Abstract
Adolescence is a critical period in brain development that coincides with the initiation of alcohol use. Nicotinic acetylcholine receptors (nAChR) have been shown to modulate ethanol behaviors in adult humans and in animal models; however, the role of these receptors in adolescent ethanol behaviors has not been explored. Throughout adolescence, nAChR expression undergoes large-scale developmental changes which may alter behavioral responses to ethanol. Here we examined the effect of varenicline, a nAChR partial agonist, on ethanol consumption, ataxia, sedation, and metabolism in adolescent male and female C57BL/6J mice. The effect of varenicline on ethanol consumption was tested through the Drinking-in-the-Dark (DID) paradigm that models binge-like ethanol consumption. To ensure that results were specific for ethanol, we also tested the effect of varenicline on saccharin consumption. Additionally, varenicline was administered 30 minutes prior to an acute injection of ethanol before being tested for ataxia on the balance beam, sedation using the loss of righting reflex, or ethanol metabolism. Varenicline dose dependently decreased ethanol consumption, but also influenced saccharin intake. Varenicline showed no significant effect on ethanol metabolism, ataxia, or sedation. Unlike its effects in adult animals, varenicline is able to reduce ethanol consumption without increasing the ataxic and sedative effects of ethanol. This work suggests that the neurobiological mechanisms of ethanol behaviors may change across the lifespan and highlights the need for more research on the role of nAChRs in ethanol behaviors throughout development.
Keywords: Ethanol, Nicotinic Acetylcholine Receptors, Ataxia, Sedation, Consumption, Varenicline
1. Introduction
Alcohol use disorders are common in the United States with a total lifetime prevalence of 30% (Hasin et al., 2007). Experimentation with alcohol often begins in early adolescence or young adulthood. Among 12-13 year olds, 3.5% have used alcohol, but this number rises to 70.2% in 21-25 year olds (Substance Abuse and Mental Health Services Administration, 2010). In 2012, 64% of high school seniors used alcohol and almost a quarter of all seniors binge drank alcohol (Johnston et al., 2013). Moreover, heavy alcohol use in adolescence is associated with increased alcohol use disorders in adulthood (Marshall, 2014). The biological factors that promote adolescent alcohol consumption are not well understood. These data highlight the need for research focused on adolescent alcohol behaviors and the underlying neurobiology.
Adolescence is a critical time in brain development that coincides with the initiation of alcohol use. During adolescence, the brain undergoes significant changes in regard to both structure and function (Spear, 2013). One receptor system that matures during this time is the nicotinic acetylcholine receptor (nAChR) system. Research has shown that the expression of nAChR subunits changes over the course of development, with greater expression of particular subunits observed in adolescent compared to adult animals (Azam et al., 2007; Doura et al., 2008). In addition to changes in expression, increased function of nAChRs in brain regions known to modulate drug behaviors has also been observed in adolescent versus adult animals (Azam et al., 2007; Kota et al., 2007). Therefore, changes in nAChRs throughout the course of adolescent development could lead to altered behavioral responses compared to adult animals.
Nicotinic receptors have received much attention recently for their link to ethanol behaviors. Ethanol has both direct and indirect effects on nAChRs that can modulate behavioral responses of this drug (Davis and de Fiebre, 2006). Human genetic variants in genes that code for nAChR subunits have been linked to binge alcohol consumption, onset of alcohol use, and age of initiation of alcohol use (Coon et al., 2014; Ehringer et al., 2007; Lubke et al., 2012; Schlaepfer et al., 2008). Moreover, pharmacological and genetic manipulation of rodent nAChRs have implicated these receptors in a variety of ethanol behaviors including consumption, reward, locomotor responses, ataxia, and sedation (Bowers et al., 2005; Dawson et al., 2013; Hendrickson et al., 2009; Kamens et al., 2009; Powers et al., 2013; Sajja and Rahman, 2013; Steensland et al., 2007).
One of the most widely used pharmacological tools to investigate the role of nAChRs in ethanol behaviors is varenicline, an α4β2 partial agonist (Rollema et al., 2007). In adults, varenicline has been shown to decrease ethanol consumption (Hendrickson et al., 2010; Kamens et al., 2010a; Steensland et al., 2007) and increase the ataxic and sedative properties of ethanol (Kamens et al., 2010b). These effects occur without influencing ethanol metabolism (Kamens et al., 2010a) or the rewarding properties of the drug (Gubner et al., 2014). Further data implicates the α4 nAChR subunit in mediating the effect of varenicline on ethanol consumption with the posterior ventral tegmental area as the site of action (Hendrickson et al., 2010). Importantly, all data regarding the role of varenicline in modulating ethanol behaviors has been performed in adult animals. Given that alcohol use begins during adolescence and that nicotinic receptors dynamically change during this time, it is important to understand the role of nAChRs in adolescent ethanol behaviors.
The aim of the current study was to examine the role of nAChRs in adolescent ethanol behaviors. Here we examine the influence of varenicline on ethanol consumption, ataxia, sedation, and metabolism in adolescent male and female C57BL/6J mice. Further, the effect of varenicline on saccharin consumption was utilized to determine if effects on ethanol consumption were specific. The results indicate that varenicline reduces ethanol consumption in adolescent animals, but also decreases consumption of the sweet solution saccharin. In contrast, no significant effects were observed on ethanol-induced ataxia, sedation, or metabolism.
2. Materials and Methods
2.1 Animals
Adolescent C57BL/6J mice purchased from The Jackson Laboratory (Bar Harbor, Maine) were used in all experiments. Mice arrived on postnatal day (PND) 25. Animals to be used in drinking studies were singly housed, while mice for all other behaviors were placed in same sex groups with 2-4 mice per cage. Food and water was available ad libitum except where noted below. Mice were allowed to acclimate to our animal facility for one week prior to testing. Animals used in drinking studies were housed in a room with a modified 12-hour light/dark cycle (lights on at 2200) to allow for drinking studies to be conducted in the dark between 1300-1500. For all other experiments, mice were housed on a standard 12-hour light/dark cycle (lights on at 0700) and behavioral studies were performed in the light between 0900-1300. All procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
2.2 Drugs
Ethyl alcohol (200 proof; Koptec) and saccharin sodium salt (Sigma-Aldrich, St. Louis, MO) were diluted in tap water for drinking studies. For injections, ethyl alcohol (20% v/v) and varenicline tartrate (graciously provided by Pfizer, Groton, CT) were mixed with physiological saline (0.9% NaCl; Baxter) and administered into the intraperitoneal (i.p.) cavity.
2.3 Drinking in the Dark (DID)
The effect of varenicline on ethanol consumption was tested with a 2-day drinking-in-the-dark (DID) procedure (Gupta et al., 2008; Kamdar et al., 2007; Kamens et al., 2017a). Briefly, naïve male and female mice (N = 24) were repeatedly tested in four 2-day DID sessions at postnatal days (PND) 32-33, 36-37, 39-40, and 43-44. On the first day, the animal's water bottle was removed from the cage 3 hours into the dark cycle and a single 20% ethanol tube was presented (Rhodes et al., 2005). The volume of fluid in the tube was read to the closest 0.1 ml at the beginning of the session and at 30 minutes, 1 hour, and 2 hours into the drinking session. At the end of the 2 hour drinking session the ethanol tube was removed and water was replaced on the cage. On day 2 of each session this protocol was repeated, but mice were given an acute injection of saline or varenicline (0.5, 1, or 2 mg/kg, (Kamens et al., 2010b, 2010a)) 30 minutes prior to the ethanol availability. Mice received saline and each of the three varenicline doses in a Latin square design. The primary dependent variable was ethanol consumption (g/kg). To ensure that the effect of varenicline was specific for ethanol, a naïve group of male and female adolescent C57BL/6J mice (N = 12/group) had access to 0.033% saccharin using the same procedure (Kamens et al., 2017a).
2.4 Balance Beam
The balance beam was used to examine the effect of varenicline on ethanol-induced ataxia (Crabbe et al., 2003; Kamens et al., 2010b; Linsenbardt et al., 2009). Briefly, naïve male and female mice were tested (N = 114). On PND 32 all mice were trained to cross the balance beam (¾ inch wide, 54.6 cm off of the ground). Mice were placed on the end of the beam and crossed the beam twice to complete their training. On PND 33, mice were moved to the test room and allowed to acclimate for at least 1 hour. Mice were pretreated with an i.p. injection of saline or varenicline (0.5, 1, or 2 mg/kg; Kamens et al., 2010b) and placed into a holding cage (n=13-17/ group). Thirty minutes after the varenicline treatment, mice received either saline or 1.5 g/kg ethanol injection and were returned to their holding cage. Ten minutes after this challenge injection, the mice were tested for ataxia on the balance beam. The primary dependent variable was the number of hindpaw slips while crossing the balance beam. Hindpaw slips were counted by a trained experimenter unaware of the animal's group assignment.
2.5 LORR
The Loss of Righting Reflex (LORR) was used to examine the effect of varenicline on ethanol-induced sedation (Crabbe et al., 2006; Kamens et al., 2012, 2010b). A subset of male and female animals tested for ataxia were left undisturbed for 7 days and then were tested for LORR on PND 39 (N = 71). Mice were moved to the testing room, weighed, and were left to acclimate for at least 1 hour. Each mouse received a pretreatment injection of either saline or varenicline (N = 17-19/group; 0.5, 1, or 2 mg/kg; Kamens et al., 2010b) and was immediately placed into a holding cage. Thirty minutes after the pretreatment, all mice were challenged with a 4.0 g/kg ethanol injection. Mice were returned to their holding cage for approximately 1 minute or until they appeared intoxicated. They were then placed on their back in a V-shaped plastic trough. When mice were unable to right themselves for 30 seconds they were determined to have lost their righting reflex. Two dependent variables were collected: latency to LORR and duration of LORR. Latency to LORR was defined as the time from ethanol injection until the animal lost its righting reflex. Duration of LORR was defined as time until the end of LORR when the animal could right itself twice in one minute.
2.6 Ethanol Metabolism
The effect of varenicline on ethanol metabolism was examined using a standard procedure (Kamens et al., 2010a, 2006). Briefly, a subset of male mice (N = 15) tested on the balance beam were left undisturbed and tested at PND 39. Before testing the mice were moved to the behavior room, weighed, and left to acclimate for 1 hour. Mice were then treated with an acute injection of saline or varenicline (N = 5/group; 1 or 2 mg/kg; Kamens et al., 2010b) and were placed directly into a holding cage where they remained for 30 minutes. Mice were then challenged with 4 g/kg ethanol and placed back into the holding cage. At 30, 60, 120, and 180 minutes following the ethanol injection a 10 μL blood sample was obtained from the tail vein. The primary dependent variable was blood ethanol concentrations (BEC) as measured by an enzymatic assay (Ehringer et al., 2009; Kamens et al., 2012).
2.7 Statistical Analysis
Data from the DID and metabolism experiments were analyzed with repeated measures analysis of variance (ANOVA). Data from balance beam and LORR experiments were analyzed with a factorial ANOVA. Independent factors included sex, varenicline dose, ethanol concentration, and saccharin concentration where appropriate. Significant effects were examined further with Tukey's post hoc test. α < 0.05 was considered significant.
3. Results
3.1 DID
Varenicline decreased ethanol consumption in adolescent C57BL/6J mice (Fig 1A). All analyses were performed with sex included as a factor. In no case did sex interact with varenicline dose suggesting that males and females responded similarly to treatment. Main effects of sex are reported where appropriate. At the 30 minute time point, the group of male and female mice that received the 2 mg/kg varenicline dose consumed significantly less ethanol than the group treated with saline (main effect of varenicline dose: F3, 138=5.5, p<0.01). There were no significant effects or interactions observed at the 60 or 120 minute time points. Graphs of ethanol and saccharin consumption split by sex can be found in Supplementary Fig 1.
Fig 1. Varenicline decreased binge-like ethanol consumption and saccharin consumption in adolescent male and female C57BL/6J mice.
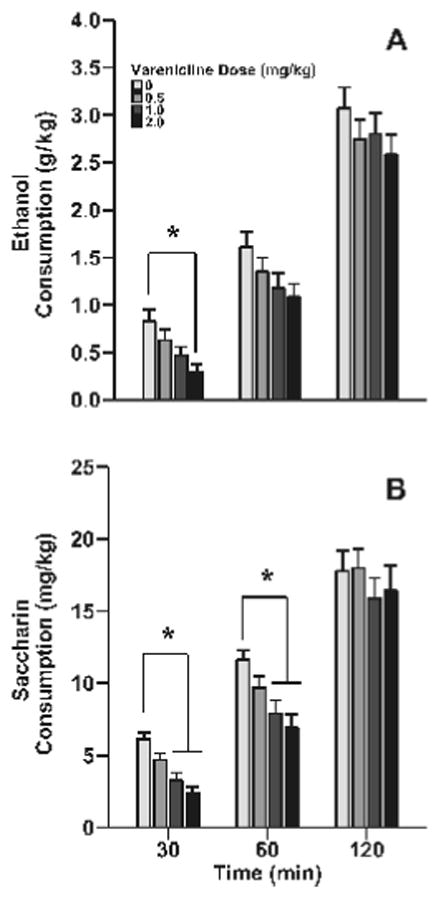
Data (mean ± SEM) represent ethanol consumption (A) and saccharin consumption (B). There was a main effect of sex on saccharin consumption such that females consumed more saccharin that males (F1, 22=9.3, p<0.01; 20.0 ± 2.4, 12.9 ± 2.0, respectively), but this did not interact with treatment, thus the data for both sexes are shown combined. N = 12 animal per dose for saccharin consumption and 24 animals per dose for ethanol consumption. Asterisks, p < 0.05 from the control group.
To ensure that the decrease in ethanol consumption was specific to this drug, we examine the effect of varenicline on saccharin consumption in the DID model. Varenicline significantly reduced saccharin consumption in adolescent male and female C57BL/6J mice (Fig 1B). At both the 30 and 60 minute time points there was a significant main effect of varenicline dose (F3, 66=15.2, p<0.001 and F3, 66=6.9, p<0.001, respectively); here both the 1 and 2 mg/kg doses of varenicline significantly reduced saccharin consumption compared to the saline treated animals (p's < 0.05). By 120 minutes there was no longer a significant main effect or interaction with varenicline dose. At 120 minutes there was, however, a significant main effect of sex (F1, 22=9.3, p<0.01) where female mice consumed more saccharin than males (20.0 ± 2.4, 12.9 ± 2.0, respectively).
3.2 Balance Beam
Varenicline had no effect on ethanol-induced ataxia measured on the balance beam (Fig 2). Treatment with varenicline had no effect on baseline ataxia (sal: 0.9 ± 0.3, 0.5 mg/kg: 0.9 ± 0.3, 1 mg/kg: 1.4 ± 0.5, 2 mg/kg: 1.3 ± 0.3 hindpaw slips), thus data were analyzed as a corrected score (Kamens et al., 2010b). There was a significant effect of varenicline dose on ethanol-induced ataxia (F3, 58=5.0, p<0.005) such that the animals that received 2 mg/kg varenicline displayed more ethanol-induced ataxia compared to animals that received either the 0.5 or 1 mg/kg dose of varenicline. Importantly, there were no significant differences between the animals that received a saline pretreatment compared to those that received a varenicline pretreatment.
Fig 2. Varenicline does not modulate ethanol-induced ataxia in adolescent male and female C57BL/6J mice.
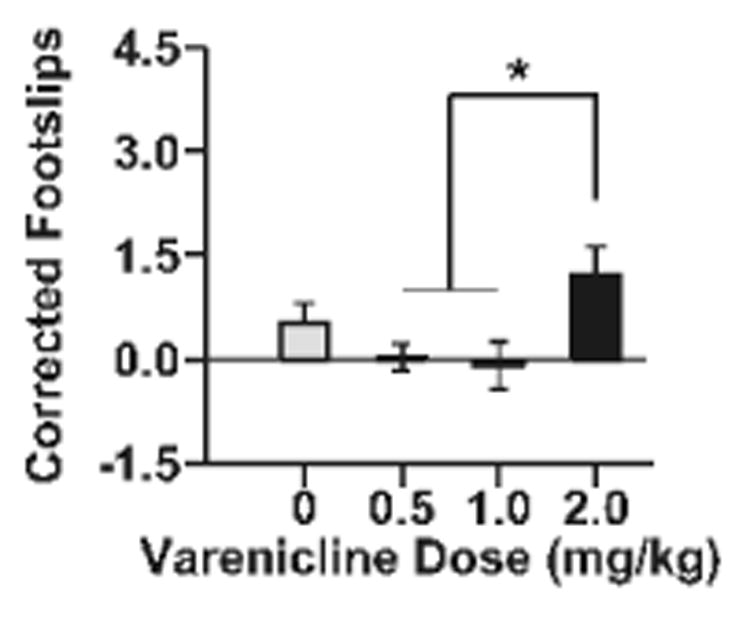
Data (mean ± SEM) represent corrected footslips (ethanol slips – baseline slips). N = 13 - 17 animals per dose. Asterisks, p < 0.05 between groups.
3.3 LORR
Varenicline had no effect on the sedative effects of ethanol in adolescent C57BL/6J mice (Fig 3). Male and female adolescent C57BL/6J mice were tested for LORR. For the dependent variable time to LORR there were no significant effects of sex, varenicline dose, or the interaction (Fig. 3A). For duration of LORR, there was a significant main effect of sex (F1, 71=4.1, p<0.05). Female mice were more sensitive to the sedative effects of ethanol having a longer duration of LORR compared to male mice (75.1 ± 4.2, 62.6 ± 3.9, respectively). There was no significant effect of varenicline dose or the interaction of these factors (Fig. 3B).
Fig 3. Varenicline had no effect on the sedative-hypnotic effects of ethanol in adolescent male and female mice.
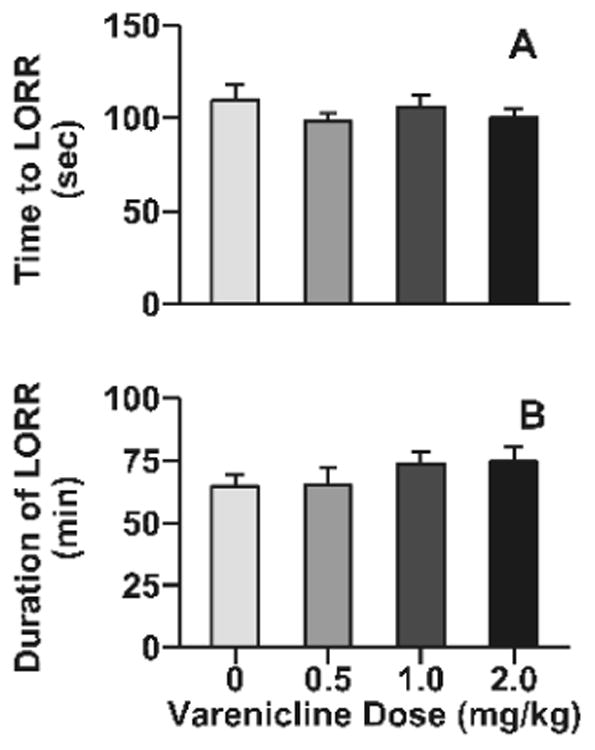
Data (mean ± SEM) represent time to LORR (A) and duration of LORR (B). For duration of LORR, there was a significant main effect of sex such that females were more sensitive to the sedative effects of ethanol compared to males (F1, 71=4.1, p<0.05; 75.1 ± 4.2, 62.6 ± 3.9, respectively), but since this did not interact with varenicline treatment the data presented combined. N = 17 – 19 animals per dose.
3.4 Metabolism
Varenicline had no effect on ethanol metabolism in adolescent male C57BL/6J mice (Fig 4). A repeated measures ANOVA was used to evaluate the effect of varenicline on ethanol metabolism. Only a significant main effect of time was observed (F3, 66=4.2, p<0.01). As expected, BECs decreased over the course of the experiment (p's < 0.05).
Fig 4. Varenicline does not alter ethanol metabolism in adolescent male mice.
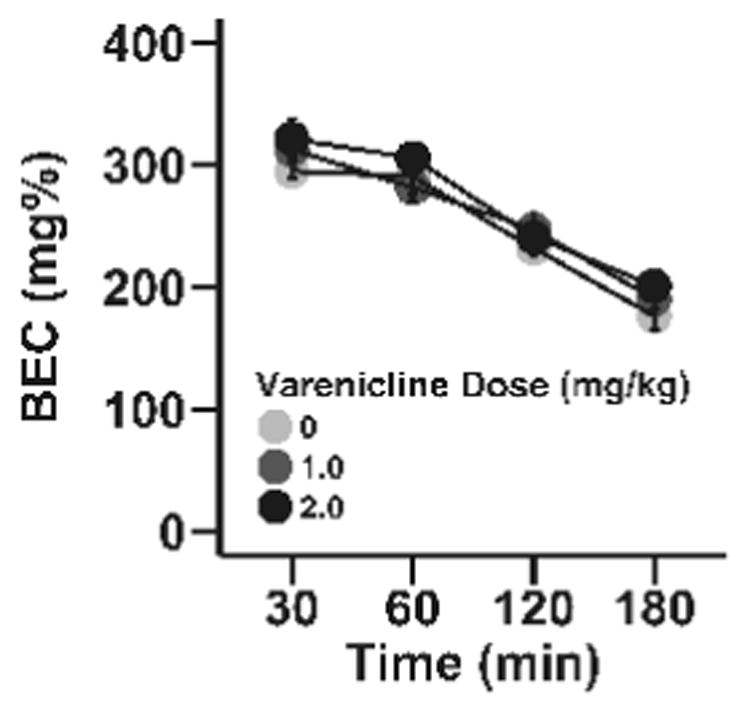
Data (mean ± SEM) represent blood ethanol concentrations (BEC) in male mice. N = 5 animals per dose.
4. Discussion
Previous studies have shown that varenicline, an α4β2 partial agonist, reduces ethanol consumption in adult humans (McKee et al., 2009), mice (Hendrickson et al., 2010; Kamens et al., 2010a), and rats (Feduccia et al., 2014; Steensland et al., 2007), and increases ethanol-induced ataxia and sedation (Fucito et al., 2011; Kamens et al., 2010b). Here we show that varenicline decreases ethanol and saccharin consumption in adolescent C57BL/6J mice, without enhancing ethanol-induced ataxia or sedative effects. These findings support the known role of nAChRs in the reduction of ethanol consumption. However, these results suggest that differences in the effect of varenicline on ethanol's aversive effects (i.e. ethanol-induced ataxia and sedation) may be related to age. The differences in behavior observed between adult and adolescent mice may be associated with normative development of the nAChR system that includes changes in the expression and function of these receptors throughout life.
The current study shows that a high dose (2 mg/kg) of varenicline decreases ethanol consumption in adolescent C57BL/6J mice, with no significant effect on ethanol metabolism. The results of this study are in line with a number of other studies indicating that varenicline can decrease ethanol consumption (Hendrickson et al., 2010; Kamens et al., 2010a; Steensland et al., 2007). In the current study, we found that varenicline significantly reduced ethanol consumption within 1 hour of treatment (30 minute pre-treatment time plus 30 minute ethanol consumption). While there also appears to be a decrease at the 60 minute time point (Figure 1), this did not reach statistical significance. Prior work in adult C57BL/6J mice has shown that varenicline reduces ethanol consumption up to 120-180 minutes after drug administration (Hendrickson et al., 2010; Kamens et al., 2010a). We know of no data that has investigated pharmacokinetic differences in varenicline across ages. What is known is that adolescent animals, in general, metabolize drugs faster than adult animals (Spear, 2007), which may contribute to the decreased duration of action in adolescent animals. Alternatively, procedural variation between studies may account for the observed differences.
Work on the role varenicline in ethanol consumption began with the recognition that there may be common genetic and neurobiological mechanisms that mediate ethanol and nicotine use. Varenicline decreases both ethanol consumption and nicotine self-administration (Coe et al., 2005; Kamens et al., 2010a; Rollema et al., 2007; Steensland et al., 2007) in adult animals, suggesting a common nAChR mechanism may underlie these behaviors. Although varenicline decreases intake of both drugs, the doses of varenicline required to influence ethanol consumption are higher than the doses that reduce nicotine intake (Coe et al., 2005; Kamens et al., 2010a; Rollema et al., 2007; Steensland et al., 2007) . While varenicline is a high affinity α4β2 partial agonist (Coe et al., 2005; Rollema et al., 2007), it is also known to interact with other nicotinic receptors (α3β4, α6β2, and α7) at high concentrations (Bordia et al., 2012; Mihalak et al., 2006) and it is possible that multiple nAChRs may mediate some of its actions.
To determine the nAChRs that underlie varenicline's reduction of ethanol consumption, researchers turned to genetically modified animals. Deletion of the α7, β2, and β4 nAChR subunits did not block the effect of varenicline on ethanol consumption suggesting that these receptor subunits are not involved (Kamens et al., 2010a; Kamens et al., 2017b; Patkar et al., 2016). In contrast, work in genetically modified animals that both increased sensitivity of the α4 subunit and knocked it out demonstrated that this subunit was necessary and sufficient for varenicline-induced reduction of ethanol consumption in adult mice (Hendrickson et al., 2011). Importantly, previous studies have reported no major changes in α4 mRNA expression throughout development in the ventral tegmental area (Azam et al., 2007), a region shown to mediate this effect (Hendrickson et al., 2010). Hence, α4-containing nicotinic receptors may be in involved in ethanol consumption throughout life, but further research would be required to test this hypothesis.
In contrast to the results observed with ethanol consumption, work examining the effect of varenicline on the consumption of sweet solutions has yielded mixed results. The current results showed that the 1 and 2 mg/kg doses of varenicline significantly reduced saccharin consumption. To ensure that reductions in ethanol intake are specific, researchers have examined the impact of varenicline on consumption of the sweet non-caloric solution, saccharin, or sweet caloric solution, sucrose. Most prior research in adult animals has reported no significant change in saccharin/sucrose consumption when pretreated with varenicline (Hendrickson et al., 2010; Kamens et al., 2010a; Steensland et al., 2007). However, increased sucrose self-administration (Wouda et al., 2011) and decreased saccharin/sucrose consumption have also been observed (Shariff et al., 2016). Indeed, there are many differences that may contribute to the inconsistent results. These include the duration of exposure, varenicline dose, and the animal paradigm used. Results in adult C57BL/6J mice has provided little evidence that varenicline can alter consumption of sweet solutions, but this is in contrast to rat models showing increased consumption, decreased consumption, or no change. These inconsistent results across species are important to take into account and suggest that it is possible that there could be differences between species in the involvement of nicotinic receptors in consumption of sweet solutions.
In the current work, we focused on adolescent animals. To our knowledge, we know of no other work that has examined the influence of varenicline on saccharin or sucrose administration during this developmental window. Our work suggests that, in adolescence, varenicline has a non-specific effect on consumption because it reduces saccharin consumption in addition to ethanol. We recently reported similar findings with a α6β2 antagonist, N,N-decane-1,10-diyl-bis-3-picolinium diiodide (bPiDI) in adolescent mice (Kamens et al., 2017a). Specifically, we observed decreased ethanol and saccharin consumption providing evidence that α6β2 nAChRs may drive this effect in adolescent animals. Future work using genetically modified α6 animals would be required to test this hypothesis.
In the current study, we found no effect of varenicline on ethanol-induced ataxia in adolescent C57BL/6J mice. While the 2 mg/kg dose of varenicline increased ataxia compared to lower varenicline doses, there was no significant difference compared to saline. These results are in contrast to our early work in adult C57BL/6J animals. In adult animals, we found that varenicline increased ethanol-induced ataxia using the same protocol employed here (Kamens et al., 2010b). Prior research from other groups has implicated cerebellar α4β2 and α7 nAChRs as mediators of ethanol-induced ataxia. In this work, intracerebellar administration of an α4β2 or α7 agonist decreased ethanol-induced ataxia (Taslim et al., 2008; Taslim and Saeed Dar, 2011). We are unaware of any studies on developmental changes in cerebellar nAChRs that may account for these differences.
In this study, no effect of varenicline was observed on ethanol-induced sedation. We have previously reported that varenicline increases ethanol-induced sedation in adult mice (Kamens et al., 2010b) and human clinical studies have reported enhanced ratings of alcohol sedation (Fucito et al., 2011). Research in knockout animal models has provided evidence that a number of nicotinic receptor subunits are involved in the sedative effects of ethanol – these include α5, α6, and α7 (Bowers et al., 2005; Kamens et al., 2012; Santos et al., 2013), but not β4 (Kamens et al., 2017b). It is possible that changes in expression and function of nAChRs throughout development (Azam et al., 2007; Doura et al., 2008; Kota et al., 2007) underlie the differences observed between adolescent and adult animals.
Alcohol use is known to start during adolescence. The work presented here suggests that the involvement of nicotinic receptors in ethanol behaviors may be dependent on the age at which the animal is tested. Further work is required to more fully characterize the role of nAChRs in ethanol behaviors and how these change throughout development. Furthermore, future work on the mechanisms through which varenicline is reducing ethanol consumption may be warranted. For example, a sensitive measure like a lick-o-meter system could be used to determine if varenicline alters how animals approach and/or consume ethanol.
Supplementary Material
Highlights.
Varenicline decreases ethanol consumption in adolescent mice
Varenicline decreases saccharin consumption in adolescent mice
Varenicline does not influence ethanol ataxia, sedation, or metabolism in adolescent mice
Effects of varenicline on ethanol behaviors appears to be age specific
Acknowledgments
The authors would like to thank Ms. Nina Riser and Mr. Alex Gechlik for technical help with this project. Varenicline was graciously provided by Pfizer. This work was supported by the National Institutes of Health [AA019447], the Pennsylvania State University College of Health and Human Development, and the Broadhurst Career Development Professorship for the Study of Health Promotion and Disease Prevention. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Hrachova M, Chin M, McIntosh JM, Quik M. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012;342:327–334. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res. 2005;29:295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O'Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Coon H, Piasecki TM, Cook EH, Dunn D, Mermelstein RJ, Weiss RB, Cannon DS. Association of the CHRNA4 Neuronal Nicotinic Receptor Subunit Gene with Frequency of Binge Drinking in Young Adults. Alcohol Clin Exp Res. 2014;38:930–937. doi: 10.1111/acer.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Ponomarev I, Prescott CA, Wahlsten D. Effects of genetic and procedural variation on measurement of alcohol sensitivity in mouse inbred strains. Behav Genet. 2006;36:536–552. doi: 10.1007/s10519-006-9067-6. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Yu CH, Schlumbohm JP, Cameron AJ, Wahlsten D. Genotypic differences in ethanol sensitivity in two tests of motor incoordination. J Appl Physiol Bethesda Md 1985. 2003;95:1338–1351. doi: 10.1152/japplphysiol.00132.2003. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health J Natl Inst Alcohol Abuse Alcohol. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Miles MF, Damaj MI. The β2 nicotinic acetylcholine receptor subunit differentially influences ethanol behavioral effects in the mouse. Alcohol Fayettev N. 2013;47:85–94. doi: 10.1016/j.alcohol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehringer MA, Clegg HV, Collins AC, Corley RP, Crowley T, Hewitt JK, Hopfer CJ, Krauter K, Lessem J, Rhee SH, Schlaepfer I, Smolen A, Stallings MC, Young SE, Zeiger JS. Association of the neuronal nicotinic receptor beta2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. 2007;144B:596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol Fayettev N. 2009;43:443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Simms JA, Mill D, Yi HY, Bartlett SE. Varenicline decreases ethanol intake and increases dopamine release via neuronal nicotinic acetylcholine receptors in the nucleus accumbens. Br J Pharmacol. 2014 doi: 10.1111/bph.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubner NR, McKinnon CS, Phillips TJ. Effects of varenicline on ethanol-induced conditioned place preference, locomotor stimulation, and sensitization. Alcohol Clin Exp Res. 2014;38:3033–3042. doi: 10.1111/acer.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Gardner PD, Tapper AR. Nicotinic acetylcholine receptors containing the α4 subunit are critical for the nicotine-induced reduction of acute voluntary ethanol consumption. Channels. 2011;5:124–127. doi: 10.4161/chan.5.2.14409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci Off J Soc Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology (Berl) 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston Lloyd D, O'Malley Patrick M, Bachman Jerald G, Schulenberg John E. Monitoring the Future National Results on Drug Use: 2012 Overview, Key Findings on Adolescent Drug Use. Institute for Social Research, University of Michigan; Ann Arbor: 2013. [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010a;208:613–626. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. The nicotinic acetylcholine receptor partial agonist varenicline increases the ataxic and sedative-hypnotic effects of acute ethanol administration in C57BL/6J mice. Alcohol Clin Exp Res. 2010b;34:2053–2060. doi: 10.1111/j.1530-0277.2010.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Ethanol-related traits in mice selectively bred for differential sensitivity to methamphetamine-induced activation. Behav Neurosci. 2006;120:1356–1366. doi: 10.1037/0735-7044.120.6.1356. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Hoft NR, Cox RJ, Miyamoto JH, Ehringer MA. The α6 nicotinic acetylcholine receptor subunit influences ethanol-induced sedation. Alcohol Fayettev N. 2012;46:463–471. doi: 10.1016/j.alcohol.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, McKinnon CS, Li N, Helms ML, Belknap JK, Phillips TJ. The alpha 3 subunit gene of the nicotinic acetylcholine receptor is a candidate gene for ethanol stimulation. Genes Brain Behav. 2009;8:600–609. doi: 10.1111/j.1601-183X.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Peck C, Garrity C, Gechlik A, Jenkins BC, Rajan A. α6β2 Nicotinic Acetylcholine Receptors Influence Locomotor Activity and Ethanol Consumption. Alcohol. 2017a;61:43–49. doi: 10.1016/j.alcohol.2017.02.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Silva C, McCarthy R, Cox RJ, Ehringer MA. No evidence of a role of the β4 subunit of the nicotinic acetylcholine receptor in alcohol-related behaviors. BMC Res Notes. 2017b;10:151. doi: 10.1186/s13104-017-2470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine Dependence and Reward Differ between Adolescent and Adult Male Mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616.initiate. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., 2nd Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubke GH, Stephens SH, Lessem JM, Hewitt JK, Ehringer MA. The CHRNA5/A3/B4 gene cluster and tobacco, alcohol, cannabis, inhalants and other substance use initiation: replication and new findings using mixture analyses. Behav Genet. 2012;42:636–646. doi: 10.1007/s10519-012-9529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EJ. Adolescent alcohol use: risks and consequences. Alcohol Alcohol Oxf Oxfs. 2014;49:160–164. doi: 10.1093/alcalc/agt180. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Patkar OL, Belmer A, Tarren JR, Holgate JY, Bartlett SE. The effect of varenicline on bingelike ethanol consumption in mice is β4 nicotinic acetylcholine receptor-independent. Neurosci Lett. 2016;633:235–239. doi: 10.1016/j.neulet.2016.09.048. [DOI] [PubMed] [Google Scholar]
- Powers MS, Broderick HJ, Drenan RM, Chester JA. Nicotinic acetylcholine receptors containing α6 subunits contribute to alcohol reward-related behaviours. Genes Brain Behav. 2013;12:543–553. doi: 10.1111/gbb.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Sajja RK, Rahman S. Nicotinic receptor partial agonists modulate alcohol deprivation effect in C57BL/6J mice. Pharmacol Biochem Behav. 2013;110:161–167. doi: 10.1016/j.pbb.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Santos N, Chatterjee S, Henry A, Holgate J, Bartlett SE. The α5 neuronal nicotinic acetylcholine receptor subunit plays an important role in the sedative effects of ethanol but does not modulate consumption in mice. Alcohol Clin Exp Res. 2013;37:655–662. doi: 10.1111/acer.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff M, Quik M, Holgate J, Morgan M, Patkar OL, Tam V, Belmer A, Bartlett SE. Neuronal Nicotinic Acetylcholine Receptor Modulators Reduce Sugar Intake. PloS One. 2016;11:e0150270. doi: 10.1371/journal.pone.0150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent Neurodevelopment. J Adolesc Health, Emerging Issues in Adolescent Health. 2013;52:S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Assessment of Adolescent Neurotoxicity: Rationale and Methodological Considerations. Neurotoxicol Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H-38A. Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. HHS Publication No. SMA 10-4586 Findings. [Google Scholar]
- Taslim N, Al-Rejaie S, Saeed Dar M. Attenuation of ethanol-induced ataxia by alpha(4)beta(2) nicotinic acetylcholine receptor subtype in mouse cerebellum: a functional interaction. Neuroscience. 2008;157:204–213. doi: 10.1016/j.neuroscience.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Taslim N, Saeed Dar M. The role of nicotinic acetylcholine receptor (nAChR) α7 subtype in the functional interaction between nicotine and ethanol in mouse cerebellum. Alcohol Clin Exp Res. 2011;35:540–549. doi: 10.1111/j.1530-0277.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer ANM, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


