Abstract
Background
Based on reports from tertiary care centers, chronic pancreatitis (CP) is considered to be a painful and debilitating disease frequently requiring invasive interventions. Our primary aim was to assess the natural course of CP in a population-based cohort using endoscopic and surgical interventions as surrogates for disease aggressiveness.
Methods
We identified all patients (n=89, alcoholic [ACP=46], non-alcoholic [NACP]=43) with newly diagnosed definite CP from Olmsted County, Minnesota between 1977 and 2006. Patients were followed until death or censoring. Medical records were reviewed at time of diagnosis and during each follow-up. Both lifetime proportions and cumulative incidence since the initial manifestation of CP were estimated and compared. Survival was estimated with Kaplan-Meier methodology.
Results
Median age at CP diagnosis was 56 years (IQR, 48–67) and 56% were male. During median follow-up of 10 years, 68 (76%) experienced pancreatic pain, but only 27 (30%) needed any invasive therapeutic intervention: 23% had endotherapy and 11% had pancreatic surgery. During the clinical course, when compared with NACP, ACP patients had significantly more (all p<0.05) pain (87 vs. 65%), recurrent acute pancreatitis (44 vs. 23%), pseudocysts (41 vs. 16%), cumulative incidence of exocrine insufficiency (60 vs. 21%), and annual hospitalizations after CP diagnosis (0.79 vs. 0.25). The cumulative risk of diabetes, calcifications, surgery and overall survival was similar in ACP and NACP.
Conclusions
Our study suggests that CP at a population level may have a milder course than that reported from tertiary centers. We confirm that ACP has a more severe phenotype than NACP.
Keywords: chronic pancreatitis, natural history, alcoholic, nonalcoholic, interventions
Introduction
Chronic pancreatitis (CP) is a pathologic fibroinflammatory process of the pancreas that can result in pain syndrome, organ dysfunction, and impaired quality of life.1 Pain, the most common and disabling feature of CP, can be highly variable among affected individuals - some experience it only intermittently with long pain-free intervals (type A), while others have constant pain with or without clusters of severe exacerbation (type B).2 In the majority of patients, especially those with type A pain, a step-up approach that starts with medical therapy is used for pain management.3 Type B pain is often the manifestation of local complications such as a pseudocyst or ductal obstruction, which may need endoscopic or surgical interventions.2 Therefore, the aggressiveness of the disease can indirectly be measured by the need of interventions.
In several natural history studies, 40–60% of CP patients are reported to have at least one endoscopic or surgical intervention during their disease course.2, 4–8 While surgery used to be the preferred intervention, in the past 2 decades there has been a shift towards using endoscopic therapy as the initial approach in appropriate candidates, reserving surgery for patients in whom endoscopic therapy is not feasible or unsuccessful.9, 10
In the US, a large prospective study from the Mayo Clinic of CP patients diagnosed between 1976 and 1982 reported that ~40% underwent at least one surgical intervention.5 In a more recent large US multicenter cross-sectional study, 61% of CP patients underwent at least one endoscopic treatment, and 31% required at least one surgical procedure.6 These studies primarily included patients referred to expert centers for evaluation and management. Thus, it is unclear if the high rate of interventions reported is reflective of the disease at a population level. In many other gastrointestinal diseases, such as acute pancreatitis, inflammatory bowel disease, and liver cirrhosis11–13, patients seen at expert centers have more severe disease when compared with community centers. This could potentially explain why a recent population study from Florida found low rates of endoscopic (11%) and surgical interventions (5%) in CP.14 However, this study was limited by the use of only inpatient administrative data and inability to capture outpatient procedures. Therefore, additional population data is needed in CP patients to confirm these findings.
We hypothesized that the natural history of CP at a population level differs from that seen at expert centers. We previously reported the incidence, prevalence, and survival of CP in Olmsted County, Minnesota from 1977 to 2006.15 This population is ideal for natural history studies, as nearly all the medical care in the county is provided at the Mayo Clinic, Olmsted Medical Center, or their affiliated hospitals; and all medical records including outpatient encounters, emergency room visits, hospitalizations, surgical procedures, autopsy examinations, and death certificates, are accessible through the Rochester Epidemiology Project. Thus, we used these data to evaluate the natural history of CP in Olmsted County, MN. Our primary aim was to assess the use of endoscopic and surgical interventions during the course of CP as a measure of aggressiveness of the disease. Our secondary aim was to evaluate the differences between alcoholic (ACP) and nonalcoholic CP (NACP) to validate results of previous studies.
Methods
Study setting and ascertainment of cases
Olmsted County is situated in southeastern Minnesota, about 128 km away from the nearest major metropolitan city (the twin cities of Minneapolis), and had a population of 144,260 inhabitants as of the 2010 US Census. The majority of the population resides in Rochester, the main urban center of this rural county. Up to 90% of county residents are white, which makes the population socioeconomically comparable with the US white population.
The details of the methodology and ascertainment of cases have been previously published.15 The Mayo Diagnostic index was used to identify all Olmsted County residents who received a diagnosis of CP from 1977 to 2006. The diagnosis of CP was considered as definite if the Mayo score was 4 or more. During the study period, 331 Olmsted County residents received a diagnosis of CP, and 100 of these fulfilled definite CP Mayo criteria. Seventeen CP cases diagnosed during an autopsy were excluded. Additional 6 clinical cases of definite CP were identified after randomly reviewing the medical records of 171 patients with acute pancreatitis (AP) and 210 with pancreatitis-not otherwise specified. Thus, 89 patients formed the final cohort for this study. The study was approved by the Institutional Review Board of the Mayo Clinic Foundation and University of Pittsburgh.
Data collection
Medical records of the 89 cases with definite CP were comprehensively reviewed by a trained data abstractor (LT) under the direction of two study investigators (DY, STC). Demographic characteristics at time of diagnosis including age, gender, and smoking status were obtained. History of heavy smoking was defined as the use of more than 1 pack of cigarettes daily. Etiology of CP was based on the treating physician’s assessment. Subjects with idiopathic CP were subdivided as early onset (≤ 35 years of age) and late onset (>35 years), as previously proposed by Layer et al.5 For comparisons, etiologies were categorized into alcoholic (ACP) or nonalcoholic (NACP).
At the time of diagnosis and during the follow-up period, presence of pain, exocrine pancreatic insufficiency, diabetes, and acute pancreatitis, were recorded. The presence at diagnosis or during the follow-up period of pancreatic calcifications, pancreatic ductal dilation, pseudocyst or fluid collections, pancreatic atrophy, and common bile duct dilation, were extracted from results of imaging studies. We defined the age at onset of CP symptoms as the earliest age at which the patient had pain, episode of AP, exocrine insufficiency or diagnosis of CP. During the entire observation period, use of endoscopic and surgical interventions for management of CP was extracted. The indication of the intervention was categorized as pain, jaundice, pseudocyst, or other. Records of hospitalizations after diagnosis of CP were reviewed, and whether they were related or not to pancreatitis was recorded. Development of pancreatic cancer during the follow-up was also noted.
Statistics
Data on clinical profile at CP diagnosis and during the clinical course including demographics, risk factors, etiology, symptoms, morphologic changes, interventions, and hospitalizations are reported. Data was initially analyzed for the whole cohort, and then stratified by etiology as ACP and NACP. Descriptive analyses are presented as proportions for categorical data, and median with interquartile range (IQR) for continuous data. Bivariate comparisons based on CP etiology were performed using Chi-square or Fischer’s exact test for categorical data and Student’s t -test or Mann-Whitney U test for continuous data.
To account for variable duration of follow-up across ACP and NACP patients, we calculated the cumulative incidence of diabetes, exocrine insufficiency, calcifications, and surgical interventions, over lifetime and since the first clinical manifestation of CP. Log-rank test was used to assess whether the etiologic groups had significantly different cumulative incidence rates of these disease specific characteristics.16 The annual rates of any hospitalization or pancreatitis-related hospitalizations since the diagnosis of CP were estimated. Poisson regression models were used to test the difference in hospitalization rates between ACP and NACP.
Kaplan-Meier methodology was used to estimate the overall survival of patients with ACP and NACP over their lifetime. The log-rank test was used to test the difference in overall survival between ACP and NACP patients. Patients were followed up until date of death or censoring. Censoring was either due to loss of follow-up, emigration, or end of follow-up period. Statistical significance was defined as p<0.05. Data analysis was performed with SAS system version 9.2 (SAS Software Institute, Cary, NC).
Results
Demographics and etiology
The final sample size for the current study was 89 patients. At the time of CP diagnosis, median age was 56 years (IQR, 48–67), 56% were male, 52% were current smokers, and 60% had history of heavy smoking. Etiology of CP was alcohol-related (52%), idiopathic (40%) or others (8%). Those with idiopathic etiology were predominantly of late-onset (92%). Patients were followed for a median of 10 years (range, 0.1–29.4 years).
When compared with NACP, ACP patients were diagnosed at a younger age (52 vs. 64, p<0.05). Current (72 vs. 28%, p<0.05) and heavy smoking (74 vs. 40%, p<0.05) was significantly more common in patients with ACP. There was a non-significant trend towards male gender predominance in the ACP group (61% vs. 51%, p=0.3) (Table 1). Patients with NACP had longer median follow-up (15 years) than those with ACP (9 years) (log-rank, p=0.01).
Table 1.
Demographics for ACP and NACP in Olmsted County from 1977–2006.
| Variable | All (n=89) |
ACP (n=46) |
NACP (n=43) |
P-value |
|---|---|---|---|---|
|
| ||||
| Age at diagnosis, median (IQR) | 56 (48,67) | 52 (40,57) | 64 (54,73) | <0.05 |
|
| ||||
| Male, n (%) | 50 (56) | 28 (61) | 22 (51) | 0.34 |
|
| ||||
| Smoking, n (%) | ||||
| Never | 22 (24) | 7 (15) | 14 (33) | <0.05 |
| Past | 22 (24) | 6 (13) | 17 (39) | |
| Current | 45 (52) | 33 (72) | 12 (28) | |
|
| ||||
| History of heavy smoking, n (%) | 51 (60) | 34 (74) | 17 (40) | <0.05 |
Clinical and morphologic features
Pain at onset or during the observation period was present in 68 (76%) patients, diabetes was noted in 36 (40%), exocrine insufficiency was noted in 27 (30%), and 49 (55%) had at least one episode of AP. At the time of CP diagnosis, 20 (22%) patients already had diabetes, 12 (13%) had exocrine insufficiency, and 37 (42%) had experienced at least one attack of AP. From CP diagnosis until the end of observation, 16 (18%) patients developed new-onset diabetes, 15 (17%) developed exocrine insufficiency, and 12 (13%) had a first-attack of AP (13%). Of 49 CP patients who had AP, 61% had recurrent AP attacks.
In the majority of patients, pancreatic calcifications (69%) and pancreatic duct dilation (57%) were noted during the disease course. Other abnormal imaging findings included pancreatic atrophy (43%), pseudocyst or fluid collections (29%), and common bile duct dilation (26%).
During the disease course, when compared with NACP, patients with ACP were more likely to have pain (87 vs. 65%, p<0.05), recurrent AP (44 vs. 23%, p<0.05), exocrine insufficiency (41 vs. 19%, p<0.05), and pseudocysts or fluid collections (41 vs. 16%, p<0.05) (Table 2). The cumulative incidence of exocrine insufficiency from birth and the onset of CP symptoms was significantly higher for ACP than in NACP patients (P<0.01; Figure 1 a–b). The cumulative incidence of diabetes and calcifications was similar for ACP and NACP patients (Figure 1 c–f).
Table 2.
Clinical profile and morphologic findings of patients with ACP and NACP.
| Variable | All (n=89) |
ACP (n=46) |
NACP (n=43) |
P-value |
|---|---|---|---|---|
|
| ||||
| Pain, n (%) | 68 (76) | 40 (87) | 28 (65) | <0.05 |
|
| ||||
| Number of AP attacks, n (%) | ||||
| Single attack | 19 (21) | 10 (21) | 9 (21) | 1.00 |
| Recurrent AP | 30 (34) | 20 (44) | 10 (23) | <0.05 |
|
| ||||
| Timing of first AP, n (%) | ||||
| Before/At diagnosis | 37 (42) | 24 (52) | 13 (30) | <0.05 |
| After diagnosis | 12 (13) | 6 (13) | 6 (14) | 0.89 |
|
| ||||
| Diabetes, n (%) | 36 (40) | 17 (37) | 19 (44) | 0.50 |
|
| ||||
| Timing of diabetes, n (%) | ||||
| Before/At diagnosis | 20 (22) | 7 (15) | 13 (30) | 0.09 |
| After diagnosis | 16 (18) | 10 (22) | 6 (14) | 0.33 |
|
| ||||
| Exocrine insufficiency, n (%) | 27 (30) | 19 (41) | 8 (19) | <0.05 |
|
| ||||
| Timing of exocrine insufficiency, n (%) | ||||
| Before/At diagnosis | 12 (13) | 7 (15) | 5 (12) | 0.68 |
| After diagnosis | 15 (17) | 12 (26) | 3 (7) | <0.05 |
|
| ||||
| Calcifications, n (%) | 62 (69) | 30 (65) | 32 (74) | 0.35 |
|
| ||||
| Pseudocysts/Fluid collections, n (%) | 26 (29) | 19 (41) | 7 (16) | <0.05 |
|
| ||||
| Pancreatic duct dilation, n (%) | 51 (57) | 28 (61) | 23 (54) | 0.50 |
|
| ||||
| Pancreas atrophy, n (%) | 38(43) | 21 (46) | 17 (40) | 0.57 |
|
| ||||
| Common bile duct dilation, n (%) | 23 (26) | 13 (30) | 10 (24) | 0.52 |
Figure 1.

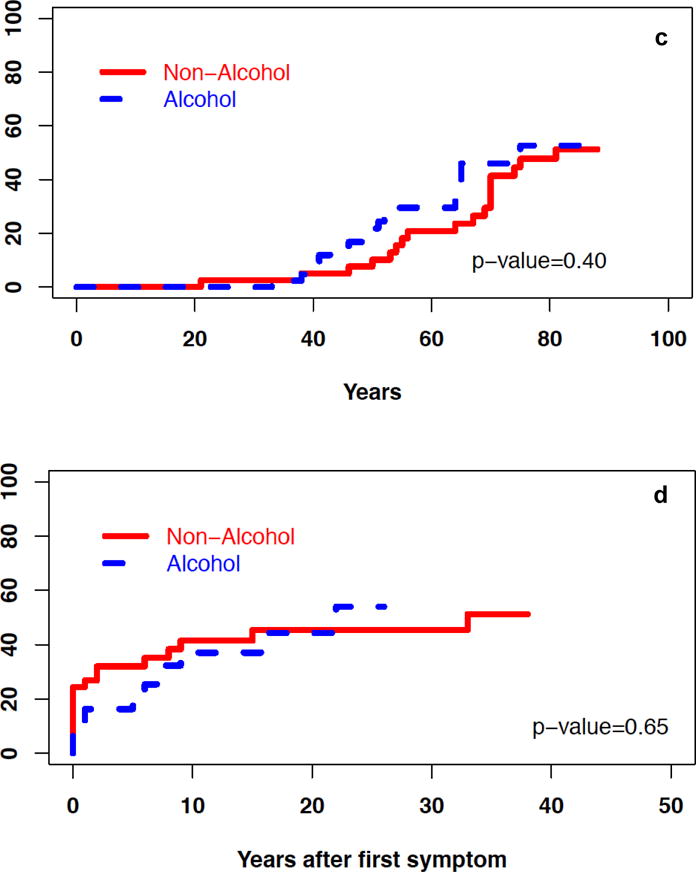
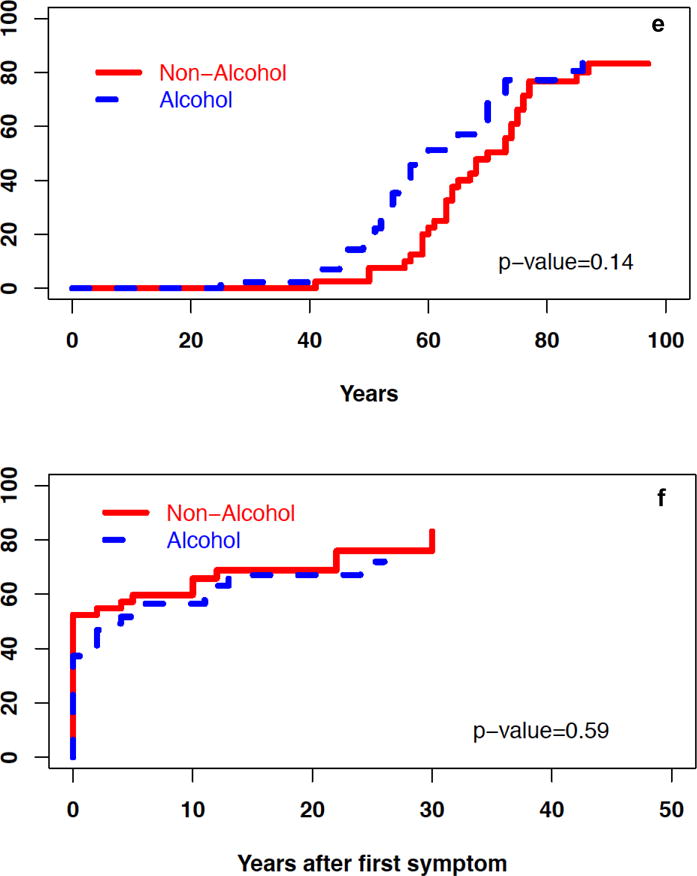

Cumulative incidence of select characteristics in patients with ACP and NACP – a) exocrine insufficiency from birth, b) exocrine insufficiency after onset of symptoms, c) diabetes from birth, d) diabetes after onset of symptoms, e) calcifications from birth, f) calcifications after onset of symptoms, g) surgical intervention after onset of symptoms.
Interventions and hospitalizations
Endoscopic or surgical interventions for CP were performed in 27 (30%) patients during the disease course. A total of 20 (23%) patients underwent at least one endoscopic intervention, 10 (11%) at least one surgical intervention, and 3 (3%) both types of interventions (Table 3). A surgical drainage was performed in 6 patients and a surgical resection in 4 patients. The indications for endoscopic and surgical interventions, and the type of surgical procedure performed, are summarized in Tables 3 and 4. The annual rate for any hospitalization after CP diagnosis was 0.78 (se=0.03), whereas for pancreatitis-related hospitalizations was 0.25 (se=0.02).
Table 3.
Interventions and indications for interventions in ACP and NACP patients.
| Variable | All (n=89) |
ACP (n=46) |
NACP (n=43) |
P-value |
|---|---|---|---|---|
|
| ||||
| Interventions, n (%) | ||||
| Endoscopy or surgery | 27 (30) | 15 (33) | 12 (28) | 0.61 |
| Endoscopy | 20 (23) | 12 (26) | 8 (19) | 0.43 |
| Surgery | 10 (11) | 5 (11) | 5 (12) | 0.88 |
|
| ||||
| Indications for endoscopic therapy (n=20) | ||||
| Pain | 8 (40) | 4 (33) | 4 (50) | |
| Jaundice | 4 (20) | 3 (25) | 1 (12) | |
| Pseudocyst | 3 (15) | 3 (25) | 0 (0) | |
| Other | 5 (25) | 2 (17) | 3 (38) | |
|
| ||||
| Indications for surgery (n=10) | ||||
| Pain | 4 (40) | 2 (40) | 2 (40) | |
| Jaundice | 5 (50) | 2 (40) | 3 (60) | |
| Pseudocyst | 2 (20) | 2 (40) | 0 (0) | |
Table 4.
Indication and type of surgical intervention on individual CP patients.
| Etiology | Age surgery (years) | Indication | Type of surgery |
|---|---|---|---|
| ACP | 53 | Pain | Resection, Whipple |
| ACP | 55 | Pain, Jaundice | Drainage, choledochoduodenostomy |
| ACP | 86 | Jaundice | Drainage, choledochoduodenostomy |
| ACP | 50 | Pseudocyst | Resection, distal pancreatectomy |
| ACP | 51 | Pseudocyst | Drainage, cystgastrostomy |
| NACP | 54 | Jaundice | Drainage, choledochoduodenostomy |
| NACP | 81 | Jaundice | Drainage, choledochoduodenostomy |
| NACP | 73 | Jaundice | Resection, Whipple |
| NACP | 50 | Pain | Resection, Whipple |
| NACP | 48 | Pain | Drainage, Puestow |
The cumulative incidence of surgical interventions since the onset of CP symptoms was also similar for patients with ACP and NACP (Table 3, Figure 1g). The annual rate of any hospitalization and pancreatitis-related hospitalizations after CP diagnosis was higher in patients with ACP vs. NACP (p<0.05) (Figure 2).
Figure 2.
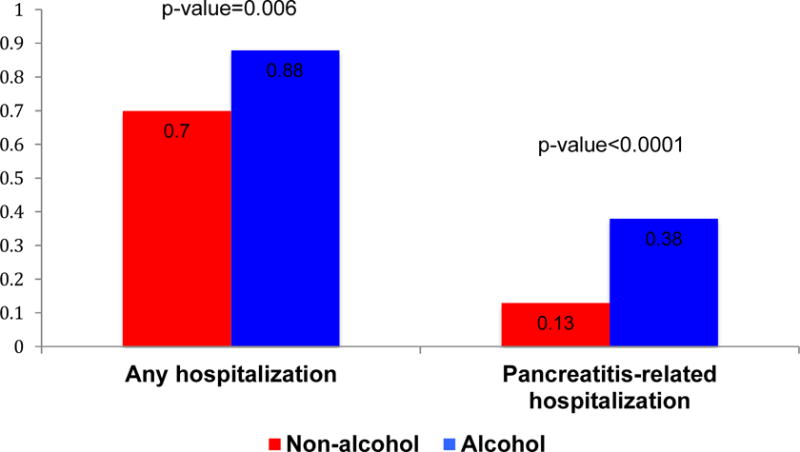
Annual rates of hospitalization after CP diagnosis in patients with ACP and NACP.
Survival
During the study follow-up, 39 of the 89 cases died. Figure 3 shows Kaplan-Meier estimates of overall survival in patients with ACP and NACP. Log-rank test demonstrated that ACP patients had similar overall survival when compared with NACP patients. A total of 3 patients had pancreatic cancer, and all occurred in the setting of ACP.
Figure 3.
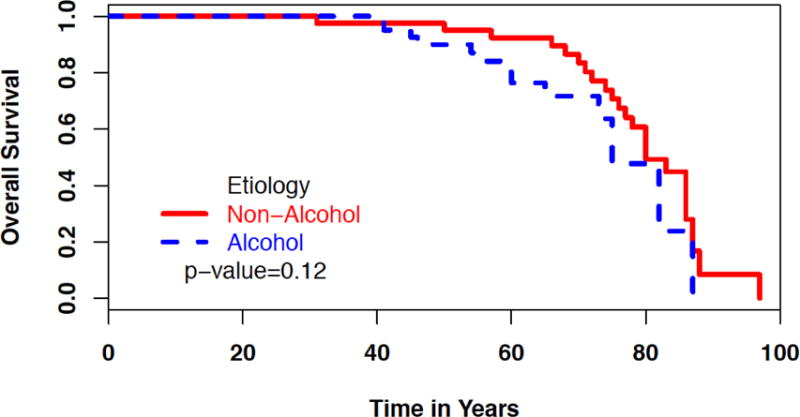
Overall survival of patients with ACP and NACP.
Discussion
In this population-based study, we found that the need for endoscopic and surgical interventions in CP was lower when compared with previous reports at expert centers. These results suggest a less aggressive course of CP at a population level. We also confirmed previously observed differences in the natural history of ACP and NACP -patients with ACP have more pain, exocrine insufficiency, pseudocysts, and hospitalizations, than those with NACP. No difference in survival was found between ACP and NACP patients.
The use of endoscopic and surgical interventions in CP has been described in several natural history studies. In studies from 1960s–1990s, 30–40% of CP patients were reported to undergo surgery.2, 4, 5 In more recent studies, 50–60% have at least one endoscopic intervention, and 30–35% undergo at least one surgical intervention.6, 17, 18 In this population study, we found that the number of CP patients that required an endoscopic (22%) or surgical (11%) intervention was lower than previously reported rates. Our results were similar after stratification by alcohol etiology suggesting that the high prevalence of late-onset idiopathic CP will not explain the observed results. The less frequent need for interventions in our study suggests a milder course of CP at a population level. This is not an isolated phenomenon for CP. In AP, the risk of organ failure, pancreatic necrosis, and mortality is significantly higher at referral centers when compared with community hospitals. For example, in a study from 3 large referral cohorts (University of Pittsburgh, Brigham and Women’s Hospital, Dutch Pancreatitis Study Group), 25% had pancreatic necrosis, 18% persistent organ failure, and 5% died.19 In contrast, in a community-based cohort from Alicante, Spain, 12% had pancreatic necrosis, 4% persistent organ failure, and 3% died.20 Similarly, a study using the National Inpatient Sample demonstrated that patients with inflammatory bowel disease admitted at high-volume centers had a more complicated disease phenotype and had two-fold greater probability of undergoing surgery than those at low-volume centers.12 In liver cirrhosis this phenomenon is even more obvious, as evaluation of patients approaching liver transplantation is always performed in tertiary care centers.13 Overall, our results are indicative that a large proportion of patients with CP do not require an intervention and are managed in the community with referral prompted by the need to management beyond local expertise.
Our results also confirm the results of previous studies that have compared the natural history of ACP and NACP. First, patients with ACP are more symptomatic than patients with NACP. In well-conducted studies from tertiary care centers and in two Japanese population studies, pain was significantly more frequent in ACP than in NACP.4, 5, 21, 22 Second, progression to exocrine insufficiency is delayed in NACP compared with ACP. Studies have previously demonstrated that the median time for developing exocrine insufficiency is shorter for ACP patients than for NACP - 5 to 10 years in ACP, 10 to 20 years in late-onset idiopathic CP, and 20 to 30 years in early-onset idiopathic CP.4, 5, 23, 24 Therefore, ACP patients have higher cumulative incidence of exocrine insufficiency when compared with patients with NACP. Third, ACP patients require more pancreatitis-related hospitalizations than those with NACP. Using administrative data, a population study from Allegheny County, Pennsylvania, demonstrated that CP patients with billing codes of alcoholism had higher risk of pancreatitis-related readmissions (HR=2.1) than those without alcoholism.25 Fourth, survival is similar in ACP and NACP. Two Danish population studies have demonstrated that definite CP carries 4 to 5-fold greater risk of death compared with matched population controls, but the risk was not different between alcoholic and nonalcoholic etiologies.18, 26 Overall these findings suggest that ACP has a more aggressive course than NACP, but without a difference in overall survival.
Strengths of our study include the use of a well-defined population cohort, ascertainment of definite CP by extensive chart review, and long follow-up. This approach overcomes important limitations of prior work in this area, but has several limitations. First, the Olmsted County population is predominantly white of Northern European decent, but is considered to be representative only of the US White population. Therefore, generalizability of our results to other racial groups may be limited. Second, our sample size was small and may have introduced type II error. For example, in contrast to previous studies, we found equal distribution of male gender between ACP and NACP patients. Third, since data was extracted retrospectively from medical records, our findings are limited to the information recorded at the time of the clinical encounter. Finally, the diagnostic methods and definitions of diabetes and exocrine insufficiency have changed over the years. This may partially explain the lower rates of pancreatic insufficiency in our cohort when compared with other natural history studies.4, 27, 28
In conclusion, our study shows that CP at a population level may have a milder course when compared to patients treated at expert centers. Recognition of this is important and should be reassuring to primary care physicians and family practitioners, as many patients with CP diagnosed in the community can be managed successfully in collaboration with a multidisciplinary team, either locally or at a referral center for evaluation and management of unexplained or intractable disease, local complications, or need for interventions. We also confirm that the natural course of ACP is more aggressive than NACP.
Acknowledgments
Research reported in this paper was supported by the Department of Medicine, University of Pittsburgh Medical Center (DY); National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health -U01DK108306 (DY), UO1 DK108288 (STC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Specific author contributions:
Study design: Jorge D. Machicado, Suresh T. Chari, Dhiraj Yadav
Data acquisition: Suresh T. Chari, Lawrence Timmons, Dhiraj Yadav
Statistical analysis: Gong Tang, Dhiraj Yadav
Drafting of the manuscript: Jorge D. Machicado, Dhiraj Yadav
Data interpretation, review of manuscript for important intellectual content, final approval of the manuscript: all authors.
Conflict of interest: None
Guarantor of the article: Dhiraj Yadav, MD MPH
References
- 1.Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D, et al. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016;16:218–224. doi: 10.1016/j.pan.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132–1140. doi: 10.1016/s0016-5085(99)70016-8. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed Ali U, Issa Y, Bruno MJ, van Goor H, van Santvoort H, Busch OR, et al. Early surgery versus optimal current step-up practice for chronic pancreatitis (escape): Design and rationale of a randomized trial. BMC Gastroenterol. 2013;13:49. doi: 10.1186/1471-230X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820–828. [PubMed] [Google Scholar]
- 5.Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–1487. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 6.Glass LM, Whitcomb DC, Yadav D, Romagnuolo J, Kennard E, Slivka AA, et al. Spectrum of use and effectiveness of endoscopic and surgical therapies for chronic pancreatitis in the united states. Pancreas. 2014;43:539–543. doi: 10.1097/MPA.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, et al. Prognosis of chronic pancreatitis: An international multicenter study. International pancreatitis study group. Am J Gastroenterol. 1994;89:1467–1471. [PubMed] [Google Scholar]
- 8.Nojgaard C. Prognosis of acute and chronic pancreatitis - a 30-year follow-up of a danish cohort. Dan Med Bull. 2010;57:B4228. [PubMed] [Google Scholar]
- 9.Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, et al. Endoscopic treatment of chronic pancreatitis: European society of gastrointestinal endoscopy (esge) clinical guideline. Endoscopy. 2012;44:784–800. doi: 10.1055/s-0032-1309840. [DOI] [PubMed] [Google Scholar]
- 10.Anderson MA, Akshintala V, Albers KM, Amann ST, Belfer I, Brand R, et al. Mechanism, assessment and management of pain in chronic pancreatitis: Recommendations of a multidisciplinary study group. Pancreatology. 2016;16:83–94. doi: 10.1016/j.pan.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anand G, Hutfless SM, Akshintala VS, Khashab MA, Lennon AM, Makary MA, et al. A population-based evaluation of severity and mortality among transferred patients with acute pancreatitis. Pancreas. 2014;43:1111–1116. doi: 10.1097/MPA.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN, McGinley EL, Binion DG. Does it matter where you are hospitalized for inflammatory bowel disease? A nationwide analysis of hospital volume. Am J Gastroenterol. 2008;103:2789–2798. doi: 10.1111/j.1572-0241.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- 13.Grattagliano I, Ubaldi E, Bonfrate L, Portincasa P. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273–2282. doi: 10.3748/wjg.v17.i18.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bliss LA, Yang CJ, Eskander MF, de Geus SW, Callery MP, Kent TS, et al. Surgical management of chronic pancreatitis: Current utilization in the united states. HPB (Oxford) 2015;17:804–810. doi: 10.1111/hpb.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: A population-based study. Am J Gastroenterol. 2011;106:2192–2199. doi: 10.1038/ajg.2011.328. [DOI] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 17.Rutter K, Ferlitsch A, Sautner T, Puspok A, Gotzinger P, Gangl A, et al. Hospitalization, frequency of interventions, and quality of life after endoscopic, surgical, or conservative treatment in patients with chronic pancreatitis. World J Surg. 2010;34:2642–2647. doi: 10.1007/s00268-010-0713-z. [DOI] [PubMed] [Google Scholar]
- 18.Nojgaard C, Bendtsen F, Becker U, Andersen JR, Holst C, Matzen P. Danish patients with chronic pancreatitis have a four-fold higher mortality rate than the danish population. Clin Gastroenterol Hepatol. 2010;8:384–390. doi: 10.1016/j.cgh.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Koutroumpakis E, Wu BU, Bakker OJ, Dudekula A, Singh VK, Besselink MG, et al. Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: A post hoc analysis of three large prospective databases. Am J Gastroenterol. 2015;110:1707–1716. doi: 10.1038/ajg.2015.370. [DOI] [PubMed] [Google Scholar]
- 20.Acevedo-Piedra NG, Moya-Hoyo N, Rey-Riveiro M, Gil S, Sempere L, Martinez J, et al. Validation of the determinant-based classification and revision of the atlanta classification systems for acute pancreatitis. Clin Gastroenterol Hepatol. 2014;12:311–316. doi: 10.1016/j.cgh.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 21.Hirota M, Shimosegawa T, Masamune A, Kikuta K, Kume K, Hamada S, et al. The sixth nationwide epidemiological survey of chronic pancreatitis in japan. Pancreatology. 2012;12:79–84. doi: 10.1016/j.pan.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Hirota M, Shimosegawa T, Masamune A, Kikuta K, Kume K, Hamada S, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in japan: Clinical significance of smoking habit in japanese patients. Pancreatology. 2014;14:490–496. doi: 10.1016/j.pan.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Ammann RW, Muellhaupt B, Meyenberger C, Heitz PU. Alcoholic nonprogressive chronic pancreatitis: Prospective long-term study of a large cohort with alcoholic acute pancreatitis (1976–1992) Pancreas. 1994;9:365–373. [PubMed] [Google Scholar]
- 24.Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, et al. Clinical and genetic characteristics of hereditary pancreatitis in europe. Clin Gastroenterol Hepatol. 2004;2:252–261. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 25.Yadav D, Muddana V, O’Connell M. Hospitalizations for chronic pancreatitis in allegheny county, pennsylvania, USA. Pancreatology. 2011;11:546–552. doi: 10.1159/000331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bang UC, Benfield T, Hyldstrup L, Bendtsen F, Beck Jensen JE. Mortality, cancer, and comorbidities associated with chronic pancreatitis: A danish nationwide matched-cohort study. Gastroenterology. 2014;146:989–994. doi: 10.1053/j.gastro.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 27.Ammann RW, Buehler H, Muench R, Freiburghaus AW, Siegenthaler W. Differences in the natural history of idiopathic (nonalcoholic) and alcoholic chronic pancreatitis. A comparative long-term study of 287 patients. Pancreas. 1987;2:368–377. doi: 10.1097/00006676-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Lankisch PG, Seidensticker F, Lohr-Happe A, Otto J, Creutzfeldt W. The course of pain is the same in alcohol- and nonalcohol-induced chronic pancreatitis. Pancreas. 1995;10:338–341. doi: 10.1097/00006676-199505000-00003. [DOI] [PubMed] [Google Scholar]


