Abstract
Background
Debate exists about whether neuromyelitis optica spectrum disorder seronegative disease represents the same immune-mediated attack on astrocytic aquaporin-4 as in seropositive disease.
Objective
We investigated whether response to common treatments for neuromyelitis optica spectrum disorder differed by serostatus, as assessed by change in annualized relapse rate.
Methods
We performed a multicenter retrospective analysis of 245 patients with neuromyelitis optica spectrum disorder who were treated with either rituximab or mycophenolate mofetil as their first-line immunosuppressive treatment for disease prevention. Patients were followed for a minimum of 6 months following treatment initiation.
Results
In those started on rituximab, the pre-treatment annualized relapse rates for seropositive and seronegative patients were 1.81 and 1.93, respectively. On-treatment annualized relapse rates significantly declined to 0.32 (seropositive; p<0.0001) and 0.12 (seronegative; p=0.0001). In those started on mycophenolate mofetil, the pre-treatment annualized relapse rates for seropositive and seronegative patients were 1.79 and 1.45, respectively. On-treatment annualized relapse rates declined to 0.29 (seropositive; p<0.0001) and 0.30 (seronegative; p<0.005).
Conclusions
In this international collaboration involving a large number of neuromyelitis optica spectrum disorder patients, treatment was effective regardless of serostatus. This suggests that treatment should not differ when considering these treatments.
Keywords: Devic’s disease, immunosuppression, relapse, neuromyelitis optica, rituximab, mycophenolate
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare, relapsing autoimmune disease of the central nervous system characterized by longitudinally extensive myelitis and optic neuritis (ON), leading to paralysis and blindness.1 NMOSD is associated with the highly- specific antibody against aquaporin-4 (AQP4) biomarker found in the sera of 58–83% of patients worldwide, depending on the assay.2,3 The new NMOSD clinical criteria stratify NMOSD by AQP4 antibody serostatus allowing for the diagnosis of seropositive patients with limited or early forms of the disease.4 AQP4 seronegative patients must meet a higher threshold with proof of multifocal inflammatory disease across the spinal cord, brainstem, and/or optic nerves. Debate continues about whether AQP4 seronegative NMOSD represents the same immune-mediated attack on astrocytic AQP4 versus a different immunological process,5,6 and studies to date comparing the clinical profile of patients by serostatus are incongruous.5,7–9 In the meantime, patients and their clinicians have had little guidance regarding the utility of commonly prescribed immunosuppressive treatments specifically for AQP4 seronegative disease, and whether response differs by treatment. The objective of this study is to retrospectively assess records of both seropositive and seronegative patients to determine if response to common immunosuppressive treatments differs by serostatus. The current study provides support that two of the most commonly used immunosuppressive medications for prevention of relapses,10,11 rituximab (RTX) and mycophenolate mofetil (MMF), are equally efficacious in AQP4 seropositive and seronegative NMOSD patients.
Patients and Methods
For this retrospective study, we reviewed records for patients meeting 2015 NMOSD revised diagnostic criteria evaluated at six international departments of neurology: the Johns Hopkins University School of Medicine (Baltimore, MD, USA), Research Institute and Hospital of National Cancer Center (Goyang, Korea), University of Texas Southwestern (Dallas, TX, USA), Mayo Clinic (Scottsdale, AZ, USA), Charite University Medicine (Berlin, Germany) and Neuroclinica (Medellín, Colombia). The study was approved by individual Institutional Review Boards, as appropriate, and shared data were deidentified for analysis. Informed consent was waived. Patients were included if complete records of their relapse and treatment history, demographic data and serostatus were available for those who received RTX or MMF as their first-line immunosuppressive treatment and were subsequently followed for a minimum of 6 months after initiation of treatment. Patients with prior exposure to glatiramer acetate were included as there are no data to suggest it is harmful in NMOSD, while those with exposure to other immunotherapies used for multiple sclerosis were excluded, including interferon beta-1, fingolimod and natalizumab.12–14 Patients on concomitant low-dose corticosteroids were excluded. Patients on prior immunosuppressive treatment with azathioprine, cyclophosphamide, methotrexate, and mitoxantrone were excluded, as were those who received both RTX and MMF in combination. No additional patients were excluded.
A relapse was defined across all centers as a new or worsening acute neurologic symptom lasting at least 24 hours, associated with a change in exam localizing to the spinal cord, optic nerve or area postrema and not explainable by fever, infection or metabolic condition. MRI was used for relapse confirmation at 4 centers (n=213), and as needed for equivocal presentations at the other two, as determined by the treating neurologist. Recent criteria have been published for use in an ongoing international NMOSD clinical trial,15 and these criteria were applied for those patients without MRI verification at the time of the event.
The primary outcome was change in annualized relapse rates (ARR) between pre- treatment ARR calculated from onset of disease and post-treatment ARR. For the purposes of this study, those patients who did not have any new inflammatory event while on treatment were considered to be in remission, and remission rates reflect the total percent of patients who remained relapse-free. Time to first relapse on treatment included only those patients in whom therapy had failed. Mann-Whitney t-tests, Wilcoxon signed-rank tests and Fisher’s exact tests were conducted as applicable using GraphPad Prism v7 software. For this analysis, findings with p-values less than 0.05 were considered significant.
Results
We analyzed 245 patients who met inclusion criteria, including 102 from the Research Institute and Hospital of National Cancer Center, 80 from Johns Hopkins University, 20 from University of Texas Southwestern, 19 from the Mayo Clinic, 13 from Charité University Medicine and 11 from Universidad de Antioquia. Median age at disease onset was 37.0 years (mean, 38.3). Patients were followed for a median of 7.9 years (mean, 8.6). One hundred forty two patients were initiated on RTX (87% seropositive) and 103 (83% seropositive) were initiated on MMF. Non-white patients were 3.5 times more likely to be seropositive compared to whites and female patients were 5.4 times more likely to be seropositive compared to males (Table 1). Time to diagnosis was significantly higher in seronegative patients. Disease duration, age at onset, pre-treatment relapse rates, and all other demographics were similar between groups (Table 1).
Table 1.
Demographic Characteristics of Patients
| Seropositive (n=208) |
Seronegative (n=37) |
p-value | |||
|---|---|---|---|---|---|
|
| |||||
| Age at Onset, median (mean, range) | 37.0 (38.3; 7.0–78.8) | 36.0 (38.6; 7.9–70.0) | 0.90 | ||
|
| |||||
| Female sex, n (%) | 191 (92%) | 25 (68%) | 0.0002* | ||
|
| |||||
| Race | |||||
| White, Non-Hispanic | 52 | 20 | 0.0007* | ||
| Non-White | 156 | 17 | |||
| Asian | 97 | 9 | |||
| Black | 44 | 5 | |||
| Hispanic | 15 | 3 | |||
|
| |||||
| Disease duration, median (mean, range) in years | 8.3 (8.8; 0.8–25.2) | 5.9 (7.5; 0.7–33.6) | 0.15 | ||
|
| |||||
| Time to initial immunotherapy, median (mean, range) in years | 0.7 (2.73; 0.0–27.9) | 3.1 (4.0; 0.0–23.4) | 0.02* | ||
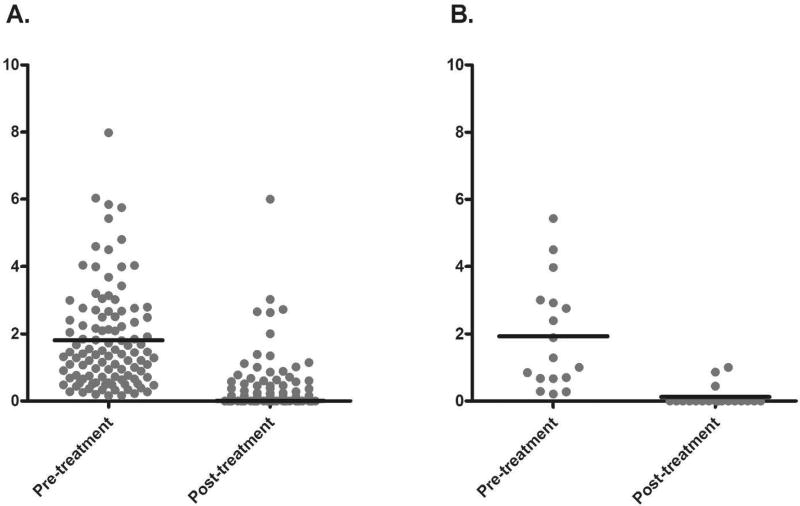
RTX was dosed at 1000 mg +/− a second dose 2 weeks later or 375 mg/m2 weekly x4 weeks to achieve B cell depletion by CD19+ and/or CD27+ counts, with repeat infusions every 6 months or upon B cell repletion defined as a discrete population of B cells measurable in the blood by flow cytometry. In this group, the pre-treatment annualized relapse rates were very similar between AQP4 seropositive and seronegative patients at 1.81 and 1.93 relapses per year, respectively. After a median treatment duration of 3.5 years (mean 4.3), the annualized relapse rates significantly dropped to 0.32 and 0.12 relapses per year, in seropositive and seronegative patients respectively (p≤0.0001, Figure 1). The remission rate among AQP4 seropositive patients was 64.2%, while among AQP4 seronegative patients was 84.2%. The slight improved response among AQP4 seronegative patients was not significantly different from AQP4 seropositive patients (p=0.12; Table 2). Among those patients who relapsed despite treatment with RTX, the median time to relapse in seropositive and seronegative patient groups was 7 months and 5 months, respectively, which is not significantly different (p=0.79).
Figure 1.
Comparison of annualized relapse rate before and after initiation of rituximab: A. patients with AQP4 seropositive NMOSD (p<0.0001), B. patients with AQP4 seronegative NMOSD (p=0.0001).
Table 2.
Rituximab Response to Treatment by Serostatus
| Seropositive (n=123) |
Seronegative (n=19) |
p-value | |
|---|---|---|---|
| Time on treatment, median (mean, range) in years | 3.5 (4.3; 0.5–10.5) | 2.4 (3.5; 0.5–10.9) | 0.29 |
| Pre-RTX ARR | 1.81 | 1.93 | 0.92 |
| Post-RTX ARR | 0.32 | 0.12 | 0.11 |
| Remission rate, % | 64.2 | 84.2 | 0.12 |
| Time to 1st relapse on treatment, median (mean, range) in years; relapse group | 0.6 (1.0, 0.1–4.7) | 0.4 (0.9, 0.2–2.2) | 0.79 |
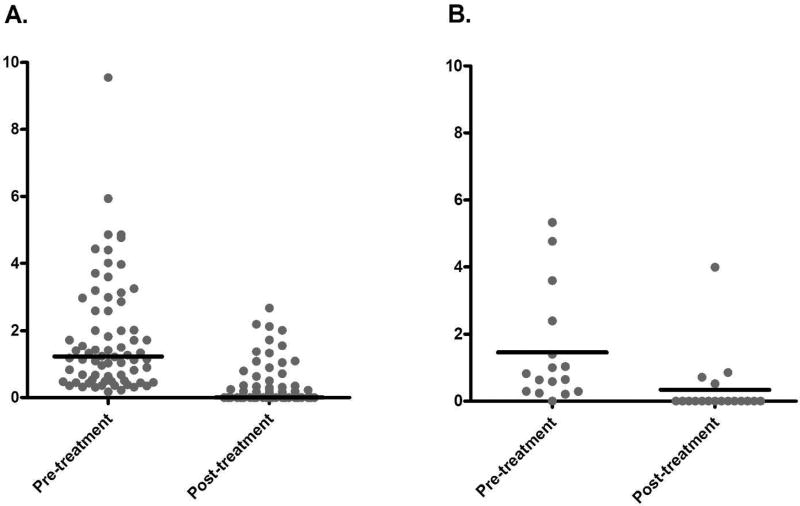
MMF was dosed at 1500–2000 mg/day +/− titration to a goal absolute lymphocyte count of 1.0–1.5 × 103/µL, or to a target weight-based dose of 30mg/kg/d. In this group, the pre- treatment annualized relapse rates were similar between AQP4 seropositive and seronegative patients at 1.79 and 1.45 relapses per year, respectively. After a median treatment duration of treatment of 3.0 years (mean 3.4), the annualized relapse rates significantly dropped to 0.29 and 0.30 relapses per year, in seropositive and seronegative patients respectively (p<0.005; Figure 2). The remission rate among AQP4 seropositive patients was 64.7%, while among AQP4 seronegative patients was 77.8% (p=0.41; Table 3). Among those patients who relapsed despite treatment with MMF, the median time to relapse was 17 months and 7.5 months, which is also not significantly different between AQP4 seropositive and seronegative patients (p=0.21).
Figure 2.
Comparison of annualized relapse rate before and after initiation of mycophenolate mofetil: A. patients with AQP4 seropositive NMOSD (p<0.0001), B. patients with AQP4 seronegative NMOSD (p=0.0039)
Table 3.
Mycophenolate Mofetil Response to Treatment by Serostatus
| Seropositive (n=85) | Seronegative (n=18) | p-value | |
|---|---|---|---|
| Time on treatment, median (mean, range) in years | 3.4 (3.0, 0.5–7.7) | 3.1 (2.6, 0.5–9.6) | 0.63 |
| Pre-MMF ARR | 1.79 | 1.45 | 0.14 |
| Post-MMF ARR | 0.29 | 0.30 | 0.41 |
| Remission rate, % | 64.7 | 77.8 | 0.41 |
| Time to 1st relapse on treatment, median (mean, range) in years; relapse group | 1.4 (1.7, 0.3–5.9) | 0.6 (0.9, 0.1–2.3) | 0.21 |
Discussion
In this multicenter analysis of a large number of NMOSD patients, there was no difference in treatment response to either RTX or MMF based on AQP4 serostatus. Both AQP4 seropositive and seronegative patients showed a beneficial response characterized by a notable reduction in the annualized relapse rate after starting either medication. Our findings in a racially diverse cohort that is more reflective of the worldwide NMOSD population supports previous findings from a local NMOSD patient cohort.9 The degree of response to both immunosuppressive treatments is also similar to previously published studies.16–18 In addition to the medications working similarly despite serostatus, there was no significant difference in degree of response between medications. The implication is that patients meeting the 2015 revised diagnostic criteria for NMOSD should respond similarly to either RTX or MMF regardless of AQP4 serostatus. While it has been previously reported that patients are responsive to immunosuppression despite serostatus, this is, to our knowledge, the first study to examine the impact of specific immunosuppressive treatments, RTX and MMF, on relapse rates by serostatus. Our data do not indicate that seropositive and seronegative patients have the same primary immunopathogenesis, but they do suggest that immunosuppression may be beneficial regardless of the serostatus. While the decision to treat and the treatment itself should not differ for seronegative patients when considering RTX and MMF, differences in treatment responses may emerge by serostatus as antigen-specific therapies become available in the future. For example, AQP4 seronegative NMOSD patients who test positive for serological antibodies to myelin-oligodendrocyte glycoprotein (MOG) may not respond as well to antigen-specific therapy targeted to AQP4 as an AQP4 seropositive NMOSD patient,19 and most of our seronegative patients were not tested for this antibody.
Several limitations are noted in this retrospective analysis. Despite the relatively large number of patients examined in this analysis of a rare disease, many patients were excluded because of failure to meet our inclusion criteria. Several sites historically used azathioprine as their first-line therapy in NMOSD, which allowed for only small numbers for inclusion from these centers. Also, in what is already a rare disease, the AQP-4 seronegative patient population is rarer yet. As such, even with our pooled resources across centers, the numbers in the seronegative group are relatively small. Another limitation of this study is such that relapses resulting from suboptimal treatment were not differentiated from those in which the medication failed despite optimal immunosuppression, as laboratory data were not made available from all sites. The medication regimens differed not only by site, but also within patients over time, so a sub-analysis was not possible in this retrospective review. This study is further limited by the inherent biases associated with retrospective studies, including information bias. Given the scarcity of the disease, a random sampling of patient records is not feasible; however, the cross-cultural inclusion of any patient meeting study criteria allows for increased generalizability of results. Relapse severity was not collected for this analysis. Because of deficits in the application of the Expanded Disability Status Scale to NMOSD, an international effort is underway to clarify relapse severity in NMOSD; once the relapse definition and severity scale have been published, we intend to address this important factor through prospective data collection. Future well-controlled studies are also indicated to prospectively validate our findings that suggest immunosuppression with RTX or MMF decreases relapse rates in patients with NMOSD, regardless of serostatus.
Acknowledgments
none
Footnotes
Declaration of Conflicting Interests: the authors declare there are no conflicts of interest
References
- 1.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53(5):1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 2.Fryer JP, Lennon VA, Pittock SJ, et al. AQP4 autoantibody assay performance in clinical laboratory service. Neurology® Neuroimmunology & Neuroinflammation. 2014;1(1):e11. doi: 10.1212/NXI.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melamed E, Levy M, Waters PJ, et al. Update on biomarkers in neuromyelitis optica. Neurology® Neuroimmunology & Neuroinflammation. 2015;2(4):e134. doi: 10.1212/NXI.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. Journal of Neuroinflammation. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jasiak-Zatonska M, Kalinowska-Lyszczarz A, Michalak S, Kozubski W. The Immunology of Neuromyelitis Optica—Current Knowledge, Clinical Implications, Controversies and Future Perspectives. Kleinschnitz C, ed. International Journal of Molecular Sciences. 2016;17(3):273. doi: 10.3390/ijms17030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akman-Demir G, Tuzun E, Waters P, et al. Prognostic implications of aquaporin-4 antibody status in neuromyelitis optica patients. J Neurol. 2011 Mar;258(3):464–70. doi: 10.1007/s00415-010-5780-4. Epub 2010 Oct 17. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Huang DH, Wu WP, et al. The role of aquaporin-4 antibodies in Chinese patients with neuromyelitis optica. J Clin Neurosci. 2013 Jan;20(1):94–8. doi: 10.1016/j.jocn.2012.06.006. Epub 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 9.Jiao Y, Fryer JP, Lennon VA, et al. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology. 2013 Oct 1;81(14):1197–204. doi: 10.1212/WNL.0b013e3182a6cb5c. Epub 2013 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS) J Neurol. 2014 Jan;261(1):1–16. doi: 10.1007/s00415-013-7169-7. Epub 2013 Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler RA, Mealy MA, Levy M. Treatment of Neuromyelitis Optica Spectrum Disorder: Acute, Preventive, and Symptomatic. Curr Treat Options Neurol. 2016 Jan;18(1):2. doi: 10.1007/s11940-015-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Kim W, Li XF, et al. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler. 2012;18:1480. doi: 10.1177/1352458512439439. [DOI] [PubMed] [Google Scholar]
- 13.Kleiter I, Hellwig K, Berthele A, et al. Failure of Natalizumab to Prevent Relapses in Neuromyelitis Optica. Arch Neurol. 2012;69:239–245. doi: 10.1001/archneurol.2011.216. [DOI] [PubMed] [Google Scholar]
- 14.Min JH, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler. 2012;18(1):113–5. doi: 10.1177/1352458511431973. [DOI] [PubMed] [Google Scholar]
- 15.Cree BA, Bennett JL, Sheehan M, et al. Placebo-controlled study in neuromyelitis optica-Ethical and design considerations. Mult Scler. 2016 Jun;22(7):862–72. doi: 10.1177/1352458515620934. Epub 2015 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison of Relapse and Treatment Failure Rates Among Patients With Neuromyelitis Optica: Multicenter Study of Treatment Efficacy. JAMA Neurol. 2014;71(3):324–330. doi: 10.1001/jamaneurol.2013.5699. [DOI] [PubMed] [Google Scholar]
- 17.Huh S, Kim S, Hyun J, et al. Mycophenolate Mofetil in the Treatment of Neuromyelitis Optica Spectrum Disorder. JAMA Neurol. 2014;71(11):1372–1378. doi: 10.1001/jamaneurol.2014.2057. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Jeong I, Hyun J, et al. Treatment Outcomes with Rituximab in 100 Patients With Neuromyelitis Optica: Influence of FCGR3A Polymorphisms on the Therapeutic Response to Rituximab. JAMA Neurol. 2015;72(9):989–995. doi: 10.1001/jamaneurol.2015.1276. [DOI] [PubMed] [Google Scholar]
- 19.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. 2015 Jan 22;2(1):e62. doi: 10.1212/NXI.0000000000000062. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]