Abstract
Background
Detection of a “central vein sign” (CVS) on FLAIR* MRI is highly specific and sensitive for multiple sclerosis (MS). We evaluated the specificity and sensitivity of simplified CVS algorithms for MS diagnosis.
Methods
MRIs from 10 participants with MS without additional comorbidities for MRI white matter abnormalities; 10 with MS and additional comorbidities for white matter abnormalities; 10 with migraine, white matter abnormalities, and no additional comorbidities; and 10 who had previously been erroneously diagnosed with MS were evaluated. 3T MRI T2-FLAIR and T2*-weighted sequences were acquired to create FLAIR* images. Three MS physician reviewers, blinded to diagnosis, evaluated two different algorithms: 1) Three lesions pre-selected on FLAIR were subsequently evaluated for CVS on FLAIR*(select3). 2) FLAIR* was evaluated for up to three lesions with CVS(select3*).
Results
For select3, average specificity across reviewers for MS was 0.98 and sensitivity 0.52 and a correct prediction of diagnosis demonstrated kappa=0.29. For select3*, specificity was 0.81, sensitivity 0.83, and kappa 0.31.
Conclusion
A simplified determination of CVS in three white matter lesions on 3T FLAIR* MRI demonstrated good specificity and sensitivity and fair inter-rater reliability for a diagnosis of MS and with further study, may be a candidate for clinical application.
Keywords: multiple sclerosis, MRI, biomarker
Introduction
The diagnosis of multiple sclerosis (MS) relies on the interpretation of clinical and radiographic data.1 MS diagnostic criteria have high specificity and sensitivity when applied in the setting of syndromes typical for demyelination.2 Yet, misdiagnosis of MS is a persistent problem that results in unnecessary medical risk and morbidity.3–5 Development of MRI criteria has facilitated earlier diagnosis,2 but their specificity is diminished when applied in patients presenting with clinical syndromes atypical for demyelination.2 Overreliance on the presence of abnormalities satisfying MS MRI criteria in patients with nonspecific or atypical clinical syndromes likely contributes frequently to misdiagnosis.3
New imaging techniques, such as the recently described “central vein sign” (CVS),6, 7 may improve the MRI differentiation of MS from other disorders. Several imaging techniques have been used for CVS detection,8 and various criteria have been proposed.9–11 The North American Imaging in Multiple Sclerosis (NAIMS) Cooperative recently published consensus recommendations for evaluation of CVS.12 Most previous studies have differentiated MS and non-MS populations based on the proportion of total lesions per MRI scan demonstrating CVS,6, 9 a method impractical for clinical application, whereas few studies have evaluated algorithms assessing for CVS in a limited number of lesions per person.7, 11 In a previous pilot study performed prior to NAIMS consensus recommendations,11 a method evaluating three lesions for CVS (select3) differentiated 10 participants with MS from 10 with migraine and white matter abnormalities when tested by a single rater. Here, this prior cohort was enlarged to 40 study participants, and both select3 and an additional simplified algorithm (select3*) were tested by three raters from three different institutions.
Methods
The study was approved by the University of Vermont Institutional Review Board. Written informed consent was obtained from all participants.
Forty participants, comprising four cohorts, participated in the study. These included: (1) 10 with a diagnosis of MS by 2010 criteria and no history of a comorbidity that may also cause MRI white matter abnormalities (“MS”); (2) 10 with a diagnosis of MS by 2010 criteria and at least one additional comorbidity known to cause MRI white matter abnormalities (“MS+”); (3) 10 with a diagnosis of migraine and a previous history of an MRI with at least 2 white matter abnormalities in any location but no additional comorbidities known to cause white matter abnormalities (“migraine”); and (4) 10 who had been previously incorrectly diagnosed with MS, who did not meet 2010 diagnostic criteria, and in whom a variety of diagnoses had been identified to explain clinical and radiographic abnormalities mistaken for MS (“misdiagnosed”). Diagnoses in the MS and MS+ and misdiagnosed cohorts had been established after evaluation by a neurologist with MS subspecialty training. Diagnoses in the migraine cohort had been established after evaluation by a neurologist. For analysis, the “total MS cohort” was defined as MS and MS+ combined and the “non-MS cohort” as migraine and misdiagnosed combined.
T2-weighted FLAIR (1 mm isotropic resolution) and T2*-weighted segmented echo-planar imaging (0.55 mm isotropic resolution) data were acquired on a 3T Philips dStream MRI with 32-channel head coil. A single-dose of gadopentetate dimeglumine (Magnevist) was injected manually, and acquisition of the T2* sequence followed immediately. FLAIR images were coregistered and resampled to the space of the T2* images using SPM8. The product of the voxelwise signal intensities, FLAIR*,13 was imported to a clinical image viewer to allow multiplanar reformatting.
NAIMS consensus recommendations12 were used to identify candidate MRI lesions for CVS evaluation: discrete ovoid lesions were included and confluent lesions excluded. The NAIMS criteria were slightly modified to allow discrete ovoid lesions 3mm or greater in at least one plane, rather than 3mm in all planes as the latter is technically challenging to assess in routine practice. A “positive” CVS required visualization of the vessel in at least two perpendicular planes to assure it was “central”; lesions with multiple vessels were excluded.
All MRIs were coded, and randomly ordered. Three trained neurologists (AJS, DO, MA), from three different institutions, all experienced in MS neuroimaging and blinded to the diagnosis in each case, served as reviewers for CVS evaluation. Two different algorithms assessing a limited number of lesions per subject were completed. The 40 MRI scans were re-ordered and re-numbered by a study collaborator (RW) before transmission to the reviewers for evaluation of the second algorithm, and a minimum of twenty-six days elapsed between reviews.
In the first algorithm – select3 – reviewers identified 3 candidate lesions restricted to the subcortical or deep white matter on the FLAIR sequence. MRIs with <3 candidate lesions were excluded from evaluation. Only after selection of all 3 lesions on FLAIR was each of the 3 pre-selected lesions identified and evaluated on the FLAIR* sequence for CVS. If a lesion demonstrated more than one vessel on FLAIR*, it was excluded, and a new lesion was selected on FLAIR and then evaluated for CVS on FLAIR*. Select3 was first developed and evaluated in the MS and migraine cohorts in this study, and with a single CVS reviewer, as published in a prior study.11
The second algorithm – select3* – was based on only evaluation of the FLAIR* sequence. Reviewers determined if there were at minimum 3 candidate lesions restricted to subcortical, deep white matter, and juxtacortical regions. Participant MRIs with <3 candidate lesions were excluded. Reviewers then determined whether at least 3 candidate lesions in these regions demonstrated CVS.
For select3, specificity was defined as the probability that scans from the non-MS cohort would have fewer than 3/3 or 2/3 pre-selected lesions demonstrating CVS. Sensitivity was defined as the probability that the MS cohort would have either 3/3 or 2/3 pre-selected lesions demonstrating CVS. For select3*, specificity was defined as the probability that reviewers were unable to identify at least 2, or at least 3, CVS+ lesions on FLAIR* in the non-MS cohort. Sensitivity was defined as the probability that up to 2 or 3 lesions with CVS were identified on FLAIR* in MS participants.
Since it was possible that there was not always agreement on whether there were 3 candidate lesions for inclusion for CVS evaluation, for the calculation of the average specificity and sensitivity across reviewers for each algorithm, any given MRI might be weighted from 0 to 3 times. Inter-reviewer agreement (Cohen’s Kappa) was quantified based on the correct diagnosis rate per reviewer.
After select3 and select3 * analysis was complete, a single reviewer blinded to diagnosis (AJS) evaluated the FLAIR brain MRIs acquired for each study participant to determine if 2010 MRI dissemination in space (DIS) crtieria1 were met.
Results
Cohort Description
Baseline demographics are presented in Table 1. There was no significant difference among the four cohorts for age (ANOVA p = 0.24). In the MS and MS+ cohorts, respectively, 8/10 and 9/10 participants were receiving disease modifying therapy (DMT) at the time of participation in the study. The comorbid conditions known to cause MRI white matter abnormalities in the MS+ cohort included: migraine (4), hypertension (3), hypertension and migraine (1), diabetes mellitus and migraine (1), diabetes mellitus and hypertension (1). In addition, 6/10 participants in this cohort had a comorbid history of tobacco use.
Table 1.
Study Participant Characteristics
| MS (n=10) | |
|---|---|
| age | 44 (16) |
| sex | 9 F/1 M |
| years since clinical onset of MS | 9 (7) |
| phenotype | 10/10 RRMS |
|
| |
| MS+ (n=10) | |
| age | 43 (9) |
| sex | 9 F/1 M |
| years since clinical onset of MS | 9 (6) |
| phenotype | 10/10 RRMS |
|
| |
| Migraine (n=10) | |
| age | 47 (13) |
| sex | 10 F |
|
| |
| Misdiagnosed (n=10) | |
| age | 53 (7) |
| sex | 9 F/1 M |
MS: multiple sclerosis without comorbidities for MRI white matter abnormalities, MS+: multiple sclerosis with additional comorbidities for MRI white matter abnormalities, Migraine: migraine with MRI white matter abnormalities without additional comorbidities for MRI white matter abnormalities, misdiagnosed: previously misdiagnosed with multiple sclerosis, n: number of participants, RRMS: relapsing-remitting multiple sclerosis
Note: values for age and years since clinical onset of MS are given as mean (standard deviation).
Mean duration of misdiagnosis in the misdiagnosed cohort was 9 years (median: 6). In this cohort, 5/10 had received DMT therapy for MS in the past. CSF evaluation, including testing for elevation in intrathecal IgG production or oligoclonal bands, was normal in 9 and had not been performed in the remaining participant. MRI of the whole spinal cord had been performed in 6/10 and was normal; only cervical spinal cord MRI had been performed in the remaining 4/10 and was normal in all cases.
The alternative diagnoses in the misdiagnosed cohort included: migraine (8), psychogenic disorder (4), trigeminal neuralgia (1), B12 deficiency (1), vertigo (1), and transient numbness (1). Diagnoses presumed responsible for abnormal brain MRI findings in this cohort included: migraine (8), small vessel ischemic disease (SVID) due to hypertension (2), SVID due to tobacco use (7), andvitamin B12 deficiency(1). Of note, 6/10 of the participants in the misdiagnosed cohort had more than one clinical diagnosis and 6/10 had more than radiographic explanation for MRI abnormalities.
Specificity and Sensitivity
Figure 1 depicts CVS in a lesion from one participant from each of the MS and MS+ cohorts with enlarged axial and sagittal views, and Figure 2 depicts candidate lesions without CVS in one participant from each of the migraine and misdiagnosed cohorts with enlarged axial and sagittal views. Table 2 presents average specificity and sensitivity for the two algorithms in the total MS and non-MS cohorts, and Table 3 presents similar data across the four cohorts considered separately.
Figure 1.
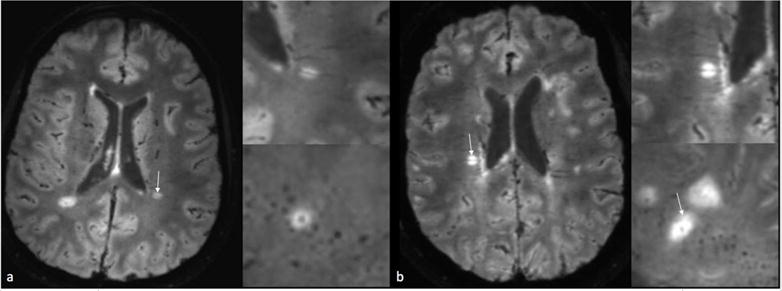
a: 3T FLAIR* MRI from a participant from the MS cohort demonstrating central vein sign b: 3T FLAIR* MRI from a participant from the MS+ cohort demonstrating central vein sign
Figure 2.
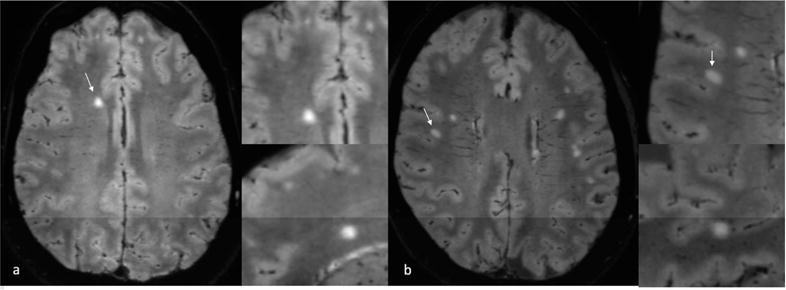
a: 3T FLAIR* MRI from a participant from the migraine cohort demonstrating a candidate lesion without central vein sign b: 3T FLAIR* MRI from a participant from the misdiagnosed cohort with diagnoses of migraine, vitamin B12 deficiency, and chronic tobacco use demonstrating a candidate lesion without central vein sign
Table 2.
Average Specificity and Sensitivity for a Diagnosis of Multiple Sclerosis Evaluating Two Algorithms for Central Vein Sign Detection
| Average Specificity (range) | Average Sensitivity (range) | |
|---|---|---|
|
| ||
| Select3 | ||
| 3/3 with CVS | 0.98 (0.95–1.00) | 0.52 (0.41–0.65) |
| 2/3 with CVS | 0.95 (0.90–1.00) | 0.81 (0.76–0.88) |
|
| ||
| Select3* | ||
| at least 3 w/CVS | 0.81 (0.68–0.95) | 0.83 (0.76–0.94) |
| at least 2 w/CVS | 0.65 (0.42–0.89) | 0.90 (0.83–1.00) |
CVS: Central Vein Sign
Table 3.
Comparison Between Individual Cohorts: Average Specificity and Sensitivity for a Diagnosis of Multiple Sclerosis Evaluating Two Algorithms for Central Vein Sign Detection
| Select3, 3/3 with CVS | Average Specificity (range) | Average Sensitivity (range) |
|---|---|---|
|
| ||
| MS vs Migraine | 1.00 (1.00–1.00) | 0.52 (0.50–0.57) |
| MS vs Misdiagnosed | 0.97 (0.90–1.00) | 0.52 (0.50–0.57) |
| MS+ vs Migraine | 1.00 (1.00–1.00) | 0.51 (0.33–0.78) |
| MS+ vs Misdiagnosed | 0.97 (0.90–1.00) | 0.51 (0.33–0.78) |
|
| ||
| Select3, 2/3 with CVS | ||
|
| ||
| MS vs Migraine | 0.97 (0.90–1.00) | 0.74 (0.63–0.88) |
| MS vs Misdiagnosed | 0.93 (0.90–1.00) | 0.74 (0.63–0.88) |
| MS+ vs Migraine | 0.97 (0.90–1.00) | 0.88 (0.86–0.89) |
| MS+ vs Misdiagnosed | 0.93 (0.90–1.00) | 0.88 (0.86–0.89) |
|
| ||
| Select3*, 3 w/CVS | ||
|
| ||
| MS vs Migraine | 0.89 (0.78–1.00) | 0.73 (0.63–0.88) |
| MS vs Misdiagnosed | 0.71 (0.44–0.90) | 0.73 (0.63–0.88) |
| MS+ vs Migraine | 0.89 (0.78–1.00) | 0.93 (0.89–1.00) |
| MS+ vs Misdiagnosed | 0.71 (0.44–0.90) | 0.93 (0.89–1.00) |
|
| ||
| Select3*, 2 w/CVS | ||
|
| ||
| MS vs Migraine | 0.67 (0.33–0.89) | 0.81 (0.67–1.00) |
| MS vs Misdiagnosed | 0.61 (0.44–0.90) | 0.81 (0.67–1.00) |
| MS+ vs Migraine | 0.67 (0.33–0.89) | 1.00 (1.00–1.00) |
| MS+ vs Misdiagnosed | 0.61 (0.44–0.90) | 1.00 (1.00–1.00) |
MS: MS with no history of a comorbidity that may also cause MRI white matter abnormalities, MS+: MS with history of at least one additional comorbidity known to cause MRI white matter abnormalities, migraine: diagnosis of migraine and a previous history of an MRI with at least 2 white matter abnormalities in any location but with no history of additional comorbidities known to cause white matter abnormalities, misdiagnosed: previously incorrectly diagnosed with MS
Per study methodology, MRIs with <3 candidate lesions in the pre-specified regions were excluded from CVS evaluation for both algorithms. For select3, in the total MS cohort, two MRIs were excluded by all three reviewers, two MRIs by two reviewers, and two MRIs by one reviewer. In the non-MS cohort, one MRIwas excluded by two reviewers and one MRI by one reviewer. As a result, rather than the 20 MRIs from the total MS and non-MS cohorts having been reviewed once by each reviewer (i.e. 60 reviews per cohort), 48 reviews of MRIs from the total MS cohort and 57 reviews of MRIs from the total non-MS cohort were entered into the calculation of average specificity and sensitivity for MS diagnosis for the select3 algorithm.
For select3*, in the total MS cohort, one participant MRI was excluded by all three reviewers, and two participant MRIs by two reviewers. In the non-MS cohort, one participant MRIs was excluded by two reviewers and one MRI by one reviewer. As a result, MRIs in from the total MS cohort reviewed 53 times, and MRIs from the non-MS cohort reviewed 57 times, were used to calculate the average specificity and sensitivity for MS diagnosis for the select3* algorithm.
In sum, 13% of reviews for select3, and 8% of reviews for select3*, were excluded.
For select3, per study methodology the three reviewers excluded a total of 4, 7, and 11 lesions respectively, due to the presence of multiple vessels on FLAIR*.
Inter-Rater Reliability Evaluation
For the inter-reviewer reliability analysis, only MRIs evaluated for CVS by all three reviewers were included. For select3, this included 14 total MS and 18 non-MS participants; for select3*, 17 total MS and 18 non-MS participants. For select3, a correct prediction of diagnosis using 3/3 CVS lesions demonstrated kappa=0.29, and using 2/3 CVS lesions, kappa=0.87. Using select3*, a correct prediction of diagnosis demonstrated kappa=0.31 with identification of at least 3 lesions with CVS, and kappa=0.74 with CVS in at least 2 lesions.
MRI Dissemination in Space Criteria
In the total MS cohort, 90% (18/20) met MRI DIS compared to 45% (9/20) in the non-MS cohort (p=0.006, Fisher’s Exact test). In the individual cohorts, DIS was met in 80% (8/10) of MS, 100% (10/10) MS+, 20% (2/10) of migraine, and 70% (7/10) of misdiagnosed participants.
Fulfillment of MRI DIS criteria based on review of brain MRI only, in the setting of identification of at least 3 lesions by select3* criteria, increased average specificity for MS from 0.81 to 0.86; average sensitivity did not change from 0.83. Fulfillment of DIS in addition to the identification of at least 2 lesions with CVS using select3* increased average specificity from 0.65 to 0.81; average sensitivity changed from 0.90 to 0.88.
Participants excluded by even one reviewer from CVS evaluation for the select3* algorithm (due to <3 candidate lesions) were further analyzed; among these, MRIs from 2 MS, 1 MS+, and 1 misdiagnosed participant fulfilled DIS criteria. MRIs from 2 excluded participants from the MS and migraine cohorts did not fulfill DIS.
Discussion
This pilot study demonstrated that the identification of CVS in only a limited number of MRI lesions provided good specificity and sensitivity for MS diagnosis. Our select3 and select3* algorithms, when performed by 3 raters at different institutions blinded to diagnosis, differentiated MS and non-MS patients with fair inter-rater reliability.
The select3 algorithm showed promise in a prior study11 when performed by a single reviewer in the MS and migraine cohorts. These participants had a single diagnosis responsible for MRI white matter abnormalities. The present study addressed whether addition of the MS+ and misdiagnosed cohorts, with multiple potential causes of MRI white matter abnormalities, might confound the specificity of CVS. To our surprise, using select3 criteria, the identification of 3/3 CVS+ lesions in the subcortical or deep white matter demonstrated excellent specificity for MS with moderate sensitivity, even in the enlarged cohort. Of note, when the criteria for MS diagnosis using this algorithm was broadened to identification of CVS in 2/3 lesions, sensitivity improved even further at the expense of only a small decrease in specificity.
Although select3 appears promising, selecting lesions on FLAIR sequences prior to evaluation for CVS on FLAIR*is time-consuming and not compatible with typical clinical workflow where multiple imaging sequences are commonly viewed simultaneously. To improve clinical applicability, we developed the select3* algorithm, and included juxtacortical lesions in an attempt to improve sensitivity compared to select3. Interestingly, select3* did not perform as well as select3. It is possible that the select3 method biased reviewers toward pre-selection of lesions on FLAIR with morphological characteristics typical of demyelination and thus likely to demonstrate CVS. Clinical application of select3 among non-MS specialist providers who may be less attentive to such characteristics might result in diminished performance. Furthermore, in several studies non-MS populations have demonstrated CVS in up to 40% of total MRI lesions.12 It is likely that select3 may have thus under-represented CVS in non-MS participants relative to select3*, since in the latter, every candidate lesion could be evaluated in the pre-specified regions until up to three with CVS were identified. Our select3* results are likely a more accurate reflection of how a limited assessment of lesions for CVS will perform if tested in a larger cohorts of patients. Future work might re-evaluate the select3 algorithm, but may also explore the effects of combining CVS assessment with stringent morphological criteria, perhaps in the context of a simultaneous assessment of FLAIR and FLAIR* sequences.
Ovoid infratentorial lesions are less common in non-MS populations with diagnoses causing supratentorial MRI abnormalities that might mimic MS, and the presence of infratentorial lesions often aids in confirming MS diagnosis. Thus, the select3 and select3* methods excluded infratentorial lesions in an attempt to best replicate a potential application of CVS with the highest probability of impact in clinical practice. We did evaluate infratentorial lesions separately for CVS in this study population, however only 2/20 participants in the non-MS cohort, compared to 16 in the total MS cohort, had such candidate lesions, providing an inadequate sample size for analysis. Future work should test whether simply ascertaining the presence of an infratentorial lesion increases specificity for MS when added to a CVS algorithm.
Since the three reviewers may have selected and evaluated different lesions for the two algorithms, it was not possible with the current dataset to evaluate lesion-wise inter-reviewer reliability of CVS determination. Instead, assessment of inter-reviewer reliability was limited to diagnosis. Our kappa values showed fair agreement using 3/3 criteria for select3 CVS and in 3-lesion criteria for select3*, and improvement in agreement when select3 criteria were expanded to 2/3 and in 2-lesion criteria for select3*. The range of specificity and sensitivity for MS between reviewers demonstrated in Tables 2 and 3 also reflects this inter-reviewer variability. It is notable that the number of participant MRIs excluded for having <3 candidate lesions also varied by algorithm, possibly due to a training effect as select3 was performed prior to select3*. More extensive pre-study training in NAIMS criteria and CVS identification may improve inter-reviewer reliability.
Our methodology differed from prior 3T MRI limited-lesion assessments of CVS.7 First, it is possible that the combined FLAIR* contrast13 provides superior visualization of central veins compared to other techniques.8, 12 Further, unlike prior studies, our algorithms excluded periventricular lesions, confluent lesions, and lesions with multiple vessels from evaluation. Doing so may diminish the likelihood of error if CVS evaluation is transitioned to clinical application. Periventricular lesions are often confluent and typically demonstrate multiple vessels on T2*-weighted imaging, and both of these factors make it difficult to identify a true “central” vessel. Further study is needed to determine if exclusion of periventricular lesions improves diagnostic accuracy. Published limited-lesion methodology7 also included evaluation for CVS in a higher number and proportion of lesions per participant than our algorithms; such a method is less practical for clinical application but has potentially better specificity – although specificity was high with our methodology as well. Inclusion of 3 reviewers from 3 different institutions in this study may have provided a more realistic evaluation of the reliability of CVS evaluation for MS diagnosis.
There were limitations to this study. Several participants were excluded from some of the evaluations due to insufficient number of candidate lesions, and we expect that rendering a firm diagnosis in very-low-lesion-load cases will remain difficult even if CVS evaluation is fully implemented in clinical practice. Indeed, as identification of two lesions in specific regions in the setting of a syndrome typical for demyelination may be sufficient for a diagnosis of MS,1 a requirement for ≥3 ovoid lesions ≥3mm in diameter and within the restricted regions evaluated in select3 and select3* may limit maximal attainable sensitivity. Addition of MRI DIS criteria to select3* when 2/3 lesion demonstrate CVS may improve diagnostic accuracy for MS, however may not improve specificity when < 3 candidate lesions are identified. Of note, fulfillment of DIS was ascertained in this study without the availability of dedicated spinal cord imaging, and additional participant MRIs may have fulfilled DIS if such imaging was available. We also did not specifically assess the proportion of lesions meeting MRI DIS in each participant for the presence of CVS since previous data3, 14 suggests that DIS may not be uniformly applied in practice, and its misapplication commonly contributes to misdiagnosis. Participants were also recruited from a convenience sample, and while migraine may be frequently mistaken for MS,3, 4, 14 unlike the misdiagnosed cohort, participants in the migraine cohort were not suspected to have MS. As MS and migraine are more common in women,15–17 fewer men participated in the study, limiting our ability to determine whether sex has an influence on CVS prevalence or determination – although there is no reason to suspect this might be the case. Finally, while CVS has demonstrated specificity for MS in a number of populations,12 the non-MS cohort in this study did not represent the full breadth of disorders18 that may be mistaken for MS.
In this study, evaluation of a limited number of MRI lesions for central veins on 3T MRI using FLAIR* differentiated participants with MS from participants with other causes of MRI white matter abnormalities. While CVS has shown promise for diagnosis of MS, developing such algorithms is a step toward clinical application. Evaluation in larger prospective cohorts undergoing evaluation for MS is needed, along with development of easily applied automated processing tools. If future studies continue to demonstrate promise for CVS as an imaging biomarker for MS, these methods might be incorporated into MS diagnostic criteria to improve the differentiation of MS from other disorders for which it is frequently mistaken.
Acknowledgments
Supported by the University of Vermont Department of Neurological Sciences, University of Vermont Department of Radiology, the University of Vermont MRI Center for Biomedical Imaging, and the Intramural Research Program of National Institute of Neurological Disorders and Stroke.
Financial Disclosures: Andrew J Solomon: consulting for Biogen, Teva, EMD Serono
Richard Watts: none
Daniel Ontaneda: grant support from NIH, NMSS, Genzyme, Novartis, Genentech, consulting from Genentech, Genzyme, Biogen Idec.
Martina Absinta: none
Pascal Sati: none
Daniel S Reich: support for collaborative research projects from the Myelin Repair Foundation and Vertex Pharmaceuticals
MS Journal Appendix for MRI methodology
| Hardware | |
|---|---|
| Field strength | 3.0T |
| Manufacturer | Philips |
| Model | Achieva dStream |
| Coil type (e g. head, surface) | Head |
| Number of coil channels | 32 |
| Acquisition sequence | ||
|---|---|---|
| Type (e.g. FLAIR, DIR, DTI, fMRI) | FLAIR | |
| Acquisition time | 5:50 | |
| Orientation | Sagittal | |
| Alignment (e.g. anterior commissure/poster commissure line) | ||
| Voxel size | 1.0×1.0×1.0 | |
| TR | 4800 | |
| TE | 366 | |
| TI | 1650 | |
| Flip angle | Multi FA sweep | |
| NEX | 2 | |
| Field of view | 240×240×180 | |
| Matrix size | 240×239×180 | |
| Parallel imaging | Yes | |
| If used, parallel imaging method: (e.g. SENSE, GRAPPA) | SENSE 2.6 (AP) × 2 (RL) | |
| Cardiac gating | No | No |
| If used, cardiac gating method: (e.g. PPU or ECG) | ||
| Contrast enhancement | Yes | |
| Acquisition sequence | |
|---|---|
| If used, provide name of contrast agent, dose and timing of scan post-contrast administration | Magnevist, single dose by weight, 4–5 minutes post-contrast |
| Other parameters: |
| Acquisition sequence | ||
|---|---|---|
| Type (e.g. FLAIR, DIR, DTI, fMRI) | T2* susceptibility weighted imaging(SWI) | |
| Acquisition time | 4:15 | |
| Orientation | Sagittal | |
| Alignment (e.g. anterior commissure/poster commissure line) | ||
| Voxel size | 0.55×0.55×0.55 | |
| TR | 54ms | |
| TE | 29ms | |
| TI | ||
| Flip angle | 10 | |
| NEX | 2 | |
| Field of view | 240×240×185 | |
| Matrix size | 436×420 | |
| Parallel imaging | Yes | |
| If used, parallel imaging method: (e.g. SENSE, GRAPPA) | SENSE 2.0 (AP) × 2.0 (RL) | |
| Cardiac gating | No | |
| If used, cardiac gating method: (e.g. PPU or ECG) | ||
| Contrast enhancement | Yes | |
| Acquisition sequence | |
|---|---|
| If used, provide name of contrast agent, dose and timing of scan post-contrast administration | Magnevist, single dose by weight, injection at the beginning of acquisition |
| Other parameters: |
| Image analysis methods and outputs | |
|---|---|
| Lesions | |
| Type (e.g. Gd-enhancing, T2-hyperintense, T1-hypointense) | T2*-FLAIR |
| Analysis method | As described in Sati P. George IC. Shea CD. Gaitan MI, Reich DS. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012:265:926–932. |
| Analysis software | In-house. Osirix DICOM viewer |
| Output measure (e.g. count or volume [ml]) | Central vessel present/absent from lesions |
| Tissue volumes | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord) | |
| Analysis method | |
| Analysis software | |
| Output measure (e.g. absolute tissue volume in ml, tissue volume as a fraction of intracranial volume, percentage change in tissue volumes) | |
| Tissue measures (e.g. MTR, DTI, T1-RT, T2-RT, T2*, T2′, 1H-MRS, perfusion, Na) | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord, normal-appearing grey matter or white matter) | |
| Analysis method | |
| Analysis software | |
| Output measure | |
| Other MRI measures (e.g. functional MRI) | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord, normal-appearing grey matter or white matter) | |
| Analysis method | |
| Analysis software | |
| Output measure | |
Other analysis details:
Footnotes
Author Contributions:
Andrew J Solomon contributed to conceptualization and study design, analysis and interpretation of the data, and drafting of the manuscript for intellectual content
Richard Watts contributed to conceptualization and study design, analysis and interpretation of the data, and drafting of the manuscript for intellectual content
Daniel Ontaneda contributed to analysis and interpretation of the data, and drafting of the manuscript for intellectual content
Martina Absinta contributed to analysis and interpretation of the data, and drafting of the manuscript for intellectual content
Pascal Sati contributed to conceptualization and study design, and drafting of the manuscript for intellectual content
Daniel S Reich contributed to conceptualization and study design, analysis and interpretation of the data, and drafting of the manuscript for intellectual content
Citations
- 1.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selchen D, Bhan V, Blevins G, et al. MS, MRI, and the 2010 McDonald criteria: a Canadian expert commentary. Neurology. 2012;79:S1–15. doi: 10.1212/WNL.0b013e318277d144. [DOI] [PubMed] [Google Scholar]
- 3.Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87:1393–1399. doi: 10.1212/WNL.0000000000003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon AJ, Klein EP, Bourdette D. “Undiagnosing” multiple sclerosis: the challenge of misdiagnosis in MS. Neurology. 2012;78:1986–1991. doi: 10.1212/WNL.0b013e318259e1b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon AJ, Weinshenker BG. Misdiagnosis of multiple sclerosis: frequency, causes, effects, and prevention. Current neurology and neuroscience reports. 2013;13:403. doi: 10.1007/s11910-013-0403-y. [DOI] [PubMed] [Google Scholar]
- 6.Mistry N, Dixon J, Tallantyre E, et al. Central Veins in Brain Lesions Visualized With High-Field Magnetic Resonance Imaging: A Pathologically Specific Diagnostic Biomarker for Inflammatory Demyelination in the Brain. JAMA neurology. 2013:1–6. doi: 10.1001/jamaneurol.2013.1405. [DOI] [PubMed] [Google Scholar]
- 7.Mistry N, Abdel-Fahim R, Samaraweera A, et al. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Multiple sclerosis (Houndmills, Basingstoke, England) 2016;22:1289–1296. doi: 10.1177/1352458515616700. [DOI] [PubMed] [Google Scholar]
- 8.Samaraweera AP, Clarke MA, Whitehead A, et al. The Central Vein Sign in Multiple Sclerosis Lesions Is Present Irrespective of the T2* Sequence at 3 T. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2017;27:114–121. doi: 10.1111/jon.12367. [DOI] [PubMed] [Google Scholar]
- 9.George IC, Sati P, Absinta M, et al. Clinical 3-tesla FLAIR* MRI improves diagnostic accuracy in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2016;22:1578–1586. doi: 10.1177/1352458515624975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mistry N, Dixon JE, Tallantyre EC, et al. 3 Tesla T2*-weighted brain MRI distinguishes multiple sclerosis from incidental white matter microangiopathic lesions. Multiple sclerosis (Houndmills, Basingstoke, England) 2013;19:8–597. [Google Scholar]
- 11.Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2016;3:82–87. doi: 10.1002/acn3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nature reviews Neurology. 2016;12:714–722. doi: 10.1038/nrneurol.2016.166. [DOI] [PubMed] [Google Scholar]
- 13.Sati P, George IC, Shea CD, Gaitan MI, Reich DS. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology. 2012;265:926–932. doi: 10.1148/radiol.12120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Kullnat J, Bourdette D, et al. Prevalence of brain magnetic resonance imaging meeting Barkhof and McDonald criteria for dissemination in space among headache patients. Multiple sclerosis (Houndmills, Basingstoke, England) 2013;19:1101–1105. doi: 10.1177/1352458512471874. [DOI] [PubMed] [Google Scholar]
- 15.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 16.Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology. 1994;44:S17–23. [PubMed] [Google Scholar]
- 17.Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet neurology. 2006;5:932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller DH, Weinshenker BG, Filippi M, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Multiple sclerosis (Houndmills, Basingstoke, England) 2008;14:1157–1174. doi: 10.1177/1352458508096878. [DOI] [PMC free article] [PubMed] [Google Scholar]


