 18 years of age, non-Hispanic White mothers, those with low educational attainment and of low socioeconomic status (as measured by use of Medicaid insurance). Cigarette smoking and pre-pregnancy diabetes were also significantly associated with presence of congenital anomalies. The rate of congenital anomalies was highest among pregnancies complicated by pregestational diabetes, 7.1 cases per 1000 live births. Anomalies occurred slightly more frequently in the earlier years of study (2006 and 2007), and in births during the summer and fall seasons.
Table 1.
(online). Number of congenital anomalies in each birth cohort, stratified by distance from residential address to PM2.5 monitor stationa
| Groups of congenital anomalies | Congenital anomaly | Number of cases and rate per 1000 live births | |||||
|---|---|---|---|---|---|---|---|
| 10 km | 7 km | 5 km | |||||
| Total number of births in the study populationb | 290173 | 210777 | 143968 | ||||
| Chromosome disorders | Suspected Chromosomal disorderc | 227 (0.8‰) | 93 (0.3‰) | 167 (0.8‰) | 69 (0.3‰) | 110 (0.8‰) | 45 (0.3‰) |
| Down Syndromec | 140 (0.5‰) | 103 (0.5‰) | 66 (0.5‰) | ||||
| Hypospadias | Hypospadias | 116 (0.4‰) | 116 (0.4‰) | 82 (0.4‰) | 82 (0.4‰) | 54 (0.4‰) | 54 (0.4‰) |
| Cleft Lip/Palate | Cleft Palate | 230 (0.8‰) | 124 (0.4‰) | 178 (0.8‰) | 95 (0.5‰) | 124 (0.9‰) | 64 (0.4‰) |
| Cleft Lip with or without Cleft Palate | 166 (0.6‰) | 132 (0.6‰) | 95 (0.7‰) | ||||
| Limb Reduction Defect | Limb Reduction Defect | 61 (0.2‰) | 61 (0.2‰) | 39 (0.2‰) | 39 (0.2‰) | 26 (0.2‰) | 26 (0.2‰) |
| Abdominal defect | Gastroschisis | 184 (0.6‰) | 114 (0.4‰) | 135 (0.6‰) | 88 (0.4‰) | 93 (0.6‰) | 64 (0.4‰) |
| Omphalocele | 28 (0.1‰) | 21 (0.1‰) | 11 (0.1‰) | ||||
| Congenital diaphragmatic hernia | 44 (0.2‰) | 27 (0.1‰) | 19 (0.1‰) | ||||
| Cyanotic congenital heart disease | Cyanotic congenital heart disease | 108 (0.4‰) | 108 (0.4‰) | 86 (0.4‰) | 86 (0.4‰) | 47 (0.3‰) | 47 (0.3‰) |
| Neural tube defect | Meningomyelocele/Spina Bifida | 126 (0.4‰) | 63 (0.2‰) | 99 (0.5‰) | 51 (0.2‰) | 77 (0.5‰) | 42 (0.3‰) |
| Anencephaly | 66 (0.2‰) | 51 (0.2‰) | 38 (0.3‰) | ||||
Subjects are counted multiple times if they have multiple congenital anomalies.
Chromosome disorders subjects are included in this table, but they are excluded in main statistical analysis
Both confirmed and pending subjects are included
Table 2.
(online). Characteristics of study population (Ohio Birth Cohort 2006–2010)
| Pregnancy and birth characteristics, 10 km cutoff cohort | Any congenital anomalya (N=784) |
No anomaly (N=289950) |
p-valueb | Congenital anomaly rate per 1,000 |
|---|---|---|---|---|
| Demographic Factors | ||||
| Maternal age | ||||
| ≤ 18 years | 81 (10.3%) | 21080 (7.3%) | <0.01 | 3.8 |
| 19–34 years | 620 (79.1%) | 234400 (81.1%) | 2.6 | |
| ≥ 35 years | 83 (10.6%) | 33686 (11.7%) | 2. 5 | |
| Race and Ethnicity | ||||
| Non-Hispanic White | 548 (69.9%) | 182378 (63.1%) | <0.01 | 3.0 |
| Non-Hispanic Black | 181 (23.1%) | 83077 (28.7%) | 2.2 | |
| Non-Hispanic Other | 18 (2.3%) | 8321 (2.9%) | 2.2 | |
| Hispanic | 37 (4.7%) | 15390 (5.3%) | 2.4 | |
| Social Behaviors & Socioeconomic Factors | ||||
| Education | ||||
| Less than high school | 191 (24.4%) | 57650 (19.9%) | <0.01 | 3.3 |
| High school graduate | 396 (50.5%) | 137913 (47.7%) | 2.9 | |
| College education | 197 (25.1%) | 93603 (32.4%) | 2.1 | |
| Tobacco use | ||||
| Yes | 189 (24.1%) | 54215 (18.8%) | <0.01 | 3.5 |
| No | 595(75.9%) | 234951 (81.3%) | 2.5 | |
| Marital Status | ||||
| Yes | 337 (43.0%) | 144282 (49.9%) | <0.01 | 2.3 |
| No | 447 (57.0%) | 144884 (50.1%) | 3.1 | |
| Low social economic status | ||||
| Yes | 378 (50.2%) | 120593 (43.6%) | <0.01 | 3.1 |
| No | 375 (49.8%) | 155842 (56.4%) | 2.4 | |
| Pre-pregnancy Diabetes | ||||
| Yes | 16 (2.0%) | 2247 (0.8%) | <0.01 | 7.1 |
| No | 768 (98.0%) | 286919 (99.2%) | 2.7 | |
| Year of Birth | ||||
| 2006 | 187 (23.9%) | 63076 (21.8%) | <0.01 | 3.0 |
| 2007 | 176 (22.5%) | 62581 (21.6%) | 2.8 | |
| 2008 | 144 (18.4%) | 58714 (20.3%) | 2.4 | |
| 2009 | 121 (15.4%) | 55897 (19.3%) | 2.2 | |
| 2010 | 156 (19.9%) | 48898 (16.9%) | 3.2 | |
| Season of conception | ||||
| Winter | 157 (20.0%) | 70745 (24.5%) | 0.04 | 2.2 |
| Spring | 201 (25.6%) | 71321 (24.7%) | 2.8 | |
| Summer | 210 (26.8%) | 73278 (25.3%) | 2.9 | |
| Fall | 216 (27.6%) | 73822 (25.5%) | 2.9 | |
| Infant gender | ||||
| Male | 482 (61.5%) | 147268 (50.9%) | <0.01 | 3.3 |
| Female | 302 (38.5%) | 141898 (49.1%) | 2.1 | |
Cases of chromosome disorders were excluded in this table in order to be consistent with main statistical analysis, whose results are shown in Tables 2–4 and Figure 1.
P-values were calculated using Chi-square test for each factor (contingency table). For comparisons where Chi-square test was not appropriate, Fisher exact test was used.
The mean PM2.5 level during the periconception period for the study population was 13.79 µg/m3 among the 10 km cohort, which was slightly lower than EPA standard during the study period of 15 µg/m3, but higher than the current standard of 12 µg/m3. (28) Births with any congenital anomaly had a higher mean PM2.5 exposure level compared to non-anomalous births across all periconception months and within each distance cohort. Table 3 demonstrates the mean PM2.5 levels in pregnancies complicated congenital anomalies compared births with no anomaly, stratified by periconception month of exposure and distance from monitor.
Table 3.
Summary statistics of PM2.5 levels in Ohio 2006 – 2010, stratified by different distance cohorts and periconception exposure periodsa
| Datasets by exposure periods | Congenital Anomalies | Normal | p-valuec | |||
|---|---|---|---|---|---|---|
| PM2.5 level | Mean (SD) | IQR (Q1, Q3) | Mean (SD) | IQR (Q1, Q3) | ||
| 10 km | 2 months before | 13.93 (3.97) | 4.54 (11.28, 15.82) | 13.86 (3.84) | 4.95 (11.08, 16.03) | 0.59 |
| 1 month before | 13.97 (4.00) | 5.02 (11.10, 16.13) | 13.77 (3.79) | 4.83 (11.04, 15.87) | 0.16 | |
| Month of conception | 13.85 (3.94) | 5.27 (10.99, 16.26) | 13.75 (3.83) | 4.88 (10.99, 15.87) | 0.44 | |
| Average of three monthsb | 13.92 (2.90) | 3.73 (11.85, 15.57) | 13.79 (2.77) | 3.50 (11.86, 15.36) | 0.23 | |
| 1 month after conception | 13.99 (3.82) | 5.40 (11.00, 16.40) | 13.65 (3.80) | 4.90 (10.90, 15.80) | 0.02 | |
| 2 months after conception | 13.58 (3.87) | 5.30(10.60, 15.90) | 13.43 (3.70) | 4.90 (10.70, 15.60) | 0.27 | |
| 7 km | 2 months before | 13.92 (3.95) | 4.70 (11.12, 15.82) | 13.91 (3.85) | 4.95 (11.13, 16.08) | 0.94 |
| 1 month before | 14.13 (4.01) | 5.14 (11.37, 16.51) | 13.81 (3.79) | 4.84 (11.10, 15.94) | 0.04 | |
| Month of conception | 13.92 (4.00) | 5.24 (11.13, 16.37) | 13.79 (3.83) | 4.92 (11.02, 15.94) | 0.40 | |
| Average of three monthsb | 13.99 (2.90) | 3.75 (11.92, 15.67) | 13.84 (2.77) | 3.52 (11.89, 15.41) | 0.21 | |
| 1 month after conception | 13.98 (3.79) | 5.40 (11.10, 16.40) | 13.65 (3.80) | 4.90 (10.90, 15.80) | 0.04 | |
| 2 months after conception | 13.64 (4.01) | 5.40 (10.60, 15.90) | 13.43 (3.70) | 4.90 (10.70, 15.60) | 0.19 | |
| 5 km | 2 months before | 14.07 (3.94) | 4.94 (11.34, 16.28) | 13.92 (3.84) | 4.97 (11.14, 16.11) | 0.44 |
| 1 month before | 14.23 (3.89) | 5.13 (11.52, 16.65) | 13.81 (3.77) | 4.87 (11.12, 15.99) | 0.03 | |
| Month of conception | 13.89 (3.97) | 5.12 (11.21, 16.32) | 13.81 (3.82) | 4.96 (11.04, 16.00) | 0.67 | |
| Average of three monthsb | 14.07 (2.86) | 3.84 (11.88, 15.72) | 13.85 (2.76) | 3.54 (11.90, 15.44) | 0.12 | |
| 1 month after conception | 13.77 (3.81) | 5.50 (10.90, 16.40) | 13.65 (3.80) | 4.90 (10.90, 15.80) | 0.55 | |
| 2 months after conception | 13.64 (3.99) | 5.00 (10.70, 15.70) | 13.43 (3.70) | 4.90 (10.70, 15.60) | 0.30 | |
PM2.5 levels are expressed in micrograms per cubic meter (µg/m3)
SD: standard deviation; IQR: Inter Quartile Range; Q1/Q3: lower/upper quartile.
Local PM2.5 level for each subject is assigned by the nearest monitoring station during that time period. This table shows the summary statistics of population average of local PM2.5 using this assignment (estimation) method.
Average of three months = 2 months before conception, 1 month before conception, and the month of conception.
P-values were calculated using two sample t-test.
3.2. Regression analyses
3.2.1. Primary results
The association between PM2.5 exposure and congenital anomalies during each periconception period is displayed in Table 4 as adjusted ORs with 95% CI for each distance cutoff (10, 7 and 5 km). We reported the adjusted odds for congenital anomalies associated with PM2.5 exposure in two ways: (1) continuous exposure level (per interquartile range increment) and (2) continuous exposure level (per 10 µg/m3 increase) for each period. Comparison of results between the three distance cutoff models shown in Table 4 allows for examination of consistency or inconsistency of findings based on distance from monitor exposure assessment. Of all the time periods and distance cutoffs under investigation, nearly all point estimates of effect demonstrate an odds ratio above 1. However, only several of the associations reached statistical significance with lower limit of the 95% confidence interval higher than 1. These modeling results are also consistent with the unadjusted comparisons shown in Table 3.
Table 4.
Adjusted OR and corresponding 95% CI for the association of any congenital anomaly and PM2.5 levels, stratified by different distance cohorts and periconception exposure periodsa
| Datasets by exposure periods | Number of cases | Number of total subjects | Continuous (per IQR increment) | Continuous (per 10 μm/m3 increment) | |
|---|---|---|---|---|---|
| 10 km | 2 months before | 782 | 287862 | 1.00 (0.89, 1.13) | 1.01 (0.80, 1.27) |
| 1 month before | 779 | 287283 | 1.06 (0.96, 1.18) | 1.14 (0.92, 1.41) | |
| Month of conception | 773 | 286566 | 1.02 (0.92, 1.13) | 1.05 (0.85, 1.29) | |
| Average of three monthsb | 779 | 287412 | 1.04 (0.92, 1.18) | 1.13 (0.80, 1.59) | |
| 1 month after | 759 | 285842 | 1.09 (1.01, 1.18) | 1.19 (1.02, 1.40) | |
| 2 months after | 759 | 285421 | 1.02 (0.95, 1.10) | 1.05 (0.91, 1.22) | |
| 7 km | 2 months before | 583 | 209007 | 0.99 (0.88, 1.11) | 1.00 (0.81, 1.25) |
| 1 month before | 580 | 208609 | 1.11 (0.98, 1.25) | 1.24 (0.97, 1.58) | |
| Month of conception | 577 | 208100 | 1.03 (0.93, 1.15) | 1.07 (0.86, 1.32) | |
| Average of three monthsb | 580 | 208703 | 1.06 (0.93, 1.20) | 1.18 (0.82, 1.68) | |
| 1 months after | 565 | 206868 | 1.09 (1.00, 1.20) | 1.20 (1.00, 1.44) | |
| 2 months after | 566 | 206565 | 1.06 (0.97, 1.17) | 1.13 (0.94, 1.37) | |
| 5 km | 2 months before | 397 | 142626 | 1.04 (0.93, 1.17) | 1.09 (0.87, 1.36) |
| 1 month before | 393 | 142349 | 1.17 (1.03, 1.34) | 1.39 (1.05, 1.83) | |
| Month of conception | 392 | 141981 | 1.03 (0.90, 1.17) | 1.05 (0.82, 1.36) | |
| Average of three monthsb | 393 | 142424 | 1.12 (0.99, 1.27) | 1.38 (0.97, 1.97) | |
| 1 months after | 393 | 141540 | 1.02 (0.90, 1.15) | 1.04 (0.81, 1.34) | |
| 2 months after | 393 | 141381 | 1.05 (0.91, 1.20) | 1.09 (0.83, 1.45) | |
OR: odds ratio; CI: confidence interval; IQR: Inter Quartile Range.
Births with a chromosome disorder were not included in these analyses.
Average of three months = 2 months before conception, 1 month before conception, and the month of conception.
We found that increasing PM2.5 exposure levels occurring one month prior to conception consistently demonstrated the highest adjOR point estimates and corresponding 95% CI lower bounds among all three preconception time windows from the 10 km model to 5 km model. In addition, this finding shown in Table 4 is consistent to unadjusted descriptive results in Table 3, which implies that this association cannot be explained by factors adjusted in the models. Therefore, these results suggest that compared to other preconception periods, increasing PM2.5 exposure during one month prior to conception is more likely to be associated with increasing risk of congenital anomalies.
We also assessed the inconsistency of results between the three distance cutoff models shown in Table 4. First, increasing PM2.5 exposure one month after conception exposure was significantly associated with congenital anomalies at 10 km and 7 km models, but not in the smaller 5 km model. Second, the association between PM2.5 exposure one month prior to conception is only statistical significant at 5% significance level for the 5 km model; however, this association became stronger with smaller distance cutoffs from the 10 km cohort to 5 km cohort, as indicated in Table 3 and Table 4. Our findings demonstrate some evidence of spatial variability of PM2.5 exposure within the 10 km cutoff, considering some inconsistency of results compared to more narrow distance cutoff cohorts.
3.2.2. Secondary outcomes – individual congenital anomalies
Tables 5, 6 and 7 (online) demonstrate the association of PM2.5 exposure with individual anomalies, grouped by organ system.(16) Considering the small number of cases for each individual anomaly, the confidence intervals shown in these tables are comparatively wider than those in Table 4 among each distance cutoff. The small sample size in these individual comparisons limits the ability to draw significant conclusions from these analyses. However, stratifying the analyses by individual anomalies in an exploratory manner does highlight several specific organ systems associated with PM2.5, such as urogenital and gastrointestinal organ systems, which may be useful for hypothesis generation and validation in larger analyses. The interpretation of modeling results for anomalies with a small number of cases may also be limited due to model overfitting, if considering a general practice of having at least 10 cases per 1 covariate parameter. Overfitting with a high ratio of covariates to cases may lead to less reliable risk estimates and exaggerated confidence intervals. For anomalies with a larger number of cases, such as abdominal defects, neural tube defects and cleft lip or cleft palate, the results should be more reliable.
Table 5.
(online). Adjusted OR and corresponding 95% CI for the association of individual congenital anomalies and PM2.5 levels for 10km cohort, stratified by periconception exposure periodsa
| 10 km cutoff dataset by exposure periods | Number of cases | Number of total subjects used | Continuous (per IQR increment) | Continuous (per 10 μm/m3 increment) | |
|---|---|---|---|---|---|
| Chromosome disorders | 2 months before | 226 | 287303 | 1.07 (0.87, 1.30) | 1.14 (0.76, 1.70) |
| 1 month before | 226 | 286727 | 1.00 (0.85, 1.18) | 1.00 (0.71, 1.41) | |
| Month of conception | 225 | 286015 | 0.92 (0.77, 1.10) | 0.84 (0.59, 1.21) | |
| Average of three months | 226 | 286856 | 0.99 (0.84, 1.18) | 0.98 (0.61, 1.60) | |
| Hypospadiasb,d | 2 months before | 111 | 146326 | 1.00 (0.77, 1.31) | 1.01 (0.59, 1.72) |
| 1 month before | 111 | 146056 | 1.16 (0.96, 1.40) | 1.36 (0.92, 2.00) | |
| Month of conception | 111 | 145695 | 1.39 (1.07, 1.81) | 1.97 (1.14, 3.38) | |
| Average of three months | 111 | 146114 | 1.27 (0.99, 1.61) | 1.97 (0.98, 3.96) | |
| Cleft Lip/Palate | 2 months before | 218 | 287295 | 1.05 (0.92, 1.21) | 1.11 (0.85, 1.46) |
| 1 month before | 216 | 286717 | 1.05 (0.88, 1.27) | 1.12 (0.76, 1.63) | |
| Month of conception | 214 | 286004 | 0.97 (0.83, 1.14) | 0.95 (0.69, 1.31) | |
| Average of three months | 216 | 286846 | 1.04 (0.89, 1.22) | 1.12 (0.71, 1.78) | |
| Cyanotic congenital heart disease | 2 months before | 101 | 287178 | 0.80 (0.60, 1.06) | 0.63 (0.36, 1.13) |
| 1 month before | 100 | 286601 | 1.17 (0.90, 1.53) | 1.39 (0.81, 2.40) | |
| Month of conception | 100 | 285890 | 0.84 (0.58, 1.22) | 0.70 (0.33, 1.49) | |
| Average of three months | 100 | 286730 | 0.90 (0.62, 1.30) | 0.74 (0.26, 2.13) | |
| Abdominal defectsc | 2 months before | 181 | 287258 | 1.03 (0.84, 1.26) | 1.06 (0.71, 1.60) |
| 1 month before | 180 | 286681 | 1.06 (0.89, 1.27) | 1.13 (0.79, 1.63) | |
| Month of conception | 176 | 285966 | 0.98 (0.79, 1.23) | 0.96 (0.61, 1.52) | |
| Average of three months | 180 | 286810 | 1.04 (0.85, 1.27) | 1.12 (0.64, 1.96) | |
| Neural tube defectsd | 2 months before | 125 | 287202 | 1.04 (0.82, 1.32) | 1.09 (0.68, 1.76) |
| 1 month before | 124 | 286625 | 0.97 (0.69, 1.36) | 0.94 (0.47, 1.90) | |
| Month of conception | 125 | 285915 | 0.99 (0.81, 1.20) | 0.97 (0.65, 1.45) | |
| Average of three months | 124 | 286754 | 1.00 (0.73, 1.38) | 1.01 (0.40, 2.52) | |
OR: odds ratio; CI: confidence interval; IQR: Inter Quartile Range.
For cases of individual congenital anomalies, births with a chromosome disorder in addition to the congenital anomaly were not included in this analysis.
Hypospadias analysis was limited to male infants and infant gender covariate was excluded from the adjusted model.
Abdominal defect models did not include pre-pregnancy diabetes due to lack of observations of that covariate in the outcome group.
Neural tube defects and Hypospadias models did not include maternal race due to lack of observations in one race category within the outcome group.
Table 6.
(online). Adjusted OR and corresponding 95% CI for the association of individual congenital anomalies and PM2.5 levels for 7km cohort, stratified by periconception exposure periodsa
| 7 km cutoff dataset by exposure periods | Number of cases | Number of total subjects used | Continuous (per IQR increment) | Continuous (per 10 μm/m3 increment) | |
|---|---|---|---|---|---|
| Chromosome disorders | 2 months before | 169 | 208591 | 1.00 (0.80, 1.26) | 1.00 (0.63, 1.60) |
| 1 month before | 169 | 208196 | 0.88 (0.72, 1.09) | 0.77 (0.50, 1.18) | |
| Month of conception | 170 | 207691 | 0.92 (0.77, 1.10) | 0.84 (0.59, 1.21) | |
| Average of three months | 169 | 208290 | 0.90 (0.73, 1.10) | 0.74 (0.42, 1.31) | |
| Hypospadiasb,d | 2 months before | 78 | 106052 | 1.02 (0.79, 1.31) | 1.03 (0.62, 1.73) |
| 1 month before | 78 | 105877 | 1.39 (1.15, 1.69) | 2.00 (1.34, 2.95) | |
| Month of conception | 78 | 105615 | 1.47 (1.06, 2.02) | 2.18 (1.13, 4.20) | |
| Average of three months | 78 | 105920 | 1.44 (1.07, 1.94) | 2.82 (1.21, 6.58) | |
| Cleft Lip/Palate | 2 months before | 168 | 208590 | 1.08 (0.93, 1.25) | 1.17 (0.86, 1.57) |
| 1 month before | 166 | 208193 | 0.99 (0.83, 1.19) | 0.99 (0.68, 1.43) | |
| Month of conception | 164 | 207685 | 0.94 (0.78, 1.13) | 0.88 (0.60, 1.29) | |
| Average of three months | 166 | 208287 | 1.01 (0.86, 1.19) | 1.02 (0.64, 1.63) | |
| Cyanotic Congenital heart disease | 2 months before | 80 | 208502 | 0.73 (0.55, 0.97) | 0.52 (0.29, 0.93) |
| 1 month before | 79 | 208106 | 1.21 (0.91, 1.59) | 1.47 (0.83, 2.62) | |
| Month of conception | 79 | 207600 | 0.91 (0.62, 1.33) | 0.82 (0.38, 1.78) | |
| Average of three months | 79 | 208200 | 0.91 (0.62, 1.33) | 0.76 (0.26, 2.23) | |
| Abdominal defectsc | 2 months before | 132 | 208554 | 1.08 (0.86, 1.35) | 1.16 (0.73, 1.84) |
| 1 month before | 131 | 208158 | 1.25 (1.00, 1.54) | 1.57 (1.01, 2.46) | |
| Month of conception | 130 | 207651 | 1.03 (0.81, 1.31) | 1.06 (0.65, 1.74) | |
| Average of three months | 131 | 208252 | 1.17 (0.95, 1.45) | 1.56 (0.85, 2.87) | |
| Neural tube defectsd | 2 months before | 98 | 208520 | 0.99 (0.72, 1.38) | 0.99 (0.51, 1.91) |
| 1 month before | 97 | 208124 | 0.96 (0.63, 1.48) | 0.93 (0.39, 2.23) | |
| Month of conception | 98 | 207619 | 1.01 (0.82, 1.25) | 1.03 (0.67, 1.58) | |
| Average of three months | 97 | 208218 | 0.99 (0.65, 1.49) | 0.96 (0.30, 3.11) | |
OR: odds ratio; CI: confidence interval; IQR: Inter Quartile Range.
For cases of individual congenital anomalies, births with a chromosome disorder in addition to the congenital anomaly were not included in this analysis.
Hypospadias analysis was limited to male infants and infant gender covariate was excluded from the adjusted model.
Abdominal defect models did not include pre-pregnancy diabetes due to lack of observations of that covariate in the outcome group.
Neural tube defects and Hypospadias models did not include maternal race due to lack of observations in one race category within the outcome group.
Table 7.
(online). Adjusted OR and corresponding 95% CI for the association of individual congenital anomalies and PM2.5 levels for 5km cohort, stratified by periconception exposure periodsa
| 5 km cutoff dataset by exposure periods | Number of cases | Number of total subjects used | Continuous (per IQR increment) | Continuous (per 10 μm/m3 increment) | |
|---|---|---|---|---|---|
| Chromosome disorders | 2 months before | 112 | 142339 | 1.04 (0.72, 1.50) | 1.08 (0.52, 2.25) |
| 1 month before | 112 | 142066 | 0.90 (0.70, 1.16) | 0.80 (0.48, 1.35) | |
| Month of conception | 113 | 141700 | 0.95 (0.77, 1.18) | 0.91 (0.59, 1.38) | |
| Average of three months | 112 | 142141 | 0.94 (0.69, 1.29) | 0.85 (0.35, 2.04) | |
| Hypospadiasb,d | 2 months before | 50 | 72296 | 1.22 (0.93, 1.59) | 1.48 (0.87, 2.54) |
| 1 month before | 50 | 72194 | 1.41 (1.08, 1.85) | 2.05 (1.17, 3.59) | |
| Month of conception | 50 | 71985 | 1.39 (0.96, 2.02) | 1.96 (0.92, 4.17) | |
| Average of three months | 50 | 72225 | 1.53 (1.10, 2.14) | 3.35 (1.31, 8.61) | |
| Cleft Lip/Palate | 2 months before | 117 | 142344 | 1.10 (0.92, 1.31) | 1.20 (0.84, 1.73) |
| 1 month before | 115 | 142069 | 0.97 (0.81, 1.17) | 0.94 (0.64, 1.38) | |
| Month of conception | 115 | 141702 | 1.02 (0.78, 1.32) | 1.04 (0.61, 1.76) | |
| Average of three months | 115 | 142144 | 1.05 (0.88, 1.26) | 1.15 (0.69, 1.91) | |
| Cyanotic Congenital heart disease | 2 months before | 43 | 142270 | 0.79 (0.60, 1.04) | 0.62 (0.35, 1.09) |
| 1 month before | 42 | 141996 | 1.28 (0.90, 1.83) | 1.66 (0.80, 3.45) | |
| Month of conception | 42 | 141629 | 0.76 (0.45, 1.30) | 0.58 (0.20, 1.71) | |
| Average of three months | 42 | 142071 | 0.90 (0.57, 1.42) | 0.75 (0.21, 2.70) | |
| Abdominal defectsc | 2 months before | 91 | 142318 | 1.11 (0.82, 1.49) | 1.23 (0.68, 2.23) |
| 1 month before | 89 | 142043 | 1.51 (1.17, 1.96) | 2.33 (1.37, 3.97) | |
| Month of conception | 88 | 141675 | 0.98 (0.72, 1.34) | 0.97 (0.52, 1.79) | |
| Average of three months | 89 | 142118 | 1.28 (0.99, 1.65) | 2.00 (0.97, 4.14) | |
| Neural tube defectsd | 2 months before | 76 | 142303 | 0.99 (0.70, 1.41) | 0.98 (0.48, 1.98) |
| 1 month before | 75 | 142029 | 1.01 (0.63, 1.61) | 1.02 (0.39, 2.66) | |
| Month of conception | 76 | 141663 | 0.95 (0.73, 1.24) | 0.90 (0.53, 1.54) | |
| Average of three months | 75 | 142104 | 0.97 (0.64, 1.48) | 0.92 (0.28, 3.04) | |
OR: odds ratio; CI: confidence interval; IQR: Inter Quartile Range.
For cases of individual congenital anomalies, births with a chromosome disorder in addition to the congenital anomaly were not included in this analysis.
Hypospadias analysis was limited to male infants and infant gender covariate was excluded from the adjusted model.
Abdominal defect models did not include pre-pregnancy diabetes due to lack of observations of that covariate in the outcome group.
Neural tube defects and Hypospadias models did not include maternal race due to lack of observations in one race category within the outcome group.
3.2.3. Sensitivity analyses
The primary adjusted models presented in this study utilized the covariate cigarette smoking as a dichotomous yes/no (1, 0) variable. In sensitivity analyses, we modeled average number of cigarettes smoked per day as a categorical covariate rather than dichotomous, which resulted in findings consistent with our initial results shown in Table 4. We also performed sensitivity analyses considering the influence of genetic disorders.
Most prior published studies examining the association of PM2.5 with congenital anomalies excluded cases of chromosome disorders. Considering the possibility that chromosome disorders could be on the causal pathway of PM2.5 exposure and congenital anomalies, we performed sensitivity analysis including and excluding live births with chromosome disorders as recorded on the US birth certificate for the study population. The primary results excluding chromosome disorders are displayed in Table 4, as described previously. For sensitivity analyses, first we examined the association of births complicated by chromosome disorders (Down syndrome or other, karyotype confirmed or pending) with PM2.5 exposure. This initial sensitivity analysis included only cases with chromosome disorders, in the absence of other congenital anomalies. As shown in Tables 5–7 (online) we found no significant risk increase of chromosome disorders with PM2.5 exposure. Then, we analyzed the association between PM2.5 and CA including cases with chromosome disorders. We found that after including the chromosome disorder subjects in the analysis, no significant risk increases were observed at any periconception periods (data not shown). However, the trends persisted, with higher point estimate and 95% CI lower bounds at one month prior to conception than other periconception time periods. Given the lack significant association between PM2.5 and chromosome disorders demonstrated in these analyses, we prefer to model the analyses excluding births complicated by chromosome disorders (results as demonstrated in Table 4).
4. Discussion
We found that exposure to increasing levels of PM2.5 in the air during some critical periods during the periconception period may be associated with a modest increased risk of a major congenital anomaly, even after adjustment for confounding influences of other factors associated with malformation risk. The association with PM2.5 exposure during the other time periods – either earlier or later – was non-significant, suggesting the times nearest to conception may be the most susceptible time of exposure for this risk. Our analysis adds depth and clarity to the current body of evidence investigating the possible association between air pollutants and risk of birth defects. We provide novel data by exploring a detailed month-by-month exposure risk assessment including the months prior to conception to assess if there is a particularly susceptible time in the periconception period when exposure to airborne PM may pose a hazard to development of fetal anomalies. The time of embryonic development, weeks 3 to10 of gestational age or weeks 1 to 8 of embryonic age, are thought to be the critical times of exposure for most teratogenic agents to risk of birth defects. This window would indeed be the most critical time if the exposure were known to have a deleterious embryonic effect with only acute high-level exposure. However, the association between air pollutants and adverse health outcomes may not be a clear immediate temporal exposure-outcome relationship. Buildup or accumulation of high concentrations of some pollutants or their metabolites over a longer period of time may pose a more notable risk for congenital anomalies if the high-level exposure occurs during the preconception period.(29) Long-term high PM2.5 exposure specifically has been shown to cause oxidative stress, inflammation, mitochondrial alteration (30) and have higher risk of deleterious health effects.(31) Therefore, exposure to increased amounts of air pollutants may also affect birth defect risk in the time period preceding pregnancy, rather than only during the first trimester, weeks 1–12 of gestation. Prior studies examining the association between PM2.5 and birth defects have only measured this relationship during a brief period of exposure in the first trimester only, and did not measure PM2.5 exposure in the months preceding conception.(3, 6, 7, 10-12, 15) In the present study we aimed to assess the specific time of exposure in the periconception period when increased pollutant exposure may be the most deleterious, by examining exposure timing of exposure in a month-by-month fashion extending from two months prior to two months after conception.
In this study, we report the association of PM2.5 exposure with any anomaly, as recorded in the US birth certificate, and also with individual malformations and malformations grouped by organ systems involved. We found a modest, but positive association with increasing levels of PM2.5 exposure one month prior to and one month after conception with risk of any congenital anomaly, when assessed as a composite variable. Additionally, we found some risk increases among individual anomalies limited to cases of hypospadias and abdominal defects, which had not been previously reported as individual outcomes associated with PM2.5 exposure.(3, 6, 7, 10-12, 15) A recent review on ambient air pollution and risk of congenital anomalies highlighted the narrow focus of the number of birth defects included in prior studies. The authors suggested future studies should focus on anomalies other than just cardiac and facial clefts, have clear definitions of case classification and use of classifications and exclusions in sensitivity analyses.(16) Few studies included the spectrum of all reported anomalies, (11, 17, 18) and most looked only at cardiac anomalies.(5, 6, 10, 19)
Some studies have also suggested that air pollutant exposure may be associated with increased risk of common fetal chromosome abnormalities, such as Trisomy 21, Down syndrome.(32) Inclusion and exclusion of chromosomal, syndromic and multiple anomalies have differed between studies,(16) contributing to significant study heterogeneity thus limiting the generalizability of the findings. To assess whether chromosome abnormalities may be in the causal pathway of PM2.5 exposure to birth defect risk, we measured the association with anomalies both including and excluding cases of chromosome disorders. We further analyzed the association between PM2.5 and chromosome disorders, with congenital anomaly cases excluded. We found no association between PM2.5 exposure and risk of fetal chromosome abnormalities, regardless of the periconception timing of exposure. Additionally, the risk of anomalies was not detectable with the inclusion of cases with concomitant chromosome disorders. Based on these findings, we feel that the preferable approach to assess particulate matter - birth defect risk is to limit the analysis to congenital anomalies without concurrent chromosome disorders.
Although the use of stationary monitors to assign individual-level exposures within a specified radius surrounding the monitor has obvious limitations, it has been widely used for measurement in prior air particulate-birth outcome assessment studies. Using this approach, measurement error due to spatial variability may lead to erroneous negative results, often biasing the risk estimates toward the null.(33) Studies including a large perimeter around a PM monitor for exposure quantification and also reporting a null association with birth defects have been a common theme in previously published studies on this topic. However, limiting analyses to narrow distance cutoffs decreases sample size. Given the low frequency of congenital anomalies in the population (3–8%),(1) investigators must balance the tradeoff between sample size and accuracy of exposure assessment when choosing the best cutoff for their studies. Of the prior published studies on PM and birth defects, reported distances from maternal residence to stationary monitors have varied greatly, with some not reporting the distance,(4, 9, 34) to others reporting distances of 10 km(18), 16 km(8), 40 km(6) or even a maximum distance as far as 50–80 km from a monitor.(10, 19) The large population-based cohort included in our study allowed us to model several relatively narrow distance cutoffs and compare their findings among air exposure-outcome assessment for birth defects. We found that use of various distance cutoffs including the commonly used 10 km distance, compared with more narrow areas with 7 and 5 km cutoffs provided some advantages and disadvantages. The consistency and inconsistency of results identified in the three exposure measurement distance cutoffs presented here are likely related to spatial heterogeneity within the larger distances and more precise exposure quantification but smaller sample size and power when using a smaller distances cutoff. An additional contributor to variation and inconsistency of results is that the location of exposure identified by maternal residential address does not account for women who moved during the pregnancy. Likewise, it also does not account for exposure at non-residential addresses, such as work or school, which may be outside of the 10 km radius of the recorded home address.
An additional challenge in the interpretation of results from prior studies is due to variability in the methods used to define high levels of PM exposure. A variety of exposure quantification strategies have been utilized modeling PM levels in the air as a continuous variable, or defining high PM exposure in a dichotomous approach considering the upper quantile or greater than mean plus interquartile range as “high”. Still, others quantified high exposure as per unit increases or per quantile increases associated with risk of congenital anomalies. These variations in measurement of exposure, analytic strategy and reporting of results make it quite challenging to interpret the results into a way that has practical generalizability.(16) Dichotomizing exposure values may be problematic and may introduce bias into the estimates of odds ratios.(13) Therefore, in this study, we provide data from an analytic approach using a continuous model of exposure assessment in an attempt to provide the most informative and non-biased results.
As we have highlighted here, there are a number of inherent limitations to this type of study aimed to measure the association between airborne particulate matter exposure and birth defect risk. These limitations are shared among prior published observational studies of similar design. However, by employing multiple methodologic approaches, we feel we provide an important breadth of data on optimal exposure ascertainment, measurement, and design characteristics that contribute to the reliability of the associations we have identified regarding PM2.5 exposure and risk of congenital malformations. The overall rate of anomalies reported in this study is lower than known population prevalence. Birth certificate records have a lower sensitivity for identifying birth defects compared to review of medical records, as not all birth defects are readily identifiable within the first few days of birth when the birth certificate is generated. Therefore some congenital anomalies that are not yet identified may be coded as no anomaly in the birth certificate, biasing the results of our study and those of many similar in design toward the null. Alternatively, some birth defects recorded on the birth certificate may not be accurately documented, which could lead to some misclassification of case or referent group status. Additionally, some defects are recorded within a category by organ system and do not provide data on the specific malformation which limits the ability to assess individual malformation exposure risks, such as in the category of congenital heart defects.
The mechanism of teratogenicity for airborne pollutant exposures are generally speculative.(16) Some hypothesized mechanisms include oxidative stress, coagulation aberrations and placental inflammation.(35) These mechanisms could affect embryogenesis through influencing migration and differentiation of neural crest cells. Additionally, some pollutants have demonstrated embryotoxicity in animal models. (16)
Future investigations should build upon the knowledge gained from this study and utilize approaches aimed to optimize scientific rigor, minimize bias, and report the most accurate assessment of risk for air pollutants and development of congenital anomalies. Specific areas of focus should include individual level exposure methods and improved knowledge on the mechanism of action of air pollutants with regards to teratogenic effects on the developing embryo and fetus. Public health efforts should continue to highlight the importance of minimizing population-level exposure to harmful particulate matter in the air. Although the increased risk of birth defects observed in our study with PM2.5 exposure in the month prior to conception is modest, the potential impact on a population basis is noteworthy as all reproductive age women have some degree of exposure.
Acknowledgments
We would like to thank Emily L. Kang, PhD, Department of Mathematical Sciences, University of Cincinnati, Cincinnati, Ohio, for her suggestions regarding the statistical analysis plan.
Funding sources: ED and LM are supported by the Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio and the March of Dimes Prematurity Research Center Ohio Collaborative; and SR is supported in part by NIH R01-HL 111829. E.H. is supported by R01ES016531, R21ES02116 and P30-ES06096 and NHH/NCRR8ULITR000077. AC is supported by NIH P30ES006096, RC4ES019755 and R01ES020349. The funding sources had no role in the study design, data collection, analysis or interpretation of data, nor decision to submit the manuscript for publication.
Submission Declaration: This work has not been published previously and is not under consideration for publication elsewhere. All authors have approved the submitted version of this manuscript. If accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
IRB approval: This study was approved by the Ohio Department of Health and Human Subjects Institutional Review Board (#2012–21). This study was exempt from review by the Institutional Review Board at the University of Cincinnati, Cincinnati, Ohio, USA.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Data source disclosure: This study includes data provided by the Ohio Department of Health which should not be considered an endorsement of this study or its conclusions.
Presentation information: This study was presented as an abstract at the 82nd Annual Meeting of the Central Association of Obstetricians and Gynecologists, Oct. 21–24, 2015, Charleston, South Carolina.
Reprints will not be available
References
- 1.Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117(1):168–83. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- 2.Christianson AH, C P, Modell B. March of Dimes: Global Report on Birth Defects, the Hidden Toll of Dying and Disabled Children. New York, NY, USA: March of Dimes Birth Defects Foundation; 2006. [Google Scholar]
- 3.Agay-Shay K, Friger M, Linn S, Peled A, Amitai Y, Peretz C. Air pollution and congenital heart defects. Environmental research. 2013;124:28–34. doi: 10.1016/j.envres.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Farhi A, Boyko V, Almagor J, Benenson I, Segre E, Rudich Y, et al. The possible association between exposure to air pollution and the risk for congenital malformations. Environmental research. 2014;135:173–80. doi: 10.1016/j.envres.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Hwang BF, Jaakkola JJ. Ozone and other air pollutants and the risk of oral clefts. Environmental health perspectives. 2008;116(10):1411–5. doi: 10.1289/ehp.11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall EG, Harris G, Wartenberg D. Oral cleft defects and maternal exposure to ambient air pollutants in New Jersey. Birth defects research Part A, Clinical and molecular teratology. 2010;88(4):205–15. doi: 10.1002/bdra.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padula AM, Tager IB, Carmichael SL, Hammond SK, Yang W, Lurmann F, et al. Ambient air pollution and traffic exposures and congenital heart defects in the San Joaquin Valley of California. Paediatric and perinatal epidemiology. 2013;27(4):329–39. doi: 10.1111/ppe.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA. Ambient air pollution and risk of birth defects in Southern California. American journal of epidemiology. 2002;155(1):17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- 9.Strickland MJ, Klein M, Correa A, Reller MD, Mahle WT, Riehle-Colarusso TJ, et al. Ambient air pollution and cardiovascular malformations in Atlanta, Georgia, 1986–2003. American journal of epidemiology. 2009;169(8):1004–14. doi: 10.1093/aje/kwp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stingone JA, Luben TJ, Daniels JL, Fuentes M, Richardson DB, Aylsworth AS, et al. Maternal exposure to criteria air pollutants and congenital heart defects in offspring: results from the national birth defects prevention study. Environmental health perspectives. 2014;122(8):863–72. doi: 10.1289/ehp.1307289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinikoor-Imler LC, Davis JA, Meyer RE, Luben TJ. Early prenatal exposure to air pollution and its associations with birth defects in a state-wide birth cohort from North Carolina. Birth defects research Part A, Clinical and molecular teratology. 2013;97(10):696–701. doi: 10.1002/bdra.23159. [DOI] [PubMed] [Google Scholar]
- 12.Schembari A, Nieuwenhuijsen MJ, Salvador J, de Nazelle A, Cirach M, Dadvand P, et al. Traffic-related air pollution and congenital anomalies in Barcelona. Environmental health perspectives. 2014;122(3):317–23. doi: 10.1289/ehp.1306802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–41. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 14.Mortimer K, Neugebauer R, Lurmann F, Alcorn S, Balmes J, Tager I. Early-lifetime exposure to air pollution and allergic sensitization in children with asthma. Journal of asthma. 2008;45(10):874–81. doi: 10.1080/02770900802195722. [DOI] [PubMed] [Google Scholar]
- 15.Tanner JP, Salemi JL, Stuart AL, Yu H, Jordan MM, DuClos C, et al. Associations between exposure to ambient benzene and PM during pregnancy and the risk of selected birth defects in offspring. Environmental research. 2015;142:345–53. doi: 10.1016/j.envres.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Vrijheid M, Martinez D, Manzanares S, Dadvand P, Schembari A, Rankin J, et al. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environmental health perspectives. 2011;119(5):598–606. doi: 10.1289/ehp.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolk H, Armstrong B, Lachowycz K, Vrijheid M, Rankin J, Abramsky L, et al. Ambient air pollution and risk of congenital anomalies in England, 1991–1999. Occupational and environmental medicine. 2010;67(4):223–7. doi: 10.1136/oem.2009.045997. [DOI] [PubMed] [Google Scholar]
- 18.Rankin J, Chadwick T, Natarajan M, Howel D, Pearce MS, Pless-Mulloli T. Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environmental research. 2009;109(2):181–7. doi: 10.1016/j.envres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Dadvand P, Rankin J, Rushton S, Pless-Mulloli T. Ambient air pollution and congenital heart disease: a register-based study. Environmental research. 2011;111(3):435–41. doi: 10.1016/j.envres.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Agency USEP. Air Data: Air Quality Data Collected at Outdoor Monitors Across the US 2015 [26 August 2015] Available from: http://www.epa.gov/airdata/
- 21.Hall ES, Connolly N, Jones DE, DeFranco EA. Integrating public data sets for analysis of maternal airborne environmental exposures and stillbirth. AMIA Annu Symp Proc. 2014;2014:599–605. [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services CfDCaP, editor. Statistics NCfH. National Center for Health Statistics. Guide to completing the facility worksheets for the certificate of live birth and report of fetal death (2003 revision) Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; US Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. Retrieved August 2015 from http://www.cdc.gov/nchs/data/dvs/GuidetoCompleteFacilityWks.pdf. 2012. [Google Scholar]
- 23.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 24.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 25.Bender R, Lange S. Adjusting for multiple testing–when and how? Journal of clinical epidemiology. 2001;54(4):343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 26.Garne E, Loane M, Dolk H, Barisic I, Addor MC, Arriola L, et al. Spectrum of congenital anomalies in pregnancies with pregestational diabetes. Birth defects research Part A, Clinical and molecular teratology. 2012;94(3):134–40. doi: 10.1002/bdra.22886. [DOI] [PubMed] [Google Scholar]
- 27.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 28.National Ambient Air Quality Standards for Particulate Matter. Final Rule. 2013;78(10) (to be codified at 40 CFR Parts 50, 51, 52, 53, and 58) [Google Scholar]
- 29.Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic & clinical pharmacology & toxicology. 2008;102(2):182–90. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicological sciences : an official journal of the Society of Toxicology. 2011;124(1):88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilker EH, Ljungman PL, Rice MB, Kloog I, Schwartz J, Gold DR, et al. Relation of long-term exposure to air pollution to brachial artery flow-mediated dilation and reactive hyperemia. The American journal of cardiology. 2014;113(12):2057–63. doi: 10.1016/j.amjcard.2014.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung MK, Lao TT, Ting YH, Wong TW, Leung TY. Seasonality of fetal trisomy 21 - have ambient air pollutants played a role? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014:1–6. doi: 10.3109/14767058.2014.924104. [DOI] [PubMed] [Google Scholar]
- 33.Goldman GT, Mulholland JA, Russell AG, Strickland MJ, Klein M, Waller LA, et al. Impact of exposure measurement error in air pollution epidemiology: effect of error type in time-series studies. Environ Health. 2011;10:61. doi: 10.1186/1476-069X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianicolo EA, Mangia C, Cervino M, Bruni A, Andreassi MG, Latini G. Congenital anomalies among live births in a high environmental risk area–a case-control study in Brindisi (southern Italy) Environmental research. 2014;128:9–14. doi: 10.1016/j.envres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environmental health perspectives. 2006;114(11):1636–42. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]


