Abstract
Epilepsy is a network disorder and each type of seizure involves distinct cortical and subcortical network, differently implicated in the control and propagation of the ictal activity. The role of the basal ganglia has been revealed in several cases of focal and generalized seizures. Here, we review the data that shows the implication of the basal ganglia in absence, temporal lobe, and neocortical seizure in animal models (rodent, cat, and non-human primate) and in human. Based on these results and the advancement of deep brain stimulation for Parkinson’s disease, basal ganglia neuromodulation has been tested with some success that can be equally seen as promising or disappointing. The effect of deep brain stimulation can be considered promising with a 76% in seizures reduction in temporal lobe epilepsy patients, but also disappointing, since only few patients have become seizure free and the anti-epileptic effects have been highly variable among patients. This variability could probably be explained by the heterogeneity among the patients included in these clinical studies. To illustrate the importance of specific network identification, electrophysiological activity of the putamen and caudate nucleus has been recorded during penicillin-induced pre-frontal and motor seizures in one monkey. While an increase of the firing rate was found in putamen and caudate nucleus during pre-frontal seizures, only the activity of the putamen cells was increased during motor seizures. These preliminary results demonstrate the implication of the basal ganglia in two types of neocortical seizures and the necessity of studying the network in order to identify the important nodes implicated in the propagation and control of each type of seizure.
Keywords: basal ganglia, epilepsy, seizures, non-human primate, neuromodulation, absence, TLE, neocortical seizures
Introduction
The basal ganglia (BG) network is a complex grouping of interconnected subcortical nuclei, with strongly established roles in motor control, cognition, and motivational behaviors (DeLong and Wichmann 2010; Lanciego et al. 2012). It receives diverse and topographically-organized inputs from the neocortex, as well as the thalamus, the hippocampus (Hpc), and the amygdala; and in turn, the BG projects back to the thalamus and the brainstem. While the BG is not directly involved in the generation of seizures, per se, it plays an important role in the propagation and control of several type of focal and generalized seizures (Deransart and Depaulis 2002). Epilepsy is a chronic disorder marked by recurrent seizures and each type of seizure is characterized by specific behavioral manifestations, electrographic signatures and histopathology. There are then, numerous animal models trying to reproduce the abnormal electrographic discharges seen in epileptic patients. These animal models have played a fundamental role in advancing our understanding of basic mechanisms underlying epileptogenesis (Grone and Baraban 2015; Pallud et al. 2008). Presently, rodents with genetic or experimentally-induced forms of epilepsy are by far the most common model (Loscher 2011). Rodent models have been especially instrumental in the discovery and preclinical development of novel anti-epileptic drugs (AEDs). However, there have been some concerns in the last few years that the efficacy of drug treatments have not substantially improved, which could, at least in part, be due to the choice of animal models (Loscher 2011). Although the rich scientific literature and the availability of rodent seizure models are compelling advantages, non-human primate (NHP) models are more suitable for translational therapies and understanding the underlining pathophysiology of seizures. Indeed, the anatomy and physiology of the NHP brain is unquestionably more similar to humans, and studying the epileptic network pathophysiology in the NHP promises to provide more advanced insights into human epilepsy etiology and treatment. In the case of temporal lobe epilepsy (TLE), NHPs are also a highly suitable model to study the various cognitive deficits associated with the seizures like memory impairments. In addition, studies of NHP could better uncover the disease pathology since prevention of epilepsy remains elusive, and current AEDs can only suppress or reduce seizures frequency. Despite these advantages, the number of NHP studies in the epilepsy field has been relatively limited compared to the numerous studies of rodents or even in patients, which is likely due to the lack of established NHP seizure models. In the late 70s and early 80s, studies used NHP model to induce generalized electrographic seizures by applying alumina gel directly on the cortex (Oakley and Ojemann 1982; Wyler et al. 1978). Despite very promising results, the use of NHP models was put on hiatus for several years and only recently regain some popularity with the use of electrical kindling in the Hpc and injections of penicillin to induce TLE and cortical seizures (Cleeren et al. 2016; Prabhu et al. 2014).
To date, despite an extensive literature, the exact mechanisms by which the BG could control ictal activity have not yet been clearly elucidated. By definition, epilepsy include many different types of seizures that could originate in various brain areas. Epilepsy is a network disease and for each type of seizure, there is a distinct neuronal network of structures and pathways that participates in the ictal spread and generalization. These functionally and anatomically connected structures are essential to the production, development, and maintenance of the seizures. However, seizures can also propagate to many more regions with anatomical connections that may not be necessarily involved in the epileptogenic network (Spencer 2002). This concept of epileptogenic networks has been introduced to illustrate the complexity of seizure dynamics, and identifying them could be particularly important in the context of epilepsy treatment (Bartolomei et al. 2017). Some of these networks, like the medial temporal/limbic network, have been well characterized but many others like the BG that has been less studied are likely involved in epilepsy disorder (Halasz and Rasonyi 2003). In this review, we present evidences of the critical role of the BG in the control and propagation of ictal activity. We will first briefly describe the BG network. Next, we will discuss animal and human studies that suggest a role for the BG in absence seizures, temporal lobe seizures (TLE), and neocortical seizures. We will also discuss the link between dopamine and epilepsy, and lastly, we will review the use of neuronal modulation of the BG as a treatment for epilepsy.
1. The Anatomy of the BG
The BG is a complex system of nuclei and pathways that acts in an integrated manner due to the unidirectional character of the major connections and the information being transformed and transmitted from the cortex via the BG to the thalamus and back to the cortex (Rektor et al. 2012). The BG are subcortical components of several circuits that also involve the thalamus and cortex. Traditionally, the BG is composed of subcortical structures of several parallel circuits (motor, oculomotor, associative, and limbic) that acts in a topographical segregated manner and connects with the thalamus and the cortex (Alexander et al. 1990). As shown in Figure 1, in primates the BG include the striatum (putamen (Put), caudate nucleus (CN), and nucleus accubens (NAcc)), the subthalamic nucleus (STN), the external and internal segments of the globus pallidus (GPe and GPi), and the substantia nigra with its dopaminergic pars compacta (SNc) and the GABAergic pars reticulata (SNr). Besides the STN that is glutamatergic, and SNc that is dopaminergic, all other subcortical structures in the BG system contain GABAergic (inhibitory) projections. The major entry points of the BG, STN and striatum, receive monosynaptic projections from the frontal cortex, whereas, the output nuclei of the BG, the GPi and SNr, project to the superior colliculus, pedunculopontine, specific nuclei in the thalamus, and brainstem. Although it is beyond this BG review to detail the implication of other structures, it should be noted that the superior colliculus has been well recognized as an important node for seizure control (Garant and Gale 1987; Soper et al. 2016). Thus, in its simplest form the BG network is a ‘braking system’ between the cortex and thalamus. Three pathways have been identified within the BG network: ‘direct’, ‘indirect’, and ‘hyperdirect’. The cortex sends monosynaptic glutamatergic projections to the striatum and STN. In the hyperdirect pathway, the STN will then project directly to the BG output (GPi and SNr). Striatal projections can travel to the GPi and SNr through two pathways: a ‘direct’ monosynaptic route or an ‘indirect’ polysynaptic route via the GPe then the STN.
Fig 1.
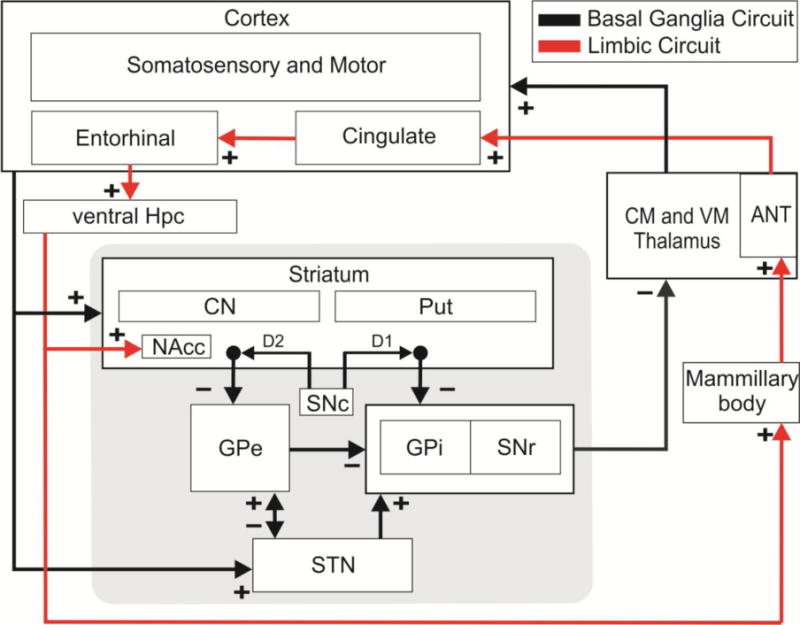
Schematic diagram of the BG (black) and limbic (red) system. The hippocampus (Hpc) innervates the nucleus accumbens (NAcc) of the striatum, and the cortex sends projections to the striatum and the subthalamic nucleus (STN) that both in turn project to the output nucleus, internal globus pallidus (GPi) and substantia nigra pars reticulate (SNr). These output nucleus connect with the central medial (CM) and ventral medial (VM) thalamus, while the mammillary body that receives direct projections from the Hpc innervate the anterior nucleus of the thalamus (ANT). Excitatory and Inhibitory connection are marked respectively with (+) and (−) signs.
Since the BG subcortical structures receive and send parallel connections that can influence multiple brain regions, the system is considered to function as a partially closed negative feedback loop (Joel and Weiner 1994). As we discussed previously, the cortico-BG-thalamic circuit is not the only network implicated in epilepsy. For example, the limbic circuit that is involved in emotion and memory functions has been heavily implicated in TLE pathophysiology. Although detailing all the limbic structures is beyond this review, the Hpc, the amygdala, and the anterior nucleus of the thalamus (AN) play a significant role in TLE. These structures will be discussed later since they have been identified as potential neuromodulation targets (Fisher et al. 2010). Between the limbic and BG circuits, the NAcc of the ventral striatum has been proposed to be the link between the two interface (Groenewegen et al. 1996; Mogenson et al. 1980). Many functional and anatomical studies have indicated that the shell and core of the NAcc may work in different neuronal circuitries (Salgado and Kaplitt 2015). In anatomical studies of rodents (Britt et al. 2012; Brog et al. 1993) and primates (Friedman et al. 2002), the ventral Hpc sends glutamatergic projection primarily to the NAcc shell of the ventral striatum (Figure 1). However, in rats and mice the projection extends to the NAcc core which differs from primates as Friedman and colleagues had noted in 2002. Since the shell and core of the NAcc work in different parallel circuits to process limbic information, it is conceivable that these anatomical differences between rodents and primates may implicate different pathways of communication downstream, and possibly different routes of seizure propagation (Akkal et al. 2007; Ito and Doya 2011; Salgado and Kaplitt 2015). Beyond the NAcc, other connections may differ between rodents and primates, such as the degree of segregation in the striatonigral and striatopallidal projections through the direct and indirect pathways (Bertran-Gonzalez et al. 2010). Although the functional relationship between the limbic and BG circuits in the context of epilepsy is still not clear, the NAcc has been proposed to be a “nodal point” of seizure propagation (Lothman et al. 1984).
2. Implication of the BG in Absence Seizures
Absence seizure or “petit mal seizure” is a childhood epilepsy syndrome characterized by the impairment of consciousness that lasts from 3 to 30 seconds and ends abruptly with an unknown cause. Typical absence seizures have a clear polygenic inheritance and are electrographically characterized by generalized abnormal cortical 2–4Hz spike-wave discharges (SWDs) (Crunelli and Leresche 2002; Yalcin 2012). In the majority of cases (2 out of 3 patients), seizures could be controlled with AED, but attention deficit may persists. While the seizures generally disappear by mid-adolescence, approximatively 10–15% of the children will later develop other seizures types, usually generalized tonic-clonic or myoclonic seizures (Crunelli and Leresche 2002). Based on the electrophysiological recordings obtained in patients and animals model, the cortex, the reticular thalamus and the relay nuclei of the thalamus have been identified to play a predominant role in the development of the SWDs (Danober et al. 1998). However, whether these typical SWDs are generated by an excessive cortical excitability (Di Pasquale et al. 1997; Luhmann et al. 1995) or by excessive thalamic oscillations (Crunelli and Leresche 1991; Liu et al. 1991; Paz et al. 2007) remain controversial.
Our understanding of the involvement of the BG in absence seizures has been greatly advanced by genetic rodent models that spontaneously display non-convulsive absences seizures, such as the GAERS (Genetic Absence Epilepsy Rats from Strasbourg) or the WAG/Rij (Wistar Albino Glaxo from Rijswijk). Pharmacological induced absences seizures could also be used on rodent and monkeys with mixed success (Venzi et al. 2015). Of these pharmacological models including gamma-hydroxybutyric acid (GHB) (Snead 1978b), pentylenetrazol (PTZ) (Cortez et al. 2016; Snead 1992) and penicillin (Avoli 1995), only GHB models have been used on NHP to induce absence seizures (Coppola and Moshe 2012). While both models generate characteristic cortical 3Hz SWDs typical of human absence seizures, very few studies have been published using these NHP models, and none of which have looked at the BG implication (Snead 1978a; Snead 1978b; Tenney et al. 2004). On the contrary, there are numerous neuropharmacological and electrophysiological evidences from rodents and human demonstrating the implication of the BG in generalized absence seizures (for review Deransart et al. 1998b). Current theories agree that in addition to the thalamus and the cortex, the BG play a major role in the control of absence seizures (Meeren et al. 2005). The concept of nigral control of seizures, initially proposed by the Gale group in 1980 for electroshock induced seizures (Gale and Iadarola 1980; Iadarola and Gale 1982), was later confirmed in rodent bicuculline model (Garant and Gale 1983), kindling (McNamara et al. 1984) and absence seizures (Depaulis et al. 1989). Thus, intranigral injection of GABAa agonist has an anti-epileptic effect in both genetic and pharmacological models of absences seizures in rats (Depaulis et al. 1989; Depaulis et al. 1988). Later, an electrophysiological study showed a high correlation between cortical SWDs and SNr bursting activity during ictal periods that terminated as soon as the SWDs had stopped (Deransart et al. 2003). Even more recently, SNr stimulation has been found to be very successful in the GAERS rats with 97% in seizures interruption (Saillet et al. 2013). Despite numerous evidence of the antiepileptic effect of SNr inhibition in absence seizure the exact pathway implicated in this control is still unclear. It has been shown that inhibition of the SNr leads to the disinhibition of the ventral medial (VM) thalamic nucleus, resulting in hyperactivity of the VM and a reduction of the regular oscillatory pattern (Paz et al. 2007). Alternatively, activation of the superior colliculus (another efference of the SNr) has a similar anti-epileptic effect in a genetic absence model (Nail-Boucherie et al. 2002). Furthermore, since the SNr receives direct and indirect GABAergic inputs from the striatum and direct glutamatergic input from the STN, the three BG pathways (direct, indirect, and hyperdirect) could also mediated the control of absence seizures.
In the GAERS rodent model, striatal output neurons stopped firing during ictal periods and displayed rebound activity following ictal activity (Slaght et al. 2004). Their results suggested that the interrupted striatal activity contributed to seizure propagation through the striatum by releasing the SNr inhibition. Thus, anti-epileptic effects were found after activating the striatum by either bilateral injection of NMDA or D1 agonist, which suggests the implication of the direct pathway (Deransart et al. 1998b). Alternatively, because the STN glutamatergic inputs is critical in the maintenance of the SNr tonic activity, changes in STN activity has been hypothesized to be able to control seizure propagation. In 2005, Paz showed that the bursting activity recorded in the STN and GP was synchronized with EEG spikes, suggesting that ictal activity propagates through the indirect pathway (Paz 2005). In addition, disinhibition of the GP by local injection of a GABAa antagonist suppresses absences seizures, whereas inhibition of GP by a GABAa agonist aggravates absence seizures (Deransart et al. 1999). They proposed a control of absence seizure mediated through the indirect pathway and suggested that the interrupted activity previously recorded in the striatum was inducing the increase of GP and STN excitation, ultimately leading to SNr activation and seizure propagation. Furthermore, it has been shown that inhibition or excitation of STN can respectively block or exacerbate seizure frequency (Deransart et al. 1996; Vercueil et al. 1998). However, recent studies have implicated the novel identified GABAergic pallido-cortical pathway in the control of absence seizures in rodent (Chen et al. 2015b). Indeed, by using computational modeling, they found that the enhancement of the inhibitory pallido-cortical pathway was able to suppress SWDs (Chen et al. 2015a). While interesting this theory presumes the existence of a direct connection between GPe and the cortex that have not yet been confirmed in NHP and human.
Current evidences have shown that inhibition of the SNr through neuromodulation of both direct and indirect pathway had a strong antiepileptic effect, and a cooperation between them has been suggested (Deransart and Depaulis 2002; Deransart et al. 2000). It has been found that intrastriatal injection of D1 and D2 agonist had an antiepileptic effect and that the simultaneous injection of both agonists had an additive effect in absence seizures reduction (Deransart et al. 2000). These results suggest that the direct and indirect pathways cooperate in the suppression of absence seizures in addition to reinforcing the key role of dopamine in the control of absence seizures (for review, see Starr 1996).
3. Implication of the BG in TLE
As the most common form of partial epilepsy, TLE is characterized by recurrent spontaneous focal seizures originating from temporal lobe structures such as the Hpc, amygdala, entorhinal cortex, piriform cortex or temporal neocortex. TLE patients could experience behavioral manifestations that are a mixture of different emotions and/or resurfaced old memories. Patients can also experience auras that are hallucinations of smells, voices or tastes and that may happen for a few seconds at the beginning of the seizure. In rodents, electrical kindling, maximal electroshock seizures (MES) or the injection of pilocarpine, lithium-pilocarpine, kainic acid, pentylenetrazol (PTZ) are common models for TLE. Some of these models reproduce chronic spontaneous recurrent seizure (SRS) and hippocampal neuronal damage that are characteristic of refractory TLE. The pilocarpine (Cavalheiro et al. 1991), lithium-pilocarpine (Andre et al. 2001), and kainic acid (Pisa et al. 1980) induce SRS and hippocampal damage. Interestingly, while amygdala kindling do produce SRS (Pinel and Rovner 1978), it was shown to not be related to hippocampal damage (Brandt et al. 2004). On the other hand, acute models such as PTZ and MES do not induce SRS and have been indicated to be poor models for AED testing (Loscher 2002). In NHP, pilocarpine (Pontes et al. 2016), alumina gel (Mayanagi and Walker 1974; Mayanagi and Walker 1975; Ribak et al. 1998), penicillin (Mayanagi and Walker 1975), kainic acid (Chen et al. 2014; Yang et al. 2015), and electrical kindling (Cleeren et al. 2016; Wada and Osawa 1976) have been used to induce TLE. Out of the previously listed methods, only NHP treated with intrahippocampal kainic acid had a change in the hippocampal neuronal volume and developed a partial SRS (Chen et al. 2014). However, the seizure frequency gradually decreased over a 3 month period, and the method required the induction of a SE that could last for more than 1 hour which endangered the animals’ life. Alternatively, the Hpc/amygdala kindling NHP model provides a progressive reflection of limbic seizure severity despite being tedious (17 months were necessary to achieve stage IV seizure generalization) (Buckmaster 2004; Cleeren et al. 2015).
The limbic circuit and the structures composing this network have been the primary focus of human and NHP studies, but while less investigated (at least in human and NHP), the BG network can also contribute to the propagation/control of TLE (Bouilleret et al. 2008). Several studies using neuropharmacological inhibition (Garant and Gale 1987), electrophysiological recordings (Bertti et al. 2010), metabolic alterations (Sperling et al. 1990), and advance imaging (Hetherington et al. 2007) have suggested an inhibitory role of the BG in TLE. However, the exact mechanisms and pathways implicated in this inhibition are still under debate.
The role of the Hpc-NAcc-pallidum pathway: The implication of this pathway is supported by the anatomical evidence of connection from the Hpc and the amygdala to the ventral pallidum via the NAcc (Haber et al. 1990; Jones and Mogenson 1980; Kahn and Shohamy 2013; Yang and Mogenson 1984). Using a kindling model in rat, Lothman (1985) demonstrated a propagation of seizures from the Hpc to the NAcc and proposed the NAcc as a ‘nodal point’ for the seizure propagation into motor pathways (Lothman et al. 1985). Seizure protection was found in a rat pilocarpine model after direct microinjection of a dopamine agonist (apomorphine) into the NAcc, (Turski et al. 1988) and more recently, a significant reduction in secondarily generalized seizures was found in a rat pilocarpine model after direct microinjection of benzodiazepine into the NAcc (Klitgaard et al. 2003). These results confirm the implication of this pathway. Interestingly, albeit smaller, comparable neuroprotective effects were found with injections into the SNr and the striatum (Klitgaard et al. 2003). The GPe that receives direct GABAergic projection from the NAcc seems to also play a major role in the control of TLE (Cheng et al. 2015; Robertson and Jian 1995). Low but not high frequency stimulation of GPe has a neuroprotective preventive effect in an amygdala-kindling model in rats with less than half of the rats (43%) showing fully kindled symptoms compared to high frequency simulated rats (89%) and control group (100%). Furthermore, the cumulative after-discharge duration and generalized seizure duration in the low frequency stimulated group was significantly shorten compared to the high frequency or control group (Cheng et al. 2015). These effects were suggested to be mediated through a normalization of the oscillatory power in the amygdala via the NAcc. Moreover, postictal motor behavior induced by hippocampal after discharges was blocked by either injection of D2 receptor agonist in the NAcc or by the inhibition of the ventral pallidum (Ma et al. 1996). Although comparable connectivity between Hpc and NAcc was found in NHP (Friedman et al. 2002) and in human fMRI study (Kahn and Shohamy 2013), the specificity on whether the NAcc-pallidum plays a similar role in regulating TLE activity as it does in rodents still needs to be determined. The results from a recent study done on amygdala-kindled monkeys lean towards this direction (Cleeren et al. 2016). By pairing electrical microsimulation during fMRI and ictal SPECT perfusion imaging, Cleeren and colleague demonstrated an implication of the pallidum among several other structures of the BG (Cleeren et al. 2016).
The role of the striato-nigral pathway: There are also many evidences supporting the implication of the striatum and the SNr in the control of TLE seizures. Studies have demonstrate the preventive effect of either bilateral injection in the rat striatum of GABA antagonist (bicuculline) or dopamine agonist (apomorphine) in a pilocarpine model, suggesting that not only dopamine but also GABAergic transmission in the striatum could modulates the seizures propagation (Turski et al. 1988; Turski et al. 1991). The preventing effect of striatal injection of bicuculline were later confirm in the amygdala-kindled seizures model (Cavalheiro et al. 1987). This preventing role of the striatum was also confirmed by an aggravation of the pilocarpine induced seizures after bilateral lesion of the striatum (Turski et al. 1987). A similar implication of the CN has been found in an amygdala-induced seizures on cats in which stimulation of the CN had an antiepileptic effect (La Grutta et al. 1971). Alternatively, in rats, early activation of the cortex and SNr highlights the important role of these structures in generalized amygdala kindled seizures (Shi et al. 2007). Control of amygdala-kindled seizures has been achieved through direct and indirect inhibition of the SNr (Deransart et al. 1998a; McNamara et al. 1984). However, based on the absence of change in the GABAergic density of the SNr found in rats after amygdala kindling, it has been suggested that the antiepileptic effect of the SNr was mediated through the connection with the striatum or the GP (Freichel et al. 2004). Interestingly, in a cat penicillin-induced hippocampal seizure model, similar seizure suppressive effects have been found with either CN or SN low frequency stimulation, and these effects were unchanged after the lesion of either CN or SN (Sabatino et al. 1989). Their results underlined the possibility that the striatum and the SN could both influence the hippocampal seizures through two different independent pathways. More recently, a decrease of the Put volume was found in TLE patients following temporal lobectomy (Postuma and Dagher 2006; Shedlack et al. 1994) and the neuronal injury in the Hpc that was evaluated by magnetic resonance spectroscopic imaging was directly correlated with the neuronal damage in the Put (Hetherington et al. 2007).
Although there is a high possibility that BG plays a role in TLE, it is not clear whether or not the BG play an active role in the control of the seizure or if their implication is related to the generalization or the recruitment of cortical areas directly connected to the BG. In line of this recruitment theory, Haut in 2008 found an implication of the BG only with certain types of seizures that were characterized by pronounced motor manifestation (Haut and Albin, 2008). Similarly, in 2002, Rektor and colleagues recorded the change in BG activity during ictal and interictal activity of eight TLE patients and found an increase of oscillatory activity in the 3–7 Hz range during seizures. However, these changes were only found when the ictal activity had spread to other cortical areas and not when the ictal activity remained localized to the seizure onset (Rektor et al., 2002). This discrepancy with the results obtain in rodents could be attributed to the anatomical differences in the Hpc projection and studying the BG network in a NHP model could probably help clarify the role of the BG in the propagation/generalization or control of TLE (Akkal et al. 2007; Ito and Doya 2011; Salgado and Kaplitt 2015). Unfortunately, to the best of our knowledge at this time, only one recent study, using imagery technique, has contributed to put in evidence an implication of the BG in a NHP model of TLE (Cleeren et al. 2016).
4. Implication of the BG in focal neocortical epilepsy
Patients with focal neocortical seizures are especially prone to pharmacological resistance and while the epileptogenic zone is restricted to a small cortical region, surgical removal of the seizure focus can only be proposed in few cases because of proximity to eloquent cortical area (Schuele and Luders 2008). Neocortical epilepsy may be caused by a lesion, tumor, or vascular malformation and may originate from the frontal, parietal, or occipital cortex. The symptoms depends on the location of the seizure focus and can vary from strange sensation to muscles spasms, or a sudden loss of consciousness. Many electrophysiological, and more recently, functional evidences have indicated the involvement of the BG in several type of neocortical seizures.
Motor and sensori-motor seizures: Based on their role in sensory-motor integration and motor control, it is not surprising to assume that epileptic discharges originating in the cortical motor areas would then influence neurons of the motor part of BG (DeLong 1990; Graybiel 1995). Thus, deoxyglucose autoradiography studies on motor seizure induced by penicillin showed the implication of Put, GP and SNr in rat (Collins et al. 1976) and in monkey (Caveness et al. 1980; Faeth et al. 1954; Hosokawa et al. 1983; Kato et al. 1980; Udvarhelyi and Walker 1965). Electrophysiological recordings have shown after discharge activity in the CN of immobilized monkey with frontal seizures (Poggio et al. 1956). Similar changes were recorded in the CN, entopeduncular nucleus, and SNr penicillin injection in the neocortex of cats (Neafsey et al. 1979) and rats (Kaniff et al. 1983; Poggio et al. 1956). In the penicillin monkey model, stimulation of the GPi was found to be pro-convulsive (Hosokawa et al. 1980); whereas, high frequency stimulation of the CN could reduce the seizure frequency in the alumina cream monkey model of motor focal seizure (Oakley and Ojemann 1982). More recently, a strong entrainment of the BG cells during motor seizures was found in a NHP model of penicillin induced motor seizure (Devergnas et al. 2012). The results suggested that the subthalamo-pallidal pathway was the main subcortical route involved in the propagation of ictal motor seizures, and the anti-epileptic effect of STN high frequency stimulation found in human and monkey are in line with this theory (Chabardes et al. 2002; Prabhu et al. 2015).
Pre-frontal seizures: Altered functional connectivity has been found between the frontal lobe and the BG in frontal lobe epilepsy patients (Braakman et al. 2013; Vytvarova et al. 2017). In the more recent study, all of the BG structures studied (CN, Put and GP) exhibited a significant and constant change in the resting state functional connectivity (Vytvarova et al. 2017). While Vytvarova and colleagues did not find significant change in the intra-BG connectivity, suggesting that the impairment was limited to the connectivity of BG-thalamic circuitry with the cortical areas, another study found a decrease in functional connectivity between the pallidum and Put in patients with frontal lobe epilepsy (Dong et al. 2016). This decrease of interaction may reflect the reduced inhibition from the striatum to the GP, leading to the facilitation of seizures. A change in connectivity between the BG, limbic, and frontal areas were also found in these imaging studies, which could account for the cognitive deficit of these patients (Braakman et al. 2011; Dong et al. 2016). The spread of neocortical ictal activity through the BG could be important for seizure termination; however, without the simultaneous recording of EEG activity the effect of interictal activity on this functional connectivity cannot be ruled out. Simultaneous recording of EEG and fMRI should help to clarify the impact of interictal activity in functional connectivity (Dong et al. 2016).
Entrainment of Put and CN cells during motor and pre-frontal seizures in NHP: Pre-frontal and motor seizures were induced in one Macaca fascicularis (male, 5.2 kg) with intra cortical injection of penicillin (Peni G sodium, Panpharma) into the pre-frontal and the motor cortex. This experiment was performed in accordance with the French guidelines on the use of living animals in scientific investigations with the approval of the local Ethical Committee for care of laboratory animals. The detailed material and method for this experiment has been previously published (Devergnas et al. 2012). Motor seizures were characterized by tonic contractions of the contralateral arm that were followed by contralateral repetitive jerks of the arm, on the contrary no obvious behavioral manifestation were observed during pre-frontal seizures. Motor and pre-frontal seizures lasted respectively 34.11 ± 25.35s and 26.33 ± 30.16s on average with a frequency of 17.05 and 17.41 seizures per hour. No difference were found between the duration or the frequency of the motor and pre-frontal seizure. During 18 motor and 18 pre-frontal seizures, we recorded 19 and 16 neurons respectively in the CN, and 14 and 13 neurons respectively in the Put (Figure 2). The firing rate of the Put cells was increased significantly during both frontal and motor seizures (Figure 2.B), while the increase firing rate of the CN cells was restricted to the frontal seizures (Figure 2.C). Not only does these results provide evidence for the implication of the BG in neocortical seizures, but it also suggest that the network involved in seizures propagation is dependent on the location of the focus. However, the explanation of the involvement of the Put cells during focal seizures is not clear yet. One theory is that it could be related to a spread of the ictal activity from the prefrontal area to the motor cortex, leading to minor motor manifestations that were not detected in our original evaluation. Further recording and analysis would be necessary to identify the difference in the BG network implicated in the propagation of pre-frontal and motor seizures in monkeys.
Fig 2.
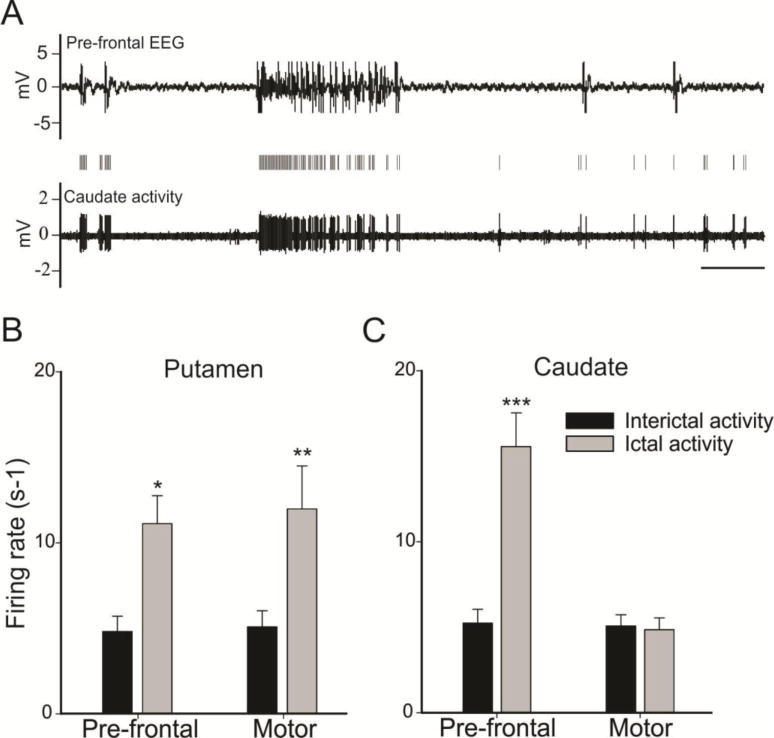
Striatum entrainment during frontal and motor seizures. A. Example of typical caudate nucleus activity during frontal seizure, scale bars 5 s. B–C. Averaged firing rate during interictal period (black bars) and ictal period (white bars) in the putamen (B) and the caudate nucleus (C) during frontal and motor seizures. Values are means ± SE compared with a Wilcoxon signed rank test for paired data (*P < 0.05, **P < 0.01, and *** P < 0.001). Devergnas A, personal observation.
5. Dopamine and epilepsy
The nigro-striatal dopaminergic pathway is a major regulator of the BG network, and its contribution in the control of epilepsy has been recognized for several years (Starr 1996). The first evidence of the role of dopamine in epilepsy came from the studies of Meldrum and collegues in 1975 showing the anti-epileptic effect of systemic apomorphine injection (a non-selective dopamine agonist) in photosensitive seizures in baboons and in audiogenic seizures in mice (Anlezark and Meldrum 1975; Meldrum et al. 1975). These antiepileptic effect were confirmed in several other models, except in the amygdala-kindled rats which was relatively unaffected by dopaminergic agonist or antagonist (Loscher and Czuczwar 1986). In a pilocarpine rats model, anti-epileptic effect were found after intracranial injections of apomorphine into the striatum, while injections of haloperidol (DA antagonist) lowered seizures threshold (Turski et al. 1988). More precisely, only D2 agonists decreased seizures frequency, while D1 agonists had little effect on seizure activity, suggesting an involvement of the BG indirect pathway (Loscher and Czuczwar 1986). The involvement of the SNc was later confirmed in a model of penicillin-induced hippocampal seizure in cat where SNc lesion induced a significant enhancement of hippocampal ictal activity (Ferraro et al. 1991). In the same study, seizures were reduced by low frequency stimulation of the SNc, which was either antagonized by systemic injection of dopamine receptor antagonist or emphasized by hippocampal injection of dopamine receptor agonist (Ferraro et al. 1991). All these findings strongly suggested that SNc represents a strategic region for the control of seizures through the indirect pathway. Recently, imaging studies have confirmed this hypothesis by showing a loss of dopaminergic D2/D3 receptor binding in the striatum of a pilocarpine rat model, and this loss of dopaminergic function in the striatum was suspected to lead to impaired seizure control (Yakushev et al. 2010). In TLE patients, a reduction in L-dopa uptake was found in bilateral CN, Put, and SN (Bouilleret et al. 2008). These results suggested that the BG dopaminergic system is involved in refractory TLE. In addition, it has been proposed that the mesio-limbic dopamine projections from the ventral tegmental area to the NAcc could play a major role in the behavioral and psychologic changes observed in MTLE patients (Ahmadi et al., 2017; Stretton et al., 2015). However, it may not be the only dopaminergic system affected by seizures, since a decrease of D2 and D3 receptor binding has also been found in the epileptogenic temporal lobe of mesial TLE patients (Werhahn et al., 2006). These results have suggested that in addition to playing a role in the control of seizure, dopamine may also be involved in the pathophysiology of epilepsy.
6. Neuromodulation of the BG as a treatment for epilepsy
About 30% of patients with epilepsy continue to suffer from seizures despite optimized medication (Laxpati et al. 2014; Sander 1993). While resective surgery for these medically intractable epilepsies can be curative, only a small proportion of these patients qualify due to unacceptable neurological or cognitive deficit. The only current option for these patients is electrical neuromodulation. Electrical deep brain stimulation (DBS) is a promising surgical alternative that could reduce abnormal frequency without significant adverse effects. Despite an extensive use of DBS in a broad range of neurologic and psychiatric disorders, including parkinsonism (DeLong and Benabid 2014), dystonia (Moro et al. 2017), obsessive compulsive disorder (Mallet et al. 2008), depression (Riva-Posse et al. 2013), and epilepsy (Chabardes et al. 2002), the mechanisms underlying the effect of electrical stimulation are poorly understood. Initially, observations that STN high frequency stimulation mimicked the effect of STN lesion in PD patients suggested that DBS was inducing a reversible inhibition (Benabid et al. 2002). However, the mechanisms of DBS have been recognized to be more complex. The parameters of stimulation, amplitude, frequency, pulse width but also waveform or polarity could significantly affect the effect of DBS (Breit et al. 2004; Vitek 2002). Since 1970s, different structures thought to modulate epilepsy have been electrically stimulated with the aim of improving severely impaired epileptic patient (Laxpati et al. 2014). In the following, the anatomical targets for electrical stimulation in epilepsy will be discussed in terms of either direct stimulation of the epileptic focus or indirect stimulation of network implicated in the control of seizure.
Direct electrical stimulation of the foci: While not directly related to the modulation of the BG, we will briefly review some studies reporting the effects of direct electrical stimulation of the foci. For patients with Mesial TLE (MTLE), using electrical stimulation instead of respective surgery to preserve the amygdala hippocampal function represents a major advancement in seizure treatment. A significant improvement was reported with direct stimulation of the Hpc using responsive neurostimulation (RNS) with a 53% reduction in seizure frequency and a recovery of memory function at 2 years. However, only 9% of these patients became seizure free over the last 3 months of the study (Heck et al. 2014). Similar outcomes were found with open-loop hippocampal neurostimulation with more than 90% of seizure reduction in six of the eleven patients, but only three of these patients became seizures free (Vonck et al. 2013). The reason for the high variability between patients is not very clear, but the location of the electrodes (Bondallaz et al. 2013) or the presence of mesial temporal sclerosis (Boon et al. 2007) could unquestionably affect the outcomes of hippocampal stimulation. In the case of neocortical seizures, surgical resection can only be proposed to the minority of the patients in whom the epileptogenic zone is located apart from eloquent cortex. In these cases, cortical electrical stimulation could be attempted. While successful in a ferric chloride-induced seizures in rats (Yao et al. 2008), low and high frequency stimulation triggered on seizures onset failed to abort motor seizures in a NHP model of penicillin-induced seizure (Blauwblomme et al. 2011).
Indirect electrical stimulation: Based on the nigral control of seizure theory and multiple evidences of the BG involvement in seizure control, several BG nuclei have been targeted for DBS. Thus, the inhibition of the SNr through direct electrical stimulation or indirect electrical modulation of the STN have been tested in animals and in small clinical case series (Franzini et al. 2008; Vesper et al. 2007; Wille et al. 2011). Chabardes and colleague found that STN stimulation had a strong effect on certain types of seizure (Chabardes et al. 2002). In this clinical study, 80% seizures reduction was obtained in 3 patients suffering from central region epilepsy, 41% reduction in the case of severe myoclonic epilepsy but no improvement was found for frontal lobe epilepsy patient (Chabardes et al. 2002). Interestingly, STN DBS not only reduced the number of seizure by 47% in a NHP model of penicillin induced-seizure, but also delayed the occurrence of the first seizure, which suggested a possible neuroprotective effect of the stimulation. Alternatively, stimulation of the striatum has also been proposed to treat epilepsy. In a NHP alumina-gel model, motor seizures were slightly reduced by continuous high frequency simulation of the head of the CN and increased when the stimulation was alternated, 10 min ON vs. 10 min OFF (Oakley and Ojemann 1982). On the contrary, in cats, low frequency stimulation of the CN has been found to inhibit hippocampal penicillin-induced seizures (La Grutta et al. 1988). Similarly, in patients, low frequency stimulation was found to reduce interictal activity and stop generalization while high frequency stimulation enhanced seizure activity (Chkhenkeli and Chkhenkeli 1997; Chkhenkeli et al. 2004). However, interpretation of these studies should be taken with precaution, since these clinical trials were not blinded, lacked any control condition, and tested a very heterogeneous population. In addition to the BG, other subcortical targets have been proposed to treat medically refractory epilepsy with mixed success. For example, the results of cerebellar stimulation were inconsistent and the effects were at best moderate, with only 33% in seizure reduction (Velasco et al. 2005; Wright et al. 1984). However, note should be made that using optogenetic methods, a recent study has shown that excitation of midline cerebellar Purkinje neurons produced a decrease in seizure frequency in a kainate mouse model (Krook-Magnuson et al. 2014). This new finding demonstrates that the cerebellum could still be considered an interesting target for TLE. With numerous widespread connection to the cortex, the thalamus is another privileged structure for DBS in epilepsy. Two of the thalamic nuclei have been explored to treat epilepsy: the centromedian nucleus (CM) and the anterior nucleus (AN) of the thalamus. The CM, which plays a central role in wakefulness and cortical excitability has strong connections with the cortex and the striatum (Smith et al. 2014). Thus, the anti-epileptic effect found with electrical modulation of the CM could be mediated by the decrease of cortical excitability through its direct cortical connection or indirectly through modulation of the striato-thalamic circuit (Velasco et al. 1987). The results from clinical trials suggest that CM stimulation may be effective for tonic-clonic seizure (Fisher et al. 1992) and for generalized tonic-clonic seizures (Velasco et al. 2006) but results were mild for patients with frontal seizures (Valentin et al. 2013). The AN, a key node in the limbic circuit (Figure 1), projects extensively to the frontal and temporal cortical area and has been a target to treat TLE. Electrical stimulation of the AN can decrease the cortical excitability, in addition to modulating the limbic network. High frequency stimulation of the AN (145Hz “ON” for 1min and “OFF” for 5min) reach 56% median reduction by year two but only 13% of the patients were seizure free for at least 6 months (Fisher et al. 2010).
The future of neuromodulation for drug resistant epilepsy: Neuromodulation has a proven record of accomplishment for its safety, efficacy, reversibility, and flexibility in a variety of neurological disorders which makes it an attractive treatment option for drug resistant epilepsy (Krishna and Lozano 2014). The results from clinical trials are encouraging with a significant decreases in seizure frequency and a preservation of cognitive function, but the rate of patients becoming seizure free, which is the ultimate goal for the patients, is still too low (Gross et al. 2015). There is then a clear need for improvement and many questions remaining: what is the exact mechanism of action, how to optimize the stimulation parameters, and what is the target? As it was mentioned earlier, while inhibition of the structure stimulated at high frequency is most likely the principal mechanism of action, other mechanisms like antidromic activation (Devergnas and Wichmann 2011; Kuriakose et al. 2010) or activation of the passing fiber (So et al. 2012) can also contribute to the global effect of DBS. In addition, since epilepsy is a network disease it is expected that DBS parameters will differ for each type of epilepsy and probably will need to be adjusted for each patients. To date, the stimulation parameters utilized in animal research and clinical trials have remained largely empirical. However, with the development of microelectrode arrays that allows several spatio-temporal stimulating patterns, more complex DBS paradigms have begun to be used in the epilepsy field. Thus, it has been shown in vitro and in vivo that asynchronous distributed multielectrode stimulations have a stronger anti-epileptic effect than regular macroelectrode stimulation in rats (Desai et al. 2016; Wagenaar et al. 2005). Additional benefit could come from the use of close-loop stimulation to stop or prevent seizure. For example, by triggering the stimulation when abnormal activity is detected, the RNS technology reached 76% in seizures reduction for TLE patients (Bergey et al. 2015). Currently, the system can only stimulate a maximum of two onset zones; however, a similar technology could certainly be applied to a different node of the network to indirectly modulate the ictal activity. An alternative approach would be to identify the state associated with the seizures and maintain the activity of the foci outside of this range by directly or indirectly modulating its electrical activity. This close-loop state control has been successfully tested in the sheep model (Stypulkowski et al. 2011; Stypulkowski et al. 2013). Using a bidirectional stimulation and recording pulse generator (Activa PC+S; Medtronic, Mineapolis, MN, USA), Stypulkowski et al. showed that the Hpc could be maintained at a certain power spectra level while using a close-loop algorithm controlling the AN stimulation. This technique will allow the maintenance of the foci activity in a less sensitive state for seizure evocation, but the behavioral and cognitive side effects of this technique have not yet been investigated. This is particularly relevant in the Hpc, since fluctuation of the power spectra have been associated with memory encoding and emotion (Li et al. 2015). Understanding the mechanisms of DBS, selecting the anatomical target, and defining the best stimulation parameters for each type of epilepsy would certainly benefit from an active combination of laboratory and clinical experience. A special note should be made on optogenetic method, which in contrary to the DBS allows the modulation of a specific pathway. While this technic has been successfully used for seizures control in rodents (Krook-Magnuson et al., 2014; Soper et al., 2016) it has not yet been used in NHP, despite the recent advances in chemogenetic and optogenetic developed NHP, especially in the BG and cortico-thalamic network (Galvan et al., 2017).”
Conclusion
There is a considerable amount of compelling evidences from animal model and clinical studies, for the implication of the BG in various forms of seizure. The BG can be essential to the development and the maintenance of the epileptic disorder, and changes in BG activity can also reflect the propagation of the ictal activity through anatomical connections. The anti-epileptic effects induced by electrical, biochemical, or metabolic modulation of the BG suggest that this network play an active part in seizure control. However, despite some promising results, neuromodulation treatment for refractory epilepsy are still not optimum. There is then a clear need for more translational research, and based on the anatomical and functional similarity between humans and NHP, the primate model could contribute to the identification of the mechanism implicated in the control of seizures.
Acknowledgments
We thank Daniel Albaugh for his fruitful comments on the manuscripts; Brigitte Piallat and Stephan Chabardes for their support and authorizing us to publish the electrophysiological data recorded under their supervision during my PhD.
Footnotes
ORCID : 0000-0002-0461-6422
The authors declare no competing financial interests
References
- Ahmadi M, Dufour JP, Seifritz E, Mirnajafi-Zadeh J, Saab BJ. The PTZ kindling mouse model of epilepsy exhibits exploratory drive deficits and aberrant activity amongst VTA dopamine neurons in both familiar and novel space. Behav Brain Res. 2017;330:1–7. doi: 10.1016/j.bbr.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. doi:27/40/10659 [pii] 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Andre V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GAbaergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. doi: 10.1002/hipo.1060. [DOI] [PubMed] [Google Scholar]
- Anlezark GM, Meldrum BS. Effects of apomorphine, ergocornine and piribedil on audiogenic seizures in DBA/2 mice. Br J Pharmacol. 1975;53:419–421. doi: 10.1111/j.1476-5381.1975.tb07378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M. Feline generalized penicillin epilepsy. Ital J Neurol Sci. 1995;16:79–82. doi: 10.1007/BF02229078. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Lagarde S, Wendling F, McGonigal A, Jirsa V, Guye M, Benar C. Defining epileptogenic networks: Contribution of SEEG and signal analysis. Epilepsia. 2017;58:1131–1147. doi: 10.1111/epi.13791. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Benazzous A, Pollak P. Mechanisms of deep brain stimulation. Mov Disord. 2002;17(Suppl 3):S73–74. doi: 10.1002/mds.10145. [DOI] [PubMed] [Google Scholar]
- Bergey GK, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–817. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the Degree of Segregation between Striatonigral and Striatopallidal Projections? Front Neuroanat. 2010;4 doi: 10.3389/fnana.2010.00136. doi:10.3389/fnana.2010.00136 136 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertti P, et al. The neurobiological substrates of behavioral manifestations during temporal lobe seizures: a neuroethological and ictal SPECT correlation study. Epilepsy Behav. 2010;17:344–353. doi: 10.1016/j.yebeh.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Blauwblomme T, Piallat B, Fourcade A, David O, Chabardes S. Cortical stimulation of the epileptogenic zone for the treatment of focal motor seizures: an experimental study in the nonhuman primate. Neurosurgery. 2011;68:482–490. doi: 10.1227/NEU.0b013e3181ff9d14. discussion 490. [DOI] [PubMed] [Google Scholar]
- Bondallaz P, et al. Electrode location and clinical outcome in hippocampal electrical stimulation for mesial temporal lobe epilepsy. Seizure. 2013;22:390–395. doi: 10.1016/j.seizure.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Boon P, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Semah F, Chassoux F, Mantzaridez M, Biraben A, Trebossen R, Ribeiro MJ. Basal ganglia involvement in temporal lobe epilepsy: a functional and morphologic study. Neurology. 2008;70:177–184. doi: 10.1212/01.wnl.0000297514.47695.48. [DOI] [PubMed] [Google Scholar]
- Braakman HM, Vaessen MJ, Hofman PA, Debeij-van Hall MH, Backes WH, Vles JS, Aldenkamp AP. Cognitive and behavioral complications of frontal lobe epilepsy in children: a review of the literature. Epilepsia. 2011;52:849–856. doi: 10.1111/j.1528-1167.2011.03057.x. [DOI] [PubMed] [Google Scholar]
- Braakman HM, et al. Frontal lobe connectivity and cognitive impairment in pediatric frontal lobe epilepsy. Epilepsia. 2013;54:446–454. doi: 10.1111/epi.12044. [DOI] [PubMed] [Google Scholar]
- Brandt C, Ebert U, Loscher W. Epilepsy induced by extended amygdala-kindling in rats: lack of clear association between development of spontaneous seizures and neuronal damage. Epilepsy Res. 2004;62:135–156. doi: 10.1016/j.eplepsyres.2004.08.008. doi:S0920-1211(04)00201-3 [pii] 10.1016/j.eplepsyres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Breit S, Schulz JB, Benabid AL. Deep brain stimulation. Cell Tissue Res. 2004;318:275–288. doi: 10.1007/s00441-004-0936-0. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. doi:10.1016/j.neuron.2012.09.040 S0896-6273(12)00990-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS. Laboratory animal models of temporal lobe epilepsy. Comp Med. 2004;54:473–485. [PubMed] [Google Scholar]
- Cavalheiro EA, Bortolotto ZA, Turski L. Microinjections of the gamma-aminobutyrate antagonist, bicuculline methiodide, into the caudate-putamen prevent amygdala-kindled seizures in rats. Brain Res. 1987;411:370–372. doi: 10.1016/0006-8993(87)91089-4. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Caveness WF, Kato M, Malamut BL, Hosokawa S, Wakisaka S, O’Neill RR. Propagation of focal motor seizures in the pubescent monkey. Ann Neurol. 1980;7:213–221. 232–215. doi: 10.1002/ana.410070304. [DOI] [PubMed] [Google Scholar]
- Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4(Suppl 3):S83–93. [PubMed] [Google Scholar]
- Chen M, et al. Critical Roles of the Direct GABAergic Pallido-cortical Pathway in Controlling Absence Seizures. PLoS Comput Biol. 2015a;11:e1004539. doi: 10.1371/journal.pcbi.1004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, et al. Identification of a direct GABAergic pallidocortical pathway in rodents. Eur J Neurosci. 2015b;41:748–759. doi: 10.1111/ejn.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, et al. High-frequency stimulation of the hippocampus protects against seizure activity and hippocampal neuronal apoptosis induced by kainic acid administration in macaques. Neuroscience. 2014;256:370–378. doi: 10.1016/j.neuroscience.2013.10.059. [DOI] [PubMed] [Google Scholar]
- Cheng H, et al. Low-frequency stimulation of the external globus palladium produces anti-epileptogenic and anti-ictogenic actions in rats. Acta Pharmacol Sin. 2015;36:957–965. doi: 10.1038/aps.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chkhenkeli SA, Chkhenkeli IS. Effects of therapeutic stimulation of nucleus caudatus on epileptic electrical activity of brain in patients with intractable epilepsy. Stereotact Funct Neurosurg. 1997;69:221–224. doi: 10.1159/000099878. [DOI] [PubMed] [Google Scholar]
- Chkhenkeli SA, et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106:318–329. doi: 10.1016/j.clineuro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Cleeren E, Casteels C, Goffin K, Janssen P, Van Paesschen W. Ictal perfusion changes associated with seizure progression in the amygdala kindling model in the rhesus monkey. Epilepsia. 2015;56:1366–1375. doi: 10.1111/epi.13077. [DOI] [PubMed] [Google Scholar]
- Cleeren E, Premereur E, Casteels C, Goffin K, Janssen P, Van Paesschen W. The effective connectivity of the seizure onset zone and ictal perfusion changes in amygdala kindled rhesus monkeys. Neuroimage Clin. 2016;12:252–261. doi: 10.1016/j.nicl.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RC, Kennedy C, Sokoloff L. Metabolic anatomy of focal motor seizures. Trans Am Neurol Assoc. 1976;101:31–34. [PubMed] [Google Scholar]
- Coppola A, Moshe SL. Animal models. Handb Clin Neurol. 2012;107:63–98. doi: 10.1016/B978-0-444-52898-8.00004-5. [DOI] [PubMed] [Google Scholar]
- Cortez MA, Kostopoulos GK, Snead OC., 3rd Acute and chronic pharmacological models of generalized absence seizures. J Neurosci Methods. 2016;260:175–184. doi: 10.1016/j.jneumeth.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. A role for GABAB receptors in excitation and inhibition of thalamocortical cells. Trends Neurosci. 1991;14:16–21. doi: 10.1016/0166-2236(91)90178-w. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol. 1998;55:27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- DeLong M, Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin EEG Neurosci. 2010;41:61–67. doi: 10.1177/155005941004100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin Trends. Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Benabid AL. Discovery of high-frequency deep brain stimulation for treatment of Parkinson disease: 2014 Lasker Award. JAMA. 2014;312:1093–1094. doi: 10.1001/jama.2014.11132. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Snead OC, 3rd, Marescaux C, Vergnes M. Suppressive effects of intranigral injection of muscimol in three models of generalized non-convulsive epilepsy induced by chemical agents. Brain Res. 1989;498:64–72. doi: 10.1016/0006-8993(89)90399-5. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Vergnes M, Marescaux C, Lannes B, Warter JM. Evidence that activation of GABA receptors in the substantia nigra suppresses spontaneous spike-and-wave discharges in the rat. Brain Res. 1988;448:20–29. doi: 10.1016/0006-8993(88)91097-9. [DOI] [PubMed] [Google Scholar]
- Deransart C, Depaulis A. The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord. 2002;4(Suppl 3):S61–72. [PubMed] [Google Scholar]
- Deransart C, Hellwig B, Heupel-Reuter M, Leger JF, Heck D, Lucking CH. Single-unit analysis of substantia nigra pars reticulata neurons in freely behaving rats with genetic absence epilepsy. Epilepsia. 2003;44:1513–1520. doi: 10.1111/j.0013-9580.2003.26603.x. [DOI] [PubMed] [Google Scholar]
- Deransart C, Le BT, Marescaux C, Depaulis A. Role of the subthalamo-nigral input in the control of amygdala-kindled seizures in the rat. Brain Res. 1998a;807:78–83. doi: 10.1016/s0006-8993(98)00745-8. [DOI] [PubMed] [Google Scholar]
- Deransart C, Marescaux C, Depaulis A. Involvement of nigral glutamatergic inputs in the control of seizures in a genetic model of absence epilepsy in the rat. Neuroscience. 1996;71:721–728. doi: 10.1016/0306-4522(95)00471-8. [DOI] [PubMed] [Google Scholar]
- Deransart C, Riban V, Le B, Marescaux C, Depaulis A. Dopamine in the striatum modulates seizures in a genetic model of absence epilepsy in the rat. Neuroscience. 2000;100:335–344. doi: 10.1016/s0306-4522(00)00266-9. [DOI] [PubMed] [Google Scholar]
- Deransart C, Riban V, Le BT, Hechler V, Marescaux C, Depaulis A. Evidence for the involvement of the pallidum in the modulation of seizures in a genetic model of absence epilepsy in the rat. Neuroscience Letters. 1999;265:131–134. doi: 10.1016/s0304-3940(99)00113-5. doi: S0304-3940(99)00113-5 [pii] [DOI] [PubMed] [Google Scholar]
- Deransart C, Vercueil L, Marescaux C, Depaulis A. The role of basal ganglia in the control of generalized absence seizures. Epilepsy Research. 1998b;32:213–223. doi: 10.1016/s0920-1211(98)00053-9. [DOI] [PubMed] [Google Scholar]
- Desai SA, Rolston JD, McCracken CE, Potter SM, Gross RE. Asynchronous Distributed Multielectrode Microstimulation Reduces Seizures in the Dorsal Tetanus Toxin Model of. Temporal Lobe Epilepsy Brain Stimul. 2016;9:86–100. doi: 10.1016/j.brs.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergnas A, Piallat B, Prabhu S, Torres N, Louis Benabid A, David O, Chabardes S. The subcortical hidden side of focal motor seizures: evidence from micro-recordings and local field potentials. Brain. 2012;135:2263–2276. doi: 10.1093/brain/aws134. [DOI] [PubMed] [Google Scholar]
- Devergnas A, Wichmann T. Cortical potentials evoked by deep brain stimulation in the subthalamic area. Front Syst Neurosci. 2011;5:30. doi: 10.3389/fnsys.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Keegan KD, Noebels JL. Increased excitability and inward rectification in layer V cortical pyramidal neurons in the epileptic mutant mouse. Stargazer J Neurophysiol. 1997;77:621–631. doi: 10.1152/jn.1997.77.2.621. [DOI] [PubMed] [Google Scholar]
- Dong L, Wang P, Peng R, Jiang S, Klugah-Brown B, Luo C, Yao D. Altered basal ganglia-cortical functional connections in frontal lobe epilepsy: A resting-state fMRI study. Epilepsy Res. 2016;128:12–20. doi: 10.1016/j.eplepsyres.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Faeth WH, Walker AE, Andy OJ. The propagation of cortical and subcortical epileptic discharge. Epilepsia. 1954;3:37–48. doi: 10.1111/j.1528-1157.1954.tb03152.x. [DOI] [PubMed] [Google Scholar]
- Ferraro G, Vella N, Sardo P, Caravaglios G, Sabatino M, Lagrutta V. Dopaminergic Control of Feline Hippocampal Epilepsy – a Nigrohippocampal Pathway. Neurosci Lett. 1991;123:41–44. doi: 10.1016/0304-3940(91)90153-K. [DOI] [PubMed] [Google Scholar]
- Fisher R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- Franzini A, Messina G, Marras C, Villani F, Cordella R, Broggi G. Deep brain stimulation of two unconventional targets in refractory non-resectable epilepsy. Stereotact Funct Neurosurg. 2008;86:373–381. doi: 10.1159/000175800. [DOI] [PubMed] [Google Scholar]
- Freichel C, Ebert U, Potschka H, Loscher W. Amygdala-kindling does not induce a persistent loss of GABA neurons in the substantia nigra pars reticulata of rats. Brain Res. 2004;1025:203–209. doi: 10.1016/j.brainres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J Comp Neurol. 2002;450:345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- Gale K, Iadarola MJ. Seizure protection and increased nerve-terminal GABA: delayed effects of GABA transaminase inhibition. Science. 1980;208:288–291. doi: 10.1126/science.6768130. [DOI] [PubMed] [Google Scholar]
- Galvan A, Caiola MJ, Albaugh DL. Advances in optogenetic and chemogenetic methods to study brain circuits in non-human primates. J Neural Transm (Vienna) 2017 doi: 10.1007/s00702-017-1697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant DS, Gale K. Lesions of substantia nigra protect against experimentally induced seizures. Brain Res. 1983;273:156–161. doi: 10.1016/0006-8993(83)91105-8. [DOI] [PubMed] [Google Scholar]
- Garant DS, Gale K. Substantia nigra-mediated anticonvulsant actions: role of nigral output pathways. Exp Neurol. 1987;97:143–159. doi: 10.1016/0014-4886(87)90289-5. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Trends Neurosci. 1995;18:60–62. [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Grone BP, Baraban SC. Animal models in epilepsy research: legacies and new directions. Nat Neurosci. 2015;18:339–343. doi: 10.1038/nn.3934. [DOI] [PubMed] [Google Scholar]
- Gross RE, Mahmoudi B, Riley JP. Less is more: novel less-invasive surgical techniques for mesial temporal lobe epilepsy that minimize cognitive impairment. Curr Opin Neurol. 2015;28:182–191. doi: 10.1097/WCO.0000000000000176. [DOI] [PubMed] [Google Scholar]
- Haber SN, Lynd E, Klein C, Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293:282–298. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- Halasz P, Rasonyi G. The network or system-oriented approach in understanding human epilepsy (comments on the article of Susan S. Spencer: “Neural networks in human epilepsy: evidence of and implications for treatment”) Epilepsia. 2003;44:625. doi: 10.1046/j.1528-1157.2003.55102.x. author reply 626. [DOI] [PubMed] [Google Scholar]
- Haut SR, Albin RL. Dopamine and epilepsy: hints of complex subcortical roles. Neurology. 2008;71:784–785. doi: 10.1212/01.wnl.0000325637.38931.27. [DOI] [PubMed] [Google Scholar]
- Heck CN, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55:432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington HP, et al. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology. 2007;69:2256–2265. doi: 10.1212/01.wnl.0000286945.21270.6d. [DOI] [PubMed] [Google Scholar]
- Hosokawa S, Iguchi T, Caveness WF, Kato M, O’Neill RR, Wakisaka S, Malamut BL. Effects of manipulation of the sensorimotor system on focal motor seizures in the monkey. Ann Neurol. 1980;7:222–229. 236–227. doi: 10.1002/ana.410070305. [DOI] [PubMed] [Google Scholar]
- Hosokawa S, Kato M, Kuroiwa Y. Topographical distribution of propagation of seizure activity in the basal ganglia during focal motor seizures in the monkey. Neuroscience Letters. 1983;38:29–33. doi: 10.1016/0304-3940(83)90105-2. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218:1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- Ito M, Doya K. Multiple representations and algorithms for reinforcement learning in the cortico-basal ganglia circuit. Curr Opin Neurobiol. 2011;21:368–373. doi: 10.1016/j.conb.2011.04.001. doi:10.1016/j.conb.2011.04.001 S0959-4388(11)00046-8 [pii] [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. doi:0306-4522(94)90536-3 [pii] [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ. Nucleus accumbens to globus pallidus GABA projection: electrophysiological and iontophoretic investigations. Brain Res. 1980;188:93–105. doi: 10.1016/0006-8993(80)90559-4. [DOI] [PubMed] [Google Scholar]
- Kahn I, Shohamy D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 2013;23:187–192. doi: 10.1002/hipo.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniff TE, Chuman CM, Neafsey EJ. Substantia nigra single unit activity during penicillin-induced focal cortical epileptiform discharge in the rat. Brain Res Bull. 1983;11:11–13. doi: 10.1016/0361-9230(83)90050-3. [DOI] [PubMed] [Google Scholar]
- Kato M, Malamut BL, Caveness WF, Hosokawa S, Wakisaka S, O’Neill RR. Local cerebral glucose utilization in newborn and pubescent monkeys during focal motor seizures. Ann Neurol. 1980;7:204–212. 230–202. doi: 10.1002/ana.410070303. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Grimee R, Vanneste-Goemaere J, Margineanu DG. Electrophysiological, neurochemical and regional effects of levetiracetam in the rat pilocarpine model of temporal lobe epilepsy. Seizure. 2003;12:92–100. doi: 10.1016/s1059131102001930. [DOI] [PubMed] [Google Scholar]
- Krishna V, Lozano AM. Brain stimulation for intractable epilepsy: Anterior thalamus and responsive stimulation Ann Indian. Acad Neurol. 2014;17:S95–98. doi: 10.4103/0972-2327.128671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro. 2014;1 doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose R, et al. The nature and time course of cortical activation following subthalamic stimulation in Parkinson’s disease. Cereb Cortex. 2010;20:1926–1936. doi: 10.1093/cercor/bhp269. [DOI] [PubMed] [Google Scholar]
- La Grutta V, Amato G, Zagami MT. The importance of the caudate nucleus in the control of convulsive activity in the amygdaloid complex and the temporal cortex of the cat. Electroencephalogr Clin Neurophysiol. 1971;31:57–69. doi: 10.1016/0013-4694(71)90289-6. [DOI] [PubMed] [Google Scholar]
- La Grutta V, Sabatino M, Gravante G, Morici G, Ferraro G, La Grutta G. A study of caudate inhibition on an epileptic focus in the cat hippocampus. Arch Int Physiol Biochim. 1988;96:113–120. doi: 10.3109/13813458809079632. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012;2:a009621. doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics. 2014;11:508–526. doi: 10.1007/s13311-014-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Long C, Yang L. Hippocampal-prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders. Biomed Res Int. 2015;2015:810548. doi: 10.1155/2015/810548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Vergnes M, Depaulis A, Marescaux C. Evidence for a critical role of GABAergic transmission within the thalamus in the genesis and control of absence seizures in the rat. Brain Res. 1991;545:1–7. doi: 10.1016/0006-8993(91)91262-y. [DOI] [PubMed] [Google Scholar]
- Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. doi:S0920121102000736 [pii] [DOI] [PubMed] [Google Scholar]
- Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Loscher W, Czuczwar SJ. Studies on the involvement of dopamine D-1 and D-2 receptors in the anticonvulsant effect of dopamine agonists in various rodent models of epilepsy. Eur J Pharmacol. 1986;128:55–65. doi: 10.1016/0014-2999(86)90557-1. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Hatlelid JM, Zorumski CF. Functional mapping of limbic seizures originating in the hippocampus: a combined 2-deoxyglucose and electrophysiologic study. Brain Res. 1985;360:92–100. doi: 10.1016/0006-8993(85)91224-7. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Mittmann T, van Luijtelaar G, Heinemann U. Impairment of intracortical GABAergic inhibition in a rat model of absence epilepsy. Epilepsy Res. 1995;22:43–51. doi: 10.1016/0920-1211(95)00032-6. [DOI] [PubMed] [Google Scholar]
- Ma J, Brudzynski SM, Leung LW. Involvement of the nucleus accumbens-ventral pallidal pathway in postictal behavior induced by a hippocampal afterdischarge in rats. Brain Res. 1996;739:26–35. doi: 10.1016/s0006-8993(96)00793-7. [DOI] [PubMed] [Google Scholar]
- Mallet L, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- Mayanagi Y, Walker AE. Experimental temporal lobe epilepsy. Brain. 1974;97:423–446. doi: 10.1093/brain/97.1.423. [DOI] [PubMed] [Google Scholar]
- Mayanagi Y, Walker AE. DC potentials of temporal lobe seizures in the monkey. J Neurol. 1975;209:199–215. doi: 10.1007/BF00312542. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Galloway MT, Rigsbee LC, Shin C. Evidence implicating substantia nigra in regulation of kindled seizure threshold. J Neurosci. 1984;4:2410–2417. doi: 10.1523/JNEUROSCI.04-09-02410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62:371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- Meldrum B, Anlezark G, Trimble M. Drugs modifying dopaminergic activity and behaviour, the EEG and epilepsy in Papio papio. Eur J Pharmacol. 1975;32:203–213. doi: 10.1016/0014-2999(75)90284-8. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. doi:0301-0082(80)90018-0 [pii] [DOI] [PubMed] [Google Scholar]
- Moro E, et al. Efficacy of pallidal stimulation in isolated dystonia: a systematic review and meta-analysis. Eur J Neurol. 2017 doi: 10.1111/ene.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nail-Boucherie K, Le-Pham BT, Marescaux C, Depaulis A. Suppression of absence seizures by electrical and pharmacological activation of the caudal superior colliculus in a genetic model of absence epilepsy in the rat. Exp Neurol. 2002;177:503–514. doi: 10.1006/exnr.2002.7997. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Chuman CM, Ward AA., Jr Propagation of focal cortical epileptiform discharge to the basal ganglia. Exp Neurol. 1979;66:97–108. doi: 10.1016/0014-4886(79)90066-9. [DOI] [PubMed] [Google Scholar]
- Oakley JC, Ojemann GA. Effects of chronic stimulation of the caudate nucleus on a preexisting alumina seizure focus. Exp Neurol. 1982;75:360–367. doi: 10.1016/0014-4886(82)90167-4. [DOI] [PubMed] [Google Scholar]
- Pallud J, Devergnas A, Chabardes S, Depaulis A. Animal models to develop surgery of focal epilepsies? Neurochirurgie. 2008;54:128–134. doi: 10.1016/j.neuchi.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Paz JT. Rhythmic Bursting in the Cortico-Subthalamo-Pallidal Network during Spontaneous Genetically Determined Spike and Wave Discharges. Journal of Neuroscience. 2005;25:2092–2101. doi: 10.1523/jneurosci.4689-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Chavez M, Saillet S, Deniau JM, Charpier S. Activity of Ventral Medial Thalamic Neurons during Absence Seizures and Modulation of Cortical Paroxysms by the Nigrothalamic Pathway. Journal of Neuroscience. 2007;27:929–941. doi: 10.1523/jneurosci.4677-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JP, Rovner LI. Experimental epileptogenesis: kindling-induced epilepsy in rats. Exp Neurol. 1978;58:190–202. doi: 10.1016/0014-4886(78)90133-4. doi:0014-4886(78)90133-4 [pii] [DOI] [PubMed] [Google Scholar]
- Pisa M, Sanberg PR, Corcoran ME, Fibiger HC. Spontaneously recurrent seizures after intracerebral injections of kainic acid in rat: a possible model of human temporal lobe epilepsy. Brain Res. 1980;200:481–487. doi: 10.1016/0006-8993(80)90938-5. doi:0006-8993(80)90938-5 [pii] [DOI] [PubMed] [Google Scholar]
- Poggio GF, Walker AE, Andy OJ. The propagation of cortical after-discharge through subcortical structures. AMA Arch Neurol Psychiatry. 1956;75:350–361. doi: 10.1001/archneurpsyc.1956.02330220014002. [DOI] [PubMed] [Google Scholar]
- Pontes JC, Lima TZ, Queiroz CM, Cinini SM, Blanco MM, Mello LE. Seizures triggered by pentylenetetrazol in marmosets made chronically epileptic with pilocarpine show greater refractoriness to treatment. Epilepsy Research. 2016;126:16–25. doi: 10.1016/j.eplepsyres.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Prabhu S, et al. Effect of subthalamic nucleus stimulation on penicillin induced focal motor seizures in primate. Brain Stimul. 2015;8:177–184. doi: 10.1016/j.brs.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Prabhu S, et al. Characteristics of a primate model of focal motor cortical seizures suitable for preclinical testing of therapies like DBS World. Journal of Neuroscience. 2014;4 [Google Scholar]
- Rektor I, Kuba R, Brazdil M. Interictal and ictal EEG activity in the basal ganglia: an SEEG study in patients with temporal lobe epilepsy. Epilepsia. 2002;43:253–262. doi: 10.1046/j.1528-1157.2002.28001.x. [DOI] [PubMed] [Google Scholar]
- Rektor I, Kuba R, Brazdil M, Chrastina J. Do the basal ganglia inhibit seizure activity in temporal lobe epilepsy? Epilepsy Behav. 2012;25:56–59. doi: 10.1016/j.yebeh.2012.04.125. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Weber P, Epstein CM, Henry TR, Bakay RA. Alumina gel injections into the temporal lobe of rhesus monkeys cause complex partial seizures and morphological changes found in human temporal lobe epilepsy. J Comp Neurol. 1998;401:266–290. [PubMed] [Google Scholar]
- Riva-Posse P, Holtzheimer PE, Garlow SJ, Mayberg HS. Practical considerations in the development and refinement of subcallosal cingulate white matter deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80:S27 e25–34. doi: 10.1016/j.wneu.2012.11.074. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;64:1019–1034. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- Sabatino M, Gravante G, Ferraro G, Vella N, Lagrutta G, Lagrutta V. Striatonigral Suppression of Focal Hippocampal. Epilepsy Neurosci Lett. 1989;98:285–290. doi: 10.1016/0304-3940(89)90415-1. [DOI] [PubMed] [Google Scholar]
- Saillet S, Gharbi S, Charvet G, Deransart C, Guillemaud R, Depaulis A, David O. Neural adaptation to responsive stimulation: a comparison of auditory and deep brain stimulation in a rat model of absence epilepsy. Brain Stimul. 2013;6:241–247. doi: 10.1016/j.brs.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Salgado S, Kaplitt MG. The Nucleus Accumbens: A Comprehensive Review Stereotact. Funct Neurosurg. 2015;93:75–93. doi: 10.1159/000368279. doi:000368279 [pii] 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- Sander JW. Some aspects of prognosis in the epilepsies: a review. Epilepsia. 1993;34:1007–1016. doi: 10.1111/j.1528-1157.1993.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Schuele SU, Luders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7:514–524. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- Shedlack KJ, Lee EK, Radtke RA, Friedman AH, Crain BJ, Boyko O, Krishnan KR. Ipsilateral subcortical atrophy associated with temporal lobectomy. Psychiatry Res. 1994;54:295–304. doi: 10.1016/0165-1781(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, McIntyre DC, Chang JY. Temporal sequence of ictal discharges propagation in the corticolimbic basal ganglia system during amygdala kindled seizures in freely moving rats. Epilepsy Research. 2007;73:85–97. doi: 10.1016/j.eplepsyres.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaght SJ, Paz T, Chavez M, Deniau JM, Mahon S, Charpier S. On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy. J Neurosci. 2004;24:6816–6825. doi: 10.1523/JNEUROSCI.1449-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, et al. The thalamostriatal system in normal and diseased states. Front Syst Neurosci. 2014;8:5. doi: 10.3389/fnsys.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead OC., 3rd Gamma hydroxybutyrate in the monkey. I. Electroencephalographic, behavioral, and pharmacokinetic studies. Neurology. 1978a;28:636–642. doi: 10.1212/wnl.28.7.636. [DOI] [PubMed] [Google Scholar]
- Snead OC., 3rd Gamma hydroxybutyrate in the monkey. II. Effect of chronic oral anticonvulsant drugs. Neurology. 1978b;28:643–648. doi: 10.1212/wnl.28.7.643. [DOI] [PubMed] [Google Scholar]
- Snead OC., 3rd Pharmacological models of generalized absence seizures in rodents. J Neural Transm Suppl. 1992;35:7–19. doi: 10.1007/978-3-7091-9206-1_2. [DOI] [PubMed] [Google Scholar]
- So RQ, Kent AR, Grill WM. Relative contributions of local cell and passing fiber activation and silencing to changes in thalamic fidelity during deep brain stimulation and lesioning: a computational modeling study. J Comput Neurosci. 2012;32:499–519. doi: 10.1007/s10827-011-0366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper C, Wicker E, Kulick CV, N’Gouemo P, Forcelli PA. Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol Dis. 2016;87:102–115. doi: 10.1016/j.nbd.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Sperling MR, Gur RC, Alavi A, Gur RE, Resnick S, O’Connor MJ, Reivich M. Subcortical metabolic alterations in partial epilepsy. Epilepsia. 1990;31:145–155. doi: 10.1111/j.1528-1167.1990.tb06299.x. [DOI] [PubMed] [Google Scholar]
- Starr MS. The role of dopamine in epilepsy. Synapse. 1996;22:159–194. doi: 10.1002/(SICI)1098-2396(199602)22:2<159::AID-SYN8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Stretton J, et al. Temporal lobe epilepsy and affective disorders: the role of the subgenual anterior cingulate cortex. J Neurol Neurosurg Psychiatry. 2015;86:144–151. doi: 10.1136/jnnp-2013-306966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stypulkowski PH, Giftakis JE, Billstrom TM. Development of a large animal model for investigation of deep brain stimulation for epilepsy. Stereotact Funct Neurosurg. 2011;89:111–122. doi: 10.1159/000323343. [DOI] [PubMed] [Google Scholar]
- Stypulkowski PH, Stanslaski SR, Denison TJ, Giftakis JE. Chronic evaluation of a clinical system for deep brain stimulation and recording of neural network activity. Stereotact Funct Neurosurg. 2013;91:220–232. doi: 10.1159/000345493. [DOI] [PubMed] [Google Scholar]
- Tenney JR, Marshall PC, King JA, Ferris CF. fMRI of generalized absence status epilepticus in conscious marmoset monkeys reveals corticothalamic activation. Epilepsia. 2004;45:1240–1247. doi: 10.1111/j.0013-9580.2004.21504.x. [DOI] [PubMed] [Google Scholar]
- Turski L, Cavalheiro EA, Bortolotto ZA, Ikonomidou-Turski C, Kleinrok Z, Turski WA. Dopamine-sensitive anticonvulsant site in the rat striatum. J Neurosci. 1988;8:4027–4037. doi: 10.1523/JNEUROSCI.08-11-04027.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, et al. Paradoxical anticonvulsant activity of the gamma-aminobutyrate antagonist bicuculline methiodide in the rat striatum. Synapse. 1991;7:14–20. doi: 10.1002/syn.890070103. [DOI] [PubMed] [Google Scholar]
- Turski L, Meldrum BS, Cavalheiro EA, Calderazzo-Filho LS, Bortolotto ZA, Ikonomidou-Turski C, Turski WA. Paradoxical anticonvulsant activity of the excitatory amino acid N-methyl-D-aspartate in the rat caudate-putamen. Proc Natl Acad Sci U S A. 1987;84:1689–1693. doi: 10.1073/pnas.84.6.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvarhelyi GB, Walker AE. Dissemination of Acute Focal Seizures in the Monkey. I. From Cortical Foci. Arch Neurol. 1965;12:333–356. doi: 10.1001/archneur.1965.00460280003001. [DOI] [PubMed] [Google Scholar]
- Valentin A, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54:1823–1833. doi: 10.1111/epi.12352. [DOI] [PubMed] [Google Scholar]
- Velasco AL, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47:1203–1212. doi: 10.1111/j.1528-1167.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- Velasco F, Carrillo-Ruiz JD, Brito F, Velasco M, Velasco AL, Marquez I, Davis R. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia. 2005;46:1071–1081. doi: 10.1111/j.1528-1167.2005.70504.x. [DOI] [PubMed] [Google Scholar]
- Velasco F, Velasco M, Ogarrio C, Fanghanel G. Electrical stimulation of the centromedian thalamic nucleus in the treatment of convulsive seizures: a preliminary report. Epilepsia. 1987;28:421–430. doi: 10.1111/j.1528-1157.1987.tb03668.x. [DOI] [PubMed] [Google Scholar]
- Venzi M, Di Giovanni G, Crunelli V. A critical evaluation of the gamma-hydroxybutyrate (GHB) model of absence seizures. CNS Neurosci Ther. 2015;21:123–140. doi: 10.1111/cns.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercueil L, Benazzouz A, Deransart C, Bressand K, Marescaux C, Depaulis A, Benabid AL. High-frequency stimulation of the subthalamic nucleus suppresses absence seizures in the rat: comparison with neurotoxic lesions. Epilepsy Research. 1998;31:39–46. doi: 10.1016/s0920-1211(98)00011-4. [DOI] [PubMed] [Google Scholar]
- Vesper J, Steinhoff B, Rona S, Wille C, Bilic S, Nikkhah G, Ostertag C. Chronic high-frequency deep brain stimulation of the STN/SNr for progressive myoclonic epilepsy. Epilepsia. 2007;48:1984–1989. doi: 10.1111/j.1528-1167.2007.01166.x. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(Suppl 3):S69–72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Vonck K, et al. A decade of experience with deep brain stimulation for patients with refractory medial temporal lobe epilepsy. Int J Neural Syst. 2013;23:1250034. doi: 10.1142/S0129065712500347. [DOI] [PubMed] [Google Scholar]
- Vytvarova E, Marecek R, Fousek J, Strycek O, Rektor I. Large-scale cortico-subcortical functional networks in focal epilepsies: The role of the basal ganglia. Neuroimage Clin. 2017;14:28–36. doi: 10.1016/j.nicl.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada JA, Osawa T. Spontaneous recurrent seizure state induced by daily electric amygdaloid stimulation in Senegalese baboons (Papio papio) Neurology. 1976;26:273–286. doi: 10.1212/wnl.26.3.273. [DOI] [PubMed] [Google Scholar]
- Wagenaar DA, Madhavan R, Pine J, Potter SM. Controlling bursting in cortical cultures with closed-loop multi-electrode stimulation. J Neurosci. 2005;25:680–688. doi: 10.1523/JNEUROSCI.4209-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, et al. Decreased dopamine D2/D3-receptor binding in temporal lobe epilepsy: an [18F]fallypride PET study. Epilepsia. 2006;47:1392–1396. doi: 10.1111/j.1528-1167.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- Wille C, Steinhoff BJ, Altenmuller DM, Staack AM, Bilic S, Nikkhah G, Vesper J. Chronic high-frequency deep-brain stimulation in progressive myoclonic epilepsy in adulthood–report of five cases. Epilepsia. 2011;52:489–496. doi: 10.1111/j.1528-1167.2010.02884.x. [DOI] [PubMed] [Google Scholar]
- Wright GD, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984;47:769–774. doi: 10.1136/jnnp.47.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler AR, Burchiel KJ, Ward AA., Jr Chronic epileptic foci in monkeys: correlation between seizure frequency and proportion of pacemaker epileptic neurons. Epilepsia. 1978;19:475–483. doi: 10.1111/j.1528-1157.1978.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Yakushev IY, et al. In vivo imaging of dopamine receptors in a model of temporal lobe epilepsy. Epilepsia. 2010;51:415–422. doi: 10.1111/j.1528-1167.2009.02272.x. [DOI] [PubMed] [Google Scholar]
- Yalcin O. Genes and molecular mechanisms involved in the epileptogenesis of idiopathic absence epilepsies. Seizure. 2012;21:79–86. doi: 10.1016/j.seizure.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Yang AC, et al. Potential Protective Effects of Chronic Anterior Thalamic Nucleus Stimulation on Hippocampal Neurons in Epileptic Monkeys. Brain Stimul. 2015;8:1049–1057. doi: 10.1016/j.brs.2015.07.041. [DOI] [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ. Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res. 1984;324:69–84. doi: 10.1016/0006-8993(84)90623-1. [DOI] [PubMed] [Google Scholar]
- Yao QH, Zhang H, Wang HW, Jing XR, Guo H, Gao GD. Low- and high-frequency electric cortical stimulation suppress the ferric chloride-induced seizures in rats. Neurosci Lett. 2008;430:187–190. doi: 10.1016/j.neulet.2007.10.024. [DOI] [PubMed] [Google Scholar]


