Abstract
Objective
Racial discrimination is increasingly recognized as a contributor to increased cardiovascular disease risk among African Americans. Previous research has shown significant overlap between racial discrimination and hostility, an established predictor of CVD risk including alterations in adrenergic receptor functioning. The present study examined the associations of racial discrimination and hostility with adrenergic receptor responsiveness.
Methods
In a sample (N=57) of young to middle-aged African American adults (51% female) with normal and mildly elevated blood pressure (BP), a standardized isoproterenol sensitivity test (CD25) was used to evaluate β-AR responsiveness, while the dose of phenylephrine required to increase mean arterial pressure (MAP) by 25 mmHg (PD25) was used to assess α1-AR responsiveness. Racial discrimination was measured using the Perceived Racism Scale and hostility was assessed using the Cook-Medley Hostility scale.
Results
In hierarchical regression models, greater racial discrimination, but not hostility, emerged as a significant predictor of decreased β-adrenergic receptor responsiveness (β = .38, p =.004). However, moderation analysis revealed that the association between racial discrimination and blunted β-adrenergic receptor responsiveness was strongest among those with higher hostility (β = .49, 95% CI [.17, .82], p =.004). In addition, hostility, but not racial discrimination, significantly predicted α1-AR responsiveness.
Conclusions
These findings suggest racial discrimination was associated with blunted β-adrenergic receptor responsiveness, providing further evidence of the potential contribution of racial discrimination to increased CVD risk among African Americans. The adverse effects of discrimination on cardiovascular health may be enhanced in individuals with higher levels of hostility.
Keywords: Adrenergic receptor responsiveness, discrimination, hostility, African Americans
INTRODUCTION
Racial discrimination has long been considered an important factor in explaining the higher incidence of hypertension and other disparities in rates of cardiovascular disease (CVD) among African Americans (1). Evidence from population-based investigations including the Cardiovascular Risk Development in Young Adults (CARDIA) and Jackson Heart Study (2, 3) has consistently shown an association between racial discrimination and hypertension prevalence. Despite an ever-increasing number of empirical studies, understanding of the mechanisms underlying this putative relationship remains limited. Indeed, recent findings have suggested that examining BP alone may be an inadequate approach to unraveling the complex linkages between racial discrimination and CVD risk (4, 5). For example, one recent meta-analysis showed that the relationship between racial discrimination and hypertensive status was more consistent than the link between discrimination and blood pressure (BP), per se. In addition, the association between racial discrimination and BP was moderated by a number of factors including age, sex and the setting in which BP measurements were obtained (i.e. clinic vs ambulatory) (6).
Several mechanisms have been implicated as being responsible for the greater CVD risk among African Americans including diminished nitric oxide bioavailability (7) and salt-sensitivity (8). In addition, research has consistently implicated heightened sympathetic nervous system (SNS) activity (9, 10). The adrenergic receptors play an important role in regulating cardiac and vascular responses to acute and chronic stressors (11, 12). Binding of epinephrine (EP) and norepinephrine (NOR) with either α1-adrenergic receptors (α1-AR) and/or β-adrenergic receptors (β-AR), trigger changes in BP via vasoconstriction and/or vasodilation, as well as through modulation of heart rate (HR) and cardiac contractility. Over time, receptor affinity diminishes or is enhanced due to repeated or prolonged catecholamine exposure (13). The resultant pattern of hyperactive α1-ARs (i.e. excessive vasoconstriction) and/or hypoactive β-ARs (i.e. blunted vasodilation and cardiac response) is an important pathway in CVD pathogenesis (14, 15). Decreased β-AR sensitivity, in particular, is especially problematic as previous research has shown that β-ARs are down-regulated in hypertension (16, 17), ischemic heart disease and chronic heart failure (18–20). There also is evidence of diminished β-AR, but enhanced α1-AR responsiveness, among African Americans (9, 21), while other research has shown that socio-demographic factors including obesity (22–24) and socioeconomic/social status (25, 26) may further impair β-AR functioning.
Psychological traits and dispositions including anxiety (27–30), depression (31) and low social support (32) also have been related to AR functioning. The presence of high levels of hostility has been shown to be related to diminished β-AR responsiveness, especially among men (13, 32). There is a well-established literature linking hostility with increased CVD incidence and mortality risk (33), and previous studies have noted a consistent positive association between hostility and racial discrimination (34, 35). Increased hostility is acknowledged as one potential response to frequent exposure to discrimination among African Americans (35–37); it also has been suggested that hostility may actually account for the relationship between discrimination and health outcomes (38). Although no studies have explored the vascular effects of both racial discrimination and hostility concurrently, Thomas and colleagues examined the interaction of ethnicity and perceived racial discrimination on the pressor response in a community-based sample of African American and White adults (39). Pressor responsiveness was defined as the difference between baseline BP and peak BP observed following administration of a 100µg dose of phenylephrine, a selective α1-AR agonist that mimics the vasoconstrictive effects of norepinephrine to produce a short-term increase in BP. While African Americans exhibited a greater BP pressor response in comparison to Whites, perceived racial discrimination was positively associated with the diastolic pressor response, irrespective of ethnicity (39).
To our knowledge, the study by Thomas, et al., is the only previous investigation of the association between racial discrimination and AR function. In the present study, we sought to further examine the relationship between racial discrimination and AR functioning in African Americans. In particular, we hypothesized that greater racial discrimination would be associated with heightened α1-AR sensitivity and blunted β-AR sensitivity. Also, because of the significant association between racial discrimination and hostility, as well as evidence linking hostility to adrenergic responsivity, we sought to examine the interactive effects of discrimination and hostility on AR responsiveness.
METHODS
Participants
Fifty-seven African American adults participating in the Biobehavioral Investigation of Hypertension (BIOH) study at Duke University Medical Center are included in the present study. Data were collected between August 1994 and August 1998 and all subjects were recruited via local media (i.e. newspapers, magazines) advertisements. Individuals with BP exceeding 160 mm Hg systolic (SBP) or 100 mm Hg diastolic (DBP), a history of antihypertensive medication or tobacco use, were excluded. The study protocol was approved by Duke University Medical Center’s Institutional Review Board, and all participants provided verbal and written consent prior to participation.
Measures
Demographic and Anthropomorphic Characteristics
Demographic and socioeconomic information was obtained by participant self-report. Income was rated on a 6-point Likert scale (1 = less than $15k, 2 = $15k – $29,999, 3 = $30k – $44,999, 4 = $45k – $59,999, 5 = $60k – $74,999 and 6 = $75k and above). Education was similarly rated on a 7-point Likert scale (1 = less than 7th grade, 2 = some high school, 3 = high school graduate, 4 = trade school, 5 = some college, 6 = college graduate and 7 = postgraduate work or degree). Clinic BP was assessed on 3 separate visits, approximately 1 week apart, to determine BP status and study eligibility. Three seated BPs were taken, 2 minutes apart, using an appropriate sized occlusion cuff, mercury column sphygmomanometer, and stethoscope. BP readings were averaged across the three visits to define clinic BP status. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Racial Discrimination
Racial Discrimination was assessed using the Perceived Racism Scale (40), a 51-item questionnaire that assesses experiences with racism across four domains: racism on the job, racism in academic settings, racism in public settings, and exposure to racist statements. Participants reported the frequency of exposure in each of these areas using a 6-point Likert scale (0 = Not Applicable, 1 = Almost never, 2 = Several times a year, 3 = Several times a month, 4 = Several times a week and 5 = Several times a day). In this study, an overall score was obtained by summing responses across the four domains (41). In the present sample, scores on the Perceived Racism Scale ranged from 0–91, with a higher score indicating a greater frequency of perceived exposure to racism over the course of one’s life. The Perceived Racism Scale has demonstrated good internal consistency and Cronbach’s alpha in the present study was .97.
Hostility
Hostility (Ho) was assessed using the Cook-Medley Hostility (Ho) Scale (42), 50-item true/false questionnaire derived from the Minnesota Multiphasic Personality Inventory. Scores range from 0 to 50 with higher scores indicating a greater degree of cynicism, mistrust of others and anger proneness. The Ho scale has shown acceptable reliability and validity and Cronbach’s alpha in the present sample was .84.
Cardiovascular Measurements During AR Responsiveness Testing
All AR responsiveness testing was conducted while participants were reclined. As previous research has shown that caffeine increases BP and HR (43), we elected to minimize the contribution of potential individual differences in BP and HR increases due to caffeine consumption by invoking a 6-hour abstinence period (44). BP was measured continuously using the Finapres Model 2300 (Ohmeda, Madison, WI) noninvasive BP monitor. This instrument utilizes the vascular unloading technique to measure SBP, DBP and mean arterial pressure (MAP) on a beat-by-beat basis, and has been validated against intra-arterial measures under various conditions including pressor responses to phenylephrine (45). Heart rate (HR) was derived via electrocardiogram (ECG) as the interval between successive R-waves.
β-AR Responsiveness
The standardized isoproterenol sensitivity test (CD25) was used to evaluate β-AR responsiveness in terms of the chronotropic dose of isoproterenol required to increase HR by 25 beats per minute (bpm) (46). Progressively increasing bolus-doses of isoproterenol (0.125, 0.25, 0.5, 1.0, 2.0, 4.0 µg) were injected into a vein until an increase in HR of at least 25 bpm was observed. HR responses following each dose were computed as the shortest 3 successive ECG R-R intervals following drug injection, compared to the shortest 3 R-R intervals at rest (pre-injection). Following each dose, the next higher dose was not injected for at least 5 minutes, or until cardiovascular activity had returned to resting levels, usually within 5–l0 minutes. The linear regression model of log-dose/HR response for each subject was used to determine CD25 exactly by interpolation. The CD25 measure provides an index of β-AR responsiveness that is inversely related to AR responsiveness (i.e., higher CD25 values are indicative of reduced or blunted β-AR responsiveness).
α1-AR responsiveness
A procedure analogous to the β-AR responsiveness test described above was used for assessing α1-AR responsiveness, using the α1-AR pharmacological agonist phenylephrine, to stimulate vascular α1-AR (47). In this test, the criterion response is defined as the dose required to increase MAP by 25 mmHg (PD25). An initial dose of 25 µg phenylephrine was used, with successive doses doubled until the 25 mmHg response was exceeded, or until a maximum dose of 800 µg. Again, at least 5-minutes, or longer if required for recovery of MAP to resting levels, preceded administration of successive doses. The linear log-dose/MAP response curve was used to determine the exact PD25 dose. The PD25 index is inversely related to vascular α1-AR responsiveness (i.e., higher PD25 indicates reduced α1-AR responsiveness, or more importantly lower PD25 indicates heightened α1-AR responsiveness).
Statistical Analysis
Descriptive statistics were computed to characterize sample characteristics and to assess initial patterns of association among the study variables. Hierarchical regression analyses were used to evaluate the effects of discrimination and hostility on β-AR responsiveness to isoproterenol (CD25) and α1-AR responsiveness to phenylephrine (PD25). Covariates were selected based on previous literature (22, 23,25,27–29, 31, 48) and included age, sex (0 = female, 1 = male), BMI, education, income and clinic BP. Model 1 included the aforementioned covariates only. Discrimination was added as the single predictor in Model 2, followed by the inclusion of hostility in Model 3 and the racial discrimination × hostility interaction in Model 4. The significant interaction of racial discrimination and hostility on CD25 was further probed in a separate model using the PROCESS conditional effects macro developed by Hayes (49). This utility provides estimates and tests of significance for simple slope effects and boot-strapped estimates of the confidence interval. All statistical analyses were conducted using the SAS 9.3 system (SAS Institute, Cary, NC) with significance set at p = .05.
RESULTS
Descriptive statistics (i.e. means, standard deviations and Pearson’s correlations) are presented in Table 1. The sample had an average age of 33.2 (standard deviation = 5.9) years and was approximately half female (51%). Participants reported an average level of education consistent with having completed some college and endorsed an average income range of between $30,000 and $44,999. In correlational analyses, age was positively associated with DBP (r = .38, p =.003). Sex was inversely associated with HR (r = −.49, p <.001) and positively correlated with PD25 (r = .27, p =.045), indicating lower HR in women and heightened α1-AR responsiveness in men. BMI was positively associated with SBP (r = .32, p =.016), DBP (r = .28, p =.036), HR (r = .28, p =.037) and CD25 (r = .28, p =.038). PD25 was inversely associated with SBP (r = −.34, p =.011), DBP (r = −.26, p =.049) and HR (r = −.28, p =.036). CD25 was positively associated with both hostility (r = .28, p =.033) and racial discrimination (r = .43, p =.001), and racial discrimination was moderately correlated with hostility (r = .50, p <.001).
Table 1.
Sample Descriptives and Correlations (N=57)
| Variable | Mean(SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age (years) | 33.12 (5.91) | - | −.10 | .10 | −.07 | .00 | .19 | .38** | .14 | .19 | .05 | .17 | .10 |
| 2. Sex (% male) | .49 | - | −.18 | −.08 | .16 | .19 | −.03 | −.49*** | .21 | .27* | .01 | .20 | |
| 3. BMI (kg/m2) | 26.39 (3.61) | - | −.17 | .10 | .32* | .28* | .28* | .28* | −.14 | .10 | −.02 | ||
| 4. Education | 5.18 (1.3) | - | .18 | −.10 | −.06 | .01 | −.17 | .00 | −.21 | .00 | |||
| 5. Income | 2.68 (1.09) | - | .05 | .16 | −.08 | .17 | −.15 | −.05 | .31* | ||||
| 6. SBP (mmHg) | 131.12 (14.22) | - | .72*** | .02 | .20 | −.34** | .02 | .01 | |||||
| 7. DBP (mmHg) | 81.96 (11.48) | - | .19 | .23 | −.26* | −.03 | .10 | ||||||
| 8. HR (bpm) | 65.42 (12.51) | - | .25 | −.28* | .21 | .05 | |||||||
| 9. CD25 (µg) | 2.26 (1.71) | - | −.11 | .28* | .43** | ||||||||
| 10. PD25 (µg) | 264.37 (154.37) | - | −.17 | .05 | |||||||||
| 11. Hostility | 21.56 (7.8) | - | .50*** | ||||||||||
| 12. Racial Discrimination | 48.12 (31.46) | - |
Income was rated on a 6 point scale: 1 = less than $15k, 2 = $15k – $29,999, 3 = $30k – $44,999, 4 = $45k – 59,999, 5 = $60k – $74,999 and 6 = $75k and above. Education was rated on a 7 point scale: 1 = less than 7th grade, 2 = some high school, 3 = high school graduate, 4 = trade school, 5 = some college, 6 = college graduate and 7 = postgraduate work or degree. CD25, dose (in micrograms) of isoproterenol to increase heart rate by 25 bpm; PD25, dose (in micrograms) of phenylephrine to increase mean arterial pressure by 25mmHg.
p ≤.001,
p ≤.01,
p ≤.05
β-AR Responsiveness
Hierarchical regression analysis was used to examine the hypothesis that racial discrimination would be independently related to β-AR responsiveness. As a reminder, higher CD25 values are indicative of reduced or blunted β-AR responsiveness.
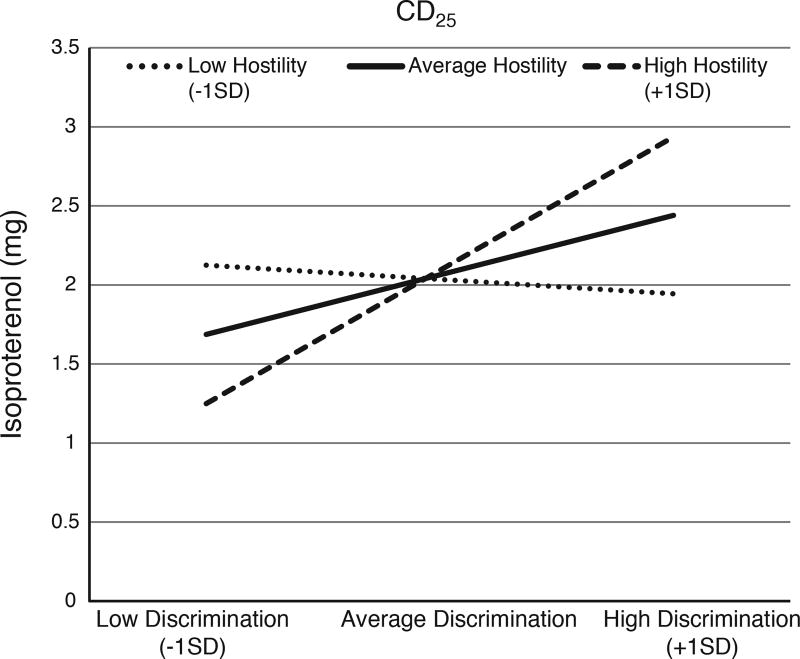
Covariates including age, sex, BMI, education, income and BP were included in the initial model (Table 2). BMI (β = .29, p = .049) was the only significant predictor in Model 1, which accounted for 10% of the variance in CD25. Discrimination emerged as a significant predictor of CD25 (β = .38, p = .004) in Model 2, explaining an additional 13% of the variance in CD25, and suggesting that greater perceived discrimination is associated with blunted β-AR responsiveness. This effect was only modestly attenuated with inclusion of hostility in Model 3, as hostility was not a significant predictor (β = .05, p = .76) and did not account for any additional variance in CD25. Importantly, the racial discrimination × hostility interaction (Model 4) was significant (β = 1.23, p = .046). As depicted in Figure 1, the effect of discrimination on CD25 was greatest among individuals with the higher (+1 standard deviation) hostility, (β = .49, 95% CI [.17, .82], p = .004); a similar trend was observed for individuals with average levels of hostility, (β = .22, 95% CI [−.11, .55], p = .18) but not among those individuals with lower hostility, (β = −.05, 95% CI [−.55, .45], p = .83).
Table 2.
Regression Model of Discrimination and Hostility Predicting CD25 (N = 57)
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Estimate | p | Estimate | p | Estimate | p | Estimate | p | |
| Age | 0.16 | .25 | 0.11 | .39 | 0.10 | .43 | 0.12 | .36 |
| Sex | 0.25 | .080 | 0.19 | .17 | 0.19 | .16 | 0.18 | .19 |
| BMI | 0.29 | .049 | 0.28 | .038 | 0.28 | .043 | 0.26 | .052 |
| Education | −0.10 | .46 | −0.09 | .46 | −0.08 | .52 | −0.06 | .63 |
| Income | 0.10 | .46 | 0.004 | .98 | 0.003 | .98 | −0.05 | .72 |
| SBP | −0.06 | .75 | −0.01 | .96 | −0.02 | .93 | 0.03 | .87 |
| DBP | 0.12 | .55 | 0.08 | .68 | 0.09 | .65 | 0.02 | .90 |
| RD | 0.38 | .004 | 0.36 | .024 | −0.54 | .25 | ||
| HO | 0.05 | .76 | −0.40 | .13 | ||||
| RD×HO | 1.23 | .046 | ||||||
|
| ||||||||
| R2 | .10 | .23 | .22 | .27 | ||||
Note: Estimates represent standardized beta coefficients. RD=racial discrimination, HO = Hostility
Figure 1. Interaction of Perceived Discrimination and Hostility on Beta-adrenergic Responsivity (i.e. CD25).
Higher CD25 values are indicative of reduced or blunted β-AR responsiveness. The effect of discrimination on CD25 was greatest among individuals with higher hostility, (β =.49, SE = .16, p =.004); compared to individuals with average (β = .22, SE = .16, p = .18) and lower (β = −.05, SE =.25, p =.83) hostility.
α1-AR responsiveness
Regression analyses were repeated with α1-AR responsiveness as the focal outcome (Table 3). As a reminder, higher PD25 indicates reduced α1-AR responsiveness, or more importantly lower PD25 indicates heightened α1-AR responsiveness. In Model 1, sex (β = 134.80, p =.004), and SBP (β = −5.68, p = .009), were the only explanatory factors in a model explaining 20% of the variance in PD25. The effects for sex and SBP were virtually unchanged in Model 2, as discrimination (β = .07, p = .91) did not account for significant additional variance in PD25. In Model 3, hostility was negatively associated with PD25 (β = −.34, p = .027), suggesting that greater hostility is associated with α1-AR responsiveness. There was no significant interaction effect for racial discrimination and hostility on PD25 (Model 4), (β = −.06, p = .92).
Table 3.
Regression Model of Discrimination and Hostility Predicting PD25 (N = 57)
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Estimate | p | Estimate | p | Estimate | p | Estimate | p | |
| Age | 0.16 | .22 | 0.16 | .23 | 0.22 | .102 | 0.22 | .107 |
| Sex | 0.44 | .002 | 0.44 | .003 | 0.40 | .005 | 0.40 | .005 |
| BMI | 0.10 | .46 | 0.10 | .47 | 0.13 | .33 | 0.13 | .34 |
| Education | 0.05 | .68 | 0.05 | .68 | 0.00 | .98 | 0.00 | .97 |
| Income | −0.22 | .091 | −0.23 | .100 | −0.28 | .039 | −0.28 | .047 |
| SBP | −0.52 | .009 | −0.52 | .010 | −0.46 | .018 | −0.46 | .020 |
| DBP | 0.08 | .69 | 0.08 | .70 | −0.02 | .91 | −0.02 | .93 |
| RD | 0.02 | .91 | 0.21 | .17 | 0.26 | .59 | ||
| HO | −0.34 | .027 | −0.31 | .25 | ||||
| RD×HO | −0.06 | .92 | ||||||
|
| ||||||||
| R2 | .20 | .18 | .25 | .23 | ||||
Note: Estimates represent standardized beta coefficients. RD = racial discrimination, HO = Hostility
DISCUSSION
Increased interest and a growing literature have heightened awareness on the importance of racial discrimination as a unique predictor of CVD risk among African Americans. The primary goal of the present study was to further examine the relationship between perceived racial discrimination and AR functioning. Consistent with our hypotheses, we found that racial discrimination was associated with diminished β-AR sensitivity, and that this effect was magnified among those with higher levels of hostility. Contrary to our expectations, hostility did not emerge as a significant predictor of β-AR responsiveness. However, hostility was associated with heightened α1-AR responsiveness.
Previous studies have suggested that exposure to racial discrimination contributes to the development of dysfunctional patterns of immune, neuroendocrine and cardiovascular regulation leading to tissue and organ damage, and ultimately disease onset (50). One ongoing criticism of past research evaluating the relationship between discrimination and health outcomes has been the failure to account for the potential influence of negative affect, such as anger and hostility, or personality factors (51). Indeed, hostility has been linked with an increased risk of CVD events and mortality (33, 52). African Americans have been shown to report higher levels of hostility compared to Whites (53, 54); and hostility has been considered to be a likely response to chronic experiences of prejudice and racial discrimination (35). Given evidence that hostility appears to be a potent predictor of diminished β-AR (i.e. vasodilatory) activity, as well as recent work linking discrimination with a heightened pressor response among African Americans, we examined whether discrimination and hostility would interactively predict AR responsiveness. While it initially appeared that the effect of racial discrimination on β-AR sensitivity was independent of hostility, results of the moderation analysis indicated that hostility enhanced the effect of racial discrimination. In particular, individuals with the highest levels of both hostility and racial discrimination exhibited the greatest reductions in β-AR responsiveness. Moreover, given our observation that higher hostility was associated with heightened α1-AR responsiveness, racial discrimination in this context would convey a combination of blunted β-AR responsiveness and heightened α1-AR responsiveness, a pattern that would favor reduced vasodilation and heightened vasoconstriction. Indeed, this hemodynamic profile has been shown to be especially common amongst African Americans and has been linked to the early onset and heightened risk of CVD (9, 15, 55). It has been postulated that reduced β-AR sensitivity among individuals higher in hostility may reflect a greater chronic stress burden and/or heightened stress-related catecholamine activity (32). Chronic stress associated with racial discrimination may be similarly related to increased catecholamine activity, with one recent study reporting positive but non-significant associations between racial discrimination assessed in childhood with overnight urinary epinephrine and norepinephrine assessed in early adulthood (56). Overall, there is limited available data regarding the association between racial discrimination and the stress hormones and further research in this area would be informative.
The present results add to a growing literature demonstrating that racial discrimination is associated with adverse patterns of vascular functioning among African Americans. It has long been established that the vascular component plays a more prominent role in pathological BP regulation among African Americans (35, 57). Yet, with some exception, there has been comparatively little research connecting discrimination to more direct measures of cardiovascular function. Notably, unfair treatment, attributed to multiple factors including race, has been found to be associated with greater intima-media thickness (IMT) among a subsample of African American women from the SWAN study (58). While this and other previous studies (59, 60) provide evidence that racial discrimination is associated with structural measures of vascular dysfunction, racial discrimination also has been linked to biochemical factors associated with local vascular regulation. For instance, Cooper and colleagues examined the relationship between perceived discrimination and Endothelin-1, a vasoconstrictive agent found throughout the central nervous system that has been implicated in vascular changes including small vessel remodeling and left ventricular hypertrophy, in a sample of African American and White adults. Notably, while there was no association among Whites; perceived discrimination was positively associated with Endothelin-1 among African Americans, even after accounting for gender, physical activity and socioeconomic status (61). Discrimination also has been linked to higher levels of C-reactive protein (CRP) among African Americans (62–65). CRP has been characterized as an important marker of both systemic and vascular-related inflammation (66). It has been suggested that heightened inflammation is one consequence of SNS hyperactivity and some work has shown a positive association between circulating levels of CRP and diminished β-AR sensitivity (48). Collectively, our results add to this prior evidence in suggesting that discrimination is associated with a broad range of both structural and functional indicators of vascular impairment among African Americans.
In contrast to our expectations, we did not find racial discrimination to predict α1-AR responsiveness. While our sample was larger than the African American subsample in the study by Thomas et al (39), it is noted that we employed a different measure of racial discrimination, incorporated a wider range of covariates, and assessed a slightly different outcome (i.e. the pressor dose of phenylephrine required to elevate blood pressure by 25 mmHg, in contrast to the prior study’s index of the absolute change in BP to a single 100µg dose of phenylephrine). In addition, our study was comprised solely of African Americans, while the study by Thomas and colleagues was composed of both African American and White adults. Thus, it is possible that these differences contributed to the lack of a significant main effect of racial discrimination as well as the non-significant interactive effect of racial discrimination and hostility on α1-AR responsiveness.
Intriguingly, we did observe a significant main effect of hostility on α1-AR responsiveness. While the association between hostility and β-AR responsiveness has been more clearly documented (13, 27,28, 32, 67), less is known regarding the relationship between hostility and α1-AR responsiveness. Indeed, some studies examining this association have reported null findings (13, 32). In contrast, hostility accounted for approximately 7% of the variance in PD25 in our sample, indicating that increasing levels of hostility were associated with enhanced α1-AR responsiveness (i.e. greater vasoconstriction). Importantly, this effect was independent of sex and clinic BP, both of which have been recently shown to account for differences in α1-AR responsiveness (21). Notably, several studies have demonstrated that African Americans exhibit heightened α1-AR responsiveness compared to Whites (9, 21) and α1-AR responsiveness also has been shown to be independent of blunted β-AR sensitivity among African Americans (68).
In addition, while we did not examine the extent to which individuals expressed their hostility in the present study, both inhibition and expression of hostile feelings (i.e. anger) have been associated with a blunted decline in nocturnal BP (41, 69), as well as delayed cardiovascular recovery following stress exposure (70). For example, in the study by Dorr et al (70), African American and White men were instructed to either express or inhibit their anger following a race- or non-race related debate. Irrespective of debate topic, African American men who expressed their anger exhibited the most prolonged elevations in BP relative to African American inhibitors, as well as relative to White men in either condition. Further, African American men who inhibited their anger also demonstrated delayed recovery in systemic vascular resistance (SVR), a measure of peripheral vasoconstriction. While recovery was only assessed up to ten minutes following the stressor in this study, other research has shown that increases in SVR may persist for as long as 45 minutes following exposure to a psychological stressor (71). It has been established that SVR plays a more prominent role in short-term and diurnal BP regulation among African Americans (9, 72, 73), and recent evidence suggests that SVR is more strongly related to an index of vascular hypertrophy among African Americans compared to Whites (74). Importantly, previous work also has shown that α1-receptors are more strongly implicated in SNS-mediated vasoconstriction (75). While it is clear that additional research is needed to further evaluate the relationship between hostility and α1-AR responsiveness, our findings do suggest that hostility may play an important role in hypertension risk among African Americans, contributing directly through heightened vasoconstriction and interactively via attenuated vasodilation.
There are several limitations which should be considered in interpreting our findings. First, given the cross-sectional nature of our study, little inference can be drawn regarding the cause-effect relationship between discrimination and hostility with AR responsivity. Our sample was relatively small and comprised entirely of young-to-middle-aged African Americans with normal or mildly elevated BP. Thus, our findings may have limited generalizability to older and/or more diverse populations or to individuals with more chronically elevated BP. While SBP emerged as a significant predictor of α1-AR responsiveness, neither SBP nor DBP approached significance in models predicting the CD25 (i.e. β-AR) response. Although we accounted for markers of cardiovascular risk (i.e. BP, HR, BMI), we did not assess physical activity in the present study. Future research should determine whether physical activity may influence the association of racial discrimination and hostility with AR functioning; however, previous studies have indicated only a weak, inverse association, particularly with β-AR responsiveness (25, 48). Lastly, while we examined β-AR responsiveness using a methodology in accord with previous studies (25, 28,29, 31, 48), it should be noted that isoproterenol is a non-selective β-AR agonist, which stimulates both increased cardiac activity and vascular dilation. Previous work has shown that changes in systemic vascular resistance mirror those in heart rate as captured by the CD25 response, using an identical protocol as the current study (13, 76). Other research also has documented both greater β-receptor sensitivity and density among hypertensive African Americans (77). Our CD25 β-AR responsiveness index may also be a manifestation of altered β-AR density and/or sensitivity, with available evidence suggesting that reduced sensitivity is the more likely mechanism (77).
Despite these limitations, there are also several strengths of the present work. Most notably, we accounted for a number of additional factors which have previously been shown to influence adrenergic receptor sensitivity. For instance, research has shown that obesity is associated with β-AR down-regulation (22). While BMI was indeed a significant predictor of CD25, its inclusion in the models did not diminish the influence of racial discrimination. Moreover, amid growing evidence that socioeconomic factors are related to AR functioning, we also considered the potential influence of income and education; however, neither of these indicators emerged as significant predictors of the CD25 response. In contrast, we did find income to be an inverse predictor of PD25, raising the possibility that SES may be more strongly related to α1-adrenergic functioning. Lastly, because we evaluated both α- and β-responsiveness, our findings provide a more complete view of the association (or lack thereof) of racial discrimination with at least one important mechanism of vascular functioning.
Overall, our findings suggest that the adverse impact of racial discrimination on vascular function is due, in part, to blunted β-AR responsiveness and that this effect is enhanced by high levels of hostility. In addition, we found that hostility was directly related to heightened α1-AR responsiveness, a pattern consistent with excessive stressor-related vasoconstriction among African Americans (68). Further research is needed to better characterize the relationship between racial discrimination and hostility with global SNS functioning, as well as to determine whether other negative psychosocial traits have a similar exacerbating influence.
Acknowledgments
Sources of Funding:
This work was supported by funding from the National Heart, Blood and Lung Institute (HL49427, HL50774, and HL121708).
Abbreviations
- AR
Adrenergic Receptors
- BP
Blood Pressure
- EP
Epinephrine
- NOR
Norepinephrine
- CVD
Cardiovascular Disease
- CD25
Dose of Isoproterenol to increase heart rate by 25 bpm
- BMI
Body Mass Index
- SBP
Systolic Blood Pressure
- DBP
Diastolic Blood Pressure
- MAP
Mean Arterial Pressure
- PD25
Dose of Phenylephrine to increase mean arterial pressure by 25 mm Hg
Footnotes
Conflicts of Interest:
The authors report no conflicts of interest.
References
- 1.Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans. A biopsychosocial model. Am Psychol. 1999;54:805–16. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- 2.Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, James SA, Robinson JC, Williams DR, Taylor HA. Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. Am J Public Health. 2012;102(Suppl 2):S258–65. doi: 10.2105/AJPH.2011.300523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Din-Dzietham R, Nembhard WN, Collins R, Davis SK. Perceived stress following race-based discrimination at work is associated with hypertension in African-Americans. The metro Atlanta heart disease study, 1999–2001. Soc Sci Med. 2004;58:449–61. doi: 10.1016/s0277-9536(03)00211-9. [DOI] [PubMed] [Google Scholar]
- 4.Paradies Y. A systematic review of empirical research on self-reported racism and health. Int J Epidemiol. 2006;35:888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- 5.Lewis TT, Williams DR, Tamene M, Clark CR. Self-Reported Experiences of Discrimination and Cardiovascular Disease. Curr Cardiovasc Risk Rep. 2014;8:365. doi: 10.1007/s12170-013-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolezsar CM, McGrath JJ, Herzig AJ, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20–34. doi: 10.1037/a0033718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–7. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 8.Peters RM, Flack JM. Salt sensitivity and hypertension in African Americans: implications for cardiovascular nurses. Prog Cardiovasc Nurs. 2000;15:138–44. doi: 10.1111/j.0889-7204.2000.080404.x. [DOI] [PubMed] [Google Scholar]
- 9.Taherzadeh Z, Brewster LM, van Montfrans GA, VanBavel E. Function and structure of resistance vessels in black and white people. J Clin Hypertens (Greenwich) 2010;12:431–8. doi: 10.1111/j.1751-7176.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114:1804–14. doi: 10.1161/CIRCRESAHA.114.302524. [DOI] [PubMed] [Google Scholar]
- 11.Poitras VJ, Pyke KE. The impact of acute mental stress on vascular endothelial function: evidence, mechanisms and importance. Int J Psychophysiol. 2013;88:124–35. doi: 10.1016/j.ijpsycho.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Toda N, Nakanishi-Toda M. How mental stress affects endothelial function. Pflügers Archiv - European Journal of Physiology. 2011;462:779–94. doi: 10.1007/s00424-011-1022-6. [DOI] [PubMed] [Google Scholar]
- 13.Suarez EC, Sherwood A, Hinderliter AL. Hostility and adrenergic receptor responsiveness: evidence of reduced beta-receptor responsiveness in high hostile men. J Psychosom Res. 1998;44:261–7. doi: 10.1016/s0022-3999(97)00201-8. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, Femminella GD, Leosco D, Bonaduce D. beta-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol. 2014;4:396. doi: 10.3389/fphys.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139:761–76. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 16.Sherwood A, Hinderliter AL. Responsiveness to alpha- and beta-adrenergic receptor agonists. Effects of race in borderline hypertensive compared to normotensive men. Am J Hypertens. 1993;6:630–5. doi: 10.1093/ajh/6.7.630. [DOI] [PubMed] [Google Scholar]
- 17.Stein CM, Nelson R, Deegan R, He H, Wood M, Wood AJ. Forearm beta adrenergic receptor-mediated vasodilation is impaired, without alteration of forearm norepinephrine spillover, in borderline hypertension. J Clin Invest. 1995;96:579–85. doi: 10.1172/JCI118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungerer M, Hartmann F, Richardt G. The sympathetic-adrenergic system in heart failure. Dtsch Med Wochenschr. 1996;121:141–5. doi: 10.1055/s-2008-1042986. [DOI] [PubMed] [Google Scholar]
- 19.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–53. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Sherwood A, Hill LK, Blumenthal JA, Johnson KS, Hinderliter AL. Race and sex differences in cardiovascular α-adrenergic and β-adrenergic receptor responsiveness in men and women with high blood pressure. Journal of Hypertension. 2017;35:975–81. doi: 10.1097/HJH.0000000000001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julius S, Valentini M, Palatini P. Overweight and hypertension : a 2-way street? Hypertension. 2000;35:807–13. doi: 10.1161/01.hyp.35.3.807. [DOI] [PubMed] [Google Scholar]
- 23.Feldstein C, Julius S. The complex interaction between overweight, hypertension, and sympathetic overactivity. J Am Soc Hypertens. 2009;3:353–65. doi: 10.1016/j.jash.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Lewis TT, Everson-Rose SA, Karavolos K, Janssen I, Wesley D, Powell LH. Hostility is associated with visceral, but not subcutaneous, fat in middle-aged African American and white women. Psychosom Med. 2009;71:733–40. doi: 10.1097/PSY.0b013e3181ad13a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Euteneuer F, Mills PJ, Rief W, Ziegler MG, Dimsdale JE. Subjective social status predicts in vivo responsiveness of beta-adrenergic receptors. Health Psychol. 2012;31:525–9. doi: 10.1037/a0025990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas KS, Nelesen RA, Ziegler MG, Natarajan L, Dimsdale JE. Influence of education and neighborhood poverty on pressor responses to phenylephrine in African-Americans and Caucasian-Americans. Biol Psychol. 2009;82:18–24. doi: 10.1016/j.biopsycho.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu BH, Dimsdale JE, Mills PJ. Psychological states and lymphocyte beta-adrenergic receptor responsiveness. Neuropsychopharmacology. 1999;21:147–52. doi: 10.1016/S0893-133X(98)00133-X. [DOI] [PubMed] [Google Scholar]
- 28.Yu BH, Kang EH, Ziegler MG, Mills PJ, Dimsdale JE. Mood states, sympathetic activity, and in vivo beta-adrenergic receptor function in a normal population. Depress Anxiety. 2008;25:559–64. doi: 10.1002/da.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang EH, Yu BH. Anxiety and beta-adrenergic receptor function in a normal population. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:733–7. doi: 10.1016/j.pnpbp.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Lee I-S, Kim K-J, Kang E-H, Yu B-H. β-adrenoceptor affinity as a biological predictor of treatment response to paroxetine in patients with acute panic disorder. Journal of Affective Disorders. 2008;110:156–60. doi: 10.1016/j.jad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Euteneuer F, Ziegler MG, Mills PJ, Rief W, Dimsdale JE. In vivo beta-adrenergic receptor responsiveness: ethnic differences in the relationship with symptoms of depression and fatigue. Int J Behav Med. 2014;21:843–50. doi: 10.1007/s12529-013-9359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes JW, Sherwood A, Blumenthal JA, Suarez EC, Hinderliter AL. Hostility, social support, and adrenergic receptor responsiveness among African-American and white men and women. Psychosom Med. 2003;65:582–7. doi: 10.1097/01.psy.0000041546.04128.43. [DOI] [PubMed] [Google Scholar]
- 33.Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009;53:936–46. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 34.Richman LS, Bennett GG, Pek J, Siegler I, Williams RB., Jr Discrimination, dispositions, and cardiovascular responses to stress. Health Psychol. 2007;26:675–83. doi: 10.1037/0278-6133.26.6.675. [DOI] [PubMed] [Google Scholar]
- 35.Anderson NB, Myers HF, Pickering T, Jackson JS. Hypertension in blacks: psychosocial and biological perspectives. J Hypertens. 1989;7:161–72. [PubMed] [Google Scholar]
- 36.Brody GH, Chen YF, Murry VM, Ge X, Simons RL, Gibbons FX, Gerrard M, Cutrona CE. Perceived discrimination and the adjustment of African American youths: a five-year longitudinal analysis with contextual moderation effects. Child Dev. 2006;77:1170–89. doi: 10.1111/j.1467-8624.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 37.Wright LB, Gregoski MJ, Tingen MS, Barnes VA, Treiber FA. Impact of Stress Reduction Interventions on Hostility and Ambulatory Systolic Blood Pressure in African American Adolescents. J Black Psychol. 2011;37:210–33. doi: 10.1177/0095798410380203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huebner DM, Nemeroff CJ, Davis MC. Do hostility and neuroticism confound associations between perceived discrimination and depressive symptoms? J Soc Clin Psychol. 2005;24:723–40. [Google Scholar]
- 39.Thomas KS, Nelesen RA, Malcarne VL, Ziegler MG, Dimsdale JE. Ethnicity, perceived discrimination, and vascular reactivity to phenylephrine. Psychosom Med. 2006;68:692–7. doi: 10.1097/01.psy.0000238214.80871.e6. [DOI] [PubMed] [Google Scholar]
- 40.McNeilly MD, Anderson NB, Armstead CA, Clark R, Corbett M, Robinson EL, Pieper CF, Lepisto EM. The perceived racism scale: a multidimensional assessment of the experience of white racism among African Americans. Ethn Dis. 1996;6:154–66. [PubMed] [Google Scholar]
- 41.Steffen PR, McNeilly M, Anderson N, Sherwood A. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosom Med. 2003;65:746–50. doi: 10.1097/01.psy.0000079380.95903.78. [DOI] [PubMed] [Google Scholar]
- 42.Cook WW, Medley DM. Proposed Hostility and Pharisaic - Virtue Scales for the MMPI. J Appl Psychol. 1954;38:414–8. [Google Scholar]
- 43.Green PJ, Kirby R, Suls J. The effects of caffeine on blood pressure and heart rate: A review. Ann Behav Med. 1996;18:201–16. doi: 10.1007/BF02883398. [DOI] [PubMed] [Google Scholar]
- 44.Mort JR, Kruse HR. Timing of blood pressure measurement related to caffeine consumption. Ann Pharmacother. 2008;42:105–10. doi: 10.1345/aph.1K337. [DOI] [PubMed] [Google Scholar]
- 45.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;13:647–55. doi: 10.1161/01.hyp.13.6.647. [DOI] [PubMed] [Google Scholar]
- 46.Cleaveland CR, Rangno RE, Shand DG. A standardized isoproterenol sensitivity test. The effects of sinus arrhythmia, atropine, and propranolol. Arch Intern Med. 1972;130:47–52. doi: 10.1001/archinte.130.1.47. [DOI] [PubMed] [Google Scholar]
- 47.Kotchen TA, Guthrie GP, Jr, McKean H, Kotchen JM. Adrenergic responsiveness in prehypertensive subjects. Circulation. 1982;65:285–90. doi: 10.1161/01.cir.65.2.285. [DOI] [PubMed] [Google Scholar]
- 48.Euteneuer F, Mills PJ, Rief W, Ziegler MG, Dimsdale JE. Association of in vivo beta-adrenergic receptor sensitivity with inflammatory markers in healthy subjects. Psychosom Med. 2012;74:271–7. doi: 10.1097/PSY.0b013e318245d762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- 50.Anderson NB, McNeilly M, Myers H. Autonomic reactivity and hypertension in blacks: a review and proposed model. Ethn Dis. 1991;1:154–70. [PubMed] [Google Scholar]
- 51.Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol. 2015;11:407–40. doi: 10.1146/annurev-clinpsy-032814-112728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matthews KA, Gump BB, Harris KF, Haney TL, Barefoot JC. Hostile behaviors predict cardiovascular mortality among men enrolled in the Multiple Risk Factor Intervention Trial. Circulation. 2004;109:66–70. doi: 10.1161/01.CIR.0000105766.33142.13. [DOI] [PubMed] [Google Scholar]
- 53.Scherwitz L, Perkins L, Chesney M, Hughes G. Cook-Medley Hostility scale and subsets: relationship to demographic and psychosocial characteristics in young adults in the CARDIA study. Psychosom Med. 1991;53:36–49. doi: 10.1097/00006842-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Barefoot JC, Peterson BL, Dahlstrom WG, Siegler IC, Anderson NB, Williams RB., Jr Hostility patterns and health implications: correlates of Cook-Medley Hostility Scale scores in a national survey. Health Psychol. 1991;10:18–24. doi: 10.1037//0278-6133.10.1.18. [DOI] [PubMed] [Google Scholar]
- 55.Calhoun DA, Mutinga ML, Collins AS, Wyss JM, Oparil S. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension. 1993;22:801–5. doi: 10.1161/01.hyp.22.6.801. [DOI] [PubMed] [Google Scholar]
- 56.Brody GH, Lei MK, Chae DH, Yu T, Kogan SM, Beach SR. Perceived discrimination among African American adolescents and allostatic load: a longitudinal analysis with buffering effects. Child Dev. 2014;85:989–1002. doi: 10.1111/cdev.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillum RF. Pathophysiology of hypertension in blacks and whites. A review of the basis of racial blood pressure differences. Hypertension. 1979;1:468–75. doi: 10.1161/01.hyp.1.5.468. [DOI] [PubMed] [Google Scholar]
- 58.Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 2003;22:300–9. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- 59.Clark R, Benkert RA, Flack JM. Large arterial elasticity varies as a function of gender and racism-related vigilance in black youth. J Adolesc Health. 2006;39:562–9. doi: 10.1016/j.jadohealth.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, Jacobs E, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN Heart Study. Psychosom Med. 2006;68:362–8. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- 61.Cooper DC, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE. The effects of ethnic discrimination and socioeconomic status on endothelin-1 among blacks and whites. Am J Hypertens. 2009;22:698–704. doi: 10.1038/ajh.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beatty DL, Matthews KA, Bromberger JT, Brown C. Everyday Discrimination Prospectively Predicts Inflammation Across 7-Years in Racially Diverse Midlife Women: Study of Women's Health Across the Nation. J Soc Issues. 2014;70:298–314. doi: 10.1111/josi.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun. 2010;24:438–43. doi: 10.1016/j.bbi.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goosby BJ, Malone S, Richardson EA, Cheadle JE, Williams DT. Perceived discrimination and markers of cardiovascular risk among low-income African American youth. American Journal of Human Biology. 2015;27:546–52. doi: 10.1002/ajhb.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, Berkman LF. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc Sci Med. 2012;75:922–31. doi: 10.1016/j.socscimed.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson TV, Abbasi A, Master VA. Systematic review of the evidence of a relationship between chronic psychosocial stress and C-reactive protein. Mol Diagn Ther. 2013;17:147–64. doi: 10.1007/s40291-013-0026-7. [DOI] [PubMed] [Google Scholar]
- 67.Fukudo S, Lane JD, Anderson NB, Kuhn CM, Schanberg SM, McCown N, Muranaka M, Suzuki J, Williams RB., Jr Accentuated vagal antagonism of beta-adrenergic effects on ventricular repolarization. Evidence of weaker antagonism in hostile type A men. Circulation. 1992;85:2045–53. doi: 10.1161/01.cir.85.6.2045. [DOI] [PubMed] [Google Scholar]
- 68.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36:945–51. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 69.Thomas KS, Nelesen RA, Dimsdale JE. Relationships between hostility, anger expression, and blood pressure dipping in an ethnically diverse sample. Psychosom Med. 2004;66:298–304. doi: 10.1097/01.psy.0000126196.82317.9d. [DOI] [PubMed] [Google Scholar]
- 70.Dorr N, Brosschot JF, Sollers JJ, Thayer JF. Damned if you do, damned if you don't: The differential effect of expression and inhibition of anger on cardiovascular recovery in Black and White males. Int J Psychophysiol. 2007;66:125–34. doi: 10.1016/j.ijpsycho.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 71.Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens. 2005;23:529–36. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- 72.Sherwood A, Hughes JW, McFetridge J. Ethnic differences in the hemodynamic mechanisms of ambulatory blood pressure regulation. Am J Hypertens. 2003;16:270–3. doi: 10.1016/s0895-7061(02)03269-7. [DOI] [PubMed] [Google Scholar]
- 73.Sherwood A, Hill LK, Blumenthal JA, Hinderliter AL. Circadian hemodynamics in men and women with high blood pressure: dipper vs. nondipper and racial differences. J Hypertens. 2017 doi: 10.1097/HJH.0000000000001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill LK, Sherwood A, Blumenthal JA, Hinderliter AL. Hemodynamics and Vascular Hypertrophy in African Americans and Caucasians With High Blood Pressure. Am J Hypertens. 2016 doi: 10.1093/ajh/hpw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Champlain J. Pre- and postsynaptic adrenergic dysfunctions in hypertension. Journal of hypertension Supplement : official journal of the International Society of Hypertension. 1990;8:S77–85. [PubMed] [Google Scholar]
- 76.Sherwood A, Hughes JW, Kuhn C, Hinderliter AL. Hostility is related to blunted beta-adrenergic receptor responsiveness among middle-aged women. Psychosom Med. 2004;66:507–13. doi: 10.1097/01.psy.0000132876.95620.04. [DOI] [PubMed] [Google Scholar]
- 77.Mills PJ, Dimsdale JE, Ziegler MG, Nelesen RA. Racial differences in epinephrine and beta 2-adrenergic receptors. Hypertension. 1995;25:88–91. doi: 10.1161/01.hyp.25.1.88. [DOI] [PubMed] [Google Scholar]