Abstract
It is now widely accepted that several gene alterations including transcription factors are critically involved in cancer progression and metastasis. Forkhead Box Class O proteins (FoxOs) including FoxO1/FKHR, FoxO3/FKHRL1, FoxO4/AFX and FoxO6 transcription factors are known to play key roles in proliferation, apoptosis, metastasis, cell metabolism, aging and cancer biology through their phosphorylation, ubiquitination, acetylation and methylation. Though FoxOs are proved to be mainly regulated by upstream phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3 K)/Akt signaling pathway, the role of FoxOs in cancer progression and metastasis still remains unclear so far. Thus, with previous experimental evidences, the present review discussed the role of FoxOs in association with metastasis related molecules including cannabinoid receptor 1 (CNR1), Cdc25A/Cdk2, Src, serum and glucocorticoid inducible kinases (SGKs), CXCR4, E-cadherin, annexin A8 (ANXA8), Zinc finger E-box-binding homeobox 2 (ZEB2), human epidermal growth factor receptor 2 (HER2) and mRNAs such as miR-182, miR-135b, miR-499-5p, miR-1274a, miR-150, miR-34b/c and miR-622, subsequently analyzed the molecular mechanism of some natural compounds targeting FoxOs and finally suggested future research directions in cancer progression and metastasis.
Keywords: Forkhead Box Class O proteins, Metastasis, Molecules, mRNAs, Natural compounds
1. Introduction
Carcinogenesis is usually recognized to comprise three stages such as tumor initiation, promotion, and progression [1,2]. Metastasis, a late step of tumor progression, is known to form a complex cascade of moving primary tumors into a secondary site through the subsequent events including migration, intravasation, dissemination and invasion [3,4]. Accumulating evidences revealed that genetic and epigenetic alterations including transcription factors, cytokines, chemokines, growth factors, and proteases within the tumor microenvironment play crucial roles in tumor progression and metastasis [5]. Of several genes involved in tumor progression and metastasis, epithelial–mesenchymal transition (EMT) proteins are known to induce dissolution of adherens and tight junctions and a loss of cell polarity with mesenchymal property, leading to highly invasiveness by a set of transcription factors, such as Slug, Snail, Twist, ZEB1, and ZEB2 [6,7]. Also, matrix metalloproteinases (MMPs) such as MMP-1, −2, −3, −7, −9, −13 and −14 are regarded to be involved in extracellular matrix (ECM) degradation, angiogenesis, migration, tumor progression and metastasis [8].
Recently, as important transcription factors, FoxOs comprising FoxO1, FoxO3, FoxO4 and FoxO6 are attractive for cancer therapy [9], since these proteins are considered as tumor suppressors that limit cell proliferation and induce apoptosis in several cancers such as rhabdomyosarcoma [10], leukemia [11], lymphoma [12], gastric cancer [13], hepatocellular carcinoma [14], breast cancer [15], prostate cancer [16] and lung cancer [17]. Nevertheless, the underlying role of FoxOs is not fully understood in cancer progression and metastasis so far. Hence, in the current review, we focus on underlying role of FoxOs in association with their related molecules and signaling pathways and the molecular mechanism of FoxOs regulating natural compounds in tumor progression and metastasis with previous experimental literatures and suggest perspectives for FoxOs targeting research.
2. FoxO family domains and their roles as tumor suppressors
2.1. FoxO family domains
Among FOX family of transcription factors including over 100 proteins identified to date, FoxO subgroup has been recognized to consists of FoxO1, FoxO3, FoxO4 and FoxO6, since FoxO1 was first identified in rhabdomyosarcomas as a forkhead in rhabdomyosarcoma (FKHR), and was located on chromosome 13 in humans [18,19]. In invertebrates, there is only one FoxO gene, termed dFoxO in the fly and daf-16 in the worm. In contrast, in mammals, there are four FoxO genes such as FoxO1 (also known as FKHR), FoxO3 (also known as FKHRL1 or FoxO2), FoxO4 (also known as AFX or MLLT7) and FoxO6 [20,21].
However, all FoxO proteins recognize two consensus sequences: 5′-GTAAA (T/C)AA-3′, known as the Daf-16 family member-binding element (DBE) [22,23] and 5′-(C/A)(A/C)AAA(C/T)AA-3′ in the IGFBP-1 promoter region as the insulin-responsive sequence (IRE) [24,25]. Also, FoxO proteins comprise four domains such as a highly conserved forkhead DBD (FHD), a nuclear localization sequence (NLS) located downstream of DBD, a nuclear export sequence (NES) and a C-terminal transactivation domain (TAD) as shown in Fig. 1. The transcriptional activity of FoxO proteins is regulated by posttranslational modifications such as phosphorylation, acetylation and ubiquitination [26,27]. FoxO1, FoxO3 and FoxO6 proteins share similar length of ∼ 650 amino-acid residues and sequence to Daf-16 that cooperates with SMAD in modulating the transcription of key metabolic and developmental genes [28], while FoxO4 sequence is shorter with approximately 500 amino-acid residues [29]. Previous evidences revealed that FoxO1 and FoxO4 are overexpressed in adipose tissue and skeletal muscle, whereas FoxO3 is predominant in various tissues of brain, kidney, heart, and spleen and FoxO6 mRNA is abundant in the developing and adult brain [30]. Notably, Han et al. demonstrated that the transcription of MDR1 gene was stimulated by FoxO1 overexpression in MCF-7/ADR cells, indicating FoxO1 is a key protein for chemoresistance. Collectively, four FoxO proteins are involved in the diverse biological activities including cell proliferation, apoptosis, reactive oxygen species (ROS) response, longevity, metabolism, cell cycle and cancer biology with high similarity in their structure, function and regulation [31].
Fig. 1.
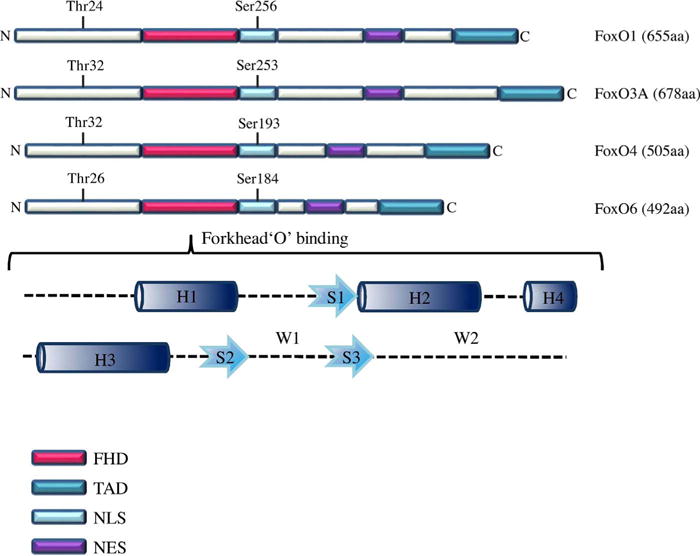
FoxO family domains. NLS, nuclear localization sequence; NES, nuclear export sequence; FHD, fork head DNA-binding domain; TAD, transactivation domain; H, α-helices; S, β-standard; W, Wing-like loops.
2.2. Regulation of FoxOs through acetylation and deacetylation
Recently the FoxO1 gene was identified a novel interaction partner of cAMP response element-binding protein (CREB)-binding protein (CBP) and its related protein p300 (CBP/p300) as histone acetyltransferases that work as coactivators of numerous transcription factors [32,33]. Daitoku et al. claimed that CBP acetylates FoxO1 to form CBP/FoxO1 complex at Lys-242, Lys-245, and Lys-262 in mice. However, FoxO1 acetylation by CBP reduces its transcriptional activity, though acetylation of transcription factors usually enhances their transactivation functions. In contrast, overexpression of sirtuin-1 (SIRT1) efficiently decreases the acetylation of FoxO1 as a transcriptional coactivator of FoxO1 in mammalian cells [34]. Similarly, Motta and his colleagues supported evidence that SIRT1 deacetylates the activity of FoxO factors including FoxO1, FoxO3, and FoxO4 [35] and so Wang et al. reported that SIRT2 suppresses adipocyte differentiation by deacetylating FoxO1 [36]. Additionally, van der Horst et al. reported that oxidative stress induced binding of CBP and acetylation of FoxO4 which was in turn deacetylated by SIRT1 [37]. Of note, FoxO acetylation in cytoplasm can induce autophagy by binding to ATG7 by dissociation from SIRT2 [38] and also enhance Akt-mediated phosphorylation of FoxO [39]. Nevertheless, further mechanistic study on the clear roles of acetylation or deacetylation of FoxOs are required in vitro and in vivo in the near future (Fig. 2).
Fig. 2.
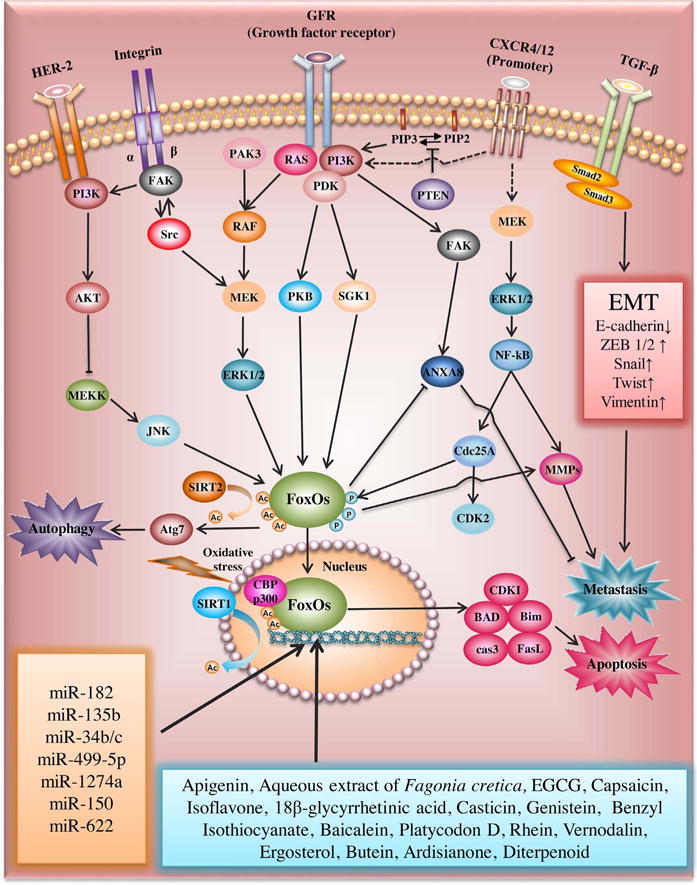
FoxOs related signaling pathway in cancer progression and metastasis.
2.3. FoxOs as tumor suppressors via inhibition of PI3K/Akt signaling pathway
Recently FoxOs have emerged as important effectors of PTEN regulated PI3 K/Akt signaling [30] and tumor suppressors [40], since PTEN inhibits the activity of PI3 K and enhances FoxOs in cancer cells as a phosphatidylinositol-3,4,5-trisphosphate (PIP(3)) phosphatase [41]. There are accumulation evidences that survival factors such as insulin-like growth factor 1 (IGF1) and neurotrophins bind to their cell surface receptors to trigger the activation of several kinases such as PI3K and Ca21/calmodulin-dependent kinase [19,42,43]. FoxOs are usually phosphorylated at Thr24, Ser256 and Ser319 by Akt in the presence of IGF1 [44]. However, growth factor withdrawal induces PI3 K/Akt pathway inactivation, FoxO dephosphorylation at its Akt sites, nuclear translocation and target gene activation [45]. Of note, Brunet et al. first reported that Akt promotes cell survival by phosphorylating and inhibiting transcription factor FoxO3 at S315, leading its retention in the cytoplasm by association with 14-3-3 proteins in 293 T cells as a downstream of insulin and insulin-like growth factor receptors [46]. Similarly, Kops and his colleagues showed that inhibition of endogenous PI3K and PKB prevents PKB-dependent phosphorylation of FoxO4/AFX [47] and also Rena et al. reported that FoxO1/FKHR, a human homologue of the DAF16 transcription factor, is rapidly phosphorylated by PKB alpha at Thr-24, Ser-256, and Ser-319 faster than BAD in IGF-1 stimulated 293T cells, implying that FoxO1 is a substrate of PKB [48].
The FoxO family such as FoxO1, FoxO3/FKHRL1 and FoxO4 are known to mediate apoptosis by activating the proapoptotic genes such as FasL and Bim [49]. Indeed, Xinbo et al. claimed that FoxOs promote apoptosis signaling by activating pro-apoptotic proteins such as BIM and BAD, death receptor ligands such as Fas, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and cyclin-dependent kinase inhibitors (CDKIs) [50]. Furthermore, FoxO1 induced G2/M arrest and apoptosis in SiHa cervical cancer as a tumor suppressor [51], and inhibited cell migration and invasion via suppression of RUNX2 in PC-3 and DU145 cells as a metastasis suppressor [52], while FoxO3 mediated BCG-induced apoptosis of human macrophages [53] and FoxO4 overexpression significantly increased apoptosis via activation of Bim, cleaved‐caspase 3, Bcl‐2‐associated X protein (Bax) and cytochrome c release in clear‐cell renal carcinoma cells [54]. Also, Abdelkader et al. reported that STI571 induced apoptosis in human CML BV173 cells via dephosphorylation of FoxO3 and accumulation of Bim [55], since FoxO dephosphorylation inducess nuclear translocation and target gene activation for apoptosis induction. In contrast, FoxO phosphorylation makes it retained in the cytoplasm for survival [56]. Thus, restoring nuclear translocation of FoxOs and their target gene activation can be an important therapeutic strategy for targeted cancer therapy [50]. Emerging evidences revealed that autophagic cell death contributes anticancer activity in several cancers [57–60]. Recently Zhao et al. claimed that the acetylated FoxO1 binds to Atg7 to induce autophagic type II cell death and also showed transfection of cytosolic FoxO1 (ΔDB) suppresses the growth of H1299 cells in nude mice, while that of nuclear FoxO1 (ΔDB-3A) enhances the growth of H1299 cells in nude mice [38]. Also, Zhu et al. found that PC3, MDA-MB-231 and HCT116 cells, while FoxO1 depletion inhibits autophagy, indicating FoxOs are closely involved in autophagy induction in several cancers. Nevertheless, the further mechanistic studies on the clear roles of FoxOs as tumor suppressors or autophagy regulators are required in vitro and in vivo in the future.
3. The interplay between FoxOs and metastasis related molecules
Cancer metastasis is a hot issue to be overcome worldwide, since over 90% of cancer-related deaths are caused by metastasis [61]. Metastasis is well known to be a complex cascade process including a number of sequential events such as local invasion into the adjacent tissue, transendothelial migration into vessels (intravasation), survival in the circulatory system, extravasation and subsequent proliferation in the organs leading to colonization [62,63]. Nevertheless, the associated cellular, genetic and biochemical determinants during metastasis cascade still remain unclear. Thus, here the molecular associations between FoxOs and metastasis related molecules were examined with previous evidences.
3.1. FoxO1 and CNR1
Alveolar rhabdomyosarcoma (ARMS) cells are characterized to be more aggressive and metastatic in children with diversity of small, round, densely packed cells around alveolar-like structures reminiscent of the lung [64]. ARMS cells were known to contain PAX3-FoxO1 or PAX7-FoxO1 fusion transcription factor along with upregulation of cannabinoid receptor 1 (CNR1/CB1) [65]. CNR1 is closely associated with a variety of different cell signaling pathways including G-proteins, adenylyl cyclase, PI3 K and mitogen-activated protein kinase (MAPK) [66] as a G-protein–coupled receptor. Usually ARMS tumors metastasize to the lung by approximately 25% of metastatic ARMS cases [67]. Interestingly, treatment with the CNR1 antagonist AM251, or loss of the CNR1 gene reduced lung metastasis formation in an ARMS metastasis mouse model injected with ADP-ribosylation factor 1 (ARF)−/− primary myoblasts and oncogene PAX3-FoxO1-IRES-GFP vector, implying metastatic potential of PAX3-FoxO1 oncogene. In contrast, Ko et al. claimed that loss of FoxO1 promotes the growth of gastric cancers including SNU-638, MKN45, SNU-216 and NCI-N87 cells and metastasis by upregulation of human epidermal growth factor receptor 2 (HER2) [68]. Also, Chen et al. suggested that activation of epidermal growth factor (EGF)/epidermal growth factor receptor (EGFR) and its downstream PI3 K/Akt induce FoxO1 nuclear exclusion and MMP9 to promote invasion in A-172 glioblastoma cells [69]. Likewise, Ding et al. also revealed that nuclear exclusion of Akt downstream FoxO1 by EGF-mediated Akt activation enhanced invasiveness via MMP7 activation in Hep-2 larynx carcinoma cells, which was inhibited by EGFR inhibitor AG1478 or by the PI3 K/Akt inhibitor LY294002 [70]. Taken together, these evidences suggest the metastatic potential of PAX3-FoxO1 oncogene, loss of FoxO1 and activation of CNR1.
3.2. FoxO1 and Cdc25A/Cdk2
It was well known that Cdk2, a downstream target of Cdc25A, directly phosphorylates FoxO1 at Ser249 to maintain FoxO1 stability [71] and inhibits the transcriptional activity of FoxO1 and FoxO3 [72]. Also, aberrant Cdc25A, which is a phosphatase and not a transcription factor, directly correlates with the metastatic phenotype such as MMP1 in breast cancer patient tissues [73]. Consistently, Cdc25A overexpression promotes invasive and metastatic ability with poor prognosis of breast, colorectal, lung, prostate, and esophageal cancers [74,75]. Also, Cdc25A, a critical regulator of cell cycle progression and checkpoint response, enhances the phosphorylation and stability of FoxO1 at Ser249, and then FoxO1 directly regulates transcription of the metastatic factor MMP1 in MDA-MD-231 breast cancer cells [73,76]. Furthermore, Cdc25A was verified to be associated with hepatocellular carcinoma [77] and breast cancer metastasis [78]. Of note, FoxO1-derived small peptide FO1-6nls was known to exert antitumor effect via and dephosphorylation of Cdk2 and FoxO1 in prostate cancers [79]. Together, these evidences indicate that Cdc25A/Cdk2 enhances the phosphorylation and stability of FoxO1 to activate MMP1 leading to invasiveness.
3.3. FoxO1 and ZEB2
Accumulating evidences revealed that zinc finger E-box-binding homeobox 2 (ZEB2) plays a critical role in the TGF-β signaling cascade and promotes tumor cell invasion and metastasis [80]. Thus, ZEB2 is overexpressed in lung metastatic nodules from hepatocellular carcinoma cells with high risk of recurrence [81]. Notably, Dong and his colleagues provided evidence that FoxO1 inhibits the invasion and metastasis of hepatocellular carcinoma by suppression of ZEB2-induced EMT [82]. However, inconsistent with previous report, Xia et al. claimed that FoxO1 induced EMT and tumor associated macrophage (TAM) infiltration leading to hepatocellular carcinoma metastasis only by transactivating ZEB2 and VersicanV1 expression [83]. Also, ZEB2 was known to promote tumor cell invasion and metastasis via inhibition of E-cadherin expression [84,85]. Thus, enhancing FoxO1 and inhibition of ZEB2 can be a potent strategy for cancer metastasis [82].
3.4. FoxO1 and HER2
Human epidermal growth factor receptor 2 (HER2/ErbB2/neu), a member of the epidermal growth factor receptor family, was overexpressed in 15–59% of advanced gastric cancer cases [86], while its gene amplification was shown in 6–35% of gastric cancer cases [87]. Emerging evidences show that HER2 expression was inversely correlated with FoxO1 activation in breast cancer cells [88], since trastuzumab suppresses proliferation of HER2-overexpressing breast cancer cells by activation of FoxO1 through inhibition of the PI3 K/Akt pathway [88]. Consistently, loss of FoxO1 was reported to promote the growth and metastasis of gastric cancer by upregulation of HER2, whereas HER2 knockdown increased FoxO1 protein expression and activation in gastric cancer cells and orthotopic xenograft tumors [68]. However, it is noteworthy that even tumor suppressor FoxO1 can mediate cisplatin resistance in gastric cancer cells by activation of FoxO1 upstream PI3 K/Akt pathway [89] and also is capable of enhancing hepatocellular carcinoma metastasis only by transactivating ZEB2 and VersicanV1 expression [83]. Likewise, HER2-induced metastasis is enhanced via FoxO1 upstream Akt/JNK/EMT signaling pathway in gastric cancer [90], implying tumor suppressor FoxO1 can be an oncogene or metastasis promoter in concert with its upstream signaling pathways, which can be a further mechanistic study in the future.
3.5. FoxO1/FoxO3 and src
Emerging evidences reveal that Src is overexpressed in the aggressive cancer cells [91,92] through the metastatic cascades via downregulation of cell adhesion molecule E-cadherin, and upregulation of MMPs [69,93]. Also, Src was reported to increase cell migration by modulating downstream effectors, such as p130Cas, PEAK, Cool-1, STAT3, and VEGF [94,95]. Notably, FoxO1 inactivation is closely associated with increased phosphorylation of Src as a known activator of Akt, ERK1/2 and STAT3 [96], since c-Src kinase activity phosphorylates FoxO1 [97]. Also, Bülow et al. demonstrated that Src tyrosine kinase blocks nuclear localization and transcription factor activity of FoxO in Lavae [98]. Thus, Li et al. provided evidence that isoflavon suppresses Akt induced phosphorylation of FoxO3 binding to the promoter of AR along with downregulation of active form Src (Tyr416) in LNCaP and C4–2 B prostate cancer cells [4]. Together, Src enhances metastasis via phosphorylation of FoxO1/FoxO3, which needs further mechanistic study in animal study and clinical trials in the future.
3.6. FoxO3 and SGKs
Serum and glucocorticoid inducible kinases (SGKs) are involved in cell survival and metastasis in close association with PI3 K/Akt signaling. Consistently, Tangir et al. claimed that SGK1 promotes c-fms- induced aggressiveness of breast cancer cells after exposure to glucocorticoids and or colony stimulating factor-1 [99]. Similarly, Dehner et al. indicated that induction of Wnt signaling blocks FoxO3-induced transcription and apoptosis in HCT116 colon cancer cells. Also, Xiaobo et al. revealed that overexpression of SGK1 promotes the growth and migration of A549 and H23 non-small cell lung cancer (NSCLC) cells [4]. Likewise, Salis et al. showed antimetastatic effect of fluvastatin may be mediated by low level of SGK1 messenger RNA (mRNA) and high levels of NDRG1 and AMPKA2 mRNA in MCF-7 and Hep3 B cancer cells [100]. Notably, Brunet et al. demonstrated that PI3K-regulated SGK1 can phosphorylate FoxO3 and inhibit its transcription factor activity, thereby inducing nuclear exclusion of FoxO3 for survival or metastatic activity [101]. Therefore, the inhibition of SGK1 is suggested as a potent strategy for metastasis prevention and treatment, since SGK1 is requisite for ras mediated glucose uptake and survival during ECM-detachment of cells from the extracellular matrix (ECM) as an important step of metastatic cascades [102]. Taken together, though SGK1 is found a target molecule for metastasis as a regulator of its downstream FoxO3 or NDRG1 as a metastasis suppressor [103], the further study is required for elucidate the association between SGK1 and FoxO3/NDRG1 during metastatic cascades.
3.7. FoxO3 and CXCR4
CXCR4, a chemokine receptor for stromal-derived-factor-1 (SDF-1) or CXCL12, is known to promote tumor invasiveness and metastasis [104–111] via degradation of extracellular matrix (ECM) proteins such as laminin, fibronectin, and collagen which contributes to the metastatic process [112–114]. Also, Tan and his colleagues reported that CXCL12 promotes invasion of Laryngeal and hypopharyngeal squamous cell carcinomas via activation of CXCR4, targeting ERK/c-Jun-dependent MMP-13 upregulation [115]. Conversely, Wang et al. demonstrated that silencing of CXCR4 led to a significant down-regulation of VEGF and MMP-9 at both the mRNA and protein levels compared to untreated control in PC-3 prostate cancer cells [116]. Thus, accumulating evidences showed that CXCL4 levels were elevated in metastatic patients with poor prognosis [117]. Notably, Dubrovska et al. claimed that CXCR4 protein level was attenuated in FoxO3 overexpressed DU145 cells, since FoxO3 binds to CXCR4 promoter by chromatin immunoprecipitation assay (ChIP) assay [117]. Collectively, CXCR4 is found to promote invasion via loss of FoxO3 in aggressive cancer cells.
3.8. FoxO4 and E-cadherin
It was well documented that mesenchymal transition (EMT) is involved in the metastatic cascade [118,119]. EMT is characterized by downregulation of E-cadherin and cytokeratins through loss of cell-cell adhesion. Also, the expression of FoxO4 at mRNA and protein levels was downregulated in metastatic tissues of gastric cancer compared to normal tissue control [13]. Consistently, Li et al. demonstrated that depletion of FoxO4 by siRNA promoted the migratory ability of H460 and A549 cells, while the expression of FoxO4 was significantly downregulated in five NSCLCs including 95C, 95D, H1299, H460 and A549 cells [120]. Furthermore, Xu et al. showed that low expression of FoxO4 correlated with a loss of E-cadherin expression in human gastric cancer tissues by IHC, implying close relationship between FoxO4 and E-cadherin [121]. Together, loss of FoxO4 is demonstrated to enhance metastasis in close association with decreased E-cadherin.
3.9. FoxO4 and annexin A8 (ANXA8)
Previous evidence demonstrated that ANXA8 inhibits the migratory and metastatic activity in cholangiocarcinoma cells via interaction with FoxO4, since activation of EGFR and its downstream PI3 K/Akt is linked to the phosphorylation of FoxO4, leading to downregulation of ANXA8 transcription [122]. Furthermore, ANXA8 was found to be closely related to FAK expression and altered F-actin dynamics including the formation of focal adhesion and loss of filopodia and stress fibers that are involved in metastatic migration [122,123]. Hence, Su et al. identified FoxO4 as a metastasis-suppressor through counteracting PI3 K/Akt signal pathway in castration-recurrent prostate cancer [124]. Likewise, Oka et al. reported ANXA8 is a target molecule for lymph node metastasis [125]. Consistently, the expression of FoxO4 and PDCD4 was decreased in highly metastatic SW620 colorectal cancer cells as direct and functional targets of miR-499-5p as a metastasis promoter [126]. Also, EGF was known to enhance PI3 K/Akt-induced phosphorylation of FoxO4 in the cytoplasm along with decreased the nuclear translocation of FoxO4, leading to inhibition of ANXA8 in SCK cells [122]. Together, these evidences indicate the relationship between FoxO4 and ANXA8 for metastasis.
3.10. FoxO6 and USP7/SOX2
Recently Hu et al. showed that overexpression of FoxO6 reduced the proliferation of A549 lung cancer cells by upregulation of ubiquitin–specific‐processing protease 7 (USP7) and p53. Consistently, ablation of FoxO6 increased the number of A549 cells and enhanced the cell proliferation, indicating that the FoxO6 acts as a tumor suppressor in regulation of lung cancer development [127]. In contrast, Wang et al. reported that FoxO6 was overexpressed in 192 gastric cancer patients with lymph node metastasis with upregulation of MMP-9. Also, multivariate analysis revealed that FoxO6 overexpression was an independent indicator for poor overall survival (OS) and recurrence free survival (RFS) in gastric cancer patients [128]. It was well documented that stem cell transcriptional regulator SOX2 stimulates cell proliferation, migration, invasion, and tumor metastasis in the variety of human malignancies such as colorectal cancer, prostate cancer, and breast cancers [129–131]. Rothenberg et al. showed that knockdown of FoxO6 using siRNA constructs dramatically reduced erlotinib-mediated induction of SOX2 in HCC827 and PC9 lung adenocarcinoma cells. As a result, using erlotinib to inhibit EGFR to kill the cancer cells increases the activity of FoxO6, which in turn promotes the survival of some cells by activating the SOX2 gene as a feedback mechanism [132]. Overall, further study is required to elucidate the dual roles of FoxO6 as a tumor suppressor or an oncogene in different types of cancers in the future.
3.11. FoxOs and miRNAs
Accumulating evidences demonstrated that FoxO transcripts are tightly regulated by the miRNA networks in cancer progression and metastasis [22,133,134]. For instance, upregulation of miR-499-5p and miR-1274a promotes metastasis in colorectal and gastric cancers by targeting FoxO4 [126,135]. Likewise, miR-150 was found to enhance the proliferation of cervical carcinoma by targeting FoxO4 [136]. Additionally, miR-135b was reported to promote proliferation and invasion of osteosarcoma cells via inhibition of FoxO1 in osteosarcoma [137] and also miR-182 was known to increase proliferation, migration and invasion in prostate cancers through suppression of FoxO1 [138]. Furthermore, other miRNAs for promoting EMT induced metastasis were reported such as mir-130b via PTEN/pAkt/HIF-1α signaling [139], miR-181b-3p via Snail stabilization targeting YWHAG tyrosine 3-monooxygenase [140] and miR-331-3p via targeting PH domain and leucine-rich repeat protein phosphatase (PH LPP) [141]. Consistently, depletion of endogenous FoxO4 can promote tumor metastasis mediated by miR-150 [120]. However, miR-622 overexpression mediated by FoxO3 was known to repress the invasiveness of lung tumor cells by inhibition of HIF-1α mediated by ERK inactivation in U0126-treated A549 cells [142]. Also, Liu et al. claimed that FoxO3-mediated transactivation of miR-34b/c as a tumor suppressor inhibits WNT/β-catenin signaling and also suppresses β-catenin dependent EMT genes in prostate cancer [16]. Together, several microRNAs such as miR-182, miR-135b, miR-499-5p, miR- 1274a, miR-150, miR-34b/c and miR-622 are suggested to be involved in metastasis in association with FoxO1, FoxO3 and FoxO4.
4. Natural compounds targeting FoxOs
FoxO transcription factors are considered to be attractive for strategy directed against human cancer in view of their pro-apoptotic effects and cell cycle arrest as tumor suppressor or metastasis suppressor [143], though they are associated with infertility, cellular degeneration, and unchecked cellular proliferation as unwanted implications [143,144]. Emerging evidences suggest beneficial effects of dietary phytochemicals in protecting against carcinogenesis phases such as initiation, promotion, progression, and metastasis of cancer, since they are frequently multi-targeted and also relatively less toxic compared to conventional anticancer agents [145,146]. Herein we analyzed antitumor mechanism of some natural compounds targeting FoxO related signalings in several cancers. As shown in Table 1, curcumin, resveratrol and phorbol 12-myristate 13-acetate induced anti-proliferative effect, cell cycle arrest and apoptosis via activation of FoxO1 in concert with several survival or apoptotic proteins such as p-ERK1/2, p27, p21, pPI3 K/p-Akt, cyclin D1, Bim, TRAIL, DR4, DR5, CDK2, CDK4, CDK6, PKCα, PKCβ and PKCδ [147–150]. Interestingly, capsaicin induces apoptosis by acetylation of FoxO1 and activation of BIM in pancreatic cancer cells [149]. Likewise, apigenin, benzyl iso-thiocyanate (BITC) aqueous extract of Fagonia cretica, epigallocatechin-3-gallate (EGCG), isoflavone, 18β-glycyrrhetinic acid, casticin, genistein, baicalein, platycodon D, rhein, vernodalin, ergosterol, butein, ardisianone and diterpenoid exerted anti-proliferative or anti-invasive effect or cell cycle arrest or apoptotic effects in several cancers via activation of FoxO3 in association with some molecules of caspase9/3, pPI3 K/pAkt, E-cadherin, ERα, P53, Bim, Bax, Bad, Bak, FasL, PTEN, p27, p21, p65, IKBα, Sirt1, GSK3ß, c-Myc, Snail, CDK1, CDK2, CDK4, CDK6, cyclin D1, E2F1, RUNX, pERK and Bcl-2 [151–167]. Notably, resveratrol induced growth arrest and apoptosis in LNCaP cells by inhibiting phosphorylation of FoxO1 FoxO3, FoxO4 for their nuclear translocation, DNA binding and transcriptional activity [148] and also isoorientin induced apoptosis in HepG2 cells via activation of FoxO4, cleaved caspase 3, cleaved PARP, Bax, cytochrome C and inhibition of pAkt and HO-1. Our study demonstrated that BITC suppressed the growth of pancreatic tumor xenografts by inhibiting phosphorylation of PI3 K, Akt, FoxO1 and FoxO3 and upregulation of Bim, p27and p21 [152] Taken together, we can suggest that these natural compounds exhibit apoptotic, anti-invasive and anti-proliferative effects not only by activation of FoxOs but also upstream PI3 K/Akt and their associated signaling pathways.
Table 1.
Natural compounds regulating FoxOs.
| Compounds | Sources | Efficacy | Molecular Mechanism | IC50(μM) | Cell line | References |
|---|---|---|---|---|---|---|
| Curcumin | Curcumalonga | Anti-proliferation | FOXO1, p-ERK1/2, p27, p21↑ Cyclin D1↓ |
10 | A549 H460 |
[147] |
| Resveratrol | Phytopolyphenol | Cell growth arrest, Apoptosis |
p-FOXO1, p-FOXO3a, p-FOXO4, p-PI3 K, p-AKT, p-Mtor, Cyclin D1↓ BimEL, BimL, BimS, p27, TRAIL, DR4, DR5↑ |
20 | LNCaP | [148] |
| Capsaicin | Chili peppers (Capsicum) | Apoptosis Cell growth inhibition |
p-FOXO1, p-JNK↓ Bim↑ |
50–150 | BxPC-3, AsPC-1, Panc-1 | [149] |
| Phorbol 12-myristate | Croton tiglium Linne | Anti-proliferation | p-FOXO1, Cyclin D3, CDK2, CDK4, CDK6, pRb, PKCζ, p-MAPK, pAKT↓ | 100 | FRO | [150] |
| 13-acetate | Cell cycle arrest | p21, p27, PKCα, PKCβ, PKCδ↑ | ARO thyroid cancer | |||
| Apigenin | Ginkgo biloba L | Anti-proliferation Apoptosis |
p-FOXO3a, p-AKT, Ki67, Cyclin D1↓, Bim↑ | 50 | LNCaP, PC-3 | [151] |
| Benzyl Isothiocyanate | Cruciferous vegetables | Apoptosis Cell growth suppression |
p-PI3-K, p-AKT, p-FOXO1, p-FOXO3a↓, p27↑, p21↑, Bim↑ | 10 | Capan-2, BxPC-3, AsPC-1 | [152] |
| Aqueous Extract | Fagonia cretica | Cell Cycle Arrest Apoptosis | FOXO3a, γ-H2AX, Bax, p53, p21↑ | 2 μg/ml | MCF-7, MDA-MB-231 | [153] |
| Epigallocatechin-3-Gallate | Grean tea | Anti-invasion | FOXO3a, E-Cadherin, γ-catenin, MTA3, Erα↑ Snail↓ | 20 μg | NF639, rel-3875, rel/CK2-5839 | [154] |
| Isoflavone | Isoflavone mixture | Cell growth inhibition Apoptosis |
p-FOXO3a, p-AKT, AR(androgen receptor) Karyopherin-α,Procaspase3↓ GSK-3β, p27, Activated caspase3↑ |
25 ∼ 50 | Pca, LNCaP, C4-2B | [155] |
| 18β-g glycyrrhetinic acid | Glycyrrhiza glaba L | Apoptosis Anti-proliferation |
p-FOXO3a, Active caspase 9, Bim, Bax, Bak, Bad↑ caspase 9, Bcl-2, Bcl-xl, p-AKT, p-GSK-3β, c-Myc↓ |
100 | MCF-7 MCF-10A |
[156] |
| Casticin | Vitex rotundifolia L | Cell cycle arrest | p-FOXO3a, CDK1, CDC25B↓ p27↑ |
2 ∼ 10 | Hep G2 PLC/PRF/5 |
[157] |
| Genistein | Iso flavonoid | Cell growth inhibition Apoptosis |
FOXO3a, p-27, PTEN, p53, p65, p50↑ p-PDK1, p-AKT, p-GSK3β, P-IKBα, SIRT1↓ |
50 | LNCaP PC-3 |
[158] |
| Baicalein | Flavonoid | Cell growth inhibition Apoptosis Ani-proliferation | FOXO3a, p-AMPKα, RUNX3, p-ERK1/2↑ | 75 | PC9, H1299, H1650, A549, H358 H1975 | [159] |
| Platycodin D | Platycodon grandiflorum | Cell Cycle Arrest Apoptosis Anti-proliferation |
FOXO3a, p53, p21, p27, Bax, Cleaved caspase3, 8, 9↑ MDM2, Bcl-2, CDK1, CDK2, CDK4, CDK6. Cyclin D1, E2F1↓ |
5 | PC3, LNCaP DU145 RWPE-1 |
[160] |
| Rhein | Rheum palmatum L | Apoptosis | p-FOXO3a, p-AKT↓ Cleaved caspase3, 7, Bim, p-elF2α, CHOP, Bid↑ |
75 | MCF-10A MCF-7 HepG2 |
[161] |
| Vernodalin | Centratherum anthelminticum seeds | Cell ctycle arrest Apoptosis Tumor suppressor |
FOXO3a, Bim, p21cip1/waf1, p21kip1↑ p-FOXO3a, Cyclin D1, Cyclin E, p-PI3 K, p-AKT↓ |
9.5 | MCF-7 MDA-MB-231 LA7 |
[162] |
| Ergosterol | Amauroderma rude | Apoptosis Anti-invasion |
FOXO3a, BimL, BimS, FasL↑ | 75 | B16 MDA-MB-231 MDA-MB-468 MCF-7 SK-BR-3 4T1 NIH 3T3 |
[163] |
| Butein | polyphenol | Tumor suppressor | FOXO3a, Cyclin D1, p27, Bax↑ p-ERK1/2, p-AKT, Bcl-2↓ |
20 | HeLa | [164] |
| Ardisianone | Croton tiglium Linne | Apoptosis Anti-proliferation Cell cycle arrest |
p-FOXO3a, p-FOXO4, Pro-caspase 9, 8, 3, 7, Bcl-2, Bcl-XL, Mcl-1, Suvivin, p-AKT, p-mTOR, p-p70A6K↓ clAP1, clAP2,AIF, GRP78, Bak, Bid↑ |
6.3 | PC-3 DU-145 |
[165] |
| Diterpenoid | Salvia miltiorrhiza | Apoptosis Tumor suppressor |
p-FOXO3a↓ TrxR1, BimEL, BimL, BimS↑ |
10 | HCT116 MEF |
[166] |
| Isoorientin | Linum usitatissimum L | Apoptosis | FOXO4, Cleaved caspase 3, Cleaved PARP, Bax, Cyt C↑ p-AKT, HO-1↓ |
40 | HepG2 | [167] |
5. Clinical trials targeting FoxOs
In recent clinical trials targeting FoxOs, Brent et al. revealed that trastuzumab and PI3 K inhibitor XL147 inhibited the HER2–PI3K–FoxO-survivin axis in trastuzumab-sensitive and –resistant breast cancer cells, leading to hypophosphorylation of FoxO and then its nuclear translocation for repressing transcription of IL-8 and survivin through preclinical study [168]. Also, Holmes et al. reported that combination of trastuzumab and lapatinib as HER2 inhibitors increased the rate of pathologic complete response (pCR) to 74% compared to trastuzumab alone (54%) or trastuzumab alone(45%) through phase II clinical trial (NCT00524303) with randomized patients with HER2-positive stage II or III invasive breast cancer, targeting nonphosphorylated FoxO and phosphorylated Stat5 [169]. Similarly, another phase 1b clinical trial was completed in HER 2 negative metastatic breast cancer with treatment of paclitaxel and/or reparixin targeting PTEN/Akt/FoxO3 axis [170]. Herein we can deduce that these clinical trials were conducted in breast cancers with combination therapy rather than single anti-HER-2 agent, targeting FoxO related multi-molecules and signalings.
6. Conclusions
The roles of FoxOs including of FoxO1, FoxO3, FoxO4 and FoxO6 have been well recognized in cell proliferation, apoptosis, ROS response, metabolism, cell cycle, longevity, and cancer biology as transcription factors with high similarity in their structure, function and regulation. It is well accepted that FoxO dephosphorylation induces nuclear translocation for apoptosis induction, while FoxO phosphorylation makes it retained in the cytoplasm for survival. Through the current review with previous literatures, we found the close molecular association between FoxO1 and Cdc25A/Cdk2 or CNR1 or ZEB2 or HER2, FoxO1/FoxO3 and Src, FoxO3 and SGKs or CXCR4, FoxO4 and E-cadherin or ANXA8 and also suggested the important role of mRNAs such as miR-182, miR-135b, miR-622, miR-34b/c miR-499-5p, miR-1274a and miR-150 in regulation of transcription factor activity of FoxOs. Among FoxOs regulating natural compounds, curcumin, and phorbol 12-myristate 13-acetate induced antiproliferative effect, cell cycle arrest and apoptosis via activation of FoxO1. Also, apigenin, aqueous extract of Fagonia cretica, EGCG, isoflavone, 18β-glycyrrhetinic acid, casticin, genistein, baicalein, platycodon D, rhein, vernodalin, ergosterol, butein, ardisianone and diterpenoid exerted antitumor effects in several cancers via activation of FoxO3. Furthermore, resveratrol induced growth arrest and apoptosis via inhibiting phosphorylation of FoxO1, FoxO3, FoxO4 and isoorientin induced apoptosis in HepG2 cells via activation of FoxO4. Herein, we can find that these natural compounds exhibit apoptotic, anti-invasive and anti-proliferative effects by activation of FoxOs, inhibition of their upstream PI3 K/Akt and their associated signaling pathways. Also, given that recent clinical trials were conducted in HER2 breast cancers with combination therapy rather than single anti-HER-2 agent, targeting FoxO related multi-molecules and signalings, we can suggest that clinical investigations by combination therapy targeting FoxOs and its related signalings are recommended rather than FoxO single target and also further mechanistic work with FoxO regulating natural compounds are requested in vitro and in animal to elucidate the immense potential of FoxOs in association with their related molecules as future research directions.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (2014R1A2A10052872) to S.H. Kim, and R01 grant CA129038 funded by National Cancer Institute, NIH, to S.K. Srivastava.
Footnotes
Disclosures
The authors disclose no conflicts.
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Moolgavkar SH, Luebeck EG. Multistage carcinogenesis and the incidence of human cancer, Genes. Chromosomes Cancer. 2003;38(4):302–306. doi: 10.1002/gcc.10264. [DOI] [PubMed] [Google Scholar]
- 2.Laconi E, Doratiotto S, Vineis P. The microenvironments of multistage carcinogenesis, Semin. Cancer Biol. 2008;18(5):322–329. doi: 10.1016/j.semcancer.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 4.Erratum. Cancer Biol Ther. 2016;17(8):881. doi: 10.1080/15384047.2016.1218721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25(1):9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 9.Coomans de Brachene A, Demoulin JB. FoxO transcription factors in cancer development and therapy. Cell Mol Life Sci: CMLS. 2016;73(6):1159–1172. doi: 10.1007/s00018-015-2112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt-Ney M, Camussi G. The PAX3-FoxO1 fusion protein present in rhabdomyosarcoma interferes with normal FoxO activity and the TGF-beta pathway. PLoS One. 2015;10(3):e0121474. doi: 10.1371/journal.pone.0121474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu H. Targeting forkhead box transcription factors FOXM1 and FoxO in leukemia (Review) Oncol Rep. 2014;32(4):1327–1334. doi: 10.3892/or.2014.3357. [DOI] [PubMed] [Google Scholar]
- 12.Vandenberg CJ, Motoyama N, Cory S. FoxO3 suppresses myc-driven lymphomagenesis. Cell Death Dis. 2016;6:e2046. doi: 10.1038/cddis.2015.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su L, Liu X, Chai N, Lv L, Wang R, Li X, Nie Y, Shi Y, Fan D. The transcription factor FoxO4 is down-regulated and inhibits tumor proliferation and metastasis in gastric cancer. BMC Cancer. 2014;14:378. doi: 10.1186/1471-2407-14-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi F, Hirata Y, Akram H, Kamitori K, Dong Y, Sui L, Tokuda M. FoxO/TXNIP pathway is involved in the suppression of hepatocellular carcinoma growth by glutamate antagonist MK-801. BMC Cancer. 2013;13:468. doi: 10.1186/1471-2407-13-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JJ, Lee HJ, Son BH, Kim SB, Ahn JH, Ahn SD, Cho EY, Gong G. Expression of FOXM1 and related proteins in breast cancer molecular subtypes. Int J Exp Pathol. 2016;97(2):170–177. doi: 10.1111/iep.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Yin J, Wang H, Jiang G, Deng M, Zhang G, Bu X, Cai S, Du J, He Z. FoxO3a modulates WNT/beta-catenin signaling and suppresses epithelial-to-mesenchymal transition in prostate cancer cells. Cell Signal. 2015;27(3):510–518. doi: 10.1016/j.cellsig.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Yin J, Wang C, Gu Y, Deng M, He Z. FoxO3a mediates the cytotoxic effects of cisplatin in lung cancer cells. Anticancer Drugs. 2014;25(8):898–907. doi: 10.1097/CAD.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 18.Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, 3rd, Emanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5(3):230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Gan B, Liu D, Paik JH. FoxO family members in cancer. Cancer Biol Ther. 2011;12(4):253–259. doi: 10.4161/cbt.12.4.15954. [DOI] [PubMed] [Google Scholar]
- 20.Carter ME, Brunet A. FoxO transcription factors. Curr Biol. 2007;17(4):R113–R114. doi: 10.1016/j.cub.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 22.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349(Pt 2):629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biggs WH, 3rd, Cavenee WK, Arden KC. Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm Genome. 2001;12(6):416–425. doi: 10.1007/s003350020002. [DOI] [PubMed] [Google Scholar]
- 24.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96(13):7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274(24):17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 26.Greer EL, Brunet A. FoxO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24(50):7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 27.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8(6):440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 28.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 29.Obsil T, Obsilova V. Structure/function relationships underlying regulation of FoxO transcription factors. Oncogene. 2008;27(16):2263–2275. doi: 10.1038/onc.2008.20. [DOI] [PubMed] [Google Scholar]
- 30.Fu Z, Tindall DJ. FoxOs, cancer and regulation of apoptosis. Oncogene. 2008;27(16):2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by Akt and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813(11):1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101(27):10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdone L, Agricola E, Caserta M, Di Mauro E. Histone acetylation in gene regulation, Briefings Funct. Genomics Proteomics. 2006;5(3):209–221. doi: 10.1093/bfgp/ell028. [DOI] [PubMed] [Google Scholar]
- 34.Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND, Hay RT, Gu W, Pestell RG. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280(11):10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. SIRT1 is critical regulator of FoxO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16(2):237–243. [PubMed] [Google Scholar]
- 36.Wang F, Tong Q. SIRT2 suppresses adipocyte differentiation by deacetylating FoxO1 and enhancing FoxO1’s repressive interaction with PPARgamma. Mol Biol Cell. 2009;20(3):801–808. doi: 10.1091/mbc.E08-06-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dansen TB, Smits LM, van Triest MH, de Keizer PL, van Leenen D, Koerkamp MG, Szypowska A, Meppelink A, Brenkman AB, Yodoi J, Holstege FC, Burgering BM. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5(9):664–672. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12(7):665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 39.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A. 2005;102(32):11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27(41):5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 41.Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1(12):1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polivka J, Jr, Janku F. Molecular targets for cancer therapy in the PI3 K/Akt/mTOR pathway. Pharmacol Ther. 2014;142(2):164–175. doi: 10.1016/j.pharmthera.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Mayer IA, Arteaga CL. The PI3 K/Akt pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]
- 44.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274(23):15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 45.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FoxO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 46.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphor-ylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 47.Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, Lam EW, Burgering BM. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002;22(7):2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J. 2001;354(Pt 3):605–612. doi: 10.1042/0264-6021:3540605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgering BM, Medema RH. Decisions on life and death: foxO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73(6):689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Zhang B, Gui LS, Zhao XL, Zhu LL, Li QW. FoxO1 is a tumor suppressor in cervical cancer. Genet Mol Res: GMR. 2015;14(2):6605–6616. doi: 10.4238/2015.June.18.3. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Pan Y, Zheng L, Choe C, Lindgren B, Jensen ED, Westendorf JJ, Cheng L, Huang H. FoxO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion. Cancer Res. 2011;71(9):3257–3267. doi: 10.1158/0008-5472.CAN-10-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haoues M, Refai A, Mallavialle A, Barbouche MR, Laabidi N, Deckert M, Essafi M. Forkhead box O3 (FoxO3) transcription factor mediates apoptosis in BCG-infected macrophages. Cell Microbiol. 2014;16(9):1378–1390. doi: 10.1111/cmi.12298. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Zhou PH, Hu W. Overexpression of FoxO4 induces apoptosis of clear-cell renal carcinoma cells through downregulation of Bim. Mol Med Rep. 2016;13(3):2229–2234. doi: 10.3892/mmr.2016.4789. [DOI] [PubMed] [Google Scholar]
- 55.Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH, Lam EW. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24(14):2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 56.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27(7):352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 57.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8(11):931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 58.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu S, Yoshida T, Tsujioka M, Arakawa S. Autophagic cell death and cancer. Int J Mol Sci. 2014;15(2):3145–3153. doi: 10.3390/ijms15023145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 61.Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol. 2015;309(7):C444–C456. doi: 10.1152/ajpcell.00188.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W, Kovacevic Z, Peng Z, Jin R, Wang P, Yue F, Zheng M, Huang ML, Jansson PJ, Richardson V, Kalinowski DS, Lane DJ, Merlot AM, Sahni S, Richardson DR. The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: new therapeutic targets. Oncotarget. 2015;6(34):35522–35541. doi: 10.18632/oncotarget.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao H, Xu E, Liu H, Wan L, Lai M. Epithelial-mesenchymal transition in colorectal cancer metastasis: a system review. Pathology, Res Pract. 2015;211(8):557–569. doi: 10.1016/j.prp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Ikeda K, Tsuta K. Effusion cytomorphology of small round cell tumors. J Cytol. 2016;33(2):85–92. doi: 10.4103/0970-9371.182529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marshall AD, Lagutina I, Grosveld GC. PAX3-FoxO1 induces cannabinoid receptor 1 to enhance cell invasion and metastasis. Cancer Res. 2011;71(24):7471–7480. doi: 10.1158/0008-5472.CAN-11-0924. [DOI] [PubMed] [Google Scholar]
- 66.Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand JP, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272(35):22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- 67.Sorensen PH, Lynch JC, Qualman SJ, Tirabosco R, Lim JF, Maurer HM, Bridge JA, Crist WM, Triche TJ, Barr FG. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children’s oncology group. J Clin Oncol. 2002;20(11):2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 68.Ko YS, Cho SJ, Park J, Kim Y, Choi YJ, Pyo JS, Jang BG, Park JW, Kim WH, Lee BL. Loss of FoxO1 promotes gastric tumour growth and metastasis through upregulation of human epidermal growth factor receptor 2/neu expression. Br J Cancer. 2015;113(8):1186–1196. doi: 10.1038/bjc.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Huang Q, Wang F. Inhibition of FoxO1 nuclear exclusion prevents metastasis of glioblastoma. Tumour Biol. 2014;35(7):7195–7200. doi: 10.1007/s13277-014-1913-1. [DOI] [PubMed] [Google Scholar]
- 70.Ding H, Zhu Y, Chu T, Wang S. Epidermal growth factor induces FoxO1 nuclear exclusion to activate MMP7-mediated metastasis of larynx carcinoma. Tumour Biol. 2014;35(10):9987–9992. doi: 10.1007/s13277-014-2067-x. [DOI] [PubMed] [Google Scholar]
- 71.Huang H, Tindall DJ. CDK2 and FoxO1: a fork in the road for cell fate decisions. ABBV Cell Cycle. 2007;6(8):902–906. doi: 10.4161/cc.6.8.4122. [DOI] [PubMed] [Google Scholar]
- 72.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphor-ylation of FoxO1 as an apoptotic response to DNA damage. Science. 2006;314(5797):294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 73.Feng X, Wu Z, Wu Y, Hankey W, Prior TW, Li L, Ganju RK, Shen R, Zou X. Cdc25A regulates matrix metalloprotease 1 through Foxo1 and mediates metastasis of breast cancer cells. Mol Cell Biol. 2011;31(16):3457–3471. doi: 10.1128/MCB.05523-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cangi MG, Cukor B, Soung P, Signoretti S, Moreira G, Jr, Ranashinge M, Cady B, Pagano M, Loda M. Role of the Cdc25A phosphatase in human breast cancer. J Clin Invest. 2000;106(6):753–761. doi: 10.1172/JCI9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer. 2007;7(7):495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 76.Ray D, Kiyokawa H. CDC25A phosphatase: a rate-limiting oncogene that determines genomic stability. Cancer Res. 2008;68(5):1251–1253. doi: 10.1158/0008-5472.CAN-07-5983. [DOI] [PubMed] [Google Scholar]
- 77.Wang XQ, Zhu YQ, Lui KS, Cai Q, Lu P, Poon RT. Aberrant Polo-like kinase 1-Cdc25A pathway in metastatic hepatocellular carcinoma. Clin Cancer Res. 2008;14(21):6813–6820. doi: 10.1158/1078-0432.CCR-08-0626. [DOI] [PubMed] [Google Scholar]
- 78.Karagoz ID, Ozaslan M, Cengiz B, Kalender ME, Kilic IH, Oztuzcu S, Gogebakan B, Demiryurek AT. CDC 25A gene 263C/T, −350C/T, and −51C/G polymorphisms in breast carcinoma. Tumour Biol. 2010;31(6):597–604. doi: 10.1007/s13277-010-0075-z. [DOI] [PubMed] [Google Scholar]
- 79.Lu H, Liu P, Pan Y, Huang H. Inhibition of cyclin-dependent kinase phosphorylation of FoxO1 and prostate cancer cell growth by a peptide derived from FoxO1. Neoplasia. 2011;13(9):854–863. doi: 10.1593/neo.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conidi A, Cazzola S, Beets K, Coddens K, Collart C, Cornelis F, Cox L, Joke D, Dobreva MP, Dries R, Esguerra C, Francis A, Ibrahimi A, Kroes R, Lesage F, Maas E, Moya I, Pereira PN, Stappers E, Stryjewska A, van den Berghe V, Vermeire L, Verstappen G, Seuntjens E, Umans L, Zwijsen A, Huylebroeck D. Few Smad proteins and many Smad-interacting proteins yield multiple functions and action modes in TGFbeta/BMP signaling in vivo. Cytokine Growth Factor Rev. 2011;22(5–6):287–300. doi: 10.1016/j.cytogfr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 81.Yang X, Wang J, Qu S, Zhang H, Ruan B, Gao Y, Ma B, Wang X, Wu N, Li X, Dou K, Li H. MicroRNA-200a suppresses metastatic potential of side population cells in human hepatocellular carcinoma by decreasing ZEB2. Oncotarget. 2015;6(10):7918–7929. doi: 10.18632/oncotarget.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong T, Zhang Y, Chen Y, Liu P, An T, Zhang J, Yang H, Zhu W, Yang X. FoxO1 inhibits the invasion and metastasis of hepatocellular carcinoma by reversing ZEB2-induced epithelial-mesenchymal transition. Oncotarget. 2017;8(1):1703–1713. doi: 10.18632/oncotarget.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia L, Huang W, Tian D, Zhang L, Qi X, Chen Z, Shang X, Nie Y, Wu K. Forkhead box Q1 promotes hepatocellular carcinoma metastasis by transactivating ZEB2 and VersicanV1 expression. Hepatology. 2014;59(3):958–973. doi: 10.1002/hep.26735. [DOI] [PubMed] [Google Scholar]
- 84.Nam EH, Lee Y, Park YK, Lee JW, Kim S. ZEB2 upregulates integrin alpha5 expression through cooperation with Sp1 to induce invasion during epithelial-mesenchymal transition of human cancer cells. Carcinogenesis. 2012;33(3):563–571. doi: 10.1093/carcin/bgs005. [DOI] [PubMed] [Google Scholar]
- 85.Sanchez-Tillo E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci: CMLS. 2012;69(20):3429–3456. doi: 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bang YJ. Advances in the management of HER2-positive advanced gastric and gastroesophageal junction cancer. J Clin Gastroenterol. 2012;46(8):637–648. doi: 10.1097/MCG.0b013e3182557307. [DOI] [PubMed] [Google Scholar]
- 87.Lin W, Kao HW, Robinson D, Kung HJ, Wu CW, Chen HC. Tyrosine kinases and gastric cancer. Oncogene. 2000;19(49):5680–5689. doi: 10.1038/sj.onc.1203924. [DOI] [PubMed] [Google Scholar]
- 88.Wu Y, Shang X, Sarkissyan M, Slamon D, Vadgama JV. FoxO1A is a target for HER2-overexpressing breast tumors. Cancer Res. 2010;70(13):5475–5485. doi: 10.1158/0008-5472.CAN-10-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park J, Ko YS, Yoon J, Kim MA, Park JW, Kim WH, Choi Y, Kim JH, Cheon Y, Lee BL. The forkhead transcription factor FoxO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric Cancer. 2014;17(3):423–430. doi: 10.1007/s10120-013-0314-2. [DOI] [PubMed] [Google Scholar]
- 90.Choi Y, Ko YS, Park J, Choi Y, Kim Y, Pyo JS, Jang BG, Hwang DH, Kim WH, Lee BL. HER2-induced metastasis is mediated by Akt/JNK/EMT signaling pathway in gastric cancer. World J Gastroenterol. 2016;22(41):9141–9153. doi: 10.3748/wjg.v22.i41.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223(1):14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 92.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6(3):209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14(7):667–678. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reynolds AB, Kanner SB, Bouton AH, Schaller MD, Weed SA, Flynn DC, Parsons JT. SRChing for the substrates of Src. Oncogene. 2014;33(37):4537–4547. doi: 10.1038/onc.2013.416. [DOI] [PubMed] [Google Scholar]
- 95.Belsches AP, Haskell MD, Parsons SJ. Role of c-Src tyrosine kinase in EGF-induced mitogenesis. Front Biosci. 1997;2:d501–18. doi: 10.2741/a208. [DOI] [PubMed] [Google Scholar]
- 96.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;33(3):122–128. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang H, Tindall DJ. Regulation of FoxO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta. 2011;1813(11):1961–1964. doi: 10.1016/j.bbamcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bulow MH, Bulow TR, Hoch M, Pankratz MJ, Junger MA. Src tyrosine kinase signaling antagonizes nuclear localization of FoxO and inhibits its transcription factor activity. Sci Rep. 2014;4:4048. doi: 10.1038/srep04048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tangir J, Bonafe N, Gilmore-Hebert M, Henegariu O, Chambers SK. SGK1, a potential regulator of c-fms related breast cancer aggressiveness. Clin Exp Metastasis. 2004;21(6):477–483. doi: 10.1007/s10585-004-4226-8. [DOI] [PubMed] [Google Scholar]
- 100.Salis O, Okuyucu A, Bedir A, Gor U, Kulcu C, Yenen E, Kilic N. Antimetastatic effect of fluvastatin on breast and hepatocellular carcinoma cells in relation to SGK1 and NDRG1 genes. Tumour Biol. 2016;37(3):3017–3024. doi: 10.1007/s13277-015-4119-2. [DOI] [PubMed] [Google Scholar]
- 101.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FoxO3a) Mol Cell Biol. 2001;21(3):952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mason JA, Schafer ZT. SGK-1 and pH LPP1. ras-mediated effectors during ECM-detachment. Cell Cycle (Georgetown Tex) 2016;15(17):2233–2234. doi: 10.1080/15384101.2016.1194136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun J, Zhang D, Bae DH, Sahni S, Jansson P, Zheng Y, Zhao Q, Yue F, Zheng M, Kovacevic Z, Richardson DR. Metastasis suppressor, NDRG1, mediates its activity through signaling pathways and molecular motors. Carcinogenesis. 2013;34(9):1943–1954. doi: 10.1093/carcin/bgt163. [DOI] [PubMed] [Google Scholar]
- 104.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, Peled A. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18(11):1240–1242. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 105.Singh S, Singh UP, Grizzle WE, Lillard JW., Jr CXCL12-CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Lab Invest. 2004;84(12):1666–1676. doi: 10.1038/labinvest.3700181. [DOI] [PubMed] [Google Scholar]
- 106.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94(11):3658–3667. [PubMed] [Google Scholar]
- 107.Barretina J, Junca J, Llano A, Gutierrez A, Flores A, Blanco J, Clotet B, Este JA. CXCR4 and SDF-1 expression in B-cell chronic lymphocytic leukemia and stage of the disease. Ann Hematol. 2003;82(8):500–505. doi: 10.1007/s00277-003-0679-0. [DOI] [PubMed] [Google Scholar]
- 108.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267(2):226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 109.Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215(3):211–213. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]
- 110.Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60(7):497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 111.Ehtesham M, Mapara KY, Stevenson CB, Thompson RC. CXCR4 mediates the proliferation of glioblastoma progenitor cells. Cancer Lett. 2009;274(2):305–312. doi: 10.1016/j.canlet.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Engl T, Relja B, Marian D, Blumenberg C, Muller I, Beecken WD, Jones J, Ringel EM, Bereiter-Hahn J, Jonas D, Blaheta RA. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 2006;8(4):290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67(1):61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 114.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15(3):657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tan CT, Chu CY, Lu YC, Chang CC, Lin BR, Wu HH, Liu HL, Cha ST, Prakash E, Ko JY, Kuo ML. CXCL12/CXCR4 promotes laryngeal and hypo-pharyngeal squamous cell carcinoma metastasis through MMP-13-dependent invasion via the ERK1/2/AP-1 pathway. Carcinogenesis. 2008;29(8):1519–1527. doi: 10.1093/carcin/bgn108. [DOI] [PubMed] [Google Scholar]
- 116.Wang Q, Diao X, Sun J, Chen Z. Regulation of VEGF, MMP-9 and metastasis by CXCR4 in a prostate cancer cell line. Cell Biol Int. 2011;35(9):897–904. doi: 10.1042/CBI20100744. [DOI] [PubMed] [Google Scholar]
- 117.Dubrovska A, Elliott J, Salamone RJ, Telegeev GD, Stakhovsky AE, Schepotin IB, Yan F, Wang Y, Bouchez LC, Kularatne SA, Watson J, Trussell C, Reddy VA, Cho CY, Schultz PG. CXCR4 expression in prostate cancer progenitor cells. PLoS One. 2012;7(2):e31226. doi: 10.1371/journal.pone.0031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bullock MD, Sayan AE, Packham GK, Mirnezami AH. MicroRNAs: critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol Cell. 2012;104(1):3–12. doi: 10.1111/boc.201100115. [DOI] [PubMed] [Google Scholar]
- 119.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 120.Li H, Ouyang R, Wang Z, Zhou W, Chen H, Jiang Y, Zhang Y, Li H, Liao M, Wang W, Ye M, Ding Z, Feng X, Liu J, Zhang B. MiR-150 promotes cellular metastasis in non-small cell lung cancer by targeting FoxO4. Sci Rep. 2016;6:39001. doi: 10.1038/srep39001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu MM, Mao GX, Liu J, Li JC, Huang H, Liu YF, Liu JH. Low expression of the FoxO4 gene may contribute to the phenomenon of EMT in non-small cell lung cancer. Asian Pac J Cancer Prev: APJCP. 2014;15(9):4013–4018. doi: 10.7314/apjcp.2014.15.9.4013. [DOI] [PubMed] [Google Scholar]
- 122.Lee MJ, Yu GR, Yoo HJ, Kim JH, Yoon BI, Choi YK, Kim DG. ANXA8 down-regulation by EGF-FoxO4 signaling is involved in cell scattering and tumor metastasis of cholangiocarcinoma. Gastroenterology. 2009;137(3):1138–1150. 1150 e1–9. doi: 10.1053/j.gastro.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 123.Sugawara M, Miyoshi H, Miura T, Tanaka H, Tsubota KI, Liu H. Dynamics of actin stress fibers and focal adhesions during slow migration in swiss 3T3 fibroblasts: intracellular mechanism of cell turning. BioMed Res Int. 2016;2016:5749749. doi: 10.1155/2016/5749749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su B, Gao L, Baranowski C, Gillard B, Wang J, Ransom R, Ko HK, Gelman IH. A genome-wide RNAi screen identifies FoxO4 as a metastasis-suppressor through counteracting PI3 K/Akt signal pathway in prostate cancer. PLoS One. 2014;9(7):e101411. doi: 10.1371/journal.pone.0101411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oka R, Nakashiro K, Goda H, Iwamoto K, Tokuzen N, Hamakawa H. Annexin A8 is a novel molecular marker for detecting lymph node metastasis in oral squamous cell carcinoma. Oncotarget. 2016;7(4):4882–4889. doi: 10.18632/oncotarget.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu X, Zhang Z, Sun L, Chai N, Tang S, Jin J, Hu H, Nie Y, Wang X, Wu K, Jin H, Fan D. MicroRNA-499-5p promotes cellular invasion and tumor metastasis in colorectal cancer by targeting FoxO4 and PDCD4. Carcinogenesis. 2011;32(12):1798–1805. doi: 10.1093/carcin/bgr213. [DOI] [PubMed] [Google Scholar]
- 127.Hu HJ, Zhang LG, Wang ZH, Guo XX. FoxO6 inhibits cell proliferation in lung carcinoma through up-regulation of USP7. Mol Med Rep. 2015;12(1):575–580. doi: 10.3892/mmr.2015.3362. [DOI] [PubMed] [Google Scholar]
- 128.Wang JH, Tang HS, Li XS, Zhang XL, Yang XZ, Zeng LS, Ruan Q, Huang YH, Liu GJ, Wang J, Cui SZ. Elevated FoxO6 expression correlates with progression and prognosis in gastric cancer. Oncotarget. 2017;8(19):31682–31691. doi: 10.18632/oncotarget.15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li J, Du L, Yang Y, Wang C, Liu H, Wang L, Zhang X, Li W, Zheng G, Dong Z. MiR-429 is an independent prognostic factor in colorectal cancer and exerts its anti-apoptotic function by targeting SOX2. Cancer Lett. 2013;329(1):84–90. doi: 10.1016/j.canlet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 130.Jia X, Li X, Xu Y, Zhang S, Mou W, Liu Y, Liu Y, Lv D, Liu CH, Tan X, Xiang R, Li N. SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J Mol Cell Biol. 2011;3(4):230–238. doi: 10.1093/jmcb/mjr002. [DOI] [PubMed] [Google Scholar]
- 131.Leis O, Eguiara A, Lopez-Arribillaga E, Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R, Martin AG. Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene. 2012;31(11):1354–1365. doi: 10.1038/onc.2011.338. [DOI] [PubMed] [Google Scholar]
- 132.Rothenberg SM, Concannon K, Cullen S, Boulay G, Turke AB, Faber AC, Lockerman EL, Rivera MN, Engelman JA, Maheswaran S, Haber DA. Inhibition of mutant EGFR in lung cancer cells triggers SOX2-FoxO6-dependent survival pathways. eLife. 2015;4 doi: 10.7554/eLife.06132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lam EW, Shah K, Brosens JJ. The diversity of sex steroid action: the role of micro-RNAs and FoxO transcription factors in cycling endometrium and cancer. J Endocrinol. 2012;212(1):13–25. doi: 10.1530/JOE-10-0480. [DOI] [PubMed] [Google Scholar]
- 134.Urbanek P, Klotz LO. Posttranscriptional regulation of FoxO expression: microRNAs and beyond. Br J Pharmacol. 2017;174(12):1514–1532. doi: 10.1111/bph.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang GJ, Liu GH, Ye YW, Fu Y, Zhang XF. The role of microRNA-1274a in the tumorigenesis of gastric cancer: accelerating cancer cell proliferation and migration via directly targeting FoxO4. Biochem Biophys Res Commun. 2015;459(4):629–635. doi: 10.1016/j.bbrc.2015.02.160. [DOI] [PubMed] [Google Scholar]
- 136.Li J, Hu L, Tian C, Lu F, Wu J, Liu L. microRNA-150 promotes cervical cancer cell growth and survival by targeting FoxO4. BMC Mol Biol. 2015;16:24. doi: 10.1186/s12867-015-0052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pei H, Jin Z, Chen S, Sun X, Yu J, Guo W. MiR-135b promotes proliferation and invasion of osteosarcoma cells via targeting FoxO1. Mol Cell Biochem. 2015;400(1–2):245–252. doi: 10.1007/s11010-014-2281-2. [DOI] [PubMed] [Google Scholar]
- 138.Wallis CJ, Gordanpour A, Bendavid JS, Sugar L, Nam RK, Seth A. MiR-182 is associated with growth, migration and invasion in prostate cancer via suppression of FoxO1. J Cancer. 2015;6(12):1295–1305. doi: 10.7150/jca.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang RM, Xu JF, Fang F, Yang H, Yang LY. MicroRNA-130b promotes proliferation and EMT-induced metastasis via PTEN/p-Akt/HIF-1alpha signaling. Tumour Biol. 2016;37(8):10609–10619. doi: 10.1007/s13277-016-4919-z. [DOI] [PubMed] [Google Scholar]
- 140.Yoo JO, Kwak SY, An HJ, Bae IH, Park MJ, Han YH. miR-181b-3p promotes epithelial-mesenchymal transition in breast cancer cells through Snail stabilization by directly targeting YWHAG. Biochim Biophys Acta. 2016;1863(7 Pt A):1601–1611. doi: 10.1016/j.bbamcr.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 141.Chang RM, Yang H, Fang F, Xu JF, Yang LY. MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting pH domain and leucine-rich repeat protein phosphatase. Hepatology. 2014;60(4):1251–1263. doi: 10.1002/hep.27221. [DOI] [PubMed] [Google Scholar]
- 142.Cheng CW, Chen PM, Hsieh YH, Weng CC, Chang CW, Yao CC, Hu LY, Wu PE, Shen CY. Foxo3a-mediated overexpression of microRNA-622 suppresses tumor metastasis by repressing hypoxia-inducible factor-1alpha in ERK-responsive lung cancer. Oncotarget. 2015;6(42):44222–44238. doi: 10.18632/oncotarget.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maiese K, Chong ZZ, Shang YC, Hou J. A FoxO in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maiese K, Chong ZZ, Shang YC, Hou J. Clever cancer strategies with FoxO transcription factors. Cell Cycle. 2008;7(24):3829–3839. doi: 10.4161/cc.7.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 146.Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10(4):236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li ZC, Zhang LM, Wang HB, Ma JX, Sun JZ. Curcumin inhibits lung cancer progression and metastasis through induction of FoxO1. Tumour Biol. 2014;35(1):111–116. doi: 10.1007/s13277-013-1013-7. [DOI] [PubMed] [Google Scholar]
- 148.Chen Q, Ganapathy S, Singh KP, Shankar S, Srivastava RK. Resveratrol induces growth arrest and apoptosis through activation of FoxO transcription factors in prostate cancer cells. PLoS One. 2010;5(12):e15288. doi: 10.1371/journal.pone.0015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pramanik KC, Fofaria NM, Gupta P, Srivastava SK. CBP-mediated FoxO-1 acetylation inhibits pancreatic tumor growth by targeting SirT. Mol Cancer Ther. 2014;13(3):687–696. doi: 10.1158/1535-7163.MCT-13-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Afrasiabi E, Ahlgren J, Bergelin N, Tornquist K. Phorbol 12-myristate 13-acetate inhibits FRO anaplastic human thyroid cancer cell proliferation by inducing cell cycle arrest in G1/S phase: evidence for an effect mediated by PKCdelta. Mol Cell Endocrinol. 2008;292(1–2):26–35. doi: 10.1016/j.mce.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 151.Shukla S, Bhaskaran N, Babcook MA, Fu P, Maclennan GT, Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3 K/Akt/FoxO pathway. Carcinogenesis. 2014;35(2):452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3 K/Akt/FoxO pathway. Clin Cancer Res. 2011;17(7):1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lam M, Carmichael AR, Griffiths HR. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FoxO3a and p53 expression. PLoS One. 2012;7(6):e40152. doi: 10.1371/journal.pone.0040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Belguise K, Guo S, Sonenshein GE. Activation of FoxO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor alpha expression reversing invasive phenotype of breast cancer cells. Cancer Res. 2007;67(12):5763–5770. doi: 10.1158/0008-5472.CAN-06-4327. [DOI] [PubMed] [Google Scholar]
- 155.Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. Regulation of Akt/FoxO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem. 2008;283(41):27707–27716. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharma G, Kar S, Palit S, Das PK. 18beta-glycyrrhetinic acid induces apoptosis through modulation of Akt/FoxO3a/Bim pathway in human breast cancer MCF-7 cells. J Cell Physiol. 2012;227(5):1923–1931. doi: 10.1002/jcp.22920. [DOI] [PubMed] [Google Scholar]
- 157.He L, Yang X, Cao X, Liu F, Quan M, Cao J. Casticin induces growth suppression and cell cycle arrest through activation of FoxO3a in hepatocellular carcinoma. Oncol Rep. 2013;29(1):103–108. doi: 10.3892/or.2012.2076. [DOI] [PubMed] [Google Scholar]
- 158.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Majid S, Igawa M, Dahiya R. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123(3):552–560. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- 159.Zheng F, Wu J, Zhao S, Luo Q, Tang Q, Yang L, Li L, Wu W, Hann SS. Baicalein increases the expression and reciprocal interplay of RUNX3 and FoxO3a through crosstalk of AMPKalpha and MEK/ERK1/2 signaling pathways in human non-small cell lung cancer cells. J Exp Clin Cancer Res. 2015;34:41. doi: 10.1186/s13046-015-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou R, Lu Z, Liu K, Guo J, Liu J, Zhou Y, Yang J, Mi M, Xu H. Platycodin D induces tumor growth arrest by activating FoxO3a expression in prostate cancer in vitro and in vivo. Curr Cancer Drug Targets. 2015;14(9):860–871. doi: 10.2174/1568009614666141128104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang J, Liu S, Yin Y, Li M, Wang B, Yang L, Jiang Y. FoxO3-mediated up-regulation of Bim contributes to rhein-induced cancer cell apoptosis. Apoptosis. 2015;20(3):399–409. doi: 10.1007/s10495-014-1071-3. [DOI] [PubMed] [Google Scholar]
- 162.Ananda Sadagopan SK, Mohebali N, Looi CY, Hasanpourghadi M, Pandurangan AK, Arya A, Karimian H, Mustafa MR. Forkhead Box Transcription Factor (FoxO3a) mediates the cytotoxic effect of vernodalin in vitro and inhibits the breast tumor growth in vivo. J Exp Clin Cancer Res. 2015;34:147. doi: 10.1186/s13046-015-0266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li X, Wu Q, Xie Y, Ding Y, Du WW, Sdiri M, Yang BB. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget. 2015;6(19):17832–17846. doi: 10.18632/oncotarget.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang L, Yang X, Li X, Li C, Zhao L, Zhou Y, Hou H. Butein sensitizes HeLa cells to cisplatin through the Akt and ERK/p38 MAPK pathways by targeting FoxO3a. Int J Mol Med. 2015;36(4):957–966. doi: 10.3892/ijmm.2015.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu CC, Wu PJ, Hsu JL, Ho YF, Hsu LC, Chang YJ, Chang HS, Chen IS, Guh JH. Ardisianone, a natural benzoquinone, efficiently induces apoptosis in human hormone-refractory prostate cancers through mitochondrial damage stress and survivin downregulation. Prostate. 2013;73(2):133–145. doi: 10.1002/pros.22548. [DOI] [PubMed] [Google Scholar]
- 166.Liu J, Mu C, Yue W, Li J, Ma B, Zhao L, Liu L, Chen Q, Yan C, Liu H, Hao X, Zhu Y. A diterpenoid derivate compound targets selenocysteine of thioredoxin reductases and induces Bax/Bak-independent apoptosis. Free Radic Biol Med. 2013;63:485–494. doi: 10.1016/j.freeradbiomed.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 167.Yuan L, Wang J, Xiao H, Xiao C, Wang Y, Liu X. Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3 K/Akt signaling pathway in HepG2 cancer cells. Toxicol Appl Pharmacol. 2012;265(1):83–92. doi: 10.1016/j.taap.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 168.Rexer BN, Arteaga CL. Optimal targeting of HER2-PI3 K signaling in breast cancer: mechanistic insights and clinical implications. Cancer Res. 2013;73(13):3817–3820. doi: 10.1158/0008-5472.CAN-13-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Holmes FA, Espina V, Liotta LA, Nagarwala YM, Danso M, McIntyre KJ, Osborne CR, Anderson T, Krekow L, Blum JL, Pippen J, Florance A, Mahoney J, O’Shaughnessy JA. Pathologic complete response after preoperative anti-HER2 therapy correlates with alterations in PTEN FoxO, phosphorylated Stat5, and autophagy protein signaling. BMC Res Notes. 2013;6:507. doi: 10.1186/1756-0500-6-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schott A GA, Perez R, Avery T, Kato G. Pilot Study to Evaluate Reparixin With Weekly Paclitaxel in Patients With HER 2 Negative Metastatic Breast Cancer ClinicalTrials.gov. 2013 doi: 10.1158/1078-0432.CCR-16-2748. NCT02001974. [DOI] [PMC free article] [PubMed] [Google Scholar]


