Abstract
Eosinophilic esophagitis (EoE) has emerged over the past 2 decades as a major cause of upper gastrointestinal morbidity. Over this time, the epidemiology of EoE has also rapidly evolved. EoE has transformed from a rare case-reportable condition to disease that is commonly encountered in the gastroenterology clinic, hospital emergency room, and endoscopy suite. The incidence and prevalence are increasing at rates that outpace increased disease recognition. Current incidence estimates range from 5 to 10 cases per 100,000, and current prevalence estimates range from 0.5 to 1 case per 1000. We review the data and potential reasons behind this increase, examine risk factors, and identify important areas for research into disease etiology. The article also discusses the progression of EoE from an inflammatory to fibrostenotic phenotype. An accurate view of the natural history of EoE is central to discussions with patients regarding disease prognosis and decisions about long-term use of medical, endoscopic, and diet therapies. Progressive remodelling appears to be gradual, but not universal, and the duration of untreated disease is the best predictor of stricture risk. Ultimately, prospective, long-term outcome studies focusing on multiple aspects of disease activity are needed to fully understand the natural history of EoE.
Keywords: Incidence, prevalence, progression, fibrosis
Eosinophilic esophagitis (EoE) is an allergen/immune-mediated disease characterized by symptoms of esophageal dysfunction and eosinophilic infiltration of the esophageal mucosa in the absence of secondary causes of eosinophilia.1, 2 The first cases of EoE were first reported in the late 1970s,3, 4 but the disease as it is recognized today was described in 3 case series in the early and mid-1990s.5–7 Since then, EoE has transformed from a rare case-reportable condition to a disease that is commonly encountered in the clinic and endoscopy suite,8 and a major cause of upper gastrointestinal morbidity and increasing health care costs.9 Over this time, our understanding of the epidemiology of EoE has also rapidly evolved. The incidence and prevalence are increasing at rates that outpace increased recognition,10–12 indicating the importance of environmental rather than genetic changes.13, 14 Descriptive epidemiology research in EoE has also matured, and there is now a focus on identifying etiologic factors. Although we know much about the pathogenesis of EoE,15 we do not fully understand why EoE develops in an individual patient.16 We review the incidence and prevalence of EoE, present potential reasons for the increase in EoE, examine possible risk factors, and discuss the natural history and possible progression of this chronic condition.
Epidemiology
Incidence of EoE and time trends
The incidence of EoE has been investigated several population-based studies, conducted primarily in North America and Europe.11, 12, 17–25 Using the most recent time point from these studies, incidence rates range from a low of 2.1/100,000/year in the Netherlands22, 26 to a high of 12.8/100,000/year in Ohio in the United States17 (Supplementary Table 1). A meta-analysis calculated an overall pooled incident rate of 3.7/100,000/year (95% CI, 1.7–6.5), though there was substantial heterogeneity.27 In this study, the incidence rate was higher in adults (7.0/100,000/year) than in children (5.1/100,000/year). When interpreting the published incidence data, it is important to recognize differences among studies performed at different centers and during different time periods. For example, proton pump inhibitor (PPI)-responsive esophageal eosinophilia may not have been excluded in some studies (there is controversy over this topic)28 and or case-finding approaches might have been used.
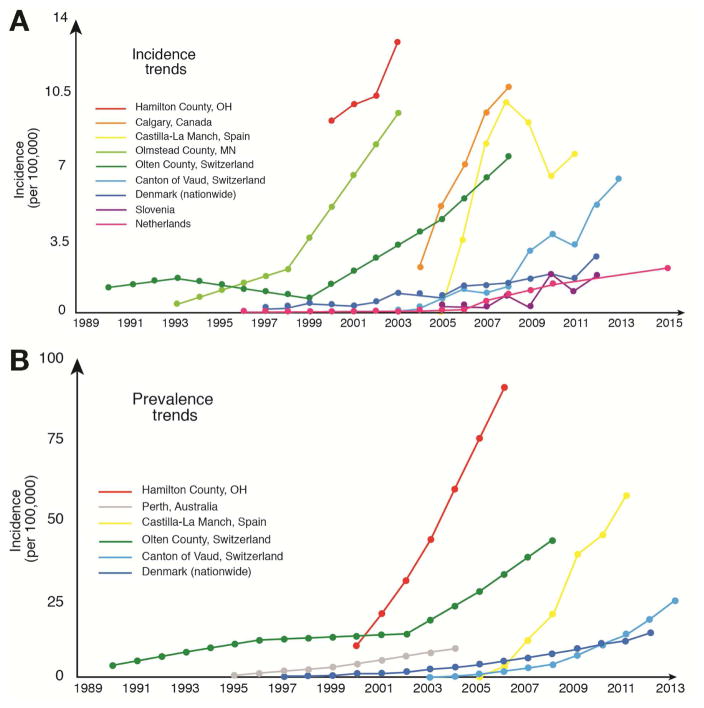
All studies that have examined incidence rates of EoE over time have concluded that the incidence of EoE is increasing rapidly (Figure 1A; Supplementary Table 1). In the first report investigating this issue, incidence increased 40% over a 4 year period (2000–2003).17 In similar analyses, incidence increased approximately 27-fold20 and 5-fold at 2 other North American centers.23 In European studies, rates of increase ranged from 6-fold to more than 100-fold.11, 12, 18, 19, 22, 24, 25 Although it is tempting to attribute this rapid change only to an associated increase in recognition of and knowledge about EoE, this is not the only explanation. Several studies have examined changes in rates of endoscopy with biopsy over the same time period as the change in rates of EoE, and have found that the increase in EoE incidence outpaces the relatively modest increase in rates of biopsy.10–12, 20, 29 In addition, other studies have retrospectively pulled archived esophageal biopsy blocks to determine if cases of EoE were previously present but missed.30, 31 Although cases of EoE were found, they were identified at rates that are far below what are currently observed. It therefore appears that the incidence of EoE is truly increasing, and is just not an artifact of increasing surveillance and detection. This information has major implications for understanding the etiology of EoE.
Figure 1.
(A) Time trends in EoE incidence from estimates in population-based studies. (B) Time trends in EoE prevalence from estimates in population-based studies.
Prevalence of EoE
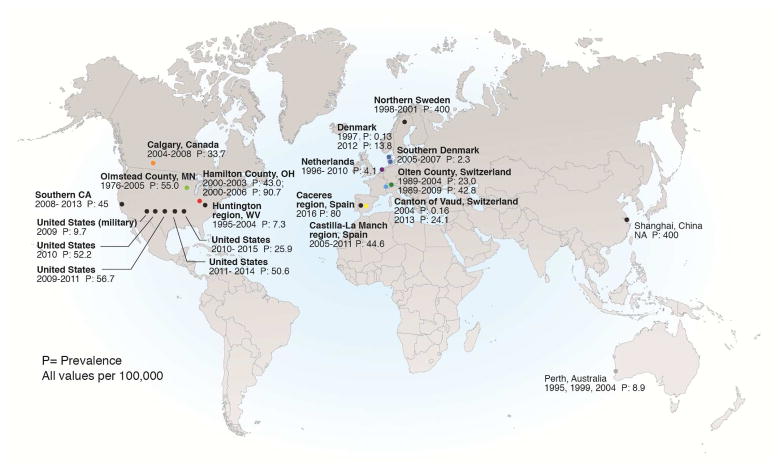
The prevalence of EoE has been investigated worldwide, but most population-based studies have been conducted in North America and Europe,11, 12, 17–24, 32–40 with select studies in Australia and Asia.41, 42 In a study in Scandinavia and a study in China, researchers performed upper endoscopies using a population-based sampling frame of asymptomatic individuals in the community.33, 42 They found a rate of esophageal eosinophilia (defined as 15–20 eosinophils per high-power field) of approximately 400/100,000. These findings should be interpreted with caution, because the studies included patients who would not meet diagnostic criteria for EoE. Other studies attempted to identify all known EoE cases within a specific population catchment area. If we use the most recent time point from these studies, prevalence values range from as low as 2.3/100,000 in Denmark21 to as high as 90.7/100,000 in Ohio (Supplementary Table 2).17 A meta-analysis estimated an overall pooled EoE prevalence of 22.7/100,000 (95% CI, 12.4–36.0), with a higher rate in adults (43.4/100,000; 95% CI, 22.5–71.2) than in children (29.5/100,000; 95% CI, 17.5–44.7), though there was substantial heterogeneity in these estimates.27 EoE is a chronic disease, so prevalence rates have increased steadily at all sites that have examined changes over time (Figure 1B).
EoE prevalence estimates vary with location (Figure 2). The prevalence tends to be on the same order of magnitude in Western Europe, North America, and Australia,12, 17, 19, 20, 22, 23, 35, 37–41, 43–45 but much lower in Japan and China.46–50 The disorder has also been reported in South American, Korea, Turkey, and the Middle East,43, 51–55 but there are still no reports from sub-Saharan Africa or India.56 The differences between high prevalences in Western countries and low prevalences in Eastern countries, despite similarities in clinical presentation and molecular features,57, 58 provides a platform for future investigations of etiologic mechanisms, especially those that focus on environmental factors.
Figure 2.
Worldwide prevalence of EoE from estimates in population-based studies.
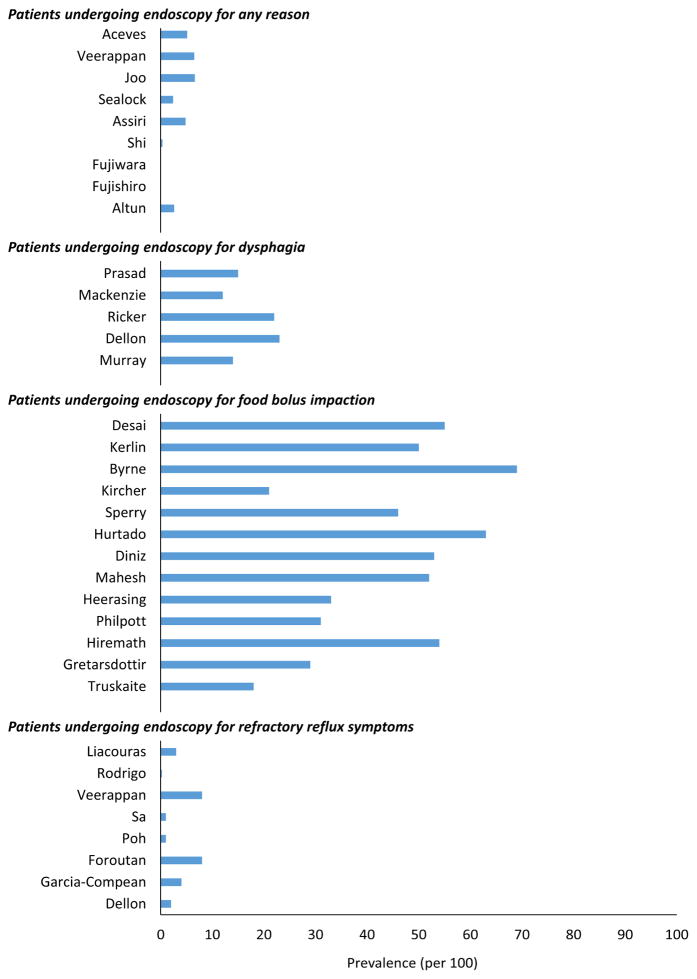
In additional to geographic variations in prevalence, the prevalence of EoE differs among clinical populations (Figure 3; Supplementary Table 3). An overall estimate of the prevalence of EoE is 0.5–1 cases per 1000 persons, yet EoE is detected in 2.4%–6.6% of patients undergoing endoscopy for any indication,51, 53, 59–61 and this rate is much lower in Japan and China (less than 0.4% of cases).46, 47, 50 In studies assessing patients undergoing endoscopy for an indication of dysphagia, rates are higher, ranging from 12% to 23%.62–66 The highest proportions of EoE, frequently above 50%, occur in patients presenting with an esophageal food bolus impaction—EoE is now the most common cause of esophageal food bolus impactions in patients presenting to emergency departments.67–79 EoE has also be detected in 1%–8% of patients undergoing endoscopy for symptoms of refractory reflux or heartburn,43, 54, 60, 65, 80–83 6% of patients undergoing endoscopy for non-cardiac chest pain,84 4% of patients undergoing endoscopy for abdominal pain,85 4% of patients with refractory aerodigestive symptoms,86 and 5% of patients with IgE-mediated food allergies.87
Figure 3.
Prevalence of EoE in special populations including patients undergoing endoscopy for any reason, for dysphagia, for food bolus impaction, or for symptoms of refractory reflux.
Risk factors
There is intense interest in learning why the incidence and prevalence of EoE are increasing.13, 88–90 Although some genetic factors have been associated with EoE,15 the rapid trends in EoE incidence indicate a role for environmental factors in disease risk.14 In a study of a large administrative database in the United States, prevalence increased steadily with age, to a peak value in individuals 30–44 years old. Prevalence then sharply decreased among older individuals;37 this trend was also observed from a population-based study in the Netherlands.22 These observations raise the question as to whether environmental changes starting 40–50 years ago contributed to disease development. One often-proposed theory is the hygiene hypothesis, which suggests that humans are losing immune tolerance from being raised in clean environments. This hypothesis is supported by the general increase in a number of allergic and autoimmune conditions.91 However, data also support a number of more specific etiologic factors (Table 1).
Table 1.
Risk Factors for EoE and Disorders Associated with EoE
| Risk factor | Comment |
|---|---|
| Aeroallergens10, 16, 94–96, 98, 99, 154 | Might cause EoE or increase disease activity; can cross react with food allergens; may explain seasonal variation in diagnosis |
| Food allergens7, 155, 156 | Directly trigger EoE; elimination can lead to disease remission |
| Helicobacter pylori109–113 | Inversely associated with EoE; decrease in H. pylori prevalence has accompanied increase in EoE prevalence over the last 20 years; mechanistic data lacking |
| Infections (herpes simplex virus; mycoplasma)115– 118 | Associated with EoE; mechanistic data lacking |
| Oral or sublingual immunotherapy120–124 | Causes or induces EoE in certain patients; baseline EoE status for reported cases usually not know prior to immunotherapy |
| Proton pump inhibitors126 | Reported to induce IgE antibodies to certain foods |
| Cold or arid climates100 | Increased odds of EoE in these climate zones, but not in temperate or tropical zones |
| Population density35, 101, 102 | Odds of EoE increase as population density decreases |
| Early life factors104–107 | Antibiotic use, Cesarean section, and preterm delivery increase the odds of pediatric EoE |
| Connective tissue disorders133 | Ehlers-Danlos, Marfan Syndrome, and Loeys- Dietz syndrome have been associated with EoE |
| Celiac disease128–132 | Associated with EoE; EoE is more common in patients with celiac disease than would be expected |
| Autoimmune conditions134, 135 | Inflammatory bowel disease, rheumatoid arthritis, IgA deficiency, multiple sclerosis, and Hashimoto’s thyroiditis associated with EoE |
Because EoE is an allergic condition, several environmental allergens have been implicated. First, food allergens trigger EoE and the disease can be put into remission by removal of specific foods, either via elimination diets or hypoallergenic elemental formulas.7, 92, 93 However, it is still not clear why foods that were tolerated over the course of human evolution would now induce EoE; the effects of farming practices, genetic modification, mass production, packaging, and other related factors have not been examined. Environmental or aeroallergens can also induce EoE—there are links between pollen season and EoE flares, seasonality of diagnosis of EoE (with more cases diagnosed during times of increase aeroallergens), and cold or arid climate zones.10, 16, 20, 94–100 Odds of EoE are higher in rural areas with lower population density,35, 101, 102 which might be explained by vegetation, pollution, or other environmental exposures.
Recently, early-life exposures have been investigated as potential risk factors for EoE.103 The first report on this topic found that antibiotics taken during the first year of life substantially increased the odds of subsequent diagnosis of pediatric EoE, and that several other factors, including Cesarean delivery and preterm birth, might also be associated with EoE.104 These results were replicated in 2 additional studies,105, 106 but were not found in a third.107 Although the mechanism of these early-life factors is not known, an intriguing possibility is that they affect the microbiome—this is an area of active research.103, 108
Infectious risk factors for EoE have also been studied. The strongest data to date show an inverse association between Helicobacter pylori and EoE; this relationship has been confirmed at several centers in different locations worldwide.109–113 This is of particular interest because the time of discovery of H pylori (in the early 1980s), along with the subsequent widespread treatment and decrease in prevalence of this infection, match the trend of increase in diagnosis of EoE. H pylori infection appears to produce a T-helper 1 cell-mediated response, so lack of H pylori might predispose people to a T-helper 2 cell-mediated immune response. 109 Lack of H pylori has also been associated with other atopic disorders,114 but there are no direct data to indicate a role for H pylori in pathogenesis of EoE. EoE might also be associated with herpes simplex virus115–117 or mycoplasma pneumoniae,118 but the mechanisms by which these would contribute to pathogenesis have not been studied—in contrast to an infectious complication of a topical steroid treatment for EoE. One study found a high rate of galactose-alpha-1,3, galactose sensitization, which is conveyed by a tick bite, in patients with EoE, but individuals without EoE (controls) had similarly high sensitization rates.119
Several other risk factors and conditions have also been associated with EoE. Oral and sublingual immunotherapy have been reported to induce EoE,120–123 and a recent systematic review estimated that EoE could develop in 2.7% of patients undergoing oral immunotherapy.124 This was an interesting observation, as it mimics the mechanism of induction of EoE in animal models.125 PPIs have been proposed as a possible factor, given that they were introduced in the 1980s, their increase in use coincides with the rise of EoE, and PPI use has been associated with formation of new food-specific IgE antibodies.126 However, there is no direct evidence to support this hypothesis. Recently, borderline low levels of vitamin D were reported in a cohort of patients with EoE,127 but no data comparing vitamin or micronutrient levels in EoE cases vs controls were presented. Finally, a number of other conditions, including celiac disease,128–132 connective tissue disorders,133 and autoimmune processes, have been associated with EoE.134, 135 However, there have not been mechanistic studies of these associations, with the exception of patients with Loeys-Dietz syndrome (and its associated mutation in the transforming growth factor beta gene), who develop eosinophilic gastrointestinal disorders.133, 136
Natural History
An accurate view of the natural history of EoE is central to discussions with patients about their prognosis and in making decisions about long-term use of medical, endoscopic, and diet therapies. Recommendations for maintenance therapy are based not only on prevention of disease relapse, but also on preventing the future consequences of EoE.1, 2 If it appears that a patient with EoE is likely to undergo spontaneous remission or experience minor consequences, the risks of long-term therapy may outweigh the benefits. Since the initial characterization of EoE in the early 1990s, investigators have approached this topic from different provider perspectives and with distinct methods. Most studies have been retrospective, single-center experiences describing variable outcome metrics. Moreover, most studies included patients who received multiple treatments for EoE, including PPI therapy, elimination diet, steroids, and esophageal dilation. In analyzing results of these studies, it is important to consider the age of the patients included and their perspectives. EoE is a relatively new disease, so uncertainties about the progression and long-term consequences of EoE can cause anxiety in patients.137
Two of the most informative studies of EoE progression came from a single-center study of adults in Switzerland.138, 139 The perspective of 1 of the first investigators to describe EoE, combined with a systematic and comprehensive approach to data collection, provided valuable insights. Once study, published 15 years ago, continues to provide the best characterization of EoE progression in adults.138 It followed 30 adults for a mean 7.2 years in the absence of medical therapy for EoE. Dysphagia and esophageal eosinophilia persisted in nearly every patient. Interestingly, the intensity of dysphagia was reduced over the follow-up period in 37% of patients, was stable in 37%, and worsened in 23%. Similarly, histopathologic findings of eosinophil density and basal zone hyperplasia decreased in most patients, although the eosinophil density increased in 20%. Subepithelial fibrosis increased on follow up in 6 of 7 biopsies (86%) evaluated for this marker of esophageal remodeling. Endoscopic abnormalities were stable but the severity of esophageal stenosis was not measured. No patient developed generalized eosinophilic infiltration of the gastrointestinal tract or an esophageal neoplasm.
The study highlighted the chronic nature of EoE in adults but also the absence of clinical progression over the follow-up period. However, there are 2 important limitations to the study. First, one third of the cohort underwent esophageal dilation. Although dilation is not expected to alter esophageal eosinophilia, it can reduce dysphagia, which could account for the substantial proportion of patients with stable or improved symptom outcomes. Esophageal dilation also makes it difficult to accurately determine stricture recurrence. Second, 50% of patients altered eating behaviors to compensate for perceived difficulties with rough-textured foods or hurried eating habits. Dietary modification may have reduced the burden of dysphagia reported.
A second study from the same center provided important insights into disease progression—it has been the only randomized study of topical steroid withdrawal performed in patients with EoE.139 In this study, 28 patients who had achieved histologic remission following a randomized, controlled trial of swallowed budesonide were randomly assigned to groups given either budesonide (0.5 mg daily, 25% of the induction dose) or placebo. In patients given placebo for 1 year, peak esophageal eosinophil counts increased from 0.7 to 65 eosinophils per high-power field. At week 50, 64% of the patients given placebo had symptom relapse, at a median 3 months following withdrawal of budesonide. As in the previous study, an increase in subepithelial fibrosis was detected in the placebo group, although this increase was not statistically significant.
Three different centers have examined long-term outcomes of EoE using a cross-sectional study design that involved collection of survey questionnaires in children and adults diagnosed with EoE at an earlier time point.140–142 All 3 studies found that most patients with EoE had mild to no symptoms several years after diagnosis. Importantly these studies assessed only symptom outcomes—they did not report endoscopic or histologic follow-up outcomes.
The first study, performed at the University of Pennsylvania, reported survey results from 53 of 140 patients with EoE over the age of 17 (mean age 21) who had been diagnosed in childhood (most during adolescence).140 Several years after their diagnosis of EoE, only 4% of patients had a positive symptom score for dysphagia, based on the Mayo Dysphagia Questionnaire, and 37% reported dysphagia during the month prior to the questionnaire. Half of the patients were actively taking PPIs, whereas 76% were following an allergy-directed diet. These proportions were typical of local practice patterns.
Using a similar study design, researchers at Indiana University surveyed 58 young adults (mean age 21) with EoE who had been diagnosed in childhood.141 After a mean follow-up period of 8 years, nearly half of the patients (47%) reported symptom resolution, despite the fact that two-thirds of the cohort did not receive active therapy for EoE (10% received topical steroids, 17% took PPIs). One third of patients reported dysphagia more than once each month and only 2% reported worsening of symptoms.
A study performed at the Mayo Clinic surveyed 59 adults (mean age, 56 years) who had been diagnosed with EoE more than 10 years ago;142 65% were taking PPIs and 7% received topical steroids. Twenty-eight percent of the subjects reported dysphagia in the month before the survey began, although 37% reported dietary restrictions. One limitation to this study is that the adults surveyed accounted for only a small proportion of the total EoE cases at the institution.
Two retrospective studies provided a more pessimistic view on the progression of EoE in adults.143, 144 One study used the Swiss EoE Database to demonstrate that the prevalence of esophageal strictures increased with longer durations of untreated disease.144 The study included 200 adults (median age, 39 years) with EoE and defined untreated disease duration as the time period from symptom onset to the diagnosis of EoE. Strictures developed in 17% of patients with a delay in EoE diagnosis of 0–2 years, in 31% with a delay of 2–5 years, in 38% with a delay of 8–11 years, in 64% with a delay of 14–17 years, and in 71% with a delay of more than 20 years. Neither age at symptom onset nor degree of esophageal eosinophilia were associated with the presence of a stricture.
Data from the University of North Carolina substantiated this concept in 379 patients with EoE (mean age, 25 years) who were classified, based on endoscopic features, into inflammatory, fibrostenotic, or mixed inflammatory/fibrostenotic phenotypes.143 Patients with an inflammatory phenotype were significantly younger than those with a mixed or fibrostenotic phenotype. The risk of developing a fibrostenotic phenotype doubled for every decade of life, and odds of developing fibrostenosis increased 5% for each year of symptoms prior to diagnosis. A study of 64 adults followed at the University of South Florida identified a greater duration of delayed diagnosis in patients with more severe esophageal strictures.145 The time period of delayed diagnosis increased from 5 years for strictures over 16 mm in diameter, to 11 years for strictures 10–16 mm, to 15 years for strictures less than 10 mm.
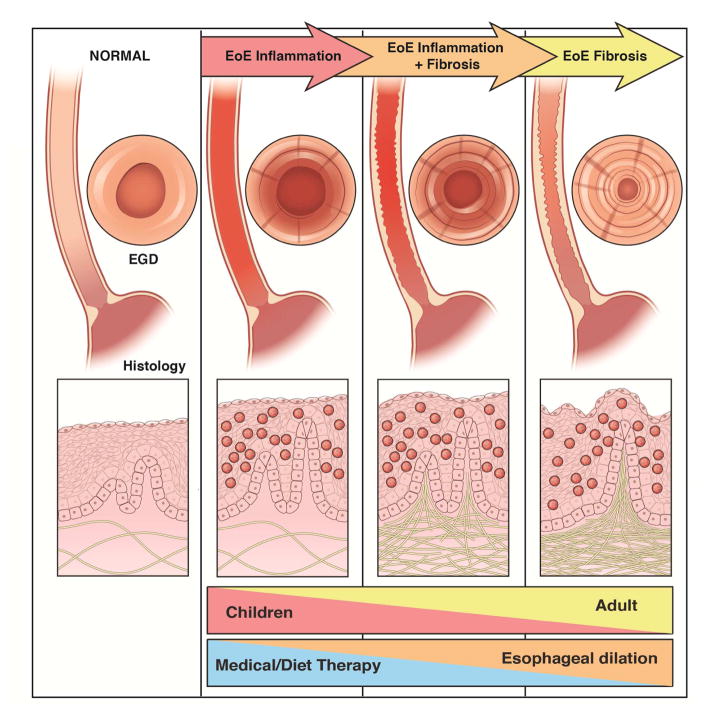
Based on these data, we created a conceptual model for progression of EoE, from development of inflammation to fibrostenosis, assessed through a combination of endoscopic and histologic outcomes (Figure 4). Medical and diet therapies to reduce mucosal inflammation would have greater utility in patients with earlier-stage disease, whereas dilation would provide more benefit patients with strictures.
Figure 4.
Progression of EoE from inflammation to fibrosis.
Two of the leading pediatric centers for treatment of EoE performed retrospective studies to determine long-term outcomes. Children’s Hospital of Philadelphia reviewed 330 pediatric cases of EoE with more than 1 year of follow-up data; the mean follow-up period was 3.2 years.146 Most patients at this center were actively treated with dietary elimination in addition to PPIs, in combination with medical therapy of concomitant allergic rhinitis and asthma. Only 3% of patients had histologic remission without continuing diet therapy and 10% had evidence for development of tolerance to foods previously identified as triggers of their EoE. Most of a subset of 24 patients who had elected to not treat their EoE had ongoing symptoms and eosinophilia, whereas 17% had progression to dysphagia after a mean follow-up time of 6 years.
Another approach to understanding the natural history of EoE was undertaken by Cincinnati Children’s Hospital, where biopsies taken from a time period prior to the recognition of EoE were reanalyzed for presence of esophageal eosinophilia.147 From this cohort, 42 children completed survey questionnaires regarding symptoms. Dysphagia was present in half of the patients at a mean time point of 15 years after their index endoscopy. Retrospective analyses of biopsies showed findings consistent with EoE, indicating interval progression in patients with unrecognized disease.
Studies have therefore reached conflicting conclusions on EoE progression. However, their findings can be reconciled by considering differences in study design and methods. Symptom-focused outcome studies have indicated a relatively benign course of the disease, with absent or only mild dysphagia in most patients, with or without use of medical or dietary therapy directed at EoE. Several years after a diagnosis of EoE, approximately 30%–50% of children transitioning to adulthood reported symptoms of dysphagia. Although patient-reported outcomes are central to the management of EoE, patients typically adapt to the slow, progressive structuring by means of modification in eating behaviors. Avoidance of specific food textures (meat, crusty bread), increased use of liquids with meals, prolonged meal times, and meticulous mastication can reduce occurrence of dysphagia from esophageal strictures.
With progressive but very gradual esophageal remodeling, esophageal stenosis would be expected to affect fewer than 50% of patients; patients would develop a mild degree of stricture over fewer than 10 years. Longer term follow-up studies might reveal more substantial effects on the prevalence and severity of dysphagia. In addition, data from trials of patients with EoE have demonstrated that even short-term medical therapy can provide several months of symptom relief.148, 149 Likewise, esophageal dilation can relieve symptoms of dysphagia for more than 1 year, even in the absence of anti-inflammatory therapy,150 and patients who undergo esophageal dilation at baseline who have an initial histologic response to anti-inflammatory treatment require fewer dilations at later time points than patients without a histologic response.151 Even sporadic use of therapy during a follow-up period could therefore reduce symptoms, even in patients not taking therapy at the time of a cross-sectional survey.
The studies reporting long-term reductions in symptoms often included patients who maintained PPI therapy. Numerous studies have demonstrated the long-term effectiveness of PPI therapy in reducing symptoms and eosinophilia (in 30%–70% of patients with symptomatic esophageal eosinophilia).152 It is therefore important to remember that study outcomes can be affected by the effectiveness of PPIs, rather than spontaneous improvement in disease activity.
Contrary to symptom-based studies, studies focusing on endoscopic outcomes have reported progression of significant fibrostenoses in most patients with over a decade of untreated EoE.143–145, 153 These studies used the time since symptom onset, rather than time since diagnosis of EoE, as a surrogate for duration of untreated disease. Although this method is subject to problems of recall accuracy, the longer time off EoE-directed treatment likely depicts a more accurate estimation of disease progression. Moreover, these studies have reported objective outcomes based on endoscopic detection of strictures rather than assessments of symptoms, which are subjective and affected by eating behavior. On the other hand, inclusion of patients with severe fibrostenoses referred to tertiary care centers may introduce selection bias, with unintentional exclusion of patients who never sought medical care due to stable or improved symptoms over time. Studies of another objective measure of disease activity, findings histopathology analyses, have uniformly corroborated the chronicity of EoE.138, 139 Spontaneous resolution of esophageal eosinophilia is uncommon in EoE, although the degree of eosinophilia may wane even in the absence of active therapy.
Future Directions
The evolution of the epidemiology of EoE over the last 2 decades is remarkable, as the incidence and prevalence have increased rapidly. EoE is now commonly encountered in the endoscopy suite and clinic, is the leading cause of food impaction, is a major cause of dysphagia, and accounts for significant health-related costs. The implications of the emergence of EoE are 2-fold. First, if current incidence and prevalence trends hold, EoE may no longer be considered a rare disease. Furthermore, the cause of the continued increase in EoE cases over the last 2–3 decades is still largely unexplained, and this directly effects epidemiology research of EoE. While the descriptive epidemiologic features of EoE have been well characterized, little is known about the etiologic epidemiologic features. It is important to learn why specific individuals develop EoE, what the initial triggers are, what changes in the environment might be responsible for the increasing incidence of EoE, and what factors predispose to its development.
EoE development involves chronic inflammation that leads to progressive fibrostenosis in many but not all patients. This process is gradual, allowing many patients to adopt coping strategies to circumvent or underestimate symptom detection. In addition to coping strategies, genetic factors and behavior factors contribute to variation in the severity and effects of disease progression. For now, the duration of untreated disease appears to be the single best predictor of stricture risk. Treatment with elimination diets or medical therapy might slow disease progression, but there are few data to support this concept, and factors that predict progression have not been identified. Spontaneous remission does occur, but it appears to be uncommon, based on data collected over the short time period that EoE has been studied. Prospective long-term outcome studies, focused on multiple aspects of disease activity, are needed to fully understand EoE progression.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by U54AI117804 (CEGIR), which is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Disease Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS, as well as R01DK101856 (ESD).
Footnotes
Potential competing interests: None of the authors report any potential conflicts of interest with this study. Dr. Dellon is a consultant for Adare, Alvio, Banner, Enumeral, GSK, Receptos, Regeneron, and Shire; receives research funding from Meritage, Miraca, Nutricia, Receptos, Regeneron, and Shire; and has received an educational grant from Banner. Dr. Hirano is a consultant for Adare, Receptos, Regeneron, and Shire and has received research funding from Receptos, Regeneron, and Shire.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves SS. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras C, Katzka DA. ACG Clinical Guideline: Evidence based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol. 2013;108:679–92. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 3.Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology. 1977;72:1312–6. [PubMed] [Google Scholar]
- 4.Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74:1298–1301. [PubMed] [Google Scholar]
- 5.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 6.Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vogtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr. 1994;124:1419–29. [PubMed] [Google Scholar]
- 7.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–12. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 8.Dellon ES, Liacouras CA. Advances in Clinical Management of Eosinophilic Esophagitis. Gastroenterology. 2014;147:1238–1254. doi: 10.1053/j.gastro.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-Care Utilization, Costs, and the Burden of Disease Related to Eosinophilic Esophagitis in the United States. Am J Gastroenterol. 2015;110:626–32. doi: 10.1038/ajg.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellon ES, Gibbs WB, Fritchie KJ, Rubinas TC, Wilson LA, Woosley JT, Shaheen NJ. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305–1313. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellon ES, Erichsen R, Baron JA, Shaheen NJ, Vyberg M, Sorensen HT, Pedersen L. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population-based estimates from Denmark. Aliment Pharmacol Ther. 2015;41:662–70. doi: 10.1111/apt.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giriens B, Yan P, Safroneeva E, Zwahlen M, Reinhard A, Nydegger A, Vavricka S, Sempoux C, Straumann A, Schoepfer AM. Escalating incidence of eosinophilic esophagitis in Canton of Vaud, Switzerland, 1993–2013: a population-based study. Allergy. 2015;70:1633–9. doi: 10.1111/all.12733. [DOI] [PubMed] [Google Scholar]
- 13.Jensen ET, Dellon ES. Environmental and infectious factors in eosinophilic esophagitis. Best Pract Res Clin Gastroenterol. 2015;29:721–9. doi: 10.1016/j.bpg.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, He H, Mukkada VA, Succop PA, Abonia JP, Foote H, Eby MD, Grotjan TM, Greenler AJ, Dellon ES, Demain JG, Furuta GT, Gurian LE, Harley JB, Hopp RJ, Kagalwalla A, Kaul A, Nadeau KC, Noel RJ, Putnam PE, von Tiehl KF, Rothenberg ME. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134:1084–1092. e1. doi: 10.1016/j.jaci.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenberg ME. Molecular, Genetic, and Cellular Bases for Treating Eosinophilic Esophagitis. Gastroenterology. 2015;148:1143–57. doi: 10.1053/j.gastro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf WA, Jerath MR, Dellon ES. De-novo onset of eosinophilic esophagitis after large volume allergen exposures. J Gastrointestin Liver Dis. 2013;22:205–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 18.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–9. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M, Beglinger C, Schoepfer AM. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128:1349–1350. e5. doi: 10.1016/j.jaci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, Locke GR, 3rd, Talley NJ. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–61. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalby K, Nielsen RG, Kruse-Andersen S, Fenger C, Bindslev-Jensen C, Ljungberg S, Larsen K, Walsted AM, Husby S. Eosinophilic Oesophagitis in Infants and Children in the Region of Southern Denmark: A Prospective Study of Prevalence and Clinical Presentation. J Pediatr Gastroenterol Nutr. 2010;51:280–2. doi: 10.1097/MPG.0b013e3181d1b107. [DOI] [PubMed] [Google Scholar]
- 22.van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. 2013;25:47–52. e5. doi: 10.1111/nmo.12009. [DOI] [PubMed] [Google Scholar]
- 23.Syed AA, Andrews CN, Shaffer E, Urbanski SJ, Beck P, Storr M. The rising incidence of eosinophilic oesophagitis is associated with increasing biopsy rates: a population-based study. Aliment Pharmacol Ther. 2012;36:950–8. doi: 10.1111/apt.12053. [DOI] [PubMed] [Google Scholar]
- 24.Arias A, Lucendo AJ. Prevalence of eosinophilic oesophagitis in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol. 2013;25:208–12. doi: 10.1097/MEG.0b013e32835a4c95. [DOI] [PubMed] [Google Scholar]
- 25.Homan M, Blagus R, Jeverica AK, Orel R. Pediatric Eosinophilic Esophagitis in Slovenia: Data From a Retrospective 2005–2012 Epidemiological Study. J Pediatr Gastroenterol Nutr. 2015;61:313–8. doi: 10.1097/MPG.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 26.Warners M, de Rooij WE, Van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Large Increase in Incidence of Eosinophilc Esophagitis Over the Last 20 Years in the Netherlands: Results from a Nationwide Pathology Database. Gastroenterology. 2017;152(Suppl 1):S862–S863. doi: 10.1111/nmo.13165. (Tu1104) [DOI] [PubMed] [Google Scholar]
- 27.Arias A, Perez-Martinez I, Tenias JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016;43:3–15. doi: 10.1111/apt.13441. [DOI] [PubMed] [Google Scholar]
- 28.Molina-Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, Hirano I, Katzka DA, Moawad FJ, Rothenberg ME, Schoepfer A, Spechler SJ, Wen T, Straumann A, Lucendo AJ. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65:524–31. doi: 10.1136/gutjnl-2015-310991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidambi T, Toto E, Ho N, Taft T, Hirano I. Temporal trends in the relative prevalence of dysphagia etiologies from 1999–2009. World J Gastroenterol. 2012;18:4335–41. doi: 10.3748/wjg.v18.i32.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeBrosse CW, Collins MH, Buckmeier Butz BK, Allen CL, King EC, Assa’ad AH, Abonia JP, Putnam PE, Rothenberg ME, Franciosi JP. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. J Allergy Clin Immunol. 2010;126:112–9. doi: 10.1016/j.jaci.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol. 2009;131:788–92. doi: 10.1309/AJCPOMPXJFP7EB4P. [DOI] [PubMed] [Google Scholar]
- 32.Buckmeier BK, Rothenberg ME, Collins MH. The incidence and prevalence of eosinophilic esophagitis. J Allergy Clin Immunol. 2008;121(Suppl 2):S71. (AB 271) [Google Scholar]
- 33.Ronkainen J, Talley NJ, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Walker MM, Agreus L. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: The population-based Kalixanda study. Gut. 2007;56:615–20. doi: 10.1136/gut.2006.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill R, Durst P, Rewalt M, Elitsur Y. Eosinophilic Esophagitis Disease in Children from West Virginia: A Review of the Last Decade (1995–2004) Am J Gastroenterol. 2007;102:2281–5. doi: 10.1111/j.1572-0241.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 35.Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, Bonis PA. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ally MR, Maydonovitch CL, Betteridge JD, Veerappan GR, Moawad FJ. Prevalence of eosinophilic esophagitis in a United States military health-care population. Dis Esophagus. 2015;28:505–11. doi: 10.1111/dote.12229. [DOI] [PubMed] [Google Scholar]
- 37.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12:589–596. e1. doi: 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maradey-Romero C, Prakash R, Lewis S, Perzynski A, Fass R. The 2011–2014 prevalence of eosinophilic oesophagitis in the elderly amongst 10 million patients in the United States. Aliment Pharmacol Ther. 2015;41:1016–22. doi: 10.1111/apt.13171. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Sheikh J. Prevalence of eosinophilic esophagitis in a population-based cohort from Southern California. J Allergy Clin Immunol Pract. 2015 doi: 10.1016/j.jaip.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Mansoor E, Cooper GS. The 2010–2015 Prevalence of Eosinophilic Esophagitis in the USA: A Population-Based Study. Dig Dis Sci. 2016;61:2928–34. doi: 10.1007/s10620-016-4204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child. 2006;91:1000–4. doi: 10.1136/adc.2006.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma X, Xu Q, Zheng Y, Zhao Y, Lu J, Wang R, Li Z, Zou D, He J. Prevalence of Esophageal Eosinophilia and Eosinophilic Esophagitis in Adults: A Population-Based Endoscopic Study in Shanghai, China. Dig Dis Sci. 2015 doi: 10.1007/s10620-014-3512-9. [DOI] [PubMed] [Google Scholar]
- 43.Sa CC, Kishi HS, Silva-Werneck AL, Moraes-Filho JP, Eisig JN, Barbuti RC, Hashimoto CL, Navarro-Rodriguez T. Eosinophilic esophagitis in patients with typical gastroesophageal reflux disease symptoms refractory to proton pump inhibitor. Clinics (Sao Paulo) 2011;66:557–61. doi: 10.1590/S1807-59322011000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther. 2010;32:712–9. doi: 10.1111/j.1365-2036.2010.04411.x. [DOI] [PubMed] [Google Scholar]
- 45.Molina-Infante J, Gonzalez-Cordero PL, Ferreira-Nossa HC, Mata-Romero P, Hernandez-Alonso M. Rising Incidence and Prevalence of Adult Eosinophilic Esophagitis in Caceres, Spain (2007–2016) Gastroenterology. 2017;152(Suppl 1):S856. doi: 10.1177/2050640617705913. (Tu1087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujiwara Y, Sugawa T, Tanaka F, Tatsuwaki H, Okuyama M, Hayakawa T, Yamamori K, Wada R, Ohtani K, Uno H, Tanigawa T, Watanabe Y, Tominaga K, Watanabe T, Takaishi O, Saeki Y, Nebiki H, Oshitani N, Sato H, Arakawa T. A multicenter study on the prevalence of Eosinophilic Esophagitis and PPI-responsive esophageal eosinophilic infiltration. Intern Med. 2012;51:3235–9. doi: 10.2169/internalmedicine.51.8670. [DOI] [PubMed] [Google Scholar]
- 47.Fujishiro H, Amano Y, Kushiyama Y, Ishihara S, Kinoshita Y. Eosinophilic esophagitis investigated by upper gastrointestinal endoscopy in Japanese patients. J Gastroenterol. 2011;46:1142–4. doi: 10.1007/s00535-011-0435-5. [DOI] [PubMed] [Google Scholar]
- 48.Kinoshita Y, Furuta K, Ishimaura N, Ishihara S, Sato S, Maruyama R, Ohara S, Matsumoto T, Sakamoto C, Matsui T, Ishikawa S, Chiba T. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2013;48:333–9. doi: 10.1007/s00535-012-0640-x. [DOI] [PubMed] [Google Scholar]
- 49.Kinoshita Y, Ishimura N, Oshima N, Ishihara S. Systematic review: Eosinophilic esophagitis in Asian countries. World J Gastroenterol. 2015;21:8433–40. doi: 10.3748/wjg.v21.i27.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi YN, Sun SJ, Xiong LS, Cao QH, Cui Y, Chen MH. Prevalence, clinical manifestations and endoscopic features of eosinophilic esophagitis: a pathological review in China. J Dig Dis. 2012;13:304–9. doi: 10.1111/j.1751-2980.2012.00593.x. [DOI] [PubMed] [Google Scholar]
- 51.Joo MK, Park JJ, Kim SH, Kim KH, Jung W, Yun JW, Lee BJ, Kim JH, Yeon JE, Kim JS, Byun KS, Lee SW, Bak YT. Prevalence and endoscopic features of eosinophilic esophagitis in patients with esophageal or upper gastrointestinal symptoms. J Dig Dis. 2012;13:296–303. doi: 10.1111/j.1751-2980.2012.00589.x. [DOI] [PubMed] [Google Scholar]
- 52.Altun R, Akbas E, Yildirim AE, Ocal S, Korkmaz M, Selcuk H. Frequency of eosinophilic esophagitis in patients with esophageal symptoms: a single-center Turkish experience. Dis Esophagus. 2013;26:776–81. doi: 10.1111/j.1442-2050.2012.01395.x. [DOI] [PubMed] [Google Scholar]
- 53.Assiri AM, Saeed A. Incidence and diagnostic features of eosinophilic esophagitis in a group of children with dysphagia and gastroesophageal reflux disease. Saudi Med J. 2014;35:292–7. [PubMed] [Google Scholar]
- 54.Foroutan M, Norouzi A, Molaei M, Mirbagheri SA, Irvani S, Sadeghi A, Derakhshan F, Tavassoli S, Besharat S, Zali M. Eosinophilic Esophagitis in Patients with Refractory Gastroesophageal Reflux Disease. Dig Dis Sci. 2010;55:28–31. doi: 10.1007/s10620-008-0706-z. [DOI] [PubMed] [Google Scholar]
- 55.Hasosah MY, Sukkar GA, Alsahafi AF, Thabit AO, Fakeeh ME, Al-Zahrani DM, Satti MB. Eosinophilic esophagitis in Saudi children: symptoms, histology and endoscopy results. Saudi J Gastroenterol. 2011;17:119–23. doi: 10.4103/1319-3767.77242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niranjan R, Thakur AK, Mishra A. Food allergy and eosinophilic esophagitis in India: Lack of diagnosis. Indian J Gastroenterol. 2016;35:72–3. doi: 10.1007/s12664-016-0629-0. [DOI] [PubMed] [Google Scholar]
- 57.Ishimura N, Shimura S, Jiao D, Mikami H, Okimoto E, Uno G, Aimi M, Oshima N, Ishihara S, Kinoshita Y. Clinical features of eosinophilic esophagitis: differences between Asian and Western populations. J Gastroenterol Hepatol. 2015;30(Suppl 1):71–7. doi: 10.1111/jgh.12746. [DOI] [PubMed] [Google Scholar]
- 58.Shoda T, Morita H, Nomura I, Ishimura N, Ishihara S, Matsuda A, Matsumoto K, Kinoshita Y. Comparison of gene expression profiles in eosinophilic esophagitis (EoE) between Japan and Western countries. Allergol Int. 2015;64:260–5. doi: 10.1016/j.alit.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Aceves SS, Newbury RO, Dohil R, Schwimmer J, Bastian JF. Distinguishing eosinophilic esophagitis in pediatric patients: clinical, endoscopic, and histologic features of an emerging disorder. J Clin Gastroenterol. 2007;41:252–6. doi: 10.1097/01.mcg.0000212639.52359.f1. [DOI] [PubMed] [Google Scholar]
- 60.Veerappan GR, Perry JL, Duncan TJ, Baker TP, Maydonovitch C, Lake JM, Wong RK, Osgard EM. Prevalence of Eosinophilic Esophagitis in an Adult Population Undergoing Upper Endoscopy: A Prospective Study. Clin Gastroenterol Hepatol. 2009;7:420–426. doi: 10.1016/j.cgh.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Sealock RJ, Kramer JR, Verstovsek G, Richardson P, Rugge M, Parente P, Vela M, El-Serag HB. The prevalence of oesophageal eosinophilia and eosinophilic oesophagitis: a prospective study in unselected patients presenting to endoscopy. Aliment Pharmacol Ther. 2013;37:825–32. doi: 10.1111/apt.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prasad GA, Talley NJ, Romero Y, Arora AS, Kryzer LA, Smyrk TC, Alexander JA. Prevalence and Predictive Factors of Eosinophilic Esophagitis in Patients Presenting With Dysphagia: A Prospective Study. Am J Gastroenterol. 2007;102:2627–32. doi: 10.1111/j.1572-0241.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 63.Mackenzie SH, Go M, Chadwick B, Thomas K, Fang J, Kuwada S, Lamphier S, Hilden K, Peterson K. Clinical trial: eosinophilic esophagitis in patients presenting with dysphagia: a prospective analysis. Aliment Pharmacol Ther. 2008;28:1140–6. doi: 10.1111/j.1365-2036.2008.03795.x. [DOI] [PubMed] [Google Scholar]
- 64.Ricker J, McNear S, Cassidy T, Plott E, Arnold H, Kendall B, Franklin K. Routine screening for eosinophilic esophagitis in patients presenting with dysphagia. Therap Adv Gastroenterol. 2011;4:27–35. doi: 10.1177/1756283X10384172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dellon ES, Speck O, Woodward K, Hores J, Madanick RD, Levinson S, Williams CG, Fritchie KJ, Woosley JT, Shaheen NJ. Prospective determination of the prevalence of PPI-responsive esophageal eosinophilia in patients with dysphagia undergoing upper endoscopy. Am J Gastroenterol. 2012;107(Suppl 1):S9. (AB 20) [Google Scholar]
- 66.Murray IA, Joyce S, Palmer J, Lau M, Schultz M. Incidence and features of eosinophilic esophagitis in dysphagia: a prospective observational study. Scand J Gastroenterol. 2016;51:257–62. doi: 10.3109/00365521.2015.1093166. [DOI] [PubMed] [Google Scholar]
- 67.Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61:795–801. doi: 10.1016/s0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 68.Kerlin P, Jones D, Remedios M, Campbell C. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol. 2007;41:356–61. doi: 10.1097/01.mcg.0000225590.08825.77. [DOI] [PubMed] [Google Scholar]
- 69.Byrne KR, Panagiotakis PH, Hilden K, Thomas KL, Peterson KA, Fang JC. Retrospective Analysis of Esophageal Food Impaction: Differences in Etiology by Age and Gender. Dig Dis Sci. 2007 doi: 10.1007/s10620-006-9499-0. [DOI] [PubMed] [Google Scholar]
- 70.Kirchner GI, Zuber-Jerger I, Endlicher E, Gelbmann C, Ott C, Ruemmele P, Scholmerich J, Klebl F. Causes of bolus impaction in the esophagus. Surg Endosc. 2011;25:3170–4. doi: 10.1007/s00464-011-1681-6. [DOI] [PubMed] [Google Scholar]
- 71.Sperry SL, Crockett SD, Miller CB, Shaheen NJ, Dellon ES. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc. 2011;74:985–91. doi: 10.1016/j.gie.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hurtado CW, Furuta GT, Kramer RE. Etiology of esophageal food impactions in children. J Pediatr Gastroenterol Nutr. 2011;52:43–6. doi: 10.1097/MPG.0b013e3181e67072. [DOI] [PubMed] [Google Scholar]
- 73.Diniz LO, Towbin AJ. Causes of Esophageal Food Bolus Impaction in the Pediatric Population. Dig Dis Sci. 2011 doi: 10.1007/s10620-011-1911-8. [DOI] [PubMed] [Google Scholar]
- 74.Mahesh VN, Holloway RH, Nguyen NQ. Changing epidemiology of food bolus impaction: is eosinophilic esophagitis to blame? J Gastroenterol Hepatol. 2013;28:963–6. doi: 10.1111/jgh.12135. [DOI] [PubMed] [Google Scholar]
- 75.Heerasing N, Lee SY, Alexander S, Dowling D. Prevalence of eosinophilic oesophagitis in adults presenting with oesophageal food bolus obstruction. World J Gastrointest Pharmacol Ther. 2015;6:244–7. doi: 10.4292/wjgpt.v6.i4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Philpott H, Nandurkar S, Thien F, Bloom S, Lin E, Goldberg R, Boyapati R, Finch A, Royce SG, Gibson PR. Seasonal Recurrence of Food Bolus Obstruction in Eosinophilic Esophagitis. Intern Med J. 2015;45:939–43. doi: 10.1111/imj.12790. [DOI] [PubMed] [Google Scholar]
- 77.Hiremath GS, Hameed F, Pacheco A, Olive A, Davis CM, Shulman RJ. Esophageal Food Impaction and Eosinophilic Esophagitis: A Retrospective Study, Systematic Review, and Meta-Analysis. Dig Dis Sci. 2015;60:3181–93. doi: 10.1007/s10620-015-3723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gretarsdottir HM, Jonasson JG, Bjornsson ES. Etiology and management of esophageal food impaction: a population based study. Scand J Gastroenterol. 2015;50:513–8. doi: 10.3109/00365521.2014.983159. [DOI] [PubMed] [Google Scholar]
- 79.Truskaite K, Dlugosz A. Prevalence of Eosinophilic Esophagitis and Lymphocytic Esophagitis in Adults with Esophageal Food Bolus Impaction. Gastroenterol Res Pract. 2016;2016:9303858. doi: 10.1155/2016/9303858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liacouras CA, Wenner WJ, Brown K, Ruchelli E. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr. 1998;26:380–5. doi: 10.1097/00005176-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Rodrigo S, Abboud G, Oh D, Demeester SR, Hagen J, Lipham J, Demeester TR, Chandrasoma P. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:435–42. doi: 10.1111/j.1572-0241.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 82.Poh CH, Gasiorowska A, Navarro-Rodriguez T, Willis MR, Hargadon D, Noelck N, Mohler J, Wendel CS, Fass R. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc. 2010;71:28–34. doi: 10.1016/j.gie.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Compean D, Gonzalez Gonzalez JA, Marrufo Garcia CA, Flores Gutierrez JP, Barboza Quintana O, Galindo Rodriguez G, Mar Ruiz MA, de Leon Valdez D, Jaquez Quintana JO, Maldonado Garza HJ. Prevalence of eosinophilic esophagitis in patients with refractory gastroesophageal reflux disease symptoms: A prospective study. Dig Liver Dis. 2011;43:204–8. doi: 10.1016/j.dld.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 84.Achem SR, Almansa C, Krishna M, Heckman MG, Wolfsen HC, Talley NJ, DeVault KR. Oesophageal eosinophilic infiltration in patients with noncardiac chest pain. Aliment Pharmacol Ther. 2011;33:1194–201. doi: 10.1111/j.1365-2036.2011.04652.x. [DOI] [PubMed] [Google Scholar]
- 85.Thakkar K, Chen L, Tatevian N, Shulman RJ, McDuffie A, Tsou M, Gilger MA, El-Serag HB. Diagnostic yield of oesophagogastroduodenoscopy in children with abdominal pain. Aliment Pharmacol Ther. 2009;30:662–9. doi: 10.1111/j.1365-2036.2009.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hill CA, Ramakrishna J, Fracchia MS, Sternberg D, Ojha S, Infusino S, Hartnick CJ. Prevalence of eosinophilic esophagitis in children with refractory aerodigestive symptoms. JAMA Otolaryngol Head Neck Surg. 2013;139:903–6. doi: 10.1001/jamaoto.2013.4171. [DOI] [PubMed] [Google Scholar]
- 87.Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract. 2017:5. doi: 10.1016/j.jaip.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonis PA. Putting the puzzle together: epidemiological and clinical clues in the etiology of eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:41–52. doi: 10.1016/j.iac.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Heine RG. Insights into the emerging epidemic of eosinophilic oesophagitis. Best Pract Res Clin Gastroenterol. 2015;29:731–7. doi: 10.1016/j.bpg.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Green DJ, Cotton CC, Dellon ES. The Role of Environmental Exposures in the Etiology of Eosinophilic Esophagitis: A Systematic Review. Mayo Clin Proc. 2015;90:1400–10. doi: 10.1016/j.mayocp.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, Flick J, Kelly J, Brown-Whitehorn T, Mamula P, Markowitz JE. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 93.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of Dietary Interventions for Inducing Histologic Remission in Patients With Eosinophilic Esophagitis: A Systematic Review and Meta-analysis. Gastroenterology. 2014;146:1639–48. doi: 10.1053/j.gastro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112:796–7. doi: 10.1016/s0091-6749(03)01715-9. [DOI] [PubMed] [Google Scholar]
- 95.Moawad FJ, Veerappan GR, Lake JM, Maydonovitch CL, Haymore BR, Kosisky SE, Wong RK. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. 2010;31:509–15. doi: 10.1111/j.1365-2036.2009.04199.x. [DOI] [PubMed] [Google Scholar]
- 96.Fahey L, Robinson G, Weinberger K, Giambrone AE, Solomon AB. Correlation Between Aeroallergen Levels and New Diagnosis of Eosinophilic Esophagitis in New York City. J Pediatr Gastroenterol Nutr. 2017;64:22–25. doi: 10.1097/MPG.0000000000001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Almansa C, Devault KR, Achem SR. A comprehensive review of eosinophilic esophagitis in adults. J Clin Gastroenterol. 2011;45:658–64. doi: 10.1097/MCG.0b013e318211f95b. [DOI] [PubMed] [Google Scholar]
- 98.Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41:451–3. doi: 10.1097/01.mcg.0000248019.16139.67. [DOI] [PubMed] [Google Scholar]
- 99.Jensen ET, Shah ND, Hoffman K, Sonnenberg A, Genta RM, Dellon ES. Seasonal variation in detection of oesophageal eosinophilia and eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;42:461–9. doi: 10.1111/apt.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. 2012;107:698–706. doi: 10.1038/ajg.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jensen ET, Hoffman K, Shaheen NJ, Genta RM, Dellon ES. Esophageal eosinophilia is increased in rural areas with low population density: results from a national pathology database. Am J Gastroenterol. 2014;109:668–75. doi: 10.1038/ajg.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elitsur Y, Aswani R, Lund V, Dementieva Y. Seasonal distribution and eosinophilic esophagitis: the experience in children living in rural communities. J Clin Gastroenterol. 2013;47:287–8. doi: 10.1097/MCG.0b013e31826df861. [DOI] [PubMed] [Google Scholar]
- 103.Jensen ET, Bertelsen RJ. Assessing Early Life Factors for Eosinophilic Esophagitis: Lessons From Other Allergic Diseases. Curr Treat Options Gastroenterol. 2016;14:39–50. doi: 10.1007/s11938-016-0083-1. [DOI] [PubMed] [Google Scholar]
- 104.Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T, Dellon ES. Early Life Exposures as Risk Factors For Pediatric Eosinophilic Esophagitis: A Pilot and Feasibility Study. J Pediatr Gastroenterol Nutr. 2013;57:67–71. doi: 10.1097/MPG.0b013e318290d15a. [DOI] [PubMed] [Google Scholar]
- 105.Radano MC, Yuan Q, Katz A, Fleming JT, Kubala S, Shreffler W, Keet CA. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2014;2:475–477. e1. doi: 10.1016/j.jaip.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 106.Jensen ET, Kuhl J, Martin LJ, Rothenberg ME, Dellon ES. Prenatal, antenatal, and early life factors are associated with risk of eosinophilic esophagitis. Gastroenterology. 2016;150(Suppl):S135–S136. (Ab 664) [Google Scholar]
- 107.Slae M, Persad R, Leung AJ, Gabr R, Brocks D, Huynh HQ. Role of Environmental Factors in the Development of Pediatric Eosinophilic Esophagitis. Dig Dis Sci. 2015;60:3364–72. doi: 10.1007/s10620-015-3740-7. [DOI] [PubMed] [Google Scholar]
- 108.Muir AB, Benitez AJ, Dods K, Spergel JM, Fillon SA. Microbiome and its impact on gastrointestinal atopy. Allergy. 2016;71:1256–63. doi: 10.1111/all.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dellon ES, Peery AF, Shaheen NJ, Morgan DR, Hurrell JM, Lash RH, Genta RM. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology. 2011;141:1586–92. doi: 10.1053/j.gastro.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elitsur Y, Alrazzak BA, Preston D, Demetieva Y. Does Helicobacter pylori Protect against Eosinophilic Esophagitis in Children? Helicobacter. 2014;19:367–71. doi: 10.1111/hel.12129. [DOI] [PubMed] [Google Scholar]
- 111.Furuta K, Adachi K, Aimi M, Ishimura N, Sato S, Ishihara S, Kinoshita Y. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J Clin Biochem Nutr. 2013;53:60–2. doi: 10.3164/jcbn.13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.von Arnim U, Wex T, Link A, Messerschmidt M, Venerito M, Miehlke S, Malfertheiner P. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43:825–30. doi: 10.1111/apt.13560. [DOI] [PubMed] [Google Scholar]
- 113.Sonnenberg A, Dellon ES, Turner KO, Genta RM. The influence of Helicobacter pylori on the ethnic distribution of esophageal eosinophilia. Helicobacter. 2016 doi: 10.1111/hel.12370. [DOI] [PubMed] [Google Scholar]
- 114.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–7. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 115.Squires KA, Cameron DJ, Oliver M, da Fonseca Junqueira JC. Herpes simplex and eosinophilic oesophagitis: the chicken or the egg? J Pediatr Gastroenterol Nutr. 2009;49:246–50. doi: 10.1097/MPG.0b013e31817b5b73. [DOI] [PubMed] [Google Scholar]
- 116.Monsanto P, Almeida N, Cipriano MA, Gouveia H, Sofia C. Concomitant herpetic and eosinophilic esophagitis--a causality dilemma. Acta Gastroenterol Belg. 2012;75:361–3. [PubMed] [Google Scholar]
- 117.Zimmermann D, Criblez DH, Dellon ES, Bussmann C, Pfeifer D, Froh M, Straumann A. Acute Herpes Simplex Viral Esophagitis Occurring in 5 Immunocompetent Individuals With Eosinophilic Esophagitis. ACG Case Rep J. 2016;3:165–8. doi: 10.14309/crj.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Srivastava MDM. Pneumoniae Is a Potential Trigger for Eosinophilic Esophagitis. J Allergy Clin Immunol. 2013;131(Suppl):AB177. [Google Scholar]
- 119.Burk CM, Beitia R, Lund PK, Dellon ES. High rate of galactose-alpha-1,3-galactose sensitization in both eosinophilic esophagitis and patients undergoing upper endoscopy. Dis Esoph. 2016;29:558–62. doi: 10.1111/dote.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ridolo E, De Angelis GL, Dall’aglio P. Eosinophilic esophagitis after specific oral tolerance induction for egg protein. Ann Allergy Asthma Immunol. 2011;106:73–4. doi: 10.1016/j.anai.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 121.Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Martinez-Gomez MJ, Ibanez MD. Possible eosinophilic esophagitis induced by milk oral immunotherapy. J Allergy Clin Immunol. 2012;129:1155–7. doi: 10.1016/j.jaci.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 122.Miehlke S, Alpan O, Schroder S, Straumann A. Induction of eosinophilic esophagitis by sublingual pollen immunotherapy. Case Rep Gastroenterol. 2013;7:363–8. doi: 10.1159/000355161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Burk CM, Dellon ES, Steele PH, Virkud YV, Kulis M, Burks AW, Vickery BP. Eosinophilic esophagitis during peanut oral immunotherapy with omalizumab. J Allergy Clin Immunol Pract. 2017;5:489–501. doi: 10.1016/j.jaip.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113:624–9. doi: 10.1016/j.anai.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 125.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Merwat SN, Spechler SJ. Might the use of acid-suppressive medications predispose to the development of eosinophilic esophagitis? Am J Gastroenterol. 2009;104:1897–902. doi: 10.1038/ajg.2009.87. [DOI] [PubMed] [Google Scholar]
- 127.Slack MA, Ogbogu PU, Phillips G, Platts-Mills TA, Erwin EA. Serum vitamin D levels in a cohort of adult and pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2015;115:45–50. doi: 10.1016/j.anai.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Verzegnassi F, Bua J, De Angelis P, Dall’oglio L, Di Leo G, Ventura A. Eosinophilic oesophagitis and coeliac disease: is it just a casual association? Gut. 2007;56:1029–1030. doi: 10.1136/gut.2006.117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thompson JS, Lebwohl B, Reilly NR, Talley NJ, Bhagat G, Green PH. Increased incidence of eosinophilic esophagitis in children and adults with celiac disease. J Clin Gastroenterol. 2012;46:e6–e11. doi: 10.1097/MCG.0b013e318221aefd. [DOI] [PubMed] [Google Scholar]
- 130.Lucendo AJ, Arias A, Tenias JM. Systematic review: the association between eosinophilic oesophagitis and coeliac disease. Aliment Pharmacol Ther. 2014;40:422–34. doi: 10.1111/apt.12859. [DOI] [PubMed] [Google Scholar]
- 131.Lucendo AJ, Arias A, Perez-Martinez I, Lopez-Vazquez A, Ontanon-Rodriguez J, Gonzalez-Castillo S, De Rezende LC, Rodrigo L. Adult patients with eosinophilic esophagitis do not show an increased frequency of the HLA-DQ2/DQ8 genotypes predisposing to celiac disease. Dig Dis Sci. 2011;56:1107–11. doi: 10.1007/s10620-010-1383-2. [DOI] [PubMed] [Google Scholar]
- 132.Jensen ET, Eluri S, Lebwohl B, Genta RM, Dellon ES. Increased risk of esophageal eosinophilia and eosinophilic esophagitis in patients with active celiac disease on biopsy. Clin Gastroenterol Hepatol. 2015;13:1426–31. doi: 10.1016/j.cgh.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abonia JP, Wen T, Stucke EM, Grotjan T, Griffith MS, Kemme KA, Collins MH, Putnam PE, Franciosi JP, von Tiehl KF, Tinkle BT, Marsolo KA, Martin LJ, Ware SM, Rothenberg ME. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2013;132:378–86. doi: 10.1016/j.jaci.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jensen ET, Martin CF, Shaheen NJ, Kappelman MD, Dellon ES. High prevalence of coexisting autoimmune conditions among patients with eosinophilic esophagitis. Gastroenterology. 2013;144(Suppl 1):S491. (Su1852) [Google Scholar]
- 135.Peterson K, Firszt R, Fang J, Wong J, Smith KR, Brady KA. Risk of Autoimmunity in EoE and Families: A Population-Based Cohort Study. Am J Gastroenterol. 2016;111:926–32. doi: 10.1038/ajg.2016.185. [DOI] [PubMed] [Google Scholar]
- 136.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, Oliva-Hemker M, Wood RA, Dietz HC. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5:195ra94. doi: 10.1126/scitranslmed.3006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taft TH, Kern E, Keefer L, Burstein D, Hirano I. Qualitative assessment of patient-reported outcomes in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2011;45:769–74. doi: 10.1097/MCG.0b013e3182166a5a. [DOI] [PubMed] [Google Scholar]
- 138.Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11. 5 years. Gastroenterology. 2003;125:1660–9. doi: 10.1053/j.gastro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 139.Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, Schoepfer A, Simon HU. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9. e1. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 140.Menard-Katcher P, Marks KL, Liacouras CA, Spergel JM, Yang YX, Falk GW. The natural history of eosinophilic oesophagitis in the transition from childhood to adulthood. Aliment Pharmacol Ther. 2013;37:114–21. doi: 10.1111/apt.12119. [DOI] [PubMed] [Google Scholar]
- 141.Bohm M, Jacobs JW, Jr, Gupta A, Gupta S, Wo JM. Most children with eosinophilic esophagitis have a favorable outcome as young adults. Dis Esophagus. 2017;30:1–6. doi: 10.1111/dote.12454. [DOI] [PubMed] [Google Scholar]
- 142.Podboy A, Pierce K, Mara K, Geno DM, Alexander J. A 10-year follow-up study of the effects of treatment on the progression of eosinophilic esophagitis in adults. Am J Gastroenterol. 2016;111(Suppl 1):S186. (ab 421) [Google Scholar]
- 143.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–85. e4. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, Straumann A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–1236. e2. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 145.Lipka S, Kumar A, Richter JE. Impact of Diagnostic Delay and Other Risk Factors on Eosinophilic Esophagitis Phenotype and Esophageal Diameter. J Clin Gastroenterol. 2016;50:134–40. doi: 10.1097/MCG.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 146.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, Liacouras CA. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–6. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 147.DeBrosse CW, Franciosi JP, King EC, Butz BK, Greenberg AB, Collins MH, Abonia JP, Assa’ad A, Putnam PE, Rothenberg ME. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol. 2011;128:132–8. doi: 10.1016/j.jaci.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, Kramer RE, Collins MH, Stucke E, Mangeot C, Jackson WD, O’Gorman M, Abonia JP, Pentiuk S, Putnam PE, Rothenberg ME. Efficacy, Dose Reduction, and Resistance to High-dose Fluticasone in Patients with Eosinophilic Esophagitis. Gastroenterology. 2014;147:324–33. e5. doi: 10.1053/j.gastro.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miehlke S, Hruz P, Vieth M, Bussmann C, von Arnim U, Bajbouj M, Schlag C, Madisch A, Fibbe C, Wittenburg H, Allescher HD, Reinshagen M, Schubert S, Tack J, Muller M, Krummenerl P, Arts J, Mueller R, Dilger K, Greinwald R, Straumann A. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut. 2016;65:390–9. doi: 10.1136/gutjnl-2014-308815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schoepfer AM, Gonsalves N, Bussmann C, Conus S, Simon HU, Straumann A, Hirano I. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–70. doi: 10.1038/ajg.2009.657. [DOI] [PubMed] [Google Scholar]
- 151.Runge TM, Eluri S, Woosley JT, Shaheen NJ, Dellon ES. Control of inflammation decreases the need for subsequent esophageal dilation in patients with eosinophilic esophagitis. Dis Esoph. 2017 doi: 10.1093/dote/dox042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lucendo AJ, Arias A, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2016;14:13–22. e1. doi: 10.1016/j.cgh.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 153.Koutlas NT, Dellon ES. Progression from an inflammatory to a fibrostenotic phenotype in eosinophilic esophagitis. Case Rep Gastroenterol. 2017 doi: 10.1159/000477391. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, DeVault KR, Wolfsen H, Raimondo M, Guarderas JC, Achem SR. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104:828–33. doi: 10.1038/ajg.2008.169. [DOI] [PubMed] [Google Scholar]
- 155.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777–82. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 156.Peterson KA, Byrne KR, Vinson LA, Ying J, Boynton KK, Fang JC, Gleich GJ, Adler DG, Clayton F. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol. 2013;108:759–66. doi: 10.1038/ajg.2012.468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.