Abstract
Objective
The infant temperament behavioral inhibition is a potent risk factor for development of an anxiety disorder. It is difficult to predict risk for behavioral inhibition at birth, however, and the neural underpinnings are poorly understood. We hypothesized that neonatal functional connectivity of the ventral attention network relates to behavioral inhibition at age 2 years beyond sociodemographic and familial factors. This hypothesis is supported by the ventral attention network’s role in attention to novelty, a key feature of behavioral inhibition.
Method
Using a longitudinal design (n=45), we measured functional connectivity in neonates with magnetic resonance imaging and behavioral inhibition at age 2 years using the Infant Toddler Social Emotional Assessment. We computed whole-brain connectivity maps for regions from the ventral attention, default mode, and salience networks. Regression analyses related these maps to behavioral inhibition at age two, co-varying for sex, social risk, and motion during scanning.
Results
Decreased neonatal functional connectivity of three connections was associated with increased behavioral inhibition at age two. One connection (between the right ventrolateral prefrontal cortex and right temporal-parietal junction) included the ventral attention network seed, while two connections (between the medial prefrontal cortex and both the right superior parietal lobule and the left lateral occipital cortex) included the default mode network seed.
Conclusion
Neonatal functional connectivity of the ventral attention and default mode networks is associated with behavioral inhibition at age two years. These results inform the developmental neurobiology of behavioral inhibition and anxiety disorders and may aid in early risk assessment and intervention.
Keywords: fMRI, infant, neonatal, behavioral inhibition, anxiety, ventral attention network
INTRODUCTION
Anxiety disorders are the most common class of psychiatric disorders and are often associated with significant functional impairment (1). Symptoms of anxiety disorders can begin in infancy or toddlerhood (2), the median age of onset of anxiety disorders is around 6–11 years (3), and most adults with anxiety disorders experienced symptoms during childhood (4). The infant temperament behavioral inhibition, characterized by increased attention and distress to novel stimuli (5, 6), is one of the most potent known risk factors for developing an anxiety disorder later in life (7). This continuity between infant temperament, symptoms in early childhood, and disorders in adulthood suggests that behavioral inhibition and anxiety disorders represent an early departure from typical brain development (8). Unfortunately, the timing and nature of this atypical brain development are poorly understood. Moreover, it is difficult to predict at birth which neonates will exhibit high behavioral inhibition during infancy (6), and behaviors in the first year of life are only moderately predictive of behavioral inhibition in boys and not predictive of behavioral inhibition in girls at age 2 years (9). Consequently, at-risk children may not be identified until many years after the onset of atypical brain development. The current study addresses these issues by identifying brain network variation near birth that is related to levels of behavioral inhibition at age two years.
Indirect evidence supports the hypothesis that the physiology of behavioral inhibition during infancy includes an overactive ventral attention network. The ventral attention network is a cortical functional brain network involved in directing attention to novel, unexpected stimuli (e.g., a stranger or a new toy) and includes portions of the right ventrolateral prefrontal cortex and right temporal-parietal junction (10). Infants with high behavioral inhibition demonstrate indirect behavioral and physiological signs of an overactive ventral attention network, including increased attention and increased right lateralized brain activity (as measured with EEG) following the presentation of novel stimuli such as toys or a stranger (11, 12). Reports of ventral attention network structural and functional abnormalities in older children and adults with anxiety disorders provide further support for this hypothesis (13, 14).
While the ventral attention network may play a role in behavioral inhibition during infancy, previous work indicates that many other brain regions and systems are associated with behavioral inhibition in older children and adults. Adolescents and adults who had high behavioral inhibition during early childhood, for example, have increased amygdala reactivity to novelty (15, 16) and altered striatal activity in response to reward (17, 18) relative to peers. Similarly, older children and adults who had high behavioral inhibition during early childhood have altered functional connectivity within and between the default mode, salience, and other brain networks (19); as well as altered connectivity between the amygdala and cortical regions that may be within these same networks (20, 21). While amygdala functional connectivity alterations associated with behavioral inhibition are apparent near birth (22), it is unknown whether altered connectivity within and between cortical brain networks is also already evident in the neonatal period. Given the central role of functional brain networks in brain organization and information processing (23), uncovering disruptions that are already present at birth is likely to have fundamental implications for the developmental neurobiology of behavioral inhibition and anxiety disorders.
The goal of this study was to identify variation in cortical functional brain networks evident soon after birth that is related to behavioral inhibition at age 2 years, in order to elucidate the neural substrate of behavioral inhibition and identify early risk factors. To achieve this goal, we used functional magnetic resonance imaging (fMRI) to relate variation in resting state functional connectivity of brain regions putatively within the ventral attention, default mode, and salience networks in 45 neonates at term-equivalent to behavioral inhibition measures at age 2 years. This prospective, longitudinal approach provides an unprecedented window into the initial stages of the developmental neurobiology of behavioral inhibition and anxiety disorders. By identifying variation in brain network connectivity shortly after birth and before temperamental characteristics of behavioral inhibition emerge, the results from this study most likely reflect variation in fetal brain development. Results cannot be attributed to early childhood experiences that are a consequence of having a behavioral inhibited temperament. Based on the indirect evidence above relating behavioral inhibition to the ventral attention network during infancy, our primary hypothesis was that functional connectivity of the ventral attention network at term equivalent would be associated with behavioral inhibition at age 2 years. We also tested whether functional connectivity of the default mode and salience networks was associated with behavioral inhibition, because of the role of these networks in older children and adults with behavioral inhibition. We previously related functional connectivity of the amygdala during the neonatal period to internalizing symptoms (including behavioral inhibition) at age 2 in a subset of this cohort, demonstrating feasibility of our approach (22). To ensure that our primary analysis was sensitive to the strongest brain-behavior relations, we performed two follow-up analyses. In a “confirmatory analysis”, we related functional connectivity among 18 regions (comprising 153 region-region pairs) at term equivalent to behavioral inhibition at age 2 years. The regions for this confirmatory analysis are frequently implicated in psychiatric illnesses and are distributed across the brain (13, 14, 19, 20, 24–26). In a final “exploratory analysis”, we examined similar relations among 216 regions, covering most of the cerebral cortex and much of the subcortex and comprising 23,220 region pairs. The primary, confirmatory, and exploratory analyses converged on a consistent set of results, suggesting that our primary, hypothesis-driven analysis detected the strongest relations between neonatal functional connectivity and behavioral inhibition at age 2 years.
METHODS
Subjects
This study was approved by the Washington University Human Studies Committee. Parental written informed consent was obtained for all subjects recruited from Washington University School of Medicine hospitals. A group of preterm infants (n=27; born at gestational age ≤30 weeks) were recruited from St. Louis Children’s Hospital Neonatal Intensive Care Unit, and a group of term infants (n=18; born at gestational age ≥36 weeks) were recruited from the adjoining mother-baby unit at Barnes-Jewish Hospital. Full-term infants underwent magnetic resonance imaging (MRI) within four days of birth and were scanned at mean post-menstrual age 39.7 weeks (±1.0 weeks). Preterm infants underwent MRI at term equivalent post-menstrual age 37.5 weeks (±1.3 weeks). Sample demographics are described in Table 1. Full inclusion and exclusion criteria, a comparison of included versus excluded subjects, and MRI acquisition parameters are provided in the Supplementary Materials.
Table 1.
Demographic Characteristics
| Male, n | 21 | 46.7% |
| Female, n | 24 | 53.3% |
|
| ||
| Race, n | ||
| Black | 21 | 46.7% |
| White | 20 | 44.4% |
| Asian | 3 | 6.7% |
| Biracial | 1 | 2.2% |
|
| ||
| Hispanic, n | 1 | 2.2% |
|
| ||
| Preterm Birth, n | 27 | 60.0% |
|
| ||
| Age at 2 year assessment, months (SD) | 28.4 (3.4) | |
|
| ||
| Behavioral Inhibition, mean (SD) | 0.95 (0.59) | |
|
| ||
| Social Risk Score, n | ||
| 0 | 19 | 42.2% |
| 1 | 5 | 11.1% |
| 2 | 7 | 15.6% |
| 3 | 10 | 22.2% |
| 4 | 4 | 8.9% |
|
| ||
| History of Maternal Affective Disorder, n | 22 | 48.9% |
Follow-up at Age Two Years
Age 2 assessments included the Infant Toddler Social Emotional Assessment (ITSEA), a 166-item parent-report measure with good psychometric properties (27). Based on study hypotheses, this analysis focused on the behavioral inhibition subscale of the ITSEA, which has acceptable internal consistency (Cronbach’s alpha = 0.77) and is within the internalizing domain. Prior work indicates that ITSEA ratings, including behavioral inhibition, correlate significantly with direct observations of infant behaviors by trained evaluators (27, 28). ITSEA behavioral inhibition scores are also highly correlated with the inhibition to novelty score of the Infant Behavior Questionnaire (IBQ), another commonly used measure of infant temperament (28). We also examined several factors that could be associated with infant behavioral inhibition including social disadvantage (29) and a maternal history of affective symptoms (30). Details of these measures are provided in the Supplementary Methods. Zero-order relations among all variables of interest in the current study are described in the Supplementary Results and Supplementary Table 1.
MRI Preprocessing
Resting state fMRI data were preprocessed with in-house software (ftp://imaging.wustl.edu/pub/raichlab/4dfp_tools/); FSL was also used for field map correction. Magnetization inhomogeneity-related distortions were corrected using a mean field map technique (31). Atlas transformation was computed using infant templates. Volumetric time series in adult Talairach atlas space (3×3×3 mm voxels) were generated, combining field map correction and atlas transformation in a single re-sampling step. Additional preprocessing included regression of nuisance waveforms derived from rigid body motion correction, cerebrospinal fluid and white matter regions, plus whole brain global signal. The data were low-pass filtered (<0.08 Hz) and spatially smoothed. Frames affected by movement (volume-to-volume head displacement ≥0.25 mm) were excluded from the resting state fMRI computations (“scrubbing”) (32). A minimum of 5 minutes of fMRI data, excluding censored frames, was required for inclusion in the analysis. Forty-five subjects survived this stringent volume-censoring threshold.
Regions of Interest
Regions-of-interest were selected from a commonly used set of regions (the “Power 264 set”) in which the functional network identities in adults are well-established (23) and which have been used in prior studies of neonates (33). The specific regions are listed in Supplementary Table 2. For the primary, hypothesis-driven analysis, we selected a region in the right ventrolateral prefrontal cortex for the ventral attention network, dorsal anterior cingulate cortex for the salience network, and medial prefrontal cortex for the default mode network. We chose these three particular seeds because they have been consistently implicated in prior studies of behavioral inhibition and anxiety disorders (13, 14, 19, 20, 24–26). Mean connectivity maps for these seed regions are presented in Supplementary Figure 1 and support the functional network assignments. For the confirmatory region-to-region based analyses, we examined relations among 18 regions from the Power 264 set that are distributed across the brain and are representative of five functional brain networks frequently implicated in neuropsychiatric diseases. These 18 regions define 153 region pairs (each of the 18 ROIs can be correlated with the 17 others, then dividing by two to avoid double counting). For a final exploratory analysis (see Supplemental Materials), we examined relations among all 214 regions in the Power 264 set that can be accurately mapped to the infant brain, plus individualized left and right amygdala regions that were the focus of a previous study using the current dataset (22). These 216 regions define 23,220 region-to-region pairs.
Statistical Analyses
Whole-brain voxel-wise analyses were performed using in-house software (www.nil.wustl.edu/labs/fidl/). All other analyses were conducted with SPSS version 22 (Armonk, NY, USA). For the primary, hypothesis-driven analysis, functional connectivity of each of the three seed regions (right ventrolateral prefrontal cortex, medial prefrontal cortex, and dorsal anterior cingulate cortex) to every other voxel in the brain was computed in each subject. Correlation coefficients were Fisher z-transformed, generating z(r) correlation maps; average maps are depicted in Supplementary Figure 1. These voxelwise whole-brain functional connectivity maps were the dependent variables in a series of regression analyses that included behavioral inhibition, sex, social risk, behavioral inhibition × sex interaction, behavioral inhibition × social risk interaction, and post-processing residual average framewise displacement. Sex and social risk were included because they were related to behavioral inhibition (see results). The three resulting voxelwise maps corresponding to behavioral inhibition in the regression model (one for each seed region) were masked to include only voxels in cortical gray matter. Each map was multiple comparisons corrected using AFNI tools as currently recommended (34, 35). Further details are provided in the Supplementary Methods.
To test whether our primary seed-based analysis was sensitive to the strongest brain-behavior relationships, we performed a confirmatory region-to-region analysis. This analysis was done to ensure that our selection of three seeds for the primary analysis captured the functional connections at term most strongly associated with behavioral inhibition at age 2 years. For this confirmatory analysis, we first computed functional connectivity for each of the 153 region pairs for each subject. Next, we performed the identical regression analysis described above. Standardized betas were computed for the association between behavioral inhibition at age 2 years and functional connectivity of each region pair at term. Betas that were significant at p<0.05, uncorrected, were examined in order to determine whether the hypothesis-driven seed-based analysis above captured the most relevant relationships between behavioral and functional connectivity. To further contextualize findings, we performed an additional exploratory analysis examining relations among 216 regions (comprising 23,220 region pairs); these exploratory results are described in the Supplementary Material.
RESULTS
Sample demographics are described in Table 1 and zero-order relations among variables measured in this study are delineated in Supplementary Table 1. Behavioral inhibition at age 2 years was significantly negatively associated with social risk scores at age 2 years (r=−0.34, p=0.02), and females had (non-significant) greater behavioral inhibition relative to males (mean difference 0.3 points, t(43)=2.0, p=0.06). History of preterm birth and history of maternal affective disorders were unrelated to behavioral inhibition at age 2 years (p>0.7 in both cases). Motion during neonatal scanning after frame censoring was also unrelated to behavioral inhibition at age 2 years (p>0.4). Based on these results, all imaging analyses below include sex, social risk, the interaction between sex and behavioral inhibition, the interaction between social risk and behavioral inhibition, and post-processing motion (framewise displacement) as covariates.
Hypothesis-Driven Seed-based Functional Connectivity Analyses
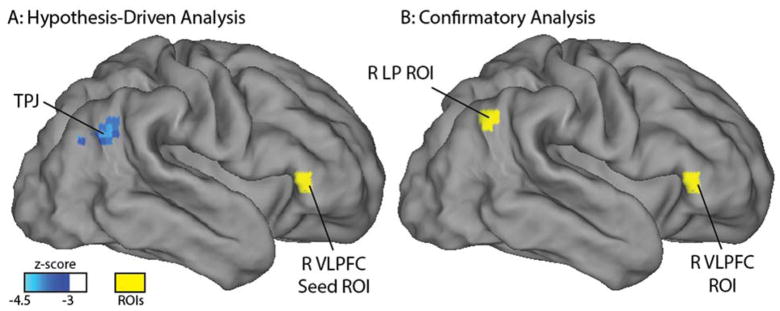
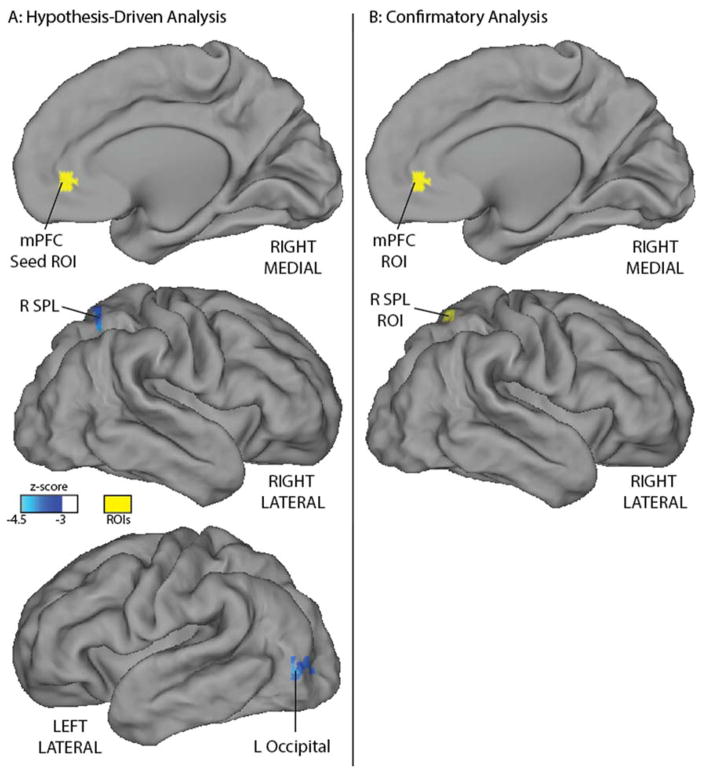
As depicted in Figure 1A, functional connectivity between the right ventrolateral prefrontal cortex seed region (located in the ventral attention network in adults) and a cluster near the right temporal-parietal junction was negatively associated with behavioral inhibition at age 2 years (−4.3 peak z-score, cluster size 1323 mm3 with all voxels z<−3.0, centered at +51 −60 +27 in Talairach coordinates). As depicted in Figure 2A, neonatal resting state functional connectivity between the medial prefrontal cortex (located in the default mode network in adults) and 2 distinct clusters was negatively associated with behavioral inhibition at age 2 years. These clusters were located within the right superior parietal lobule (−4.1 peak z-score, cluster size 1188 mm3, centered at +26 −59 +52) and in the left lateral occipital cortex (−4.0 peak z-score, cluster size 1053 mm3, centered at −42 −81 −2). The whole-brain analysis with the dorsal anterior cingulate cortex seed (from the salience network in adults) did not detect any relations surviving multiple comparison correction.
Figure 1.
Both hypothesis-driven and confirmatory analyses indicate that ventral attention network resting state functional connectivity evident soon after birth birth is related to behavioral inhibition at age 2 years. Panel A depicts a whole-brain regression relating functional connectivity soon after birth between a right ventrolateral prefrontal cortex region-of-interest (in yellow) and the rest of the brain to behavioral inhibition at age 2 years. The regression controls for sex, social risk, post-processing residual motion, the interaction between sex and behavioral inhibition, and the interaction between social risk and behavioral inhibition; and includes stringent whole-brain multiple comparisons correction. Panel B depicts the two regions from the confirmatory analysis in which functional connectivity at term equivalent was most strongly related to behavioral inhibition at age 2 years (as determined by the highest standardized beta weight), controlling for the same factors as in the hypothesis-driven analysis. The two analyses converge on a similar result – functional connectivity between the right ventrolateral prefrontal cortex and a region near the right temporal parietal junction -- right lateral parietal cortex is related to age 2 years behavioral inhibition. Note that this right temporal parietal junction location overlaps with the seed map of the right ventrolateral prefrontal cortex (see Supplementary Figure 1A), suggesting that this location is within the ventral attention network. TPJ: temporal parietal junction; R LP: right lateral parietal; R VLPFC: right ventrolateral prefrontal cortex; ROI: region-of-interest.
Figure 2.
Both hypothesis-driven and confirmatory analyses indicate that neonatal resting state functional connectivity of a default mode network seed is related to behavioral inhibition at age 2 years. Panel A depicts a whole-brain regression relating functional connectivity soon after birth birth between a medial prefrontal cortex region-of-interest (in yellow) and the rest of the brain to behavioral inhibition at age 2 years, controlling for the same covariates and including the same multiple comparison correction as in Figure 1. Panel B depicts the region pair from the confirmatory analysis that had the second highest effect size relating functional connectivity near birth to behavioral inhibition at age 2 years, with the same covariates. Both the hypothesis-driven and confirmatory analysis identified a connection between the medial prefrontal cortex and right superior parietal lobule that is related to age 2 behavioral inhibition. The hypothesis-driven analysis additionally identified a significant relation between behavioral inhibition and resting state connectivity between the medial prefrontal cortex and left lateral occipital cortex. mPFC: medial prefrontal cortex; R SPL: right superior parietal lobule; ROI: region-of-interest.
Confirmatory Region-of-Interest Functional Connectivity Analyses
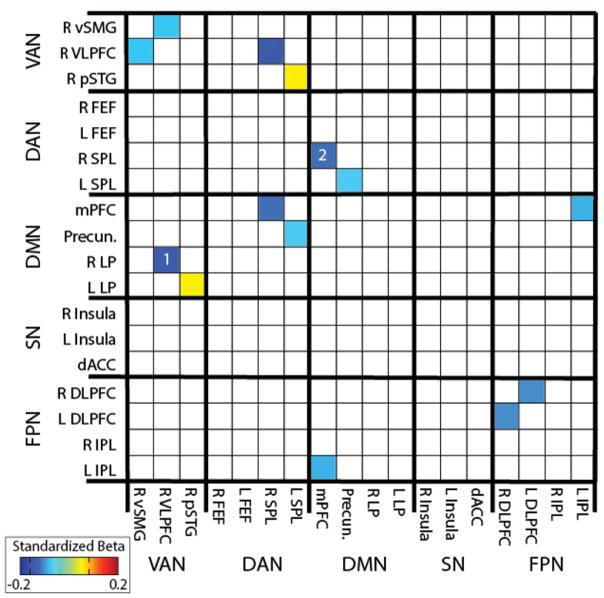
We performed an additional confirmatory analysis to verify that the three seeds we selected for the primary analysis captured the strongest relations between functional connectivity at term-equivalent and behavioral inhibition at age 2 years. For this analysis, the goal was to determine whether we would have obtained stronger results if we had chosen to use other seed regions that are commonly used in studies of neuropsychiatric disorders. In this confirmatory analysis, we examined functional connectivity among 18 regions-of-interest (yielding 153 region-region pairs) from five functional brain networks commonly implicated in neuropsychiatric illnesses; three of these 18 regions had been used as seeds in the hypothesis-driven analysis described above. Figure 2 depicts standardized beta weights for the regression analyses relating each of these region-region pair functional connectivity values during the neonatal period to behavioral inhibition at age 2 years. Only standardized beta weights in which p<0.05 are presented. Of the 153 region pairs examined, the region pair with largest effect size included the right ventrolateral prefrontal cortex region that had been used as a seed for the ventral attention network and a region in the right lateral parietal cortex, near the temporal-parietal junction, that maps to the default mode network in adults (standardized beta = −0.14, indicating that for every 1 standard deviation increase in behavioral inhibition, the connectivity value decreases by 0.14). The region pair with the second largest effect size included the medial prefrontal cortex region that had been used as a seed for the default mode network and a region in the right superior parietal lobule that maps to the dorsal attention network (standardized beta = −0.11). Figures 1B and 2B depict these 2 connections with results from the whole-brain analyses side by side, to illustrate that both the primary and confirmatory analyses converge on very similar results. In the Supplementary Results and Supplementary Figure 2, results from the whole-brain, hypothesis-driven analyses are further contextualized within an exploratory analysis examining relations among 216 regions-of-interest that cover most of the brain (yielding 23,220 region pairs).
DISCUSSION
The goal of this study was to examine associations between functional connectivity of cortical functional brain networks evident soon after birth and behavioral inhibition at age two years. Hypothesis-driven and confirmatory analyses converged on a common set of results: functional connectivity of a right ventrolateral prefrontal cortex seed (which maps to the ventral attention network in adults) and a medial prefrontal cortex seed (which maps to the default mode network in adults) near birth was associated with behavioral inhibition at age 2 years. Notably, neonatal connectivity was related to behavioral inhibition at age 2 years beyond information provided by socio-demographic factors, as all analyses controlled for these other factors.
The current results provide the first direct evidence for the previously hypothesized link between the ventral attention network and behavioral inhibition. Previous authors have hypothesized that behavioral inhibition is associated with an overactive ventral attention network because behavioral inhibition includes increased attention to novel stimuli, the putative function of the ventral attention network (13, 36). In addition, infants with behavioral inhibition have increased right lateralized brain activity, as measured with EEG, in response to novel stimuli relative to peers (37); the ventral attention network is a plausible contributor to this increased activity given that it is also right-lateralized. The current results also indicate that previously identified functional connectivity alterations associated with behavioral inhibition in the default mode network in childhood and adulthood (19–21) are already evident in early infancy. Given the role of the default mode network in self-focused processes, previous authors have suggested that default mode network connectivity alterations in behavioral inhibition may reflect an increased internal focus (19); the current results suggest that the precursors of this process are evident at birth.
The continuity of the infant temperament behavioral inhibition in infancy, symptoms of anxiety in childhood, and increased risk for anxiety disorders throughout the lifespan suggest that behavioral inhibition and anxiety disorders represent an early departure from typical brain development (38). The current results suggest that this departure from typical brain development may have its origin in fetal brain development, since alterations in functional connectivity are already evident soon after birth. We previously demonstrated, in this same cohort, that functional connectivity of the amygdala during the neonatal period is associated with internalizing symptoms including behavioral inhibition at age 2 years (22). It appears, therefore, that disruptions in the ventral attention network, the default mode network, and amygdala functional connectivity may represent the initial stage in the cascade that leads to behavioral inhibition and potentially anxiety disorders. Other problems may emerge as functional connectivity continues to develop, and other forces may turn early risk into more clinically symptomatic anxiety disorders. Longitudinal studies are required to test this hypothesis.
Identifying the first stages in the pathway to an anxiety disorder provides an opportunity for early identification and intervention of high-risk individuals. Previously identified risk factors are only moderately predictive at birth of behavioral inhibition and anxiety disorders in later childhood (6, 29, 30), and so most children are not identified until many years after the initial departure from typical brain development. The current results suggest that functional connectivity soon after birth could serve as a biomarker for guiding early interventions such as parent training and early socialization of at-risk infants. Given that the current study suggests that the origins of behavioral inhibition may lie in fetal brain development, functional connectivity shortly after birth could also serve as an outcome measure for perinatal interventions targeting risk for anxiety disorders (39).
The current results should be interpreted in light of a few limitations. The borders of functional brain networks in infants are the subject of ongoing work, and so it is difficult to definitively state the functional network identities of regions. We label regions based on adult network identities, but there are almost certainly differences in functional network organization of infants versus adults. Results should be interpreted, therefore, as reflecting properties of regions that become parts of particular functional networks in later childhood and adulthood rather than definitively residing in those networks at birth. Current work generally supports the hypothesis that adult network identities can be applied to newborn data, although local connections and bilateral connections between homologous regions are much stronger than longer-range anterior/posterior connections (40). Whole-brain fMRI analyses have historically been contaminated by unacceptably high false positive rates; the current study utilized a conservative correction criterion defined by our own dataset as currently recommended by experts (34, 35). Contamination of functional connectivity by sub-millimeter frame-to-frame motion is also a major source of artifacts (32); we used strict motion-correction procedures in this study. Finally, behavioral inhibition was assessed by maternal report, and these results could be extended by combining maternal report with laboratory observational measures.
In summary, decreased functional connectivity of regions within the ventral attention and default mode networks at term equivalent was associated with behavioral inhibition at age 2 years. Neonatal functional connectivity was related to behavioral inhibition beyond socio-demographic factors including sex and social risk. These data potentially identify the earliest stage of the neurodevelopmental cascade leading to behavioral inhibition and anxiety disorders. These results have fundamental implications for the neurobiology of anxiety disorders and could aid early risk stratification and intervention.
Supplementary Material
Figure 3.
Confirmatory analysis relating functional connectivity among 18 regions-of-interest at term equivalent to behavioral inhibition at age 2 years. The color of each square in the 18 × 18 matrix represents the standardized beta weight of the relation between functional connectivity and behavioral inhibition, controlling for sex, social risk, residual motion, sex × behavioral inhibition interaction, and social risk × behavioral inhibition interaction. Only beta weights in which p<0.05, uncorrected, are depicted. White numbers indicate the two connections with the largest effect sizes relating functional connectivity to behavioral inhibition; similar connections were identified in the hypothesis-driven analysis. VAN: ventral attention network; DAN: dorsal attention network; DMN: default mode network; SN: salience network; FPN: fronto-parietal network; R: right; L: left; vSMG: ventral supramarginal gyrus; VLPFC: ventrolateral prefrontal cortex; pSTG: posterior superior temporal gyrus; FEF: frontal eye field; SPL: superior parietal lobule; mPFC: medial prefrontal cortex; Precun: precuneus; LP: lateral parietal; dACC: dorsal anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; IPL: inferior parietal lobule.
Acknowledgments
GRANT FUNDING
This work was supported by the National Institutes of Health (K23MH109983, K23MH105179, R01HD057098, R01HD061619, KL2TR000250, UL1TR000448, K02NS089852, R01NS06424, R01NS32970), Intellectual and Developmental Disabilities Research Center at Washington University (P30HD062171), McDonnell Center for Systems Neuroscience, Taylor Family Institute, Parker Fund for Young Investigators in Psychiatry, McDonnell Foundation Collaborative Activity Award, Child Neurology Foundation, Cerebral Palsy International Research Foundation, Dana Foundation, and Doris Duke Foundation.
Footnotes
DISCLOSURES
The authors have no conflicts of interest to report.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard KR, Aase H, Torgersen S, Reichborn-Kjennerud T, Oerbeck B, Myhre A, et al. Continuity in features of anxiety and attention deficit/hyperactivity disorder in young preschool children. European child & adolescent psychiatry. 2014;23(9):743–52. doi: 10.1007/s00787-014-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mian ND, Wainwright L, Briggs-Gowan MJ, Carter AS. An ecological risk model for early childhood anxiety: the importance of early child symptoms and temperament. J Abnorm Child Psychol. 2011;39(4):501–12. doi: 10.1007/s10802-010-9476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagan J, Reznick JS, Snidman N, Gibbons J, Johnson MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Dev. 1988;59(6):1580–9. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- 6.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 7.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1066–75. e1. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. Journal of child psychology and psychiatry, and allied disciplines. 2007;48(7):631–48. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 9.Fox NA, Snidman N, Haas SA, Degnan KA, Kagan J. The relation between reactivity at 4 months and Behavioral Inhibition in the second year: Replication Across Three Independent Samples. Infancy: the official journal of the International Society on Infant Studies. 2015;20(1):98–114. doi: 10.1111/infa.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, et al. Frontal activation asymmetry and social competence at four years of age. Child Dev. 1995;66(6):1770–84. [PubMed] [Google Scholar]
- 12.Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child development. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- 13.Sylvester CM, Barch DM, Corbetta M, Power JD, Schlaggar BL, Luby JL. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(12):1326–36. e5. doi: 10.1016/j.jaac.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35(9):527–35. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300(5627):1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35(4):1538–46. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev Psychopathol. 2014;26(1):229–43. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(24):6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taber-Thomas BC, Morales S, Hillary FG, Pérez-Edgar KE. ALTERED TOPOGRAPHY OF INTRINSIC FUNCTIONAL CONNECTIVITY IN CHILDHOOD RISK FOR SOCIAL ANXIETY. Depression and anxiety. 2016;33(11):995–1004. doi: 10.1002/da.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy AK, Benson BE, Degnan KA, Perez-Edgar K, Pine DS, Fox NA, et al. Alterations in amygdala functional connectivity reflect early temperament. Biological psychology. 2014;103:248–54. doi: 10.1016/j.biopsycho.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clauss JA, Seay AL, VanDerKlok RM, Avery SN, Cao A, Cowan RL, et al. Structural and functional bases of inhibited temperament. Social cognitive and affective neuroscience. 2014;9(12):2049–58. doi: 10.1093/scan/nsu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers CE, Sylvester CM, Mintz C, Kenley JK, Shimony JS, Barch DM, et al. Neonatal Amygdala Functional Connectivity at Rest in Healthy and Preterm Infants and Early Internalizing Symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2017;56(2):157–66. doi: 10.1016/j.jaac.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional Network Organization of the Human Brain. Neuron. 2011;72(4):665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sylvester CM, Barch DM, Harms MP, Belden AC, Oakberg TJ, Gold AL, et al. Early Childhood Behavioral Inhibition Predicts Cortical Thickness in Adulthood. J Am Acad Child Adolesc Psychiatry. 2016;55(2):122–9. e1. doi: 10.1016/j.jaac.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 26.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. The American journal of psychiatry. 2006;163(6):1091–7. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 27.Carter AS, Briggs-Gowan MJ, Jones SM, Little TD. The Infant-Toddler Social and Emotional Assessment (ITSEA): factor structure, reliability, and validity. Journal of abnormal child psychology. 2003;31(5):495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- 28.Carter AS, Little C, Briggs-Gowan MJ, Kogan N. The infant-toddler social and emotional assessment (ITSEA): Comparing parent ratings to laboratory observations of task mastery, emotion regulation, coping behaviors, and attachment status. Infant Mental Health Journal. 1999;20(4):375–92. [Google Scholar]
- 29.Slopen N, Fitzmaurice G, Williams DR, Gilman SE. Poverty, food insecurity, and the behavior for childhood internalizing and externalizing disorders. J Am Acad Child Adolesc Psychiatry. 2010;49(5):444–52. doi: 10.1097/00004583-201005000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Cote SM, Boivin M, Liu X, Nagin DS, Zoccolillo M, Tremblay RE. Depression and anxiety symptoms: onset, developmental course and risk factors during early childhood. J Child Psychol Psychiatry. 2009;50(10):1201–8. doi: 10.1111/j.1469-7610.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 31.Gholipour A, Kehtarnavaz N, Gopinath K, Briggs R, Panahi I. Average field map image template for Echo-Planar image analysis. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2008;2008:94–7. doi: 10.1109/IEMBS.2008.4649099. [DOI] [PubMed] [Google Scholar]
- 32.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–51. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyser CD, Dosenbach NU, Smyser TA, Snyder AZ, Rogers CE, Inder TE, et al. Prediction of brain maturity in infants using machine-learning algorithms. Neuroimage. 2016;136:1–9. doi: 10.1016/j.neuroimage.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox RW, Reynolds RC, Taylor PA. AFNI and clustering: False positive rates redux. bioRxiv [serial on the Internet] 2016 [Google Scholar]
- 35.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson HA, Pine DS, Fox NA. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40(1):207–24. doi: 10.1038/npp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Dev. 1996;67(2):523–40. [PubMed] [Google Scholar]
- 38.Pine DS, Fox NA. Childhood antecedents and risk for adult mental disorders. Annu Rev Psychol. 2015;66:459–85. doi: 10.1146/annurev-psych-010814-015038. [DOI] [PubMed] [Google Scholar]
- 39.Hunter SK, Mendoza JH, D’Anna K, Zerbe GO, McCarthy L, Hoffman C, et al. Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. Am J Psychiatry. 2012;169(6):616–24. doi: 10.1176/appi.ajp.2012.11091365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ. Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cereb Cortex. 2016;26(1):322–33. doi: 10.1093/cercor/bhu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.