Abstract
A previous experiment showed that blue light (as a component of white light) protected against low temporal frequency dependent eye growth. This experiment investigated the role of temporal contrast. White leghorn chicks were exposed to white (with blue) or yellow (without blue) LED lighting modulated at either low (0.2 Hz) or high (10 Hz) temporal frequencies. Four cone contrast conditions were used: low (16%), medium (32%), medium-high (60%) and very-high (80%). Chicks were exposed to the lighting condition for 3 days (mean 680 lux). Exposure to high temporal frequencies, with very high temporal contrast, reduced eye growth, regardless of spectral content. However, at low temporal frequencies, eye growth was dependent on the illuminant. At lower temporal contrast levels, eye growth increased regardless of temporal or spectral characteristics. To conclude, very high temporal contrast, white light, provides a “stop” signal for eye growth that overrides temporal cues for growth that manifest in yellow light.
Keywords: Myopia, Emmetropization, Color, Temporal, Contrast, Blue Light
1. Introduction
During the first months of development, neonates undergo a critical period of eye growth called emmetropization. If the processes that underlie emmetropization are successful, the result is an emmetropic eye; one in which the refractive power of the eye results in a focused retinal image. The processes involved in emmetropization are guided by a variety of cues, including retinal defocus [1–6] and changes in color and luminance contrast [7–12]. Each of these cues are dependent on the spatial [10, 11, 13, 14], temporal [13, 15–22] and spectral [23] properties of the stimulus. When emmetropization fails to match the axial length of the eye to its refractive power, and the eye grows too long for its optics, myopia arises.
1.1 Dependence on spatial contrast
Form-deprivation experiments demonstrate that spatial frequency cues regulate eye growth. When eyes are occluded or sutured shut, spatial contrast and structure are reduced, and this results in a dramatic increase in eye growth leading to form-deprivation myopia [24–32]. Surprisingly, exposing form-deprived chicks to intermediate (0.86 cycles deg−1), high (4.3 cycles deg−1), and mixed spatial-frequency environments for as little as 20 minutes a day can reduce this form-deprivation myopia [11]. A similar reliance on intermediate spatial frequencies for lens compensation has been found by Diether & Wildsoet [33]. This dependence on spatial frequency implies an innate dependence on spatial contrast.
Several papers support the view that spatial contrast is an important cue for guiding eye growth. In the experiment mentioned above, Schmid and Wildsoet [11] examined the effect of 20 minutes of exposure to different levels of spatial contrast in form-deprived chicks. They found that restricted spatial contrast environments (9–78%) produced the same inhibition of form-deprivation myopia as normal cage environments, which suggests that even low spatial contrast influences emmetropization. Two related experiments, which used a cone apparatus and lenses to manipulate defocus, support this hypothesis and provide measures of spatial contrast required for normal emmetropization. Schmid [12] found that targets with spatial contrast of only 4.2% prevented the development of high myopia, with normal emmetropization occurring at a contrast level of 47.5%. Similarly, Diether & Wildsoet [33] noted that spatial contrast of at least 65% was required to produce the anticipated refractive shifts when an animal wore positive lenses (focusing the image in front of the retina). Considered together, these results support the idea that while low spatial contrast influences emmetropization, more moderate contrast in the retinal image is required for normal emmetropization, and even higher spatial contrast is required for lens induced defocus.
1.2 Dependence on temporal contrast
Temporal characteristics can differentially affect eye growth. For example, high temporal frequency stroboscopic illumination produces less myopic (more hyperopic) refractions in form-deprived chicks [20, 21, 34, 35]. It has been suggested that high temporal frequency stimulation of the retina signals that the eye is in focus and that the optimal eye length has been achieved [34, 36]. If detection of high temporal frequencies provides a cue to slow eye grow, then the detection of low temporal frequencies could indicate that the eye is hyperopically (image behind the retina) defocused and encourage growth. Longer eyes are associated with the development of myopia.
Previous research supports that temporal stimuli can influence eye growth. Crewther [17] examined the effects of lower temporal frequencies, by exposing chicks to temporal frequencies of 1, 2, and 4 Hz and found that exposure to low temporal frequencies lead to a myopic shift in refraction. Di [22] also found a myopic shift when guinea pigs were exposed to temporal frequency modulations of 0.5 Hz and 5 Hz. The guinea pigs exposed to the 0.5 Hz flicker stimulus were more myopic than those exposed to 5 Hz. Mice also became myopic when exposed to 2 Hz flicker [37]. In addition, a recent study from our laboratory has shown that chicks eyes grow 145 μm more at low temporal frequencies than at high temporal frequencies and become almost 2D more myopic after only 3 days [23]. Thus there is mounting evidence that exposure to low temporal frequency flicker produces more myopic refractions.
Several experiments [17, 20, 22, 38] have investigated how temporal information is used in emmetropization, but interpretation is complicated with use of a variety of illuminants. Schwahn & Schaeffel [20] used a Xenon light source with a square-wave that varied in its temporal frequency and duty cycle, Crewther [17] used a halogen light source that was pulsed for 250 ms, and Di [22], in one experiment, used a narrow band, monochromatic, green light of 505 nm, with a square-wave temporal profile (duty cycle 50%), and in a second experiment used stroboscopic white light [38]. Gawne et al. [39] used monochromatic red (628 ± 10 nm) and blue (464 ± 10 nm) light with a pseudo-random temporal profile and Rucker et al [23] used white and yellow LEDs (both consisted of red (615 ± 20 nm) and green (515 ± 35 nm) components, with a blue component (465 ± 35 nm) present in white). These different light sources have different spectral profiles. Xenon light sources have a strong blue component, while Halogen lights have a warm white color spectrum, and monochromatic lights and LEDs have particular hues depending on their wavelength and the combinations of LEDs used.
Interpretation of how frequency affects emmetropization is also complicated by the use of different waveforms some sinusoidal some non-sinusoidal. The non-sinusoidal temporal stimulation used in prior experiments has the drawback that the stimulation is not narrow-band at the modulation frequency presented but contains other temporal frequencies that stimulate the retina at lower and higher frequencies than experimenters report. For example, square-wave stimuli provide ample stimulation at higher harmonics (three and five times the frequency of the square wave) and pulsed stimuli contain a broad range of temporal frequency information. A sinusoidally modulated stimulus does not have the issues mentioned above; the stimulus energy of a sinusoidal waveform is concentrated at the stimulus frequency and little to no information is present outside of the signal frequency. It is known that the vertebrate retina responds linearly to sinusoidally flickering light [40] and that linearity holds for both horizontal cells [41] and retinal ganglion X cells, but Y cells show non-linear responses that increase with input temporal contrast [42]. Our choice of a sinusoidal input in this experiment over other types of temporally varying input minimizes non-linear responses in the retina.
In a previous experiment, Rucker et al., [23] examined the effect of exposing chicks to light modulated sinusoidally at different temporal frequencies, with fixed temporal contrast (80%). Two spectral conditions were used: 1) A White spectral condition that was a mixture of all the LED sources (red, green and blue light); 2) A Yellow spectral condition that contained red and green light but did not contain blue light. The illuminance of the white and yellow light sources was matched at 680 lux. In the Yellow condition, the results were consistent with previous research, low temporal frequency stimulation (0.2, 1, and 2 Hz) produced longer eyes while high temporal frequencies shorter eyes (5 and 10 Hz). At high temporal frequencies the spectral content of the light source did not affect eye growth, but at low temporal frequencies exposure to white light protected against the increase in eye length seen with exposure to yellow light. The current experiment will investigate the temporal contrast level required to achieve these protective effects on eye growth at high and low temporal frequencies.
1.3 Chick as an animal model for the role of color in emmetropization
In this paper we used the chick as a model system to study the stimulus dependent mechanisms in emmetropization following a tradition of nearly forty years of research. Among the animals models of myopia described by Schaeffel and Feldkaemper [43] the chick is notable for its practical benefits and for the translation of results to other animal species. Chicks are easy to breed and relatively inexpensive to keep, thus making relatively large sample sizes economical and allowing for the detection of experimental manipulations with small effect sizes. The chick choroid responds to lens-induced defocus in only 15 minutes [44], with measurable changes in eye length in three days. The chick has a visual acuity of about double that of other non-primate species used in myopia research [43, 45] and a spatial contrast sensitivity function comparable to 2–3 month-old children [11, 45, 46]. In addition, the temporal vision of the chick is qualitatively similar to humans [47], unlike other alternative animal models for myopia such as the tree shrew or guinea pig [48, 49] that have a temporal sensitivity function that is skewed towards high temporal frequencies. The temporal sensitivity of the chicks is of particular importance to the experiment presented here, given that temporal stimulation was a key variable manipulated in this experiment.
Because the spectral composition of the stimulus was a key variable in our experiment, it is worth considering how the chick color system differs from that of other animal models used for myopia research and the human visual system. Humans possess trichromatic vision, so the most general findings would be produced if researchers used an old-world, trichromatic, higher-order primate or ape as an animal model. However, using higher-order primates or apes adds complex ethical issues and practical concerns, such as the time required for rearing primates, slow responses to defocus, and the expenses associated with higher-order primate or ape care. Chicks are at least tetrachromatic [50] but there are differences between chick and human photoreceptors that include the presence of oil droplets, double cones, a short-wavelength sensitive photoreceptor (peak sensitivity: 460 nm), and an ultraviolet sensitive photoreceptor (peak sensitivity: 420 nm). Nevertheless, the avian oil-droplet absorption and photoreceptor sensitivity have been characterized precisely [8, 51] and there is evidence that double-cones do not contribute to chromatic vision [52]. Alternative animal models, such as tree shrew and guinea pig, are dichromatic, making their use in color vision studies more limited. Taken together, the richness of color signals from the chick retina, combined with their practicality, fast response to defocus, and translatable results, make chick a good choice for studying the role of color vision in emmetropization.
It is well documented that the chick, guinea pig, and tree shrew eye can emmetropize in monochromatic light without a signal from longitudinal chromatic aberration (LCA) [39, 53–59]. In monochromatic light, cone contrast is the same in all cone types, increasing and decreasing in tandem with focus and defocus, despite differences in absolute values of cone excitation with the different colored lights. There are two hypotheses regarding ways that monochromatic stimuli could signal that the eye is in focus. A simple emmetropization mechanism could aim to maximize spatial contrast (luminance contrast) in the retinal image by modulating the growth rate to retinal contrast [60]. From this perspective, eye growth would decrease gradually as retinal spatial contrast (luminance contrast) increases. In support of this idea, several studies have shown that the level of form-deprivation myopia is dependent on the degree of visual occlusion [60–62]. However Tran et al. have disagreed [10] and have suggested that defocus could trigger an “all-or-none” response or a threshold for inducing eye growth from image degradation.
There is also evidence that a signal from longitudinal chromatic aberration provides a color cue for emmetropization in chicks [8, 63]. First, Wallman & Rucker [8] showed that lens compensation in white light was more accurate than in monochromatic red or blue light, evidence the chromatic dispersion of white light provides a defocus cue for emmetropization. Second, Wallman & Rucker [63] showed that the chick eye can compensate for chromatic simulations of hyperopic and myopic defocus. Chick eyes grew more when spatial contrast was greater for the blue component of the image than for the red component. This provided evidence that the chick eye was responding to the signed changes in color contrast created by chromatic dispersion in the presence of defocus.
The experiment presented in this paper builds upon previous results that showed that blue light as a component of white light [23] protects against increased eye growth with exposure to light modulated with very high temporal contrast at low temporal frequencies. The results also showed that exposure to very high temporal contrast, at high temporal frequencies reduced growth. The primary goals of this experiment are to determine the threshold temporal contrast levels for these protective effects and their spectral dependency.
Methods
2.1 Animals
White leghorn chicks (Gallus gallus domesticus, Cornell K strain; Cornell University, Ithaca, NY) were hatched in-house. Before being exposed to experimental conditions, the birds were kept in a 12-hour light/12-hour dark cycle; animals were maintained under fluorescent lighting (Sylvania Soctron XP 4100K 30 W) at an intensity of approximately 300 lux. The birds were supplied with food and water ad libitum. In these experiments, chicks were 12 days of age at the start of the experiment. Use of animals in this study was in compliance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the NECO Institutional Animal Care and Use Committee.
2.2 Stimuli
During the experiment, chicks were exposed to one of four temporal contrasts, one of two temporal frequencies, and one of two spectral conditions. The numbers of chicks in each condition is shown in Table 1.
Table 1.
Numbers of birds in each temporal contrast condition, and each temporal frequency condition, in White and Yellow lighting conditions.
| Light | |||||
|---|---|---|---|---|---|
|
| |||||
| Temporal Contrast % | Temporal Frequency (Hz) | White | Yellow | ||
|
| |||||
| 0.2 | 10 | 0.2 | 10 | ||
| 16 | 16 | 13 | 14 | 11 | |
| 32 | 16 | 15 | 13 | 12 | |
| 60 | 12 | 14 | 12 | 12 | |
| 80 | 11 | 12 | 10 | 11 | |
The four temporal contrast conditions were: low (16%), medium (32%), medium-high (60%) and very-high (80%). The two temporal frequency conditions were: low (0.2 Hz) or high frequency (10 Hz) sinusoidal waveforms.
The White (with blue) and Yellow (without blue) illumination conditions were set at a mean illumination level of 680 lux (controlled digitally with minor adjustments being made with neutral density filters) when measured with the light source placed on the wire mesh roof of the cage and the light sensor on the floor. Stimuli were generated by a modulated light source using light emitting diodes (LEDs) that contained red, green and blue components (White) or only red and green components (Yellow). The term “stimulus” refers to the spectral and temporal characteristics of the background illumination. The mean irradiances were 50 μW/cm2 for the red, green and blue components of each light source, which is equivalent to 214 “chick lux” for red, 191 “chick lux” for green, and 64 “chick lux” for blue as in an earlier experiment [23]. Since chicks have different wavelength sensitivities (Figure 2) to humans we refer to illuminance corrected for the chick photopic sensitivity function [64] as “chick lux”, which differs from “human lux” as a function of wavelength.
A follow up temporal contrast condition of 70% at 10Hz in White light was added to the experiment after the main data collection to test a specific hypothesis suggested by the results.
2.3 Illuminants
The illuminants were Lamina Titans RGB LEDs (Lamina Ceramics) driven by an eight channel, 12-bit Access I/O, USB-DA12-8A digital to analog converter with waveform generator functionality connected to BuckPucks (LuxDrive: 3021 D-E-500) that provided a linear current output over a range of 1.6 – 4.3 V. Light output was calibrated and a sinusoidal output produced digitally using lookup tables, and confirmed by measurement of illuminance output (Newport Model 818-SL serial number: 6915). Luminance flicker was produced with in-phase sinusoidal modulation of red (615 nm half-bandwidth 20 nm), green (515 nm half-bandwidth 35 nm), and blue (465 nm half-bandwidth 35 nm) LEDs in the White condition and only the red and green LEDs in the Yellow condition.
Figure 1 shows the spectral sensitivity of the five chick cone types [8]. From this figure we can see that all three LEDs stimulate the double- (D) cones by varying amounts. Since the double cone has a broad spectral sensitivity, it is not thought to contribute to detection of color only to detection of brightness [65]. In addition to stimulating the double cone, the red LED (615 nm) stimulates long (L)-wavelength sensitive cones, the green LED (515 nm) stimulates medium-(M) and short- (S-) wavelength sensitive cones, and the blue LED (465 nm) stimulates S- and ultraviolet- (UV) sensitive cones to some degree. As a result, both the Yellow and White conditions stimulate all cone types (except UV-cones in Yellow) but the White condition excites the S-cones much more than the Yellow condition.
Figure 1.
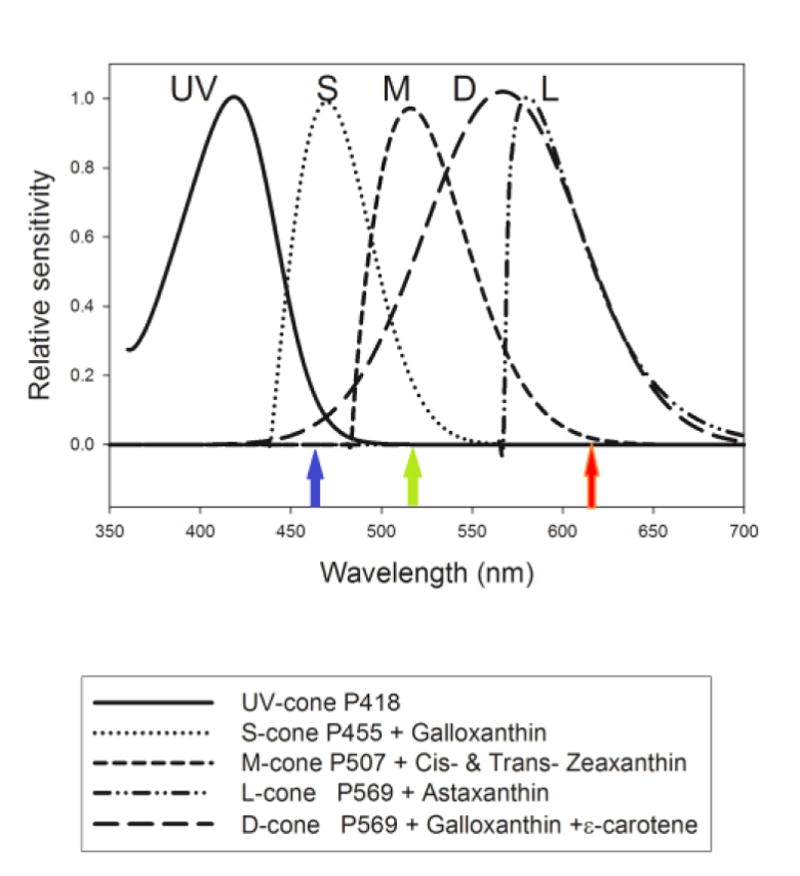
Graph of chick cone spectral sensitivity showing long- (L), middle- (M), short- (S), ultraviolet- (UV) sensitive and double- (D) cones (Rucker & Wallman, 2008). Peak spectral output of the red, green and blue LEDs are indicated by arrows of the relevant color.
An important factor in the design of the stimuli is that White and Yellow are designed to produce equal temporal contrast at the cone level to each of the cone types. This is achieved despite the shorter focal length of blue light in White that arises as a result of longitudinal chromatic aberration. In this way, the stimuli are similar to monochromatic stimuli; they provide no color signals for defocus from longitudinal chromatic aberration. However, unlike stimuli with monochromatic spatial contrast, stimuli with temporal contrast are not affected by blur, temporal contrast will not increase as focus improves. As a result, these temporal contrast stimuli lack both color and luminance contrast cues for emmetropization and are therefore open-loop stimuli.
For the purposes of this experiment, temporal cone contrast was calculated as Michelson Contrast comparing the maximum cone excitation (Lmax) and minimum cone excitation (Lmin) produced by the temporal stimulus [(Lmax-Lmin)/(Lmax+Lmin)].
2.4 Procedures
Chicks were randomly assigned to a stimulus condition and while in this condition they experienced the assigned temporal modulation of the illuminant for eight hours per day. The experimental data collection used a block-randomized design. Typically, chicks were exposed to a stimulus condition with five to eight birds to a cage. Cages were made of wire mesh with a plastic base (29″x17″x 17″). When not in the experimental environment, the chicks were in the dark in a light- and sound-proof chamber for 16 hours overnight. Both eyes were exposed to the temporally modulated illuminant and no lenses were used at any point during the study. This cycle continued for a period of 72 hours, after which the chicks were placed back into their brooders.
2.5 Measurements
At the beginning and end of the exposure period refraction and ocular biometry were measured. For both instruments, birds were placed under anesthesia using 1.5% isoflurane in oxygen with a 2 L/min oxygen flow rate. Measurements of the ocular components were made using a Lenstar biometer (Lenstar LS 900: Haag-Streit AG, Konig, Switzerland). For each bird, the Lenstar measurement was obtained from the average of five measurements. Three measurements of corneal curvature were made for each eye using calibrated images of the Lenstar mires as described previously [23] and below. Refraction was measured with a Hartinger Refractometer (Zeiss, Jena, Germany). Measurements were excluded if clear mires could not be imaged. For refraction each bird’s refractive error was estimated from the average of three measurements.
Measurements of corneal curvature were made using images of the Lenstar alignment rings, taken during the measurements of the ocular components. The images collected show the circular alignment mires positioned directly in the center of the pupil. These images were uploaded into Image J. A rectangular outline was drawn around the mires, and the dimensions of the rectangle were used to determine the radius of curvature in the horizontal and vertical meridians. Corneal curvature was calibrated using ball bearings of known radius, and the refractive index of 1.33749. These calibrations were used to convert the dimensions of the rectangle into corneal radii and corneal power measures [66]. Finally, corneal power was described by Fourier decomposition (rectangular form) with spherical power component M and power vectors J0 (cosine Jackson cross cyl [JCC]) and J45 (sine JCC) as described by Thibos et al. [67]. This method of analysis enabled us to compare changes in meridional power among birds. Changes in corneal power (not refractive correction) along these meridians were calculated as the difference in the pre- and post- measures of corneal power.
2.6 Analysis
The effects of the illumination conditions on the dimensions of the ocular components (anterior chamber depth, lens, vitreous chamber depth, choroidal thickness, eye length), refraction and corneal curvature were calculated as the mean change between pre- and post-measurements. Measurements were made in both eyes and the average change in the two eyes was used in analysis. Eye length was calculated as the distance from the anterior cornea to the posterior surface of the posterior sclera.
The statistics program R [68] was used to compute a three-way ANOVA with Type III sums of squares [69]. Type III sums of squares were used to protect against the effects of the modest imbalance in our design. Frequency (0.2, 10 Hz), temporal contrast (low: 16%, medium: 32%, high: 60%, and very high: 80%) and the spectral content of the light (White and Yellow) were factors in the ANOVA. We looked at the effect of each of these factors on refraction, corneal curvature and ocular dimensions (axial length, vitreous depth, choroid thickness, and anterior chamber depth). Partial Eta-squared (η2partial) was computed [70] to provide a measure of effect size; η2partial provides an estimate of the variance explained by a given factor after the variance explained by the other predictors is excluded. As a guideline, a η2partial effect size of 0.06 is considered a medium effect, 0.14 is considered a large effect. Tukey’s HSD test [71] or Scheffe post hoc tests were used to compare pair-wise differences of frequency, temporal contrast and spectral content.
3.0 Results
Changes in ocular components in each of the temporal frequency, contrast, and lighting conditions are shown in Table 2.
Table 2.
Table shows changes in eye length, refraction, choroidal thickness, anterior chamber depth (CAC) and vitreous chamber depth over three days when the eye is exposed to stimuli of 0.2 and 10 Hz (Freq) modulated at 16, 32, 60 and 80% (Temporal Contrast) in White and Yellow (Light) conditions. Ocular parameters are measured in millimeters (mm), refractions in diopters (D) and standard errors are shown (se).
| Freq | Contrast | Light | Δ Eye Length (mm) | ± se | Δ Refraction (D) | ± se | Δ Choroid | ± se | Δ CAC | ± se | Δ Vitreous | ± se |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 16 | White | 0.283 | 0.019 | 0.03 | 0.44 | −0.018 | 0.009 | 0.040 | 0.005 | 0.148 | 0.007 |
| 10 | 16 | White | 0.241 | 0.012 | −0.78 | 0.41 | −0.021 | 0.006 | 0.031 | 0.006 | 0.130 | 0.007 |
| 0.2 | 32 | White | 0.252 | 0.017 | −0.04 | 0.45 | −0.026 | 0.011 | 0.028 | 0.008 | 0.142 | 0.007 |
| 10 | 32 | White | 0.279 | 0.014 | −1.59 | 0.37 | −0.017 | 0.007 | 0.060 | 0.012 | 0.143 | 0.004 |
| 0.2 | 60 | White | 0.248 | 0.018 | −0.17 | 0.39 | −0.043 | 0.011 | 0.032 | 0.011 | 0.150 | 0.005 |
| 10 | 60 | White | 0.295 | 0.029 | −0.79 | 0.50 | −0.018 | 0.013 | 0.030 | 0.007 | 0.152 | 0.008 |
| 0.2 | 80 | White | 0.205 | 0.024 | −0.65 | 0.90 | −0.047 | 0.014 | 0.027 | 0.006 | 0.131 | 0.007 |
| 10 | 80 | White | 0.179 | 0.036 | 0.07 | 0.38 | −0.052 | 0.020 | −0.001 | 0.009 | 0.120 | 0.007 |
|
| ||||||||||||
| 0.2 | 16 | Yellow | 0.288 | 0.014 | −1.00 | 0.27 | −0.026 | 0.010 | 0.053 | 0.006 | 0.158 | 0.004 |
| 10 | 16 | Yellow | 0.256 | 0.017 | −0.11 | 0.65 | −0.026 | 0.009 | 0.032 | 0.007 | 0.137 | 0.008 |
| 0.2 | 32 | Yellow | 0.280 | 0.018 | −0.67 | 0.68 | −0.017 | 0.008 | 0.052 | 0.011 | 0.146 | 0.009 |
| 10 | 32 | Yellow | 0.218 | 0.029 | −0.14 | 0.41 | −0.043 | 0.014 | 0.042 | 0.009 | 0.120 | 0.011 |
| 0.2 | 60 | Yellow | 0.267 | 0.014 | −0.74 | 0.46 | −0.027 | 0.008 | 0.037 | 0.006 | 0.148 | 0.004 |
| 10 | 60 | Yellow | 0.250 | 0.008 | −1.45 | 0.66 | −0.021 | 0.008 | 0.028 | 0.006 | 0.135 | 0.005 |
| 0.2 | 80 | Yellow | 0.277 | 0.022 | −1.79 | 0.91 | −0.015 | 0.011 | 0.032 | 0.018 | 0.146 | 0.011 |
| 10 | 80 | Yellow | 0.171 | 0.031 | −0.93 | 0.45 | −0.032 | 0.008 | 0.023 | 0.008 | 0.101 | 0.012 |
3.1 Effect of Temporal Contrast
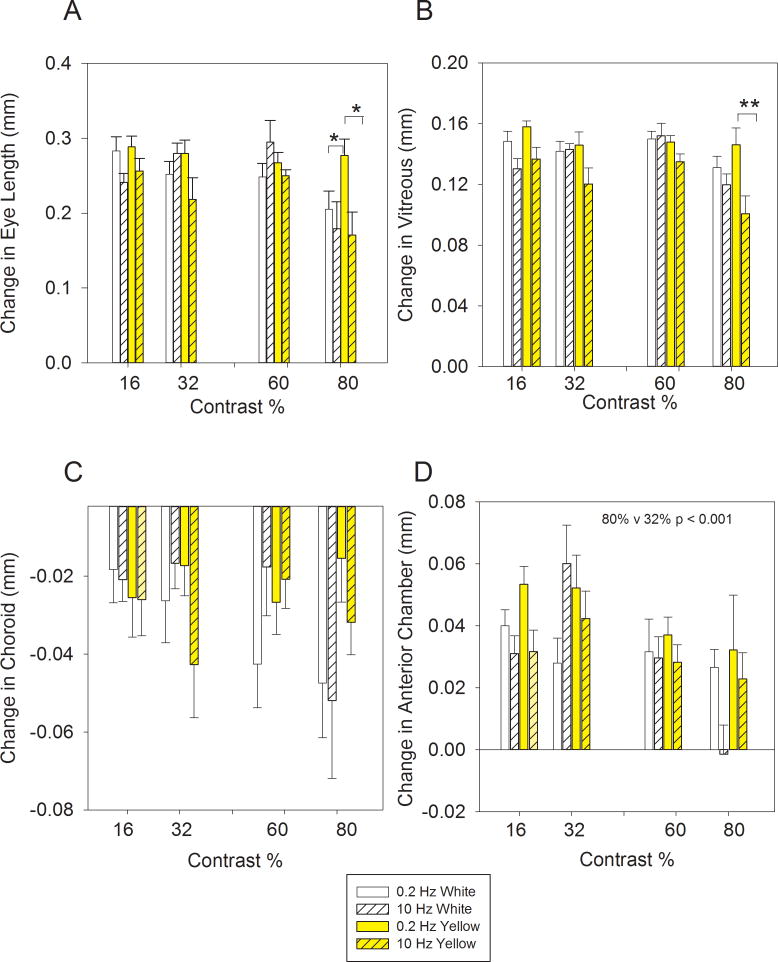
Higher temporal contrast decreased eye growth (Figure 2A: ANOVA: Eye length: F(3,189)=7.01, p<0.001,η2partial = 0.101). An example of this effect is seen at 10 Hz, when the eye grew less (0. 205 ± 0.024 mm; p < 0.01) with exposure to high contrast white light (80%) than with exposure to medium contrast (32%) white light (0.279 ± 0.014 mm). Exposure to very high temporal contrast makes the eye grow less.
Figure 2.
Change in ocular components over three days with exposure to white (solid white or black/white hatched) or yellow (solid yellow or yellow/black hatched) light modulated at 0.2 Hz (solid) or 10 Hz (hatched) with temporal contrast of 16, 32, 60 or 80%). *, **, indicate p levels < 0.05 and 0.01, respectively. Error bars indicate ± standard error.
A: At 10 Hz the eye length grew less with 80% temporal contrast with exposure to both white and yellow light, but at 0.2 Hz this effect was only seen in white light. B: At 10 Hz the vitreous chamber grew less with 80% temporal contrast with exposure to both white and yellow light, but at 0.2 Hz this effect was only seen in white light. C: Choroids thinned with exposure to temporal modulation. D: Growth of the anterior chamber decreased with high temporal contrast and high temporal frequencies. With exposure to white light with 80% temporal contrast there was no change in growth of the anterior chamber at 10 Hz (bar is only just visible on the graph) compared to the large increase seen at lower temporal contrast levels. Statistics are provided for when data is collapsed across lighting conditions in the 10 Hz condition.
Higher temporal contrast produced less vitreous chamber growth (Figure 2B: ANOVA: Vitreous: F(3,189)=7.33, p <0.001, η2partial = .098). Temporal contrast did not show either a three-or two-way interaction with either frequency or illumination. One post-hoc comparison is worth considering. Collapsing the data across frequency and lighting, an increase in temporal contrast from 60% to 80% caused the vitreous chamber to grow less by 0.022 ± 0.6 mm (p < 0.001). This result shows that there is a very high temporal contrast threshold necessary for the eye to slow its vitreal growth.
Higher temporal contrast produced shallower anterior chambers (Figure 2D: ANOVA: Ant. Chamber: p < 0.001, F (3,189) = 6.877,η2partial = 0.096). The anterior chamber depth was the only ocular component that showed a temporal frequency by temporal contrast interaction (ANOVA: F(3,189)=2.87, p < 0.05, η2partial = 0.043). Collapsing the data across lighting conditions, the anterior chamber was 0.043 ± 0.008 mm shallower at 80% contrast than it was at 32% contrast in the 10 Hz condition (p < 0.001). A reduction in anterior chamber growth can be seen with other very high temporal contrast/high temporal frequency combinations (80% contrast [10 Hz] v 32% contrast [0.2 Hz]: p < 0.01; 80% v 16% contrast [0.2 Hz]: p < 0.001). Shallower anterior chamber depths produced by very high temporal contrast and high temporal frequency combinations tend to make the eye less myopic.
To explore the threshold for temporal contrast that is required to influence eye growth and to see if the transition is graded we used a 70% temporal contrast, 10Hz, White light condition. We found that the 70% temporal contrast condition produced 0.280 ± 0.015 mm eye growth, almost the same as the eye growth with exposure to 60% temporal contrast (0.295 ± 0.029 mm; p = 0.66). Thus, there was a sharp threshold for eye growth at very high temporal contrast levels. To explore the threshold for temporal contrast that is required to influence vitreous chamber depth and to see if there is any grading of the transition between 60 and 80% temporal contrast we used a 70% temporal contrast stimulus, at 10Hz, in the White light condition. There was a small decrease in vitreous chamber growth at 70% temporal contrast in White (0.136 ± 0.022 mm; 60% v 70%) that was intermediate between that with exposure to 60% (0.152 ± 0.008 mm) and 80% (0.120 ± 0.007 mm) temporal contrast. Although the contrast threshold for change in vitreous chamber depth was high, the vitreous chamber showed a gradual decrease in depth with increasing temporal contrast as temporal contrast increased from 60 to 80%.
3.2 Effect of Frequency
High frequency temporal stimulation produced less eye growth than low frequency temporal stimulation but there was an interaction with the spectral content of the illuminant (Figure 2A: ANOVA: Eye length: F(1,189)=6.13, p<0.05,η2partial = 0.033). Collapsing the data across temporal contrast conditions, the eye grew more in Yellow at 0.2 Hz than at 10 Hz (0.055 mm; p < 0.01). Post-hoc comparisons at 80% temporal contrast, showed that the eye grew more in Yellow at 0.2 Hz than at 10 Hz (Difference: 0.106 ± 0.033 mm; Scheffe post hoc: p = 0.02), and more than in White at 10Hz (Difference: 0.098 ± 0.032 mm; p < 0.05). There was no difference in eye growth with exposure to White at 10 and 0.2 Hz (Difference: 0.026 mm; p = 0.9). At very high temporal contrast levels, exposure to yellow light at low temporal frequencies caused more eye growth than exposure to yellow light at high temporal frequencies or white light at any frequency.
High frequency temporal stimulation produced less vitreous chamber growth than low frequency temporal stimulation and there was an interaction with the spectral content of the illuminant (Figure 2B: ANOVA: Vitreous: F(1,189) = 7.01, p<0.01,η2partial = 0.026). Collapsing the data across contrast conditions, the vitreous grew more with exposure to yellow light at 0.2 Hz than at 10 Hz (Difference: 0.026 ± 0.005 mm: p < 0.001). Post-hoc comparisons showed that with exposure to yellow light, at 80% temporal contrast, the vitreous chamber depth grew more at 0.2 Hz than at 10 Hz (Difference: 0.045 ± 0.012 mm Scheffe post hoc: p < 0.01). There was no difference in vitreous chamber growth with exposure to white light at these frequencies (Difference: 0.006 ± 0.005 mm; p = 0.58). In summary, yellow light caused greater vitreous chamber growth at low temporal frequencies; a result not seen with exposure to white light.
3.3 Choroidal Thickness
Figure 2C shows that the choroid tends to thin in response to temporal frequency stimulation, though there was no evidence of a difference in choroidal thinning between conditions (ANOVA: Temporal Contrast: p = 0.34; Frequency: p = 0.84; Light: p = 0.42). At 10 Hz, choroids thinned with exposure to white and yellow light at all contrast levels (apart from 60% White) (Scheffe: p < 0.01 to p < 0.001). At 0.2 Hz, choroids thinned with exposure to white and yellow light at all temporal contrasts (apart from 80% Yellow) (Scheffe: p < 0.01 to <0.001). Choroidal thinning tends to make the eye more myopic.
3.4 Effects of Light on Refraction
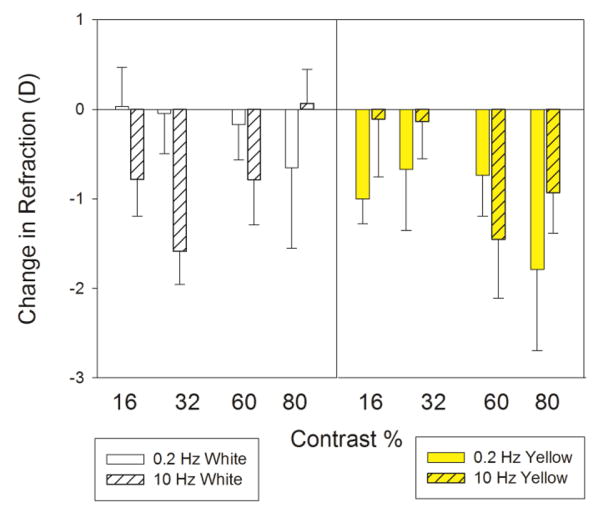
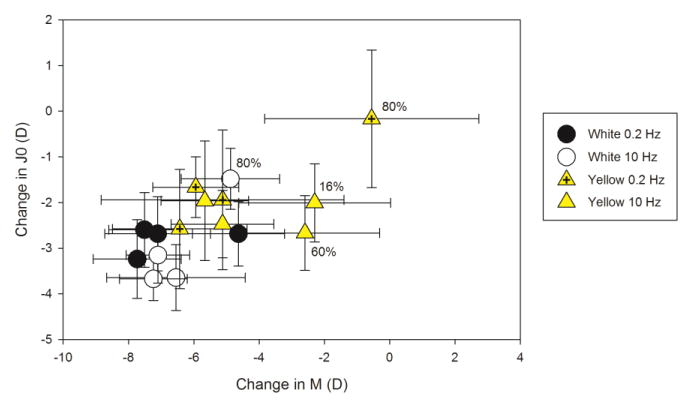
Figure 3 presents the refraction data. In addition to the expected myopic shift with emmetropization, birds exposed to yellow light became slightly more myopic (-0.24 D; p < 0.05) than those exposed to white light. When the effect of frequency is considered, the myopic shift is particularly noticeable at low temporal frequencies with exposure to yellow light. The cause of this effect was due to changes in corneal power. Corneal astigmatic (J0) and corneal spherical power (M) was reduced with exposure to white light (Tukey HSD: p < 0.05 both) compared to that with exposure to yellow light (see Figure 4) making the eye less myopic.
Figure 3.
Change in ocular refraction over three days with exposure to white (left) or yellow (right) light modulated at 0.2 (solid) or 10 Hz (hatched) with temporal contrast of 16 %, 32 %, 60 % or 80 %. Overall, refractions became more myopic with exposure to yellow light than with exposure to white light. Error bars indicate ± standard error.
Figure 4.
Change in corneal refractive components over three days with exposure to white (solid black or white) or yellow (solid yellow or yellow/black cross) light modulated at 0.2 (solid black or yellow/black cross) or 10 Hz (solid white or yellow) with temporal contrast of 16, 32, 60 or 80 %. Temporal contrasts of outlying conditions are indicated. Corneal power (M) and astigmatic power (J0) was reduced with exposure to white light making the eye less myopic. Error bars indicate ± standard error.
4.0 Discussion
Temporal frequency and temporal contrast operate as cues to control eye growth that are dependent on the spectral characteristics of the lighting. Consistent with the results from prior experiments [16, 20, 21, 23], we showed that eye growth reduction could be induced by high temporal frequency stimulation, with stimuli with very high temporal contrast. The key finding in this experiment is that these protective effects of retinal stimulation on eye growth only occur at very high temporal contrast levels of around 80%. In addition, consistent with the results of an earlier experiment [23], exposure to very high temporal contrast white light protected the eye from increased growth at low temporal frequencies. Increased eye growth is seen with exposure to low temporal frequency yellow light, even when the eye is exposed to very high temporal contrast. When temporal contrast is reduced from this optimal level, the eye grows more, irrespective of the spectral and temporal characteristics of the illuminant. Thus, in white light, high temporal contrast stimulation might be construed as an all or nothing “stop” signal that overrides frequency cues.
4.1 Temporal Frequency and Spectral Dependence
The design of our experiment allowed us to investigate whether the effect of temporal frequency depends on the temporal contrast and spectral characteristics of the light source. We can interpret our results in terms of an eye that is too small at birth, but emmetropization can also occur when the eye is too large at birth as in ostrich [72] and falcons [73, 74]. This means that the eye must be able to detect whether it is too small or too big, and a coherent theory on how the sign of defocus is detected must be part of any explanation of the emmetropization mechanisms.
A signal for the sign of defocus may arise from longitudinal chromatic aberration as described in several manuscripts [7, 63, 75]. As a result of the shorter focal length of blue light, hyperopic defocus produces a color signal that arises from an increase in retinal spatial contrast available to the short-wavelength sensitive-cones compared to long-wavelength sensitive-cones. Change in myopic defocus produces a change in luminance contrast arising from retinal spatial contrast available to the long- and middle-wavelength sensitive cones. However, in this experiment, all cone types were exposed to the same temporal contrast and temporal contrast is unaffected by defocus, so there was no color contrast or luminance contrast signal available to the emmetropization mechanism in either the White or Yellow conditions. Nevertheless, in White, the presence of the shorter-focal plane of blue light provides a signal that slows growth. This result confirms previous findings [23].
4.1 Temporal Contrast Dependence
The threshold temporal contrast growth response found in this experiment and that of Tran et al. [10] is inconsistent with the graded spatial contrast response found in other form-deprivation experiments [60–62]. Even in the vitreous, the temporal contrast threshold was high, with a graded response only being seen between 60 and 80% temporal contrast. There are two possible explanations. First, an increase in spatial contrast sensitivity with spatial contrast adaptation may have played a role, as suggested in other experiments [76–78]. It is possible that the “stop” signal is activated at lower spatial contrast levels with spatial contrast adaptation increasing as spatial blur increases. In this way, an eye wearing a dense diffuser would become more sensitive to lower levels of spatial contrast when the diffuser is removed for brief periods. Alternatively, the emmetropization mechanism may respond differently to spatial and temporal contrast showing a graded response to spatial contrast and a threshold response to temporal contrast. Further work is required to clarify these options.
These results suggest that very high temporal contrast, high temporal frequency stimulation is an indicator that the eye is in focus, even in the absence of color cues from longitudinal chromatic aberration and luminance contrast cues. In the natural world, these results imply that when a chick eye is too small at birth, and the retinal image is defocused, the eye will grow in response to low temporal frequency retinal stimulation until temporal contrast reaches a level of around 80% as image sharpness increases with focus. As the retinal image becomes clearer, higher spatial frequencies are detected, along with higher frequency temporal stimulation, and a signal to “stop” growth is produced. In white light, the signal to “stop” eye growth will be present at lower temporal frequencies (when the eye is smaller) than in yellow light, though 80% temporal contrast is still a threshold requirement.
In white light, the shorter focal plane of blue light provides a signal for myopic defocus (image focused in front of the retina), which appears to override the low temporal frequency signal for growth. Since retinal temporal contrast was equal and constant for all cone types in this experiment, and there was no color signal or luminance contrast signal that could be used to detect the optimal plane of focus. These results suggest that longitudinal chromatic aberration provides a light vergence cue for emmetropization. Light vergence is the degree of convergence or divergence of rays at edges that are blurred by defocus. This vergence signal arises through the ability of the eye to detect the myopic vergence of blue light on the retina, as it does in accommodation [79, 80]. Although most animals have eyes that are too small at birth, some animals have eyes that are too large. In this case, the eye will be dependent on the detection of myopic vergence of light to slow eye growth.
In more general terms, these results suggest that it is important that the developing child is exposed to white light, such as natural sun light (without blue light reducing filters) or artificial lighting that is rich in blue light (daylight bulbs). A light source containing blue, green and red components will provide both color contrast, luminance contrast, and vergence cues for the emmetropization mechanism, and increase the probability that the eye will emmetropize normally and prevent the development of myopia.
4.3 Choroidal Effects
We found that choroidal changes were not dependent on temporal frequency, temporal contrast, or the color of the light and were not associated with eye length changes. Experiments that used lenses to induce defocus have shown highly predictable changes in choroidal thickness with defocus [4, 5, 81, 82]. Nevertheless, it is clear that these correlations between choroidal thickness and eye length changes can be disassociated [8, 19, 83]. Our experiment shows that temporal exposure can be added to this list of experimental conditions that dissociate changes in choroidal thickness from eye length changes. One possible explanation for this dissociation is that the relatively small changes we measured (43 μm) may reflect observed changes in choroidal blood flow with flicker [84, 85] ( but not by [86]), rather than changes in choroidal lacunae volume [87] in response to defocus.
5. Conclusion
In conclusion, emmetropization mechanisms are sensitive to the temporal frequency, temporal contrast, and spectral properties of the stimulus. Exposure to high temporal frequencies and temporal contrast reduces eye growth, regardless of spectral content, providing an inhibitory “stop” signal and protecting the eye against myopia. However, exposure to low temporal frequencies increases eye growth, in yellow but not white light, even when the stimulus is of high temporal contrast, thereby increasing the risk of myopia. Temporal contrast below the optimal level increases eye growth and increases the risk of myopia, regardless of temporal or spectral characteristics of the stimulus. White light provides both color contrast, luminance contrast and light vergence cues for the sign of defocus that enable accurate emmetropization and prevent myopia development.
Acknowledgments
We would like to thank Li Deng who provided statistical guidance on experimental design. Christopher Taylor also provided expertise on statistical analysis methods. We would also like to thank Arabella Arabejo and Hannah Edwards for helping in data collection. Finally, we would like to thank Phil Kruger for reviewing the manuscript before submission.
Footnotes
This experiment was previously presented at ARVO 2016 and ECVP 2016
NIH Disclaimer:
Research reported in this publication was supported by the National Eye Institute of the National Institutes of Health under Award Number R01EY023281 and by the NIH T35 Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28(5):639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffel F, et al. Developing eyes that lack accommodation grow to compensate for imposed defocus. Vis Neurosci. 1990;4(2):177–83. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- 3.Wallman J, McFadden S. Monkey eyes grow into focus. Nat Med. 1995;1(8):737–9. doi: 10.1038/nm0895-737. [DOI] [PubMed] [Google Scholar]
- 4.Wallman J, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 5.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis Res. 1995;35(9):1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 6.Schaeffel F, Diether S. The growing eye: an autofocus system that works on very poor images. Vision Res. 1999;39(9):1585–9. doi: 10.1016/s0042-6989(98)00304-6. [DOI] [PubMed] [Google Scholar]
- 7.Rucker F, Wallman J. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012;12(6):23. doi: 10.1167/12.6.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: Differential responses to short and long wavelengths. Vision Res. 2008;48(19):1980–91. doi: 10.1016/j.visres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith EL, 3rd, Hung LF, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of Long-Wavelength Lighting on Refractive Development in Infant Rhesus Monkeys. Invest Ophthalmol Vis Sci. 2015;56(11):6490–6500. doi: 10.1167/iovs.15-17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran N, et al. The significance of retinal image contrast and spatial frequency composition for eye growth modulation in young chicks. Vision Res. 2008;48(15):1655–62. doi: 10.1016/j.visres.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid KL, Wildsoet CF. Contrast and spatial-frequency requirements for emmetropization in chicks. Vision Res. 1997;37(15):2011–21. doi: 10.1016/s0042-6989(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 12.Schmid KL, et al. The effect of manipulations to target contrast on emmetropization in chick. Vision Res. 2006;46(6–7):1099–107. doi: 10.1016/j.visres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Zhi Z, et al. The effect of temporal and spatial stimuli on the refractive status of guinea pigs following natural emmetropization. Invest Ophthalmol Vis Sci. 2013;54(1):890–7. doi: 10.1167/iovs.11-8064. [DOI] [PubMed] [Google Scholar]
- 14.Avila NV. PhD Dissertation. University of Newcastle; 2008. Spatial frequency and eye growth in the developing chick. [Google Scholar]
- 15.Zhu X. Temporal integration of visual signals in lens compensation (a review) Exp Eye Res. 2013;114:69–76. doi: 10.1016/j.exer.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kee CS, et al. Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48(3):957–62. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crewther SG, et al. Low frequency temporal modulation of light promotes a myopic shift in refractive compensation to all spectacle lenses. Exp Eye Res. 2006;83(2):322–8. doi: 10.1016/j.exer.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Crewther DP, Crewther SG. Refractive compensation to optical defocus depends on the temporal profile of luminance modulation of the environment. Neuroreport. 2002;13(8):1029–32. doi: 10.1097/00001756-200206120-00010. [DOI] [PubMed] [Google Scholar]
- 19.Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42(24):2651–68. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- 20.Schwahn HN, Schaeffel F. Flicker parameters are different for suppression of myopia and hyperopia. Vision Res. 1997;37(19):2661–73. doi: 10.1016/s0042-6989(97)00114-4. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb MD, Wallman J. Retinal activity modulates eye growth: Evidence from rearing in stroboscopic illumination. Society of Neuroscience Abstracts. 1987;13:1297. [Google Scholar]
- 22.Di Y, et al. Effects of chronic exposure to 0.5 Hz and 5 Hz flickering illumination on the eye growth of guinea pigs. Curr Eye Res. 2013;38(11):1182–90. doi: 10.3109/02713683.2013.807931. [DOI] [PubMed] [Google Scholar]
- 23.Rucker F, et al. Blue Light Protects Against Temporal Frequency Sensitive Refractive Changes. Invest Ophthalmol Vis Sci. 2015;56(10):6121–31. doi: 10.1167/iovs.15-17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266(5597):66–8. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 25.Raviola E, Wiesel TN. The mechanism of lid-suture myopia. Acta Ophthalmol Suppl. 1988;185:91–2. doi: 10.1111/j.1755-3768.1988.tb02675.x. [DOI] [PubMed] [Google Scholar]
- 26.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987;6(8):993–9. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- 27.Troilo D, et al. Differences in eye growth and the response to visual deprivation in different strains of chicken. Vision Res. 1995;35(9):1211–6. doi: 10.1016/0042-6989(94)00230-j. [DOI] [PubMed] [Google Scholar]
- 28.Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus) Invest Ophthalmol Vis Sci. 2000;41(8):2043–9. [PubMed] [Google Scholar]
- 29.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124(1):154–7. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 30.Norton TT. Experimental myopia in tree shrews. In: Bock G, Widdows K, editors. Myopia and the control of eye growth. Wiley; Chichester: 1990. pp. 178–94. [PubMed] [Google Scholar]
- 31.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201(4362):1249–51. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987;28(8):1225–35. [PubMed] [Google Scholar]
- 33.Diether S, Wildsoet CF. Stimulus requirements for the decoding of myopic and hyperopic defocus under single and competing defocus conditions in the chicken. Invest Ophthalmol Vis Sci. 2005;46(7):2242–52. doi: 10.1167/iovs.04-1200. [DOI] [PubMed] [Google Scholar]
- 34.Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686(2):169–81. doi: 10.1016/0006-8993(95)00370-6. [DOI] [PubMed] [Google Scholar]
- 35.Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42(3):575–83. [PubMed] [Google Scholar]
- 36.Gawne TJ, et al. Society for Neuroscience. Washington D.C: 2014. How does the neural retina process optical blur? Insights from emmetropization. [Google Scholar]
- 37.Yu Y, et al. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Res. 2011;46(2):80–7. doi: 10.1159/000323179. [DOI] [PubMed] [Google Scholar]
- 38.Di Y, et al. The effect of various levels of stroboscopic illumination on the growth of guinea pig eyes. Clin Exp Optom. 2014;97(1):55–61. doi: 10.1111/cxo.12079. [DOI] [PubMed] [Google Scholar]
- 39.Gawne TJ, et al. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2016;155:75–84. doi: 10.1016/j.exer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapley R. Linear and nonlinear systems analysis of the visual system: why does it seem so linear? A review dedicated to the memory of Henk Spekreijse. Vision Res. 2009;49(9):907–21. doi: 10.1016/j.visres.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tranchina D, et al. Linear information processing in the retina: a study of horizontal cell responses. Proc Natl Acad Sci U S A. 1981;78(10):6540–2. doi: 10.1073/pnas.78.10.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shapley RM, Victor JD. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978;285:275–98. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom. 2015;98(6):507–17. doi: 10.1111/cxo.12312. [DOI] [PubMed] [Google Scholar]
- 44.Zhu X, et al. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46(7):2238–41. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]
- 45.Schmid KL, Wildsoet CF. Assessment of visual acuity and contrast sensitivity in the chick using an optokinetic nystagmus paradigm. Vision Res. 1998;38(17):2629–34. doi: 10.1016/s0042-6989(97)00446-x. [DOI] [PubMed] [Google Scholar]
- 46.Banks MS, Salapatek P. Acuity and contrast sensitivity in 1-, 2-, and 3-month-old human infants. Invest Ophthalmol Vis Sci. 1978;17(4):361–5. [PubMed] [Google Scholar]
- 47.Jarvis JR, et al. Measuring and modelling the photopic flicker sensitivity of the chicken (Gallus g. domesticus) Vision Res. 2002;42(1):99–106. doi: 10.1016/s0042-6989(01)00268-1. [DOI] [PubMed] [Google Scholar]
- 48.Callahan TL, Petry HM. Psychophysical measurement of temporal modulation sensitivity in the tree shrew (Tupaia belangeri) Vision Res. 2000;40(4):455–8. doi: 10.1016/s0042-6989(99)00194-7. [DOI] [PubMed] [Google Scholar]
- 49.Armitage JA, et al. Postnatal development of flicker sensitivity in guinea pigs. Clin Exp Optom. 2001;84(5):270–275. doi: 10.1111/j.1444-0938.2001.tb05037.x. [DOI] [PubMed] [Google Scholar]
- 50.Osorio D, Vorobyev M, Jones CD. Colour vision of domestic chicks. J Exp Biol. 1999;202(Pt 21):2951–9. doi: 10.1242/jeb.202.21.2951. [DOI] [PubMed] [Google Scholar]
- 51.Lind O, Kelber A. Avian colour vision: effects of variation in receptor sensitivity and noise data on model predictions as compared to behavioural results. Vision Res. 2009;49(15):1939–47. doi: 10.1016/j.visres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Osorio D, Vorobyev M. A review of the evolution of animal colour vision and visual communication signals. Vision Res. 2008;48(20):2042–51. doi: 10.1016/j.visres.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Wildsoet CF, et al. Chromatic aberration and accommodation: their role in emmetropization in the chick. Vision Res. 1993;33(12):1593–603. doi: 10.1016/0042-6989(93)90026-s. [DOI] [PubMed] [Google Scholar]
- 54.Rohrer B, Schaeffel F, Zrenner E. Longitudinal chromatic aberration and emmetropization: results from the chicken eye. J Physiol. 1992;449:363–376. doi: 10.1113/jphysiol.1992.sp019090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002;42(21):2409–17. doi: 10.1016/s0042-6989(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 56.Li W, et al. The effect of spectral property and intensity of light on natural refractive development and compensation to negative lenses in guinea pigs. Invest Ophthalmol Vis Sci. 2014;55(10):6324–32. doi: 10.1167/iovs.13-13802. [DOI] [PubMed] [Google Scholar]
- 57.Liu R, et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011;92(6):447–53. doi: 10.1016/j.exer.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Wang F, et al. Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Curr Eye Res. 2011;36(2):103–11. doi: 10.3109/02713683.2010.526750. [DOI] [PubMed] [Google Scholar]
- 59.Jiang L, et al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus) Vision Res. 2014;94:24–32. doi: 10.1016/j.visres.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 60.Bartmann M, Schaeffel F. A simple mechanism for emmetropization without cues from accommodation or colour. Vision Res. 1994;34(7):873–6. doi: 10.1016/0042-6989(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 61.Smith EL, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 2000;40(4):371–81. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 62.Bowrey HE, et al. The relationship between image degradation and myopia in the mammalian eye. Clin Exp Optom. 2015;98(6):555–63. doi: 10.1111/cxo.12316. [DOI] [PubMed] [Google Scholar]
- 63.Rucker FJ, Wallman J. Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eyegrowth. Vision Res. 2009;49(14):1775–83. doi: 10.1016/j.visres.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen DM, Goldsmith TH. Appearance of a Purkinje shift in the developing retina of the chick. J Exp Zool. 1984;229(2):265–71. doi: 10.1002/jez.1402290212. [DOI] [PubMed] [Google Scholar]
- 65.Jones CD, Osorio D. Discrimination of oriented visual textures by poultry chicks. Vision Res. 2004;44(1):83–9. doi: 10.1016/j.visres.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 66.Howland HC, Sayles N. Photokeratometric and Photorefractive Measurements of Astigmatism in Infants and Young Children. Vision Res. 1985;25(1):73–81. doi: 10.1016/0042-6989(85)90082-3. [DOI] [PubMed] [Google Scholar]
- 67.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74(6):367–75. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 68.Team, R.C. R: A language and environment for statistical computing. 2016. [Google Scholar]
- 69.Fox J, Weisberg S. An {R} Companion to Applied Regression. 2. Thousand Oaks CA: Sage; 2011. [Google Scholar]
- 70.JC . Educational and psychological measurement. 1973. Eta-squared and partial eta-squared in fixed factor ANOVA designs. [Google Scholar]
- 71.Mendiburu Fd. agricolae: Statistical Procedures for Agricultural Research. R package version 1.2–3. 2015. [Google Scholar]
- 72.Ofri R, et al. Development of the refractive state in eyes of ostrich chicks (Struthio camelus) Am J Vet Res. 2001;62(5):812–5. doi: 10.2460/ajvr.2001.62.812. [DOI] [PubMed] [Google Scholar]
- 73.Andison ME, Sivak JG, Bird DM. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. J Comp Physiol A. 1992;170(5):565–74. doi: 10.1007/BF00199333. [DOI] [PubMed] [Google Scholar]
- 74.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 75.Rucker FJ. The role of luminance and chromatic cues in emmetropisation. Ophthalmic Physiol Opt. 2013;33(3):196–214. doi: 10.1111/opo.12050. [DOI] [PubMed] [Google Scholar]
- 76.Yeo AC, Atchison DA, Schmid KL. Effect of text type on near work-induced contrast adaptation in myopic and emmetropic young adults. Invest Ophthalmol Vis Sci. 2013;54(2):1478–83. doi: 10.1167/iovs.12-11496. [DOI] [PubMed] [Google Scholar]
- 77.Diether S, Gekeler F, Schaeffel F. Changes in contrast sensitivity induced by defocus and their possible relations to emmetropization in the chicken. Invest Ophthalmol Vis Sci. 2001;42(12):3072–9. [PubMed] [Google Scholar]
- 78.Diether S, Schaeffel F. Long-term changes in retinal contrast sensitivity in chicks from frosted occluders and drugs: relations to myopia? Vision Res. 1999;39(15):2499–510. doi: 10.1016/s0042-6989(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 79.Del Aguila-Carrasco AJ, et al. Accommodation Responds to Optical Vergence and Not Defocus Blur Alone. Invest Ophthalmol Vis Sci. 2017;58(3):1758–1763. doi: 10.1167/iovs.16-21280. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, et al. Accommodation to wavefront vergence and chromatic aberration. Optom Vis Sci. 2011;88(5):593–600. doi: 10.1097/OPX.0b013e3182112d99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nickla DL. Transient increases in choroidal thickness are consistently associated with brief daily visual stimuli that inhibit ocular growth in chicks. Exp Eye Res. 2007;84(5):951–9. doi: 10.1016/j.exer.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 82.Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41(6):1249–58. [PubMed] [Google Scholar]
- 83.Park TW, Winawer J, Wallman J. Further evidence that chick eyes use the sign of blur in spectacle lens compensation. Vision Res. 2003;43(14):1519–31. doi: 10.1016/s0042-6989(03)00180-9. [DOI] [PubMed] [Google Scholar]
- 84.Shih YF, et al. The choroidal blood flow response after flicker stimulation in chicks. J Ocul Pharmacol Ther. 1997;13(3):213–8. doi: 10.1089/jop.1997.13.213. [DOI] [PubMed] [Google Scholar]
- 85.Lovasik JV, Kergoat H, Wajszilber MA. Blue flicker modifies the subfoveal choroidal blood flow in the human eye. Am J Physiol Heart Circ Physiol. 2005;289(2):H683–91. doi: 10.1152/ajpheart.01187.2004. [DOI] [PubMed] [Google Scholar]
- 86.Garhofer G, et al. Influence of diffuse luminance flicker on choroidal and optic nerve head blood flow. Curr Eye Res. 2002;24(2):109–13. doi: 10.1076/ceyr.24.2.109.8164. [DOI] [PubMed] [Google Scholar]
- 87.Liang H, et al. Structural and elemental evidence for edema in the retina, retinal pigment epithelium, and choroid during recovery from experimentally induced myopia. Invest Ophthalmol Vis Sci. 2004;45(8):2463–74. doi: 10.1167/iovs.03-1009. [DOI] [PubMed] [Google Scholar]