Abstract
Environmental and dietary stimuli have always been implicated in brain development and behavioral responses. The gut, being the major portal of communication with the external environment, has recently been brought to the forefront of this interaction with the establishment of a gut–brain axis in health and disease. Moreover, recent breakthroughs in germ-free and antibiotic-treated mice have demonstrated the significant impact of the microbiome in modulating behavioral responses in mice and have established a more specific microbiome–gut–behavior axis. One of the mechanisms by which this axis affects social behavior is by regulating myelination at the prefrontal cortex, an important site for complex cognitive behavior planning and decision-making. The prefrontal cortex exhibits late myelination of its axonal projections that could extend into the third decade of life in humans, which make it susceptible to external influences, such as microbial metabolites. Changes in the gut microbiome were shown to alter the composition of the microbial metabolome affecting highly permeable bioactive compounds, such as p-cresol, which could impair oligodendrocyte differentiation. Dysregulated myelination in the prefrontal cortex is then able to affect behavioral responses in mice, shifting them towards social isolation. The reduced social interactions could then limit microbial exchange, which could otherwise pose a threat to the survival of the existing microbial community in the host and, thus, provide an evolutionary advantage to the specific microbial community. In this review, we will analyze the microbiome–gut–behavior axis, describe the interactions between the gut microbiome and oligodendrocytes and highlight their role in the modulation of social behavior.
Electronic supplementary material
The online version of this article (10.1007/s13311-017-0597-9) contains supplementary material, which is available to authorized users.
Key Words: Gut microbiome • myelin plasticity • social behavior • metabolites • oligodendrocytes • prefrontal cortex
Introduction
While neurogenesis and gliogenesis occur during the early stages of development, the formation of myelin around the axons is a long process that continues to develop well into adult life [1]. Myelin is the specialized membrane of cells called oligodendrocytes, which derive from progenitor cells. The differentiation of these progenitors into myelinating oligodendrocytes is defined by a complex and long-lasting transcriptional program, which is orchestrated by modification of the epigenome, caused by intrinsic and environmental factors. One of the major portals of environmental exposure for the mammalian body is the gut, and the role of a gut–brain axis in health and disease has recently been established, with multiple implications for brain physiology and pathology, as well as behavioral responses [2–4].
The most protracted changes in myelination occur in the prefrontal cortex (PFC), the association cortex of the frontal lobe, which exhibits late myelination of its axonal connections [1]. PFC has a crucial role in adjusting an organism’s behavior to the environment and shapes social behavior [5, 6]. Therefore, changes in the development and function of the PFC could significantly affect behavioral responses of mice and humans. In this review, we will describe the importance of PFC myelination in shaping social behavior and highlight the concept of myelin plasticity in the adult PFC. We will then decipher the microbiome–gut–behavior axis and provide the latest evidence regarding the crosstalk between microbiota, PFC oligodendrocytes, and social behavior.
Myelin Plasticity and Social Behavior
Myelin Formation in the PFC Shapes and is Shaped By Social Experiences
While neurons have long been considered central nervous system cells that can adapt in response to environmental stimuli, oligodendrocytes have also been shown to adapt in response to environmental changes. More specifically, social experiences in early life have been shown to shape myelin formation in the PFC [7, 8]. This is of high relevance, as this is also the brain region where external stimuli are integrated and translated into several complex behaviors [9]. Myelin changes in the PFC have been reported in a wide range of psychiatric illnesses, including autism, anxiety, schizophrenia, and depression, and abnormal myelination has been associated with numerous behavioral phenotypes, including social avoidance [10, 11]. In many instances, early life experiences have been associated with long-lasting white matter changes and suggest that myelination changes induced by childhood events are relatively stable and possibly unfavorably modulate the onset of psychiatric disorders [12–15].
Behavioral Responses can be Modified By Exogenous Regulators of Myelin Plasticity
The adult PFC has also been shown to undergo myelination in adult jugglers, further underlying the role of myelin plasticity as an adaptation to novel experiences [16]. Protracted social isolation of adult mice was also shown to impair myelination and induce epigenetic changes in oligodendrocytes, which were highlighted by ultrastructural studies of the PFC [11]. From a mechanistic standpoint, the way by which external experiences modulate the transcriptional program remain unclear. However, it was recently reported that clemastine, a muscarininc inhibitor, was sufficient to prevent the onset of social avoidance behavior induced by social isolation by favoring myelin formation and the deposition of correct epigenomic marks in oligodendrocytes [17]. Among the factors that could affect myelination in the PFC, the gut microbiome has recently emerged as a key player in the regulation of myelin formation and the pathogenesis of neurobehavioral disorders [18–23].
The Microbiome–Gut–Behavior Axis: A Novel Regulatory Pathway of PFC Myelination and Social Behavior
The microbiome–gut–behavior axis is an intricate network of dynamic and bidirectional signaling pathways that provide a link between dietary and environmental stimuli, myelination in the PFC, and behavioral responses. The gut–brain axis is broadly defined as inclusive of gut microbiota, gut epithelium, liver, enteric, parasympathetic and sympathetic nervous systems, brain and spinal cord, neuroendocrine connections, metabolites, cytokines, neuropeptides, and signaling molecules [24, 25]. The majority of gut–brain communication, however, is being facilitated via 4 distinct routes: 1) vagal afferents; 2) gut hormones; 3) cytokines; and 4) microbial metabolites [24, 26, 27]. Microbial metabolites have recently come to the forefront of the gut–brain axis after the demonstration of a surprisingly large effect of the gut microbiome on mammalian blood metabolites, especially short-chain fatty acids (e.g., butyrate) and amino acid degradation products (e.g., phenols and indoles) [28].
The gut–brain axis has been implicated in the regulation of microglial activity [29], blood–brain barrier integrity [30], hippocampal neurogenesis [31], myelination [20, 21], neuroendocrine response to stress, and neurotransmitter production [24, 25, 32]. The regulatory effect of the gut–brain axis on behavior in mice has been uncovered more recently and shown to be dependent on the formation of bioactive microbial metabolites, such as p-cresol, a phenol produced by microbial degradation of dietary tyrosine (Fig. 1) [21].
Fig. 1.
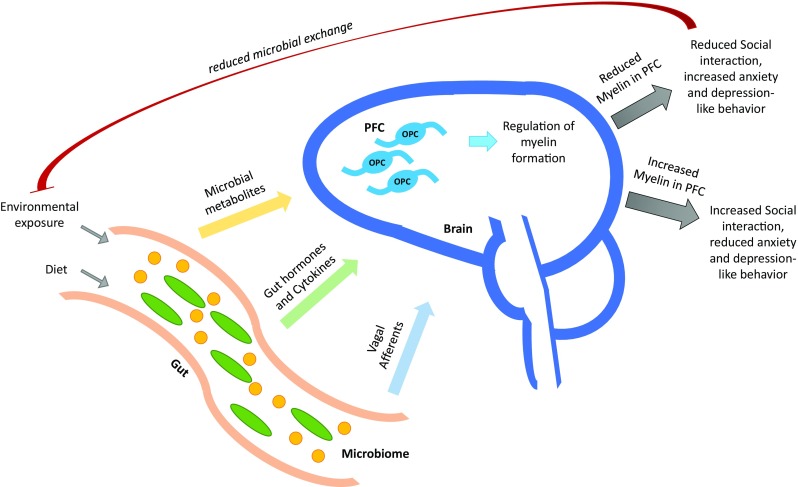
The microbiome–gut–behavior axis. The gut is one of the major portals of environmental exposure of the mammalian body and is the site where the gut microbiome integrates the environmental and dietary signals to produce certain secondary messengers that can reach and affect brain development and behavior. These second messengers can be in the form of unique microbial metabolites, gut hormones, cytokines, and vagal afferent signals. One well-studied mechanism of modulation of behavioral responses by the gut microbiome involves certain microbial metabolites that can reach the brain and affect myelin formation in the prefrontal cortex (PFC) of the brain by interfering with differentiation of oligodendrocyte progenitor cells (OPC), possibly via modulating Sox10 or MYRF. Increased production of these metabolites by the microbial community of the gut can inhibit OPC differentiation and reduce the formation of myelin in the PFC. Reduced myelination in the PFC is then associated with increased anxiety and depression-like behavior and reduced social interaction. This social isolation could then benefit the microbial community by limiting incoming competition from the external environment, as social isolation could also lead to less microbial exchange. However, decreased production of these metabolites by the gut microbial community would allow OPCs in the PFC to differentiate and increase myelination, which could lead to increased social interactions, and reduced anxiety and depressive-like behaviors, at least in mice
The Effect of the Gut Microbiome on Social Behavior
The profound impact of the gut microbiota on the modulation of behavioral responses has recently been recognized mainly through work on germ-free (GF) and antibiotic-treated mice [21, 32]. GF mice have been found to have reduced anxiety-like and depressive behaviors [33, 34] and have altered stress and amygdala-dependent fear responses [35]. In addition to these changes, GF mice, particularly males, exhibit autism-like social impairments accompanied by reduced preference for novel social situations and increased repetitive behaviors [33]. Interestingly, patients with autism spectrum disorder have been found to have altered gut microbiome with differences in species richness and diversity versus controls [3, 36]. Moreover, it was recently shown that the transfer of gut microbiota from socially withdrawn mice was sufficient to induce the same phenotype in the recipient mice [21], suggesting that gut microbiota have a central role in the modulation of their host’s social behavior. This behavioral effect could, in turn, provide the microbial community with an evolutionary advantage by eliminating incoming competition from the external environment, as socially withdrawn mice that do not interact with other mice also have less microbiome exchange.
Myelination is the Link Between the Gut and Social Behavior
Current evidence in mouse models suggests that the microbiome–gut–behavior axis exerts its regulatory effect on social behavior mainly by affecting myelination in the PFC [20, 21]. Gacias et al. [21] demonstrated that daily gavage of antibiotics could protect nonobese diabetic (NOD) mice from social avoidance and despair-like behaviors that were observed when NOD mice were gavaged with water, suggesting a role of the gut microbiota in determining the behavioral response of mice to a stressful stimulus. To further corroborate that, the transfer of gut microbiota from water-gavaged NOD donors, which included members of the Clostridiales order and Lachnospiraceae and Ruminococcaceae families, to microbiota-depleted C57BL/6 recipients was sufficient to induce the same socially withdrawn behavioral phenotype. Interestingly, it was shown that socially withdrawn mice had impaired myelination in the PFC with a reduction in all major myelin gene transcripts, which was then linked to a disturbed gut microbial metabolome. More specifically, socially withdrawn mice had changes in their gut microbiome that allowed the production of increased concentrations of p-cresol, a highly permeable product of microbial degradation of dietary tyrosine, which can inhibit oligodendrocyte differentiation in vitro [21].
However, p-cresol is not the only microbial metabolite that could potentially mediate the effects of the gut microbiome on myelination and behavior. Several other studies have investigated the role of butyrate, a short-chain fatty acid produced by the gut microbiome via dietary fiber fermentation, has been implicated in the regulation of microglial activity [37], which, in turn, could regulate myelination. Butyrate could also directly affect oligodendrocyte progenitor cell (OPC) differentiation and myelination as it has histone deacetylase inhibitory activity similar to valproic acid, a mood stabilizing drug that can inhibit OPC differentiation in vitro and in vivo [38]. These findings emphasize the importance of the microbiome–gut–behavior axis in modulating myelination and social behaviors and emphasize the role of microbial metabolites as one of the main routes of gut microbiota–oligodendrocyte communication.
The Microbiome–Gut–Behavior Axis Targets PFC Myelination Via Regulation of Myelin Regulatory Factor and Sry-Related HMg-Box Gene 10
The importance of gut microbiota in regulating PFC myelination was also shown by Hoban et al. [20]. GF mice exhibited hypermyelinated axons and marked upregulation of genes linked to myelination at the PFC. At the molecular level, myelin regulatory factor (MYRF) was identified as a major driver of the observed hypermyelination, which was also accompanied by increased Sry-related HMg-box gene 10 (Sox10) expression in GF mice. Interestingly, Sox10 was found to be downregulated by p-cresol, the microbial metabolite identified by Gacias et al. [21]. Interestingly, p-cresol has been found to be absent in GF mice [28], a finding that could potentially explain the observed increase in Sox10 expression in those mice.
Hoban et al. [20] also found that neural activity-induced transcriptional pathways were upregulated in the PFC of GF mice. This is in agreement with a previous report of hyperactive amygdala (which connects to the PFC via glutamatergic projections) in GF mice [39]. This increased neural activity could also play a role in regulating myelin formation at the PFC in GF mice, as myelination has been shown to be regulated by neuronal activity and glutamate release from synaptic vesicles [40, 41]. These data reinforce the central role of microbiota in regulating myelin plasticity and bring the PFC at the center of the microbiome–gut–behavior axis.
Microbial Neurotherapeutics: Modulation of the Microbiome–Gut–Behavior Axis
The existence of a microbiome–gut–behavior axis in various neurobehavioral diseases brings out the potential of therapeutic manipulation of the gut microbiome. The effort to leverage the therapeutic effect of microbes is still in the early stages of development and further research is needed to establish such an effect. Preliminary evidence has shown that daily supplementation with Lactobacillus and Bifidobacterium strains could exert a positive effect in mood, anxiety, and cognitive symptoms of depression, with the most benefit observed in anxiety-related symptoms [42]. Distinct probiotic preparations, however, may display batch effects and differ among various manufacturers, thereby highlighting the need for a better understanding of the probiotic-mediated modulation of microbial communities to regulate behavior. In addition to probiotics, a future microbial neurotherapeutic intervention could be targeting the early-life microbiome. It is now possible to restore the microbiome of newborn infants delivered by cesarean section to resemble that of infants delivered vaginally [43], and, thus, potentially correct the aberrant early-life microbiome composition that has been associated with deregulated brain development in other studies [3].
Finally, a future consideration for potential intervention on the gut microbiome in humans is direct fecal microbiota transplantation, a procedure that has been successfully performed for the treatment of antibiotic-resistant gastrointestinal infections. In mice, transfer of microbiota from socially withdrawn mice to microbiota-depleted recipients was sufficient to transfer the behavioral phenotype, suggesting that fecal microbiome transplantation could effectively manipulate the microbiome–gut–behavior axis [21]. Despite the fact that evidence to support the effectiveness of this method for the treatment of neurological disorders in humans is scant, the increased interest on microbiota characterization in neurological disorders highlights the need for additional studies on the therapeutic efficacy of such microbial neurotherapeutic interventions in the future.
Conclusion
The microbiome–gut–behavior axis, as part of the gut–brain axis, is being increasingly recognized as an important regulator of PFC myelination and a modulator of social behavior. PFC is a key brain region for driving behavioral responses, which has also been implicated in depression and autism [20]. Current evidence suggests that microbial transfer can transmit social behaviors in mice and unique microbial metabolites, such as p-cresol, are at the core of this microbiome–gut–behavior axis [21]. Such microbial metabolites modulate myelin plasticity at the PFC by changing the expression of MYRF and Sox10 at the molecular level. This alteration in myelination at the PFC then regulates behavioral responses to stressful or social stimuli. Moreover, regulation of the amygdala–PFC glutamatergic activity by the gut microbiome could also influence myelin plasticity at the PFC and modulate anxiety-related and social behaviors [20]. More research is needed, however, to further elucidate the components of the microbiome–gut–behavior axis with the ultimate goal of identifying potential probiotics and microbial neurotherapeutic interventions that could rescue the microbiome–gut–behavior axis deregulation that has been observed in different neurobehavioral diseases.
Electronic Supplementary Material
(PDF 1224 kb)
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2003;31(3–5):373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- 2.Kanji S, Fonseka TM, Marshe VS, Sriretnakumar V, Hahn MK, Müller DJ. The microbiome-gut-brain axis: implications for schizophrenia and antipsychotic induced weight gain. Eur Arch Psychiatry Clin Neurosci 2017. [DOI] [PubMed]
- 3.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanji J, Hoshi E. Behavioral planning in the prefrontal cortex. Curr Opin Neurobiol. 2001;11(2):164–170. doi: 10.1016/S0959-4388(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 6.Franklin TB, Silva BA, Perova Z, et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat Neurosci. 2017;20(2):260–270. doi: 10.1038/nn.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikusui T, Kiyokawa Y, Mori Y. Deprivation of mother-pup interaction by early weaning alters myelin formation in male, but not female. ICR mice. Brain Res. 2007;1133(1):115–122. doi: 10.1016/j.brainres.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience–dependent oligodendrocyte maturation and myelination. Science. 2012;337(September):1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace DL, Han M-H, Graham DL, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12(2):200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regenold WT, D’Agostino CA, Ramesh N, Hasnain M, Roys S, Gullapalli RP. Diffusion-weighted magnetic resonance imaging of white matter in bipolar disorder: a pilot study. Bipolar Disord. 2006;8(2):188–195. doi: 10.1111/j.1399-5618.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Dietz K, DeLoyht JM, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15(12):1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta MA, Golembo NI, Nosarti C, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 13.Rutter M, Colvert E, Kreppner J, et al. Early adolescent outcomes for institutionally-deprived and non-deprived adoptees. I: Disinhibited attachment. J Child Psychol Psychiatry Allied Discip. 2007;48(1):17–30. doi: 10.1111/j.1469-7610.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 14.Nelson CA, Bos K, Gunnar MR, Sonuga-Barke EJS. The neurobiological toll of early human deprivation. Monogr Soc Res Child Dev. 2011;76(4):127–146. doi: 10.1111/j.1540-5834.2011.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonuga-Barke EJ, Schlotz W, Kreppner J. Differentiating developmental trajectories for conduct, emotion, and peer problems following early deprivation. Monogr Soc Res Child Dev. 2010;75(1):102–124. doi: 10.1111/j.1540-5834.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- 16.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Dupree JL, Gacias M, et al. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J Neurosci. 2016;36(3):957–962. doi: 10.1523/JNEUROSCI.3608-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156(9):3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoban AE, Stilling RM, Ryan FJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6(4):e774. doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gacias M, Gaspari S, Santos PMG, et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife 2016;5. [DOI] [PMC free article] [PubMed]
- 22.Flight MH. Neurodevelopmental disorders: The gut–microbiome–brain connection. Nat Rev Neurosci. 2013;15(2):65. [DOI] [PubMed]
- 23.Han Y, Li Q, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. 2017;11:120. doi: 10.3389/fncel.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017;74:3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;227:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Ochoa-Reparaz J, Mielcarz DW, Begum-Haque S, Kasper LH. Gut, bugs, and brain: Role of commensal bacteria in the control of central nervous system disease. Ann Neurol. 2011;69(2):240–247. doi: 10.1002/ana.22344. [DOI] [PubMed] [Google Scholar]
- 27.Burokas A, Moloney RD, Dinan TG, Cryan JF. Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol. 2015;91:1–62. doi: 10.1016/bs.aambs.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosher KI, Wyss-Coray T. Go with your gut: microbiota meet microglia. Nat Neurosci. 2015;18(7):930–931. doi: 10.1038/nn.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel L, Prat A. One more role for the gut: microbiota and blood brain barrier. Ann Transl Med. 2016;4(1):15. doi: 10.3978/j.issn.2305-5839.2015.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry. 2015;78:e7–39. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Luczynski P, Neufeld KAMV, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. 2016;19(8):1–17. doi: 10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19(2):146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoban AE, Stilling RM, Moloney G, et al. The microbiome regulates amygdala-dependent fear recall. Mol Psychiatry 2017. [DOI] [PMC free article] [PubMed]
- 36.Strati F, Cavalieri D, Albanese D, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5(1):24. doi: 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol. 2004;141(5):874–880. doi: 10.1038/sj.bjp.0705682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen S, Sandoval J, Swiss VA, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11(9):1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stilling RM, Ryan FJ, Hoban AE, et al. Microbes & neurodevelopment—Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015;50:209–220. doi: 10.1016/j.bbi.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Tomassy GS, Dershowitz LB, Arlotta P. Diversity matters: a revised guide to myelination. Trends Cell Biol. 2016;26:135–147. doi: 10.1016/j.tcb.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333(6049):1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017;16:14. doi: 10.1186/s12991-017-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22(3):250–253. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)


