Abstract
Objective
To determine if changes in PET avidity correlated with histologic response, and were independently associated with outcome.
Background
The implications of metabolic response to neoadjuvant therapy as measured by repeat PET imaging remains ill-defined for patients with gastric and gastroesophageal junction (GEJ) cancers.
Methods
We identified patients with gastric and gastroesophageal junction (GEJ) adenocarcinoma who were evaluated with PET imaging prior to and following neoadjuvant treatment, and subsequently underwent curative resections. Spearman rank correlation and Cox proportional hazards regression were used evaluate standardized uptake value (SUV) and histologic response, pathologic parameters, and disease-specific survival (DSS).
Results
From 2002 to 2013, 192 patients met our inclusion criteria. The median SUVmax response was 57.3% (range: −110 – 100%) for patients with GEJ cancers, with a corresponding median pathologic treatment response of 80% (range: 0–100%). The median SUVmax response was 32.5% (−230 – 100%) for patients with gastric cancers, with a corresponding median pathologic treatment response of 35% (range: 0 – 100%). The Spearman correlation between SUVmax response and histologic response was significant for patients with GEJ (rho=0.19, p=0.04) and gastric (rho=0.44, p<0.0001) cancers. For patients with GEJ (p<0.0001–0.046) and gastric cancers (p=0.0003–0.016), histopathologic response and tumor staging predicted DSS. SUVmax response failed to demonstrate a relationship with DSS when entered into multivariable models containing conventional pathologic variables.
Conclusions
Following completion of neoadjuvant therapy for gastric and GEJ adenocarcinoma, histopathologic staging remains the best predictor of outcome. Repeat post-treatment/pre-operative PET imaging for the purpose of prognostication is of limited value.
Introduction
18F-FDG PET/CT imaging has historically been used to improve staging and identify occult metastatic disease for patients with gastrointestinal malignancies.[1] More recently 18F-FDG PET/CT imaging has been used to deliver actionable information regarding tumor response to therapy. For gastric and gastroesophageal junction (GEJ) cancers, the increasing incorporation of neoadjuvant chemotherapy/chemoradiotherapy into the treatment plans of patients with clinically locally advanced disease has paved the way for repeat 18F-FDG PET/CT imaging to assess in vivo metabolic responses to therapy, as measured by changes in PET avidity, prior to definitive resection and pathologic evaluation. In 2007, the MUNICON II trial documented the utility of early repeat 18F-FDG PET/CT imaging in defining responders to treatment (>35% decrease in SUV uptake), and established this to be predictive of major histologic response and prognostic of survival.[2] More importantly, the MUNCON trial identified PET non-responders to have a very poor prognosis. The Alliance for Clinical Trials in Oncology group is currently seeking to randomize PET non-responders to either continuation or to changing neoadjuvant therapy prior to surgical resection, to see if outcome in this very high-risk cohort can be improved.
Repeat 18F-FDG PET/CT imaging has been suggested be prognostic in patients with GEJ and gastric cancer. Numerous studies have established an association between alterations in PET avidity and patient outcomes[3–7]. However, alterations in PET avidity following completion of neoadjuvant therapy can only be useful if the information is superior or supplemental to the forthcoming histopathologic evaluation of the resected tumor. No study to date has considered SUV response in the context of other known pathologic predictors of outcome, which will be imminently available to all treating physicians when repeat PET imaging is obtained after completion of neoadjuvant therapy and just prior to resection. We therefore sought to evaluate the prognostic utility of repeat pre-operative 18F-FDG PET/CT imaging in patients with GEJ and gastric adenocarcinoma. Our aims were to correlate changes in SUVmax with histopathologic response, to determine if changes in SUVmax after neoadjuvant therapy predicted outcomes, and to evaluate the ability of changes in SUVmax response to predict outcomes in the context of histopathologic data.
Methods
Patients
We retrospectively reviewed our prospectively maintained database of all patients admitted to MSKCC for gastric and GEJ (Siewert II & III) adenocarcinoma from January 2002 until December 2013. Patients imaged prior to 2002 were not included in the study because of alterations in PET imaging technology, which complicates comparisons. Patients receiving neoadjuvant therapy (chemotherapy or chemotherapy and radiation therapy) who were evaluated with both pre-treatment and subsequently post-treatment preoperative 18F-FDG PET/CT imaging, and who subsequently underwent resection with curative intent were identified. PET scans were obtained as a component of routine clinical care, or, in some patients, in the context of a prospective clinical trial. Patient, treatment (chemotherapy and radiotherapy), and histopathologic variables were extracted. Date and status at last follow-up were recorded. Patients were excluded if there was any evidence of M1 disease during staging.
PET Evaluation
PET/CT images were retrieved from our picture archiving and communication system (PACS) and analyzed using AW Software (GE Healthcare). All PET/CT images were reviewed (by two radiologists, VB & HS) for hypermetabolic and non-hypermetabolic lesions using transaxial, coronal, and sagittal planes, as well as maximum intensity projection (MIP) images. Using a volumetric region of interest (ROI), SUVmax for the primary lesion and any metastatic foci were obtained. SUVmax for the gastric fundus and SUV mean values for liver were also calculated for normalization. Care was taken not to include any physiologic uptake or adjacent organs in the ROI. Uptake time (defined as time elapsed from injection of radiopharmaceutical to commencement of image acquisition) was noted for each time point and outliers (>20 min difference in post-injection time) were excluded from the study. Foreign PET/CT scans were also imported to our AW workstation and analyzed similarly to local PET/CT scans. Patients with foreign PET/CT scans with image quality issues, unavailable critical parameters (e.g. time of injection) or problems with SUV calculations were excluded from the study (Supp. Figure 1). Of note, all demographic data on patients including treatments, endoscopy and biopsy results were available to the readers (VB and HS) at the time of image assessment. SUVmax was recorded for all available PET scans. The baseline scan and the pre-operative scan were used to determine the percentage change, which is reported as SUVmax response.
Statistics
All analyses were performed stratified by site (GEJ and Gastric). The stratification of gastric and GE junction patients was performed to account for their different pre-surgery treatment regimens. Nearly all patients with gastric cancers received chemotherapy alone, while nearly all patients with GEJ cancers received both chemotherapy and radiation therapy. We postulated that this difference might be associated with a difference in SUV response in the primary tumor, histologic response in the primary tumor, and outcome, and therefore analyzed these cohorts of patients separately.
Descriptive statistics were calculated for clinical, histopathologic and outcome variables. Medians and ranges were used for continuous variables, while frequencies and percent change were used to summarize categorical variables. Pathology stage was grouped as follows: stage 0–I, stage II, and stage III. T stage was grouped into T0–T1b vs. T2, vs. T3–T4b. For descriptive analyses, tumor type was grouped into diffuse versus other, and WHO type was grouped as signet ring versus other. Histologic tumor response was dichotomized by its empirical median value separately for each cohort. The SUVmax response was correlated with histologic tumor response using Spearman’s rank correlation and visualized using scatter plots. For outcome analyses, SUVmax was examined in five ways: 1) raw SUVmax at baseline, 2) raw SUVmax pre-surgery, 3) SUVmax response (percent change), 4) SUVmax dichotomized by its empirical median, and 5) SUVmax dichotomized by previously established cut off of 35%.[2]
Kaplan Meier methods were used to model the relationship between dichotomized histologic tumor response, and SUVmax response with DSS for each site. Univariate Cox proportional hazards regression was used to assess the relationship between imaging and pathologic parameters, and disease-specific survival (DSS). DSS was defined as the time between resection and death, or date of recurrence for those patients alive with recurrence at the time of last follow up; patients alive and recurrence-free were censored at last follow up. The interaction between PET response and histologic tumor response was assessed for each cohort using Cox models. We examined the relationship between the change in SUVmax and histologic tumor response in bivariate Cox analysis. We also examined the univariate relationship between site and DSS. In each cohort, multivariate models were built for PET response variables controlling for clinical and pathologic factors. Due to sample size constraints, clinical and pathologic variables significant at p<0.05 were entered into a backwards selection model (stay criteria=0.10). Due to the inherent dependence of overall stage group on T stage and N stage, we only included T stage and N stage in the backward selection. Variables remaining after selection were included with PET/CT imaging parameters. P-values below 0.05 were considered statistically significant. All analyses were performed using SAS 9.2.
Results
We identified 373 patients with GEJ and gastric cancers who received neoadjuvant therapy for locally advanced (≥T3 or N1+) cancers, had pre-treatment and pre-operative PET imaging and underwent curative resections at our institution during the study period. One hundred and ninety-two patients, 120 with GEJ cancers and 72 with gastric cancers met our eligibility criteria and were included in the study. Patients had a median age of 63 years (range: 24–84 years), and 73% (140/192) were men. Patient demographic and histopathologic data are reported in Table 1, stratified by tumor location (GEJ vs. Gastric). Neoadjuvant chemotherapeutic regimens for patients with GEJ cancers consisted of cisplatin and irinotecan, while patients with gastric cancers were treated with epirubicin, cisplatin or oxaliplatin and 5FU. PET scans were performed as a component of routine clinical care in 171 patients, and in the context of a prospective clinical trial in 21 patients. Median follow-up time for patients alive and disease-free at last evaluation was 36 months (range: 2.6–133.8 months).
Table 1.
Demographic and Histopathologic Data
| GEJ (n=120) | Gastric (n=72) | |
|---|---|---|
| Median age in years (range) | 63 (24–84) | 64 (34–83) |
|
| ||
| Gender | ||
| Male | 96 (80%) | 44 (61%) |
| Female | 24 (20%) | 28 (39%) |
|
| ||
| Race | ||
| White | 112 (93%) | 51 (71%) |
| Other | 7 (6%) | 19 (26%) |
| Unknown | 1 (1%) | 2 (3%) |
|
| ||
| Overall Survival | ||
| Alive | 62 (52%) | 46 (64%) |
| Deceased | 58 (48%) | 26 (36%) |
|
| ||
| Recurrence | ||
| No recurrence (NED & DOC) | 63 (53%) | 47 (65%) |
| Recurrence (DOD & AWD) | 57 (47%) | 25 (35%) |
|
| ||
| Preop EUS Stage Group | ||
| T1–2 N0* | 5 (4%) | 1 (1%) |
| T3–4 N0* | 22 (18%) | 15 (21%) |
| Tany N+ | 71 (59%) | 24 (33%) |
| Unknown/EUS not done | 22 (18%) | 32 (44%) |
| Preop EUS T Stage | 2 (2%) | 0 (0) |
| 0-T1b | 7 (6%) | 11 (6%) |
| T2 | 89 (74%) | 36 (50%) |
| T3–4b | 22 (18%) | 32 (44%) |
| Unknown/EUS not done | 24 (20%) | 14 (19%) |
| Preop EUS N Stage | 71 (59%) | 24 (33%) |
| N0 | 25 (21%) | 34 (47%) |
| N+ | ||
| Unknown/EUS not done | ||
|
| ||
| Path Stage Group | ||
| 0–I | 36 (30%) | 21 (29%) |
| II | 37 (31%) | 26 (36%) |
| III | 47 (39%) | 25 (35%) |
|
| ||
| Path T Stage | ||
| T0-T1b | 33 (27%) | 19 (26%) |
| T2 | 27 (23%) | 11 (15%) |
| T3-T4b | 60 (50.0%) | 42 (59%) |
|
| ||
| Path N Stage | ||
| N0 | 65 (54%) | 31 (43%) |
| N1+ | 55 (46%) | 41 (57%) |
|
| ||
| Grade | ||
| Well/Mod | 63 (52%) | 19 (26%) |
| Poor | 56 (47%) | 53 (74%) |
| NA | 1 (1%) | 0 (0%) |
|
| ||
| LVI | ||
| No | 83 (69%) | 32 (44%) |
| Yes | 37 (31%) | 40 (56%) |
|
| ||
| Subtypes | ||
| Diffuse | 17 (14%) | 24 (33%) |
| Signet Ring | 14 (12%) | 22 (30%) |
SUVmax response and Histologic tumor response
GEJ: The median SUVmax on the baseline and pre-operative 18F-FDG PET/CT images for patients with GEJ cancers was 10.2 (range: 2 – 34) and 4.1 (range: 0–26.1), respectively. The median number of days between 18F-FDG PET/CT imaging was 119 days. The median SUVmax response was 57.3% (range: −110 −100%) for patients with GEJ cancers, with a corresponding median histologic tumor response of 80% (range: 0 – 100%). The Spearman correlation between SUVmax response and histologic tumor response was significant (rho=0.19, p=0.04) for patients with GEJ cancers (Sup. Fig 2A).
Gastric: The median SUVmax on the baseline and pre-operative 18F-FDG PET/CT images for patients with gastric cancers was 7.4 (range: 2.3 – 28.9) and 4.5 (range: 0 – 33.2), respectively. The median number of days between 18F-FDG PET/CT imaging was 85 days. The median SUVmax response was 32.5% (range: −230 – 100%), with a corresponding median histologic tumor response of 35% (range: 0 – 100%). The Spearman correlation between SUVmax response and histologic tumor response was significant (rho=0.44, p<0.0001) for patients with gastric cancers (Sup. Fig 2B).
Disease Specific Survival for GEJ Cancers
Histologic tumor response (continuous and dichotomized) was significantly associated with DSS (p=0.01, p=0.01 respectively) (Figure 1A). T stage (T3–4, vs. T0–1, p=0.01), N stage (p<0.0001), AJCC stage (III–IV vs. 0–I, p<0.0001), and LVI (p<0.0001) were also found to be significantly associated with DSS (Table 2). However, none of the SUVmax variables were significantly associated with DSS (p=0.46 – 0.88) (Table 2, Figure 1B).
Figure 1.
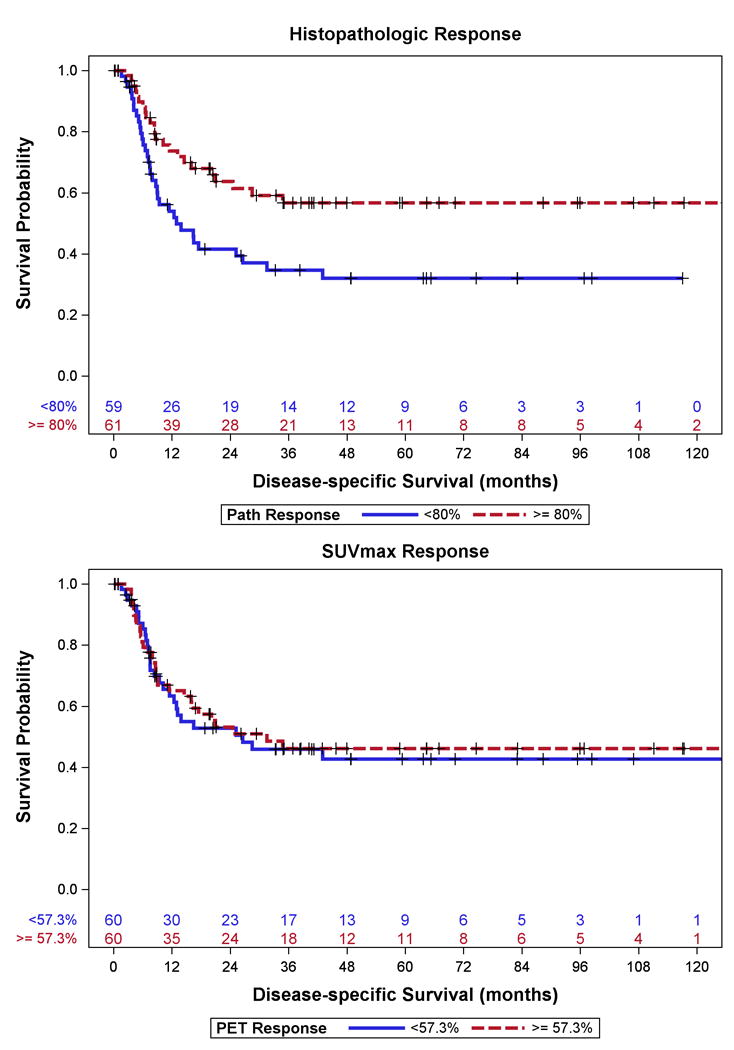
Disease–specific Survival for Patients with GEJ Cancer stratified by median (A) Histopathologic Response and (B) SUVmax Response
Table 2.
Univariate DSS for GEJ
| N (#events) | HR | [95% CI] | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Modality | Variable | Level | ||||
| PET | SUVmax (Baseline) | 120 (57) | 1.00 | [0.97 – 1.04] | 0.88 | |
| SUVmax (PreOp) | 120 (57) | 1.03 | [0.95 – 1.13] | 0.46 | ||
| PET Response (continuous) | 120 (57) | 1.00 | [1.00 – 1.01] | 0.54 | ||
| SUVmax Response (Median Cut) | < 57.3% | 60 (28) | 1.08 | [0.64 – 1.81] | 0.78 | |
| ≥ 57.3% | 60 (29) | REF | ||||
| SUVmax (Published Cut) | < 35% | 38 (18) | 1.08 | [0.62 – 1.89] | 0.78 | |
| ≥ 35% | 82 (39) | REF | ||||
|
| ||||||
| Pathology | T Stage | T0–T1b | 33 (9) | REF | ||
| T2 | 27 (15) | 2.14 | [0.94 – 4.90] | 0.07 | ||
| T3–T4 | 60 (33) | 2.44 | [1.17 – 5.11] | 0.018 | ||
| Overall Stage | 0–I | 36 (7) | REF | |||
| II | 37 (18) | 2.44 | [1.02 – 5.84] | 0.046 | ||
| III–IV | 47 (32) | 6.52 | [2.86 – 14.87] | <.0001 | ||
| N Stage | N0 | 65 (20) | REF | |||
| N1+ | 55 (37) | 3.84 | [2.21 – 6.67] | <.0001 | ||
| Pathologic Responseˆ | 120 (57) | 0.90 | [0.84 – 1.00] | 0.012 | ||
| < 80% | 59 (34) | 2.00 | [1.18 – 3.40] | .010 | ||
| ≥ 80% | 61 (23) | REF | ||||
| LVI | No | 83 (29) | REF | |||
| Yes | 37 (28) | 4.12 | [2.42 – 7.00] | <.0001 | ||
HR in increments of 10 units
Disease Specific Survival for Gastric Cancers
Histologic tumor response (continuous) was significantly associated with DSS (p=0.02) (Figure 2A). Similar to patients with GEJ cancers, T-stage (T3–T4 vs. T0–1, p=0.01), N-stage (p=0.0006), AJCC stage (III-IV vs. 0–I p=0.0003), and LVI (p=0.002) were significantly associated with DSS for patients with gastric cancers (Table 3). No SUVmax variable was found to be significantly associated with DSS (p=0.10 – 0.52) (Table 3, Figure 2B).
Figure 2.
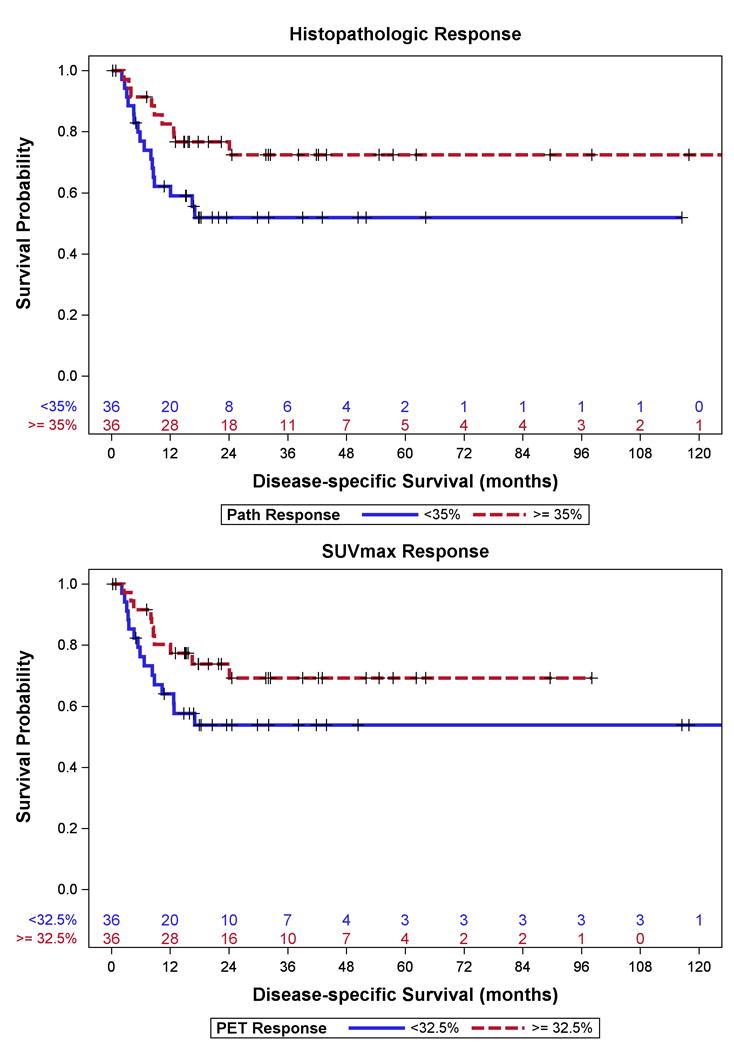
Disease Specific Survival for Patients with Gastric Cancer stratified by median (A) Histopathologic Response and (B) SUVmax Response
Table 3.
Univariate DSS for Gastric
| N (#events) | HR | [95% CI] | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Modality | Variable | Level | ||||
| PET | SUVmax (Baseline) | 72 (25) | 0.98 | [0.93 – 1.04] | 0.52 | |
| SUVmax (PreOp) | 72 (25) | 1.02 | [0.97 – 1.07] | 0.50 | ||
| PET Response (continuous) | 72 (25) | 1.00 | [0.99 – 1.00] | 0.14 | ||
| SUVmax Response (Median Cut) | < 32.5% | 36 (15) | 1.88 | [0.84–4.18] | 0.12 | |
| ≥ 32.5% | 36 (10) | REF | ||||
| SUVmax (Published Cut) | < 35% | 38 (16) | 1.99 | [0.88 – 4.52] | 0.10 | |
| ≥ 35% | 34 (9) | REF | ||||
|
| ||||||
| Pathology | T Stage | T0–T1b | 19 (2) | REF | ||
| T2 | 11 (1) | 0.77 | [0.07 – 8.52] | 0.83 | ||
| T3–T4 | 42 (22) | 6.69 | [1.57 – 28.50] | 0.010 | ||
| Overall Stage | 0–I | 21 (2) | REF | |||
| II | 26 (4) | 1.96 | [0.36 – 10.70] | 0.44 | ||
| III–IV | 25 (19) | 15.2 | [3.51 – 65.89] | 0.0003 | ||
| N Stage | N0 | 31 (3) | REF | |||
| N1+ | 41 (22) | 8.30 | [2.48 – 27.81] | 0.0006 | ||
| Pathologic Responseˆ | 72 (25) | 0.86 | [0.77 – 0.97] | 0.016 | ||
| < 35% | 36 (16) | 2.20 | [0.97 – 4.98] | 0.06 | ||
| ≥ 35% | 36 (9) | REF | ||||
| LVI | No | 32 (4) | REF | |||
| Yes | 40 (21) | 5.30 | [1.81 – 15.47] | 0.002 | ||
HR in increments of 10 units
Bivariate and Multivariable Analyses
In bivariate models with histologic tumor response and SUVmax response as predictors, histologic tumor response remained a significant predictor (p=0.007 and p=0.03, continuous), while SUVmax response failed to reach significance (p=0.28 and p=0.55, continuous) for patients with GEJ and gastric cancers, respectively. Figure 3 depicts histologic tumor response against changes in SUVmax response for each patient grouped by status at last follow-up. For illustrative purposes, only those with greater than two years of follow up were considered ANED. Of note, there was no significant relationship between disease site (GEJ vs. gastric) and DSS on univariate analysis (p=0.12).
Figure 3.
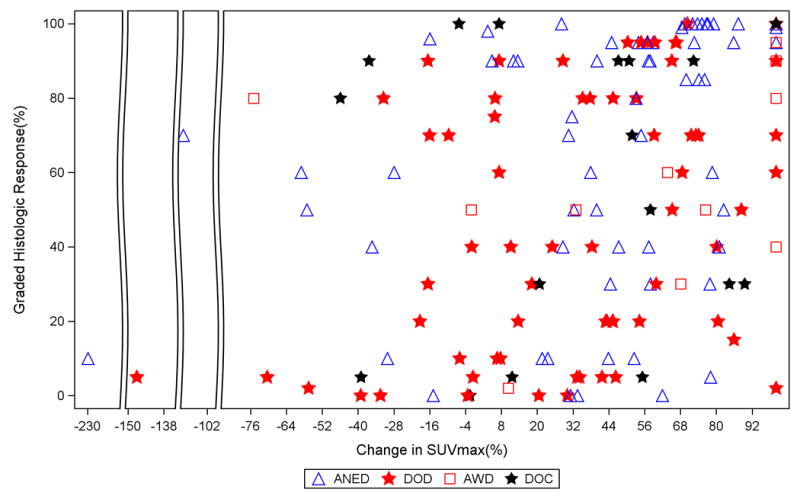
Histologic tumor response and SUVmax response by status at last follow up.
Baseline pathologic multivariable models were built for GEJ and gastric patients separately. T stage, N stage, LVI and histologic tumor response were entered into backwards selection models. After selection, only N stage (p=0.0005) and LVI (p=0.001) remained significant predictors of DSS for patients with GEJ cancers. After selection, only T stage (p=0.02) and N stage (p=0.002) remained significant predictors for patients with gastric cancers. SUVmax response was then entered into these baseline models, and failed to show any significant relationship with DSS for patients with GEJ (p=0.20) or gastric cancers (p=0.66) (Table 4).
Table 4.
Multivariate Analyses
| HR | [95% CI] | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| Model | Parameter | Level | |||
| GE Junction | LVI | No | REF | ||
| Baseline Pathologic Model | Yes | 2.78 | [1.57 – 4.93] | 0.0005 | |
| N Stage | N0 | REF | |||
| N1+ | 2.71 | [1.49 – 4.92] | 0.0010 | ||
|
| |||||
| Gastric | T Stage | T0–T1b | REF | ||
| Baseline Pathologic Model | T2 | 0.40 | [0.04 – 4.51] | 0.0217 | |
| T3–T4 | 3.87 | [0.89 – 16.89] | |||
| N Stage | N0 | REF | |||
| N1+ | 7.09 | [2.08 – 24.22] | 0.0018 | ||
|
| |||||
| GE Junction | SUVmax Response | 1.00 | [1.00 – 1.01] | 0.20 | |
| Pathologic Model + SUVmax Response | LVI | No | REF | ||
| Yes | 2.97 | [1.66 – 5.30] | 0.0002 | ||
| N Stage | N0 | REF | |||
| N1+ | 2.27 | [1.50 – 4.92] | 0.0010 | ||
|
| |||||
| Gastric | SUVmax Response | 1.00 | [0.99 – 1.01] | 0.66 | |
| Pathologic Model + SUVmax Response | T Stage | T0–T1b | REF | ||
| T2 | 0.40 | [0.04 – 4.51] | 0.0253 | ||
| T3–T4 | 3.87 | [0.89 – 16.89] | |||
| N Stage | N0 | REF | |||
| N1+ | 7.05 | [2.06 – 24.10] | 0.0019 | ||
Discussion
We have evaluated the utility of repeat pre-operative 18F-FDG PET/CT imaging in the context of histopathologic data by reviewing the PET/CT scans, pathology and outcomes for patients with GEJ and gastric adenocarcinoma treated at our institution since 2002. In doing so, our first aim was to correlate changes in SUVmax with histologic tumor response. Assessing response to neoadjuvant therapy can be difficult with conventional CT imaging or endoscopy.[8, 9] Metabolic change as seen on PET scan may be the most sensitive and quantitative method to determine response to treatment. Numerous studies investigating the predictive value of FDG-PET scanning after completion of preoperative therapy in patients with gastroesophageal cancers have identified a positive correlation with histologic response.[5–7, 10] Moreover, PET response criteria (PERCIST) have been proposed to standardize assessment of PET responses.[11] Components of the proposed PERCIST criteria include assessing normal reference tissue values, using a consistent PET protocol, using a fixed small region of interest in the most active region of metabolically active tumors, assessing tumor size, and deferring cases that do not have 18F-FDG avidity or are technically unsuitable. We evaluated all PET images in the present study using similar criteria. In doing so, we identified a significant relationship between SUVmax response and histologic tumor response for patients with GEJ cancers (rho=0.19, p=0.04) and gastric cancers (rho=0.44, p<0.0001). We suspect that this relationship was weaker for patients with GEJ cancers because of the near-uniform use of radiation as a component of neoadjuvant therapy in this cohort of patients. Radiotherapy has previously been demonstrated to obfuscate the relationship between metabolic response and histologic tumor response in patients with esophageal cancer when added to chemotherapy-only protocols.[3, 12]
Our second aim in undertaking this study was to determine if associations exist between changes in SUVmax after neoadjuvant therapy and outcomes for patients with GEJ and gastric cancers. Many studies have demonstrated a significant association between the change in PET and clinical outcomes. For example, Javeri et. al. examined 151 consecutive patients with GEJ adenocarcinoma treated at MD Anderson Cancer Center using chemoradiation, and reported an SUV response of >52% to be associated with improved overall survival. However, traditional staging variables such as T-stage and N-stage were not included in the multivariate analysis.[13] At our own institution, we previously conducted a prospective trial evaluating the role of PET response to predict treatment response in patients with esophageal cancer and identified that a decrease in SUV >60% was associated with improved disease-free and overall survival, albeit not quite achieving statistical significance.[14] It is important to note however that this study also did not evaluate PET responses in a multivariable fashion in the context of histopathologic data. In our present study, which is the largest from our institution, we found associations between histologic tumor response and DSS for patients with both GEJ and gastric cancers, but failed to identify any significant correlation with SUVmax variables (baseline, pre-operative or response). A significant strength of the present study is that we exhausted multiple attempts to identify a correlation with changes in SUVmax response and DSS, including the use of SUVmax as a continuous variable, use of median values, and the use of a pre-defined cut-off value of 35%. A significant problem with interpreting responses on PET imaging has been that clear and reproducible cut-off values are lacking. SUVmax response will need to be standardized if it is to play any role in counseling patients regarding their individual outcomes, although we believe it is exceedingly unlikely that biologically relevant cut-points exist.
In order to evaluate the ability of changes in SUVmax response to predict outcomes in the context of histopathologic data, we first generated bivariate models using histologic tumor response and SUVmax response for patients with GEJ and gastric cancers. In doing so, we identified a prognostic role for histologic response but again failed to identify a prognostic role for SUVmax response. Similarly, Lordick et al. demonstrated histologic response drives the association with improved survival using bivariate PET/histology in patients undergoing resection for GEJ cancers. (2) Interestingly, Javari et al. identified SUV response as a significant predictor of OS on bivariate analysis, although histologic response was not significant. Perhaps this disparate result stems from the author’s reliance on a previously determined cut-point of 52% for change in SUV, which was derived from 24 patients with esophageal squamous cell carcinoma by searching for an SUV cut-off that would result in a sensitivity of 100% for a histologic response.
Next we attempted to determine if SUVmax response could be predictive of outcome when histopathologic data is taken into account. In order to accomplish this aim, we built multivariate histopathologic models separately for gastric and GEJ cancers. Of note, histologic response was no longer significant when pathologic variables were also entered into the model. Following construction of the histopathologic models, we added in SUVmax response and again failed to identify a significant role for repeat PET in predicting outcomes for patients when traditional pathologic variables are considered. To date, our study is the first to evaluate PET data following completion of neoadjuvant therapy in the context of conventional postoperative pathologic staging parameters. However, it is not entirely surprising that SUVmax response did not significantly predict outcomes for patients with GEJ and gastric cancers when pathologic variables are given consideration. Ott et al. undertook a multivariate analysis on 50 patients undergoing resection for GEJ cancer that included PET response (after 2 weeks of induction therapy), histologic response and pathologic variables. Similar to our results, Ott et al. identified that only pathologic nodal status was predictive of overall survival.[15] Moreover, a recent study of 400 patients with esophageal and GEJ cancers from two large centers in the United Kingdom demonstrated that survival is strongly predicted by pathologic stage after neoadjuvant therapy.[16]
Our study has important limitations that should be clearly stated. First, our study is retrospective in nature and therefore susceptible to biases inherent to all retrospective studies. Second, it is worth noting that we did not attempt to use extreme outliers (e.g. >95% SUVmax response) in order to achieve statistical significance in a small subpopulation of patients, as this would almost certainly be useless in any prospectively evaluated population given the retrospective nature of our study. We did however analyze PET SUVmax data as a continuous variable, using median cut-off values, as well as a previously published cut-off value (35%). Third, we did not set a minimum SUVmax requirement for study entry, but rather provided latitude for the reviewing nuclear medicine physicians to include any patient they felt had PET avid tumors after normalization. Finally, this study does not address the issue of early repeat PET/CT imaging for the purposes of altering treatment. The use of repeat PET/CT imaging at pre-defined time points tied to clinical decisions has not been addressed in the present study. Although a small subset of patients in this study (N=21) had multiple PET/CTs throughout the course of their pre-operative treatment in the context of a prospective clinical trial, these studies in the majority of patients were not obtained at regular intervals and were therefore not comparable between patients. Furthermore, we have noticed that, in some patients undergoing multiple PET/CTs during neoadjuvant treatment, there is variability such that the pre-operative SUVmax may have been higher than SUVmax measured during treatment.
Summary and Conclusions
Post-treatment PET is often utilized as a quantitative measure of response to neoadjuvant therapy for patients with GEJ and gastric cancers. If the repeat imaging is undertaken prior to resection, and not at pre-defined time points tied to clinical decision making, the value of SUVmax response must be considered in the context of conventional pathologic staging data. To date, no study has evaluated pre-operative PET data in the context of conventional pathologic staging parameters. We therefore sought to ascertain whether quantification of SUVmax response yields independent prognostic information. We have demonstrated that changes in SUVmax correlate with histologic response. However, despite the use of bivariate and multivariate models we were unable to define a prognostic role for SUVmax response. It is exceedingly unlikely that post-therapy/pre-operative PET will be evaluated in a meaningful prospective manner given the current focus on identifying non-responders early in the neoadjuvant treatment course. We therefore conclude that the routine use of repeat pre-operative PET imaging following neoadjuvant therapy for locoregionally advanced adenocarcinoma of the stomach and GEJ is of limited prognostic value, and should be abandoned, unless being performed in the context of a research setting to assess its value in redirecting neoadjuvant therapy for non-responders.
Supplementary Material
Supplementary Figure 1. Consort Diagram for Study Inclusion.
Supplementary Figure 2. Scatter Plot for PET response versus Histologic Tumor Response for GEJ (A) and Gastric (B)
Acknowledgments
Debra Goldman and Mithat Gonen were partly supported by an NIH Core Grant P30 CA008748
References
- 1.Smyth EC, Shah MA. Role of (1)(8)F 2-fluoro-2-deoxyglucose positron emission tomography in upper gastrointestinal malignancies. World J Gastroenterol. 2011;17:5059–5074. doi: 10.3748/wjg.v17.i46.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 3.Gillham CM, Lucey JA, Keogan M, et al. (18)FDG uptake during induction chemoradiation for oesophageal cancer fails to predict histomorphological tumour response. Br J Cancer. 2006;95:1174–1179. doi: 10.1038/sj.bjc.6603412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MK, Ryu JS, Kim SB, et al. Value of complete metabolic response by (18)F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer. 2007;43:1385–1391. doi: 10.1016/j.ejca.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Levine EA, Farmer MR, Clark P, et al. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg. 2006;243:472–478. doi: 10.1097/01.sla.0000208430.07050.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swisher SG, Erasmus J, Maish M, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–1785. doi: 10.1002/cncr.20585. [DOI] [PubMed] [Google Scholar]
- 7.Vallbohmer D, Holscher AH, Dietlein M, et al. [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg. 2009;250:888–894. doi: 10.1097/sla.0b013e3181bc9c0d. [DOI] [PubMed] [Google Scholar]
- 8.Peng HQ, Halsey K, Sun CC, et al. Clinical utility of postchemoradiation endoscopic brush cytology and biopsy in predicting residual esophageal adenocarcinoma. Cancer. 2009;117:463–472. doi: 10.1002/cncy.20051. [DOI] [PubMed] [Google Scholar]
- 9.Sarkaria IS, Rizk NP, Bains MS, et al. Post-treatment endoscopic biopsy is a poor-predictor of pathologic response in patients undergoing chemoradiation therapy for esophageal cancer. Ann Surg. 2009;249:764–767. doi: 10.1097/SLA.0b013e3181a38e9e. [DOI] [PubMed] [Google Scholar]
- 10.Flamen P, Van Cutsem E, Lerut A, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13:361–368. doi: 10.1093/annonc/mdf081. [DOI] [PubMed] [Google Scholar]
- 11.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klaeser B, Nitzsche E, Schuller JC, et al. Limited predictive value of FDG-PET for response assessment in the preoperative treatment of esophageal cancer: results of a prospective multi-center trial (SAKK 75/02) Onkologie. 2009;32:724–730. doi: 10.1159/000251842. [DOI] [PubMed] [Google Scholar]
- 13.Javeri H, Xiao L, Rohren E, et al. The higher the decrease in the standardized uptake value of positron emission tomography after chemoradiation, the better the survival of patients with gastroesophageal adenocarcinoma. Cancer. 2009;115:5184–5192. doi: 10.1002/cncr.24604. [DOI] [PubMed] [Google Scholar]
- 14.Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21:428–432. doi: 10.1200/JCO.2003.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4692–4698. doi: 10.1200/JCO.2006.06.7801. [DOI] [PubMed] [Google Scholar]
- 16.Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32:2983–2990. doi: 10.1200/JCO.2014.55.9070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Consort Diagram for Study Inclusion.
Supplementary Figure 2. Scatter Plot for PET response versus Histologic Tumor Response for GEJ (A) and Gastric (B)


