Abstract
The prevalence of inflammatory bowel disease (IBD) has increased worldwide and IBD has been demonstrated to promote the development of colorectal cancer. The Rac family of proteins are involved in key mitogenic pathways. However, to the best of our knowledge, no prior studies have investigated the expression and role of Rac on colitis and colitis-associated cancer (CAC). In the current study, Rac expression in patients with colitis was analyzed according to the expression value from NCBI GEO database (GDS3268). EHT-1864, the specific inhibitor of Rac, was intraperitoneally injected to treat mice with dextran sulfate sodium (DSS)-induced acute and chronic colitis and mice with azoxymethane (AOM)/DSS-induced CAC. Furthermore, immune cell infiltration and the expression of several inflammatory cytokines in colon tissues were analyzed by flow cytometry, immunofluorescence, and ELISAs. We demonstrated the upregulation of the Rac family of proteins in colitis. Inhibition of Rac by EHT-1864 treatment was found to have an efficient inhibitory effect on DSS-induced acute and chronic colitis and AOM/DSS-induced CAC development. We also observed that downregulation of Rac family protein expression markedly prevented macrophage and myeloid-derived suppressor cell (MDSC) infiltration in colon tissues and suppressed pro-inflammatory cytokine expression. Our study established a foundation for understanding the role of Rac in colitis and CAC and to provide a novel strategy and target for colitis and CAC therapy.
Keywords: Colitis, colitis-associated cancer, Rac, EHT-1864
Introduction
Inflammatory bowel disease (IBD) comprises two major forms, Crohn’s disease and ulcerative colitis (UC), and is characterized by dysregulated intestinal inflammation. The prevalence of IBD has increased worldwide, and currently, it affects millions of patients. Dysregulated immune responses in the intestinal mucosa cause an overproduction of pro-inflammatory cytokines, such as interleukin (IL)-6, IL-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, and contribute to the development of colitis [1-3]. Immune cells, including dendritic cells, macrophages, neutrophils, and T cells, together with the aforementioned pro-inflammatory cytokines, collaborate to perpetuate and sustain inflammatory responses in the colon, which eventually lead to tissue damage and the development of colitis [4,5]. To date, there has been no optimal treatment for IBD, and the treatment options currently available consist of corticosteroids, 5-aminosalicylates, and immunosuppressant agents, such as antibodies against TNFα [6-8]. While 5-aminosalicylates and corticosteroids are beneficial for many patients with IBD, for the majority, they are not effective in the long term, and the short- and long-term side effects of immunosuppressive drugs limit their clinical use [6-8]. Therefore, identifying and developing novel therapeutic targets and strategies for IBD treatment are essential.
The Rac family of proteins are small monomeric GTPases involved in key mitogenic pathways, providing a link between the cell surface and transcriptional events [9]. Rac family proteins are involved in the regulation of various diseases, including chronic kidney disease (CKD) [10] and immune complex-mediated acute lung injury [11]. Thus, the Rac inhibitors EHT-1864 and NSC23766 have been used as efficient agents for inhibiting prostate smooth muscle contraction and prostate stromal cell growth [12], inhibiting breast cancer cell proliferation [13] and attenuating albumin excretion as well as glomerular and tubulo-interstitial damage [10]. However, to the best of our knowledge, there is no study to investigate the potential role of Rac on colitis and colitis-associated cancer (CAC).
In the present study, we aimed to investigate the potential role of Rac on colitis and CAC. The expression of Rac in colitis was analyzed according to the data from NCBI GEO database. EHT-1864, a Rac specific inhibitor, was intraperitoneally injected into mice with dextran sulfate sodium (DSS)-induced acute and chronic colitis and into mice with azoxymethane (AOM)/DSS-induced CAC. Furthermore, immune cell infiltration and the expression of several inflammatory cytokines in colon tissues were analyzed by flow cytometry, immunofluorescence (IF) and an ELISA. Our study aimed to establish a foundation for understanding the role of Rac in colitis and CAC, and to identify novel strategies and targets for the treatment of colitis and CAC.
Materials and methods
Mice and treatment
20-23 g weight female C57BL/6 mice were purchased from the Beijing HFK Bioscience Co., LTD (Beijing, China). All of the animal studies were approved by the Animal Experiment Committee of the Southern Medical University. EHT-1864 (Selleck, 10 mg/kg) were intraperitoneally injected once a day with 1.0 ml disposable sterilized syringe. And equal amount of DMSO were intraperitoneally injected as control group.
Acute colitis model
3% dextran sulfate sodium (DSS) (MP Biomedical) were dissolved in sterile, distilled water and used to feed C57BL/6 mice to establish acute colitis model, lasting for 7 days. Then with a 7 days interval, regular drinking water were performed. The hemoccult and stool consistency was observed and recorded according to the rules by previous study [14].
Chronic colitis model
Three cycles of 2% DSS are performed to establish DSS-induced chronic colitis model in C57BL/6 mice. In one cycle, 2% DSS was added for feeding mice for 7 days and with a 14 days interval, regular drinking water were performed. The hemoccult and stool consistency was observed and recorded according to the rules by previous study [14].
Colitis associated cancer model
Colitis associated cancer (CAC) model was established as the previous study described [15]. C57BL/6 mice were injected intraperitoneally with 10 mg/kg AOM (Sigma-Aldrich). 7 days later, 2% DSS are performed to treat mice, lasting for 7 days. Then, regular drinking water are performed for 14 days. In total, three cycles of 2% DSS treatment were needed. After sacrifice, the colon were collected and the tumor multiplicity were counted and the tumor area were measured by Vernier caliper.
Enzyme-linked immunosorbent assay (ELISA)
To measure the expression of several cytokines in colon tissues, colon tissues were collected and lysed in RIPA lysis buffer (Beyotime, Beijing, China) containing 1% protease inhibitor cocktail (Sigma-Aldrich). After measurement of protein concertation with BCA kit, mouse IL-6 ELISA kit, mouse TNF-α ELISA kit, mouse IFN-γ ELISA kit and mouse IL-1β ELISA kit were purchased from Neobioscience (Shanghai, China) and performed according to the manufacturer’s instructions.
Western blotting
To measure the expression of Rac family in colon tissues, colon tissues were collected and lysed in RIPA lysis buffer (Beyotime, Beijing, China) containing 1% protease inhibitor cocktail (Sigma-Aldrich). Then, the protein concertation were measured with BCA kit and 20 μg total protein were loaded for western blotting. After blocking with 5% non-fat milk in TBS/T buffer, the membrane were incubated with anti-Rac1 antibody, anti-Rac2 antibody and anti-Rac3 antibody (CST, MA, USA). The GAPDH was used as a loading control.
Flow cytometry
Flow cytometry were employed to investigate the immune cells infiltration in colon tissue as the previous study [16]. After sacrifice the mice, colon tissues were collected and digested in PBS containing 0.2% Collagenase D at 37°C for 30 mins. Then, the specific antibodies were added to label the isolated cells at room temperature for 1 hour. After washing the cells with PBS containing 2% fetal calfserum, the cells were analyzed on a BD FACSCalibur.
Histological staining and immunofluorescence
The colon tissues were clipped into 1 cm length section and fixed in 4% paraformaldehyde for 48 hours, followed embedding in paraffin. Then, the cutted 4 μm paraffin section were deparaffinized in xylene, and rehydrated in PBS (pH 7.4). Hematoxylin-Eosin staining kit (Beyotime, Beijing, China) were used for histological staining and the histological score were analyzed as previous study [14]. Meanwhile, after antigen retrieval, the specific antibody against iNOS, F4/80 and Ly6G were added and incubated at 37°C for 1 hour. After washing with PBS for 3 times, the goat anti-rabbit IgG-FITC and goat anti-mouse-TR antibody (Santa Cruz) were added and incubated at 37°C for 1 hour. The cell nuclei were stained by DAPI (Beyotime, Beijing, China). All specimens were evaluated using Olympus B×600 microscope and Spot Fiex camera.
Statistical analysis
All statistical analysis was performed by the Student’s t-tests and calculations were performed using SPSS v19.0 (SPSS Inc., Chicago, IL, USA). All experiments were repeated three to six times. P<0.05 was considered statistically significant.
Results
Rac family proteins were upregulated in colitis tissues
To investigate the expression of Rac family proteins in colitis, we collected the expression value of Rac1, Rac2 and Rac3 from NCBI GEO database (GDS3268), which including the expression profiles of 70 inflamed normal colon tissues and 63 flamed colonic tissues from UC patients. As shown in Figure 1A and 1B, Rac1 and Rac2 expression were significantly upregulated in colitis tissues of patients, meanwhile Rac3 expression was not dramatically increased (Figure 1C). Furthermore, real-time PCR was employed to determine the expression of Rac1, Rac2 and Rac3 in the colon tissues of chronic colitis mice and control mice. We found both of Rac1, Rac2 and Rac3 expression were upregulated in colitis (Figure 1D). Collectively, these results demonstrated the upregulation of Rac family proteins in colitis.
Figure 1.
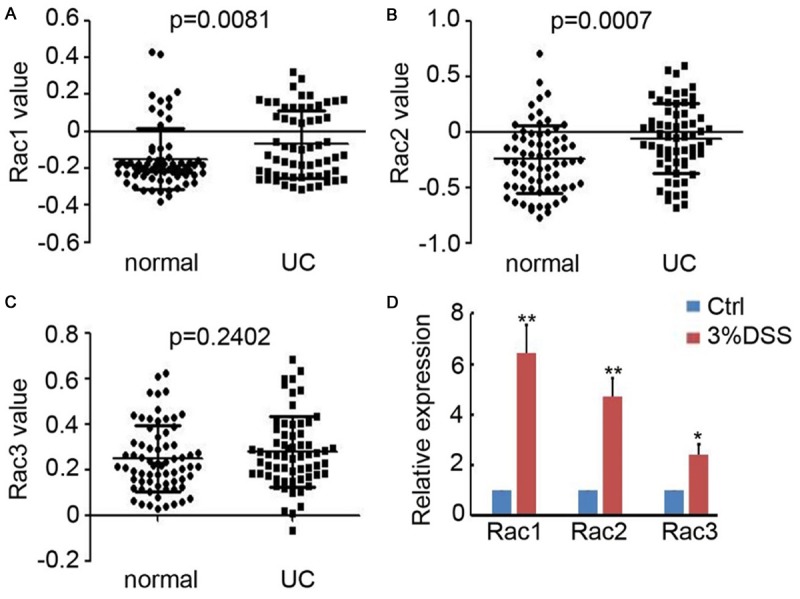
Upregulation of Rac family proteins in colitis tissues. A-C. Rac1, Rac2 and Rac3 expression in normal colon tissues and the flamed colonic tissues from UC patients were collected from GEO database (GDS3268) and analyzed. (n=70 for normal group; n=63 for UC group). D. Real-time PCR was performed to detect Rac1, Rac2 and Rac3 expression in the colonic tissue of mice with or without 2% DSS treatment. The relative expression of Rac1, Rac2 and Rac3 was analyzed. (n=4, *, P<0.05; **, P<0.01).
Inhibition of Rac family proteins impairs acute colitis in mice
To determine the role of Rac in colitis, the specific inhibitor EHT-1864 was employed to reduce Rac expression in the colon tissues of chronic colitis mice. As presented in Figure 1A, Rac1, Rac2, and Rac3 expression was evaluated and we determined that EHT-1864 efficiently inhibits the expression of Rac1, Rac2, and Rac3 (Figure 2A and 2B). As depicted in Figure 2C-E, colon length was observed recovered after EHT-1864 treatment for 5 days. Furthermore, less inflammation-associated rectal bleeding and soft stool was observed in the EHT-1864 treatment group, compared with DMSO injected group (Figure 2F and 2G). Hematoxylin and eosin (H&E) staining revealed that treatment with 3% DSS induced severe damage to the colonic mucosa, including an extensive loss of crypt structures, epithelial cell denudation and large areas of inflammatory cell infiltration (Figure 2H). Notably, the administration of EHT-1864 markedly impaired colonic inflammation, which was also reflected in the pathological assessment of colitis severity scores (Figure 2H). ELISA results indicated that EHT-1864 efficiently inhibited IL-6, TNF-α, INF-γ, and IL-1β expression in the colonic tissue of mice with acute colitis (Figure 2I), which were determined to be the pro-inflammatory cytokines involved in colitis development. These data demonstrate the preventive role of EHT-1864 on acute colitis in mice.
Figure 2.
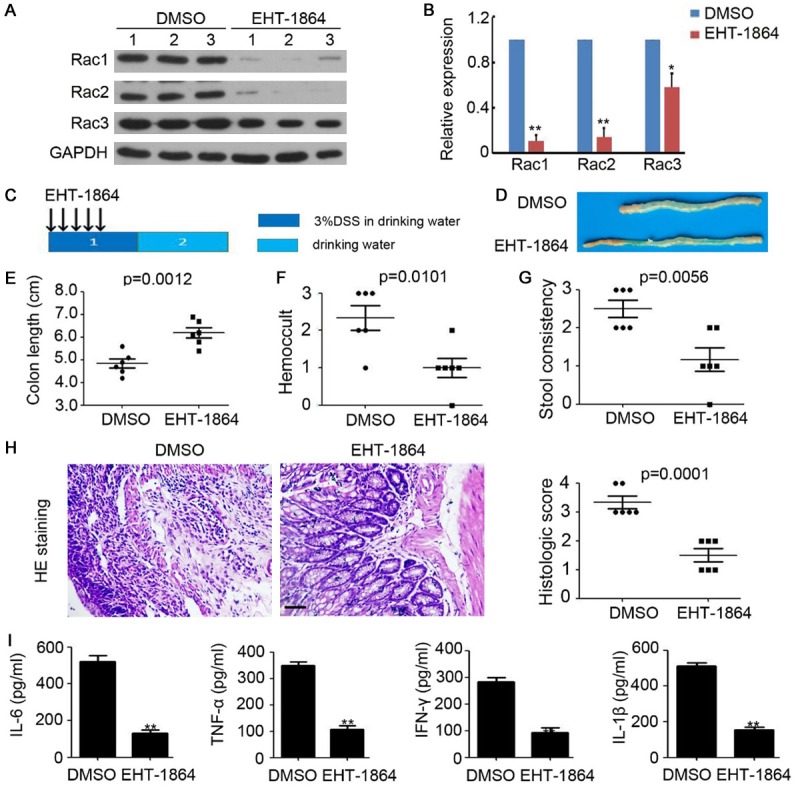
Inhibition of Rac impairs acute colitis in mice. A. Western blotting analysis of Rac1, Rac2 and Rac3 expression in colonic tissue of acute colitis mice. GAPDH was used as a loading control. B. The relative expression of Rac1, Rac2 and Rac3 was analyzed. n=3, **, P<0.01. C. Schema of acute colitis model and EHT-1864 treatment. D. The representative macroscopic photos of colon after DMSO or EHT-1864 injection in acute colitis mice. E. Colon length of acute colitis mice. n=6, **, P<0.01. F. Hemoccult of acute colitis mice. n=6, **, P<0.01. G. Stool consistency of acute colitis mice. n=6, **, P<0.01. H. HE staining of colonic tissue of acute colitis mice. The histologic score was calculated and analyzed. n=5, **, P<0.01. I. ELISA analysis of IL-6, TNF-α, INF-γ and IL-1β expression in colonic tissue of acute colitis mice. n=3, **, P<0.01.
Inhibition of Rac impairs chronic colitis in mice
Next, Rac family proteins expression were downregulated by EHT-1864 in the mice with 2% DSS-induced chronic colitis. The treatment regimen lasted for 5 days and involved a 16-day interval (Figure 3A). We demonstrated the therapeutic role of EHT-1864 on chronic colitis in mice (Figure 3B and 3C), which was accompanied by reduced inflammation-associated rectal bleeding and soft stool (Figure 3D and 3E). Furthermore, H&E staining was performed, which revealed reduced damage to the colonic mucosa with attenuated loss of crypt structures and epithelial cell denudation, and smaller areas of inflammatory cell infiltration in the Rac-downregulated mice (Figure 3F). Notably, comparatively lower expression levels of IL-6, TNF-α, INF-γ, and IL-1β were observed in colonic tissues obtained from EHT-1864-treated mice with chronic colitis (Figure 3G). These findings further demonstrated the preventive role of EHT-1864 on chronic colitis in mice.
Figure 3.
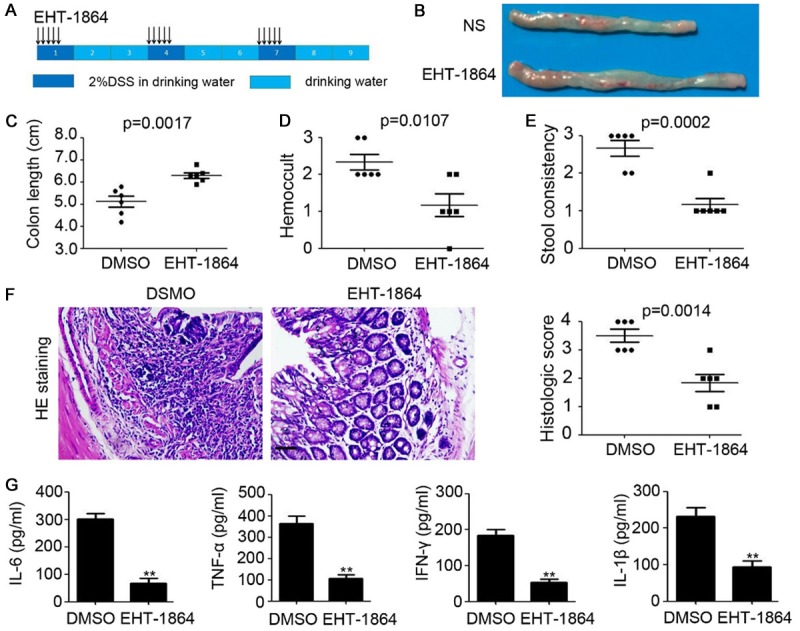
Inhibition of Rac impairs chronic colitis in mice. A. Schema of chronic colitis model and EHT-1864 treatment. B. The representative macroscopic photos of colon after DMSO or EHT-1864 injection in chronic colitis mice. C. Colon length of chronic colitis mice. n=6, **, P<0.01. D. Hemoccult of chronic colitis mice. n=6, **, P<0.01. E. Stool consistency of chronic colitis mice. n=6, **, P<0.01. F. HE staining of colonic tissue of chronic colitis mice. The histologic score was calculated and analyzed. n=5, **, P<0.01. G. ELISA analysis of IL-6, TNF-α, INF-γ and IL-1β expression in colonic tissue of chronic colitis mice. n=3, **, P<0.01.
Inhibition of Rac blocks pro-inflammatory cell recruitment in colon tissues
To determine the changes to the inflammatory microenvironment, flow cytometry was employed to examine leukocyte infiltration into the colonic tissues of chronic colitis mice. As presented in Figure 4A and 4B, reduced numbers of myeloid-derived suppressor cells (MDSC; Ly6G+CD11b+) and macrophages (F4/80+) were observed in the colons of mice with chronic colitis following EHT-1864 treatment for 7 days (Figure 4A) and for 14 days (Figure 4B). However, EHT-1864 had no significant effect on the infiltration of CD4+ T cells, CD8+ T cells or natural killer cells (Figure 4A and 4B). IF staining also indicated that EHT-1864 prevents the infiltration of pro-inflammatory MDSCs [inducible nitric oxide synthase (iNOS) positive; Figure 4C and 4D] and macrophages (iNOS positive; Figure 4E and 4F) into colon tissues. These data indicated that inhibition of Rac impairs the development and progression of colitis via blocking the recruitment of MDSCs and macrophages in colon tissues.
Figure 4.
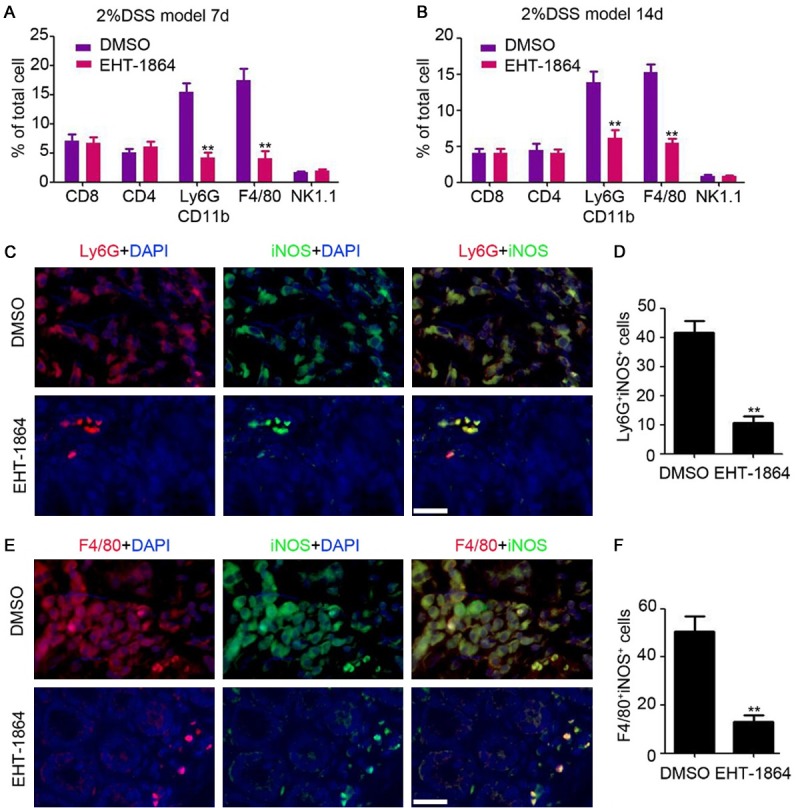
Inhibition of Rac blocks pro-inflammation cells recruitment in colon tissue. A, B. Flow cytometry analysis of CD8+ T cells, CD4+ T cells, Ly6G+CD11b+ MDSC cells, F4/80+ macrophages and NK1.1+ NK cells in colonic tissue of chronic colitis mice after DSS feeding for 7 days and 14 days. n=3, **, P<0.01. C. Immunofluorescent staining analysis of iNOS and Ly6G expression in colonic tissue of chronic mice. Scale bar = 100 μm. D. The number of iNOS and Ly6G positive cells were counted and analyzed. n=4, **, P<0.01. E. Immunofluorescent staining analysis of iNOS and F4/80 expression in colonic tissue of chronic mice. Scale bar = 100 μm. F. The number of iNOS and F4/80 positive cells were counted and analyzed. n=4, **, P<0.01.
Inhibition of Rac impairs CAC formation in mice
The following experiments directly investigated the role of Rac in CAC development in mice. The mice were injected with 10 mg/kg AOM once, followed by three cycles of 2% DSS exposure to elicit colitis (Figure 5A). EHT-1864 was injected over three cycles, lasting 5 days with a 16-day interval in one cycle (Figure 5A). However, colonic tumors could still be subsequently detected in both the DMSO- and the EHT-1864-treated mice (Figure 5B), EHT-1864 significantly inhibited the multiplicity of colonic tumors (P=0.0009, Figure 5C) and the tumor burden (P=0.0004, Figure 5D), accompanied with recovery of colon length (P=0.0010, Figure 5E). H&E staining also demonstrated a reduction in the tumor area present in the colonic tissues of EHT-1864-treated mice (P<0.0001, Figure 5F). PCNA staining indicated that more proliferative cells were present in the colonic tissues of the DMSO-treated mice (Figure 5G). Collectively, the findings suggest that inhibition of Rac significantly impaired CAC formation in mice.
Figure 5.
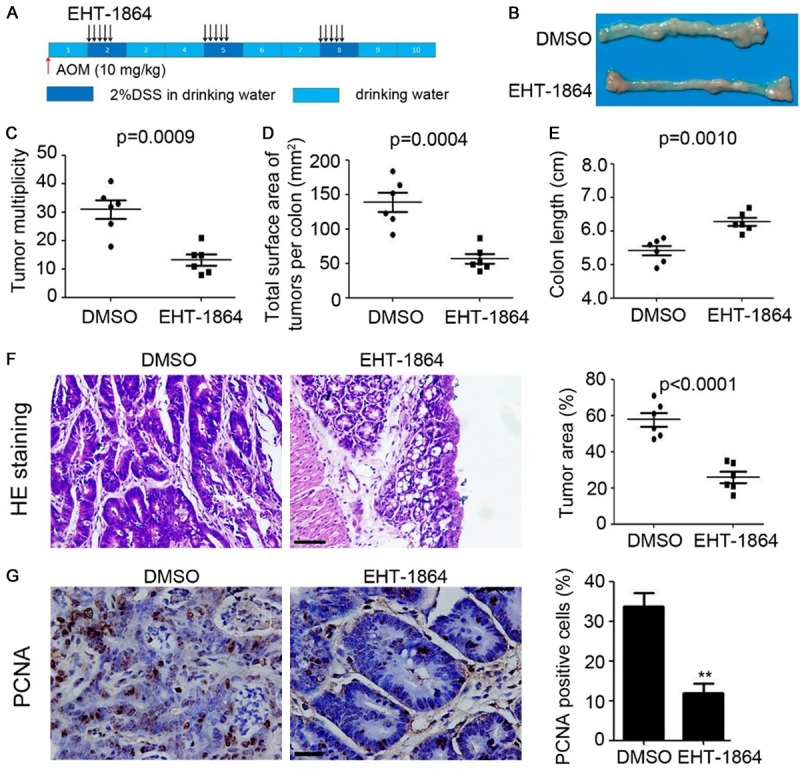
Inhibition of Rac impairs CAC formation in mice. A. Schema of CAC model and EHT-1864 treatment. B. The representative macroscopic photos of colon after DMSO or EHT-1864 injection in CAC mice. C. Tumor multiplicity of CAC mice. n=6, **, P<0.01. D. Total surface area of tumors per colon of CAC mice. n=6, **, P<0.01. E. Colon length of CAC mice. n=6, **, P<0.01. F. HE staining of colonic tissue of CAC mice. The tumor area was calculated and analyzed. n=5, **, P<0.01. G. Immunostaining of PCN expression in colonic tissue of CAC mice. The percent of PCNA positive cells were analyzed. n=5, **, P<0.01.
Inhibition of Rac prevents inflammation during CAC formation
Next, the inflammatory microenvironment in the colonic tissues of CAC mice was evaluated by IF staining and ELISA analysis. As presented in Figure 6A-D, inhibition of Rac prevented pro-inflammatory MDSC (iNOS positive, Figure 6A and 6B) and macrophage (iNOS positive, Figure 6C and 6D) infiltration in colon tissues. Furthermore, the inhibitory role of EHT-1864 on the expression of IL-6, TNF-α, INF-γ, and IL-1β was demonstrated in the colonic tissues of CAC mice (Figure 6E-H). Collectively, these findings indicated that inhibition of Rac prevents CAC formation by impairing the pro-inflammation microenvironment.
Figure 6.
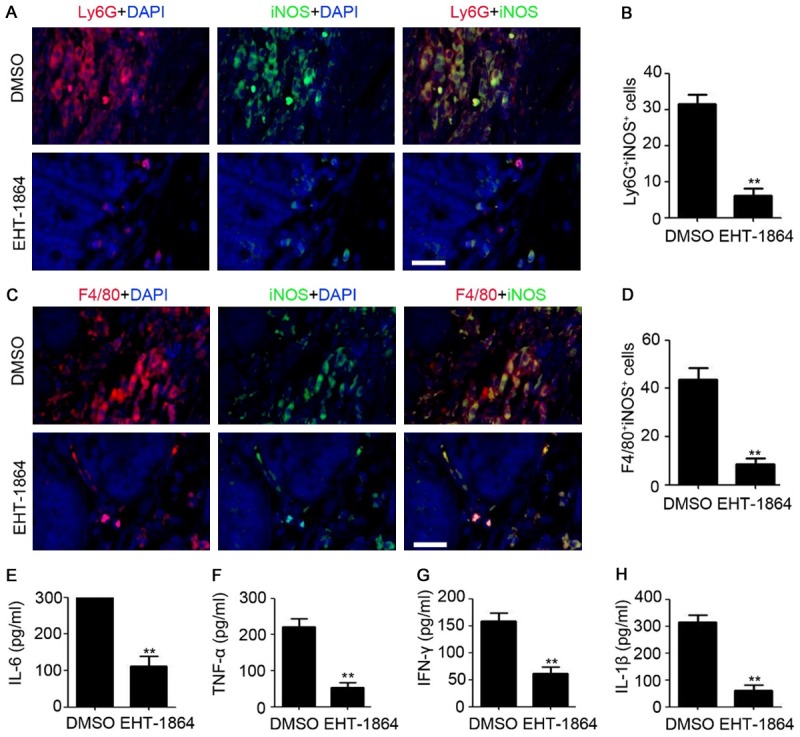
Inhibition of Rac prevents inflammation during CAC formation. A. Immunofluorescent staining analysis of iNOS and Ly6G expression in colonic tissue of CAC mice. Scale bar = 100 μm. B. The number of iNOS and Ly6G positive cells were counted and analyzed. n=4, **, P<0.01. C. Immunofluorescent staining analysis of iNOS and F4/80 expression in colonic tissue of CAC mice. Scale bar = 100 μm. D. The number of iNOS and F4/80 positive cells were counted and analyzed. n=4, **, P<0.01. E-H. ELISA analysis of IL-6, TNF-α, INF-γ and IL-1β expression in colonic tissue of CAC mice. n=3, **, P<0.01.
Discussion
Rac family proteins are potent regulators of cellular signaling that control migration and inflammation [9]. Rac is important for activating the NF-κB response downstream of integrin activation, such as after phagocytosis [17]. Rac-1 and RhoA have been identified as mediators of podocyte dysfunction in CKD; thus, the inhibition of Rho-GTPases may be a novel approach to the treatment of CKD [10]. Rac-guanine nucleotide exchange factors serve crucial pro-inflammatory roles in lung inflammation through promoting the pulmonary recruitment of monocytes and lymphocytes [18]. Rac1 GTPase signaling has previously been demonstrated to be markedly upregulated in the inflamed colonic mucosa of patients with IBD, when compared with the healthy colonic mucosa [19], as well as increased in experimentally induced mucosal wounds [19]. We identified that the Rac inhibitor EHT-1864 efficiently inhibited Rac1, Rac2, and Rac3 expression in the colon tissues of chronic colitis mice. Furthermore, the inhibition of Rac by EHT-1864 resulted in the attenuation of colitis and CSC formation in mice. Investigation of the underlying mechanism indicated that fewer macrophages and MDSCs were present in the colon tissues following Rac inhibition, often accompanied by the downregulation of pro-inflammatory cytokines. These results are consistent with the preventive role of Rac inhibition on inflammation that has been demonstrated in previous studies [10,18,20,21].
DSS is an efficient chemical molecule used for promoting inflammation in the colon through inducing apoptosis of colon epithelial cells in mice [22]. As DSS-induced colitis has been considered to closely mimic the clinical and morphological features of human UC [1,23], DSS-induced colitis in mice has been a widely used and accepted animal model for studying human UC. To elucidate the potential role of Rac in IBD, we used a DSS-induced colitis mouse model. The findings indicated that inhibition of Rac by EHT-1864 suppresses the symptoms of DSS-induced colitis, such colon length shortening, bloody feces and stool consistency. Increasing evidence has demonstrated the association between colitis and colon cancer [24-26], due to the persistence and renewal of mutagenic processes [27,28]. Prior clinical studies have also determined that patients with IBD have a higher risk of colorectal cancer development compared with the healthy population [29]. Our study demonstrated that inhibition of Rac significantly impairs AOM/DSS-induced CAC formation and progression, as evidenced by reduced tumor multiplicity and area. The effect of Rac on CAC may be attributed to the promotive role of Rac on colitis.
EHT-1864 has been used as an efficient agent in various diseases [10,12,13]. However, the effect of EHT-1864 on colitis and CAC has not been previously investigated. In the present study, we demonstrated that EHT-1864 treatment has an efficient inhibitory effect on DSS-induced acute and chronic colitis and AOM/DSS-induced CAC formation. We also found that the injection of EHT-1864 notably prevents pro-inflammatory cell infiltration in colon tissues and pro-inflammatory cytokine expression via downregulating Rac family protein expression. This study established a foundation for understanding the preventive roles of EHT-1864 in colitis and CAC, and elucidate novel strategies and targets for colitis and CAC therapy. However, the DSS-induced colitis model of IBD does not precisely recapitulate the conditions present in human IBD [1,23]. Thus, there is a long way to go before EHT-1864 can be used as a therapeutic agent in a clinical setting.
In conclusion, we demonstrated the upregulation of Rac family protein in colitis and the prevention role of EHT-1864, the specific inhibitor of Rac, in colitis and CAC. It is suggested Rac family protein are potential therapeutic targets for colitis and CAC, that acts via regulation of the immune microenvironment in mice. Notably, the present study provided a solid foundation for understanding the expression and function role of Rac in colitis and CAC, and identified a novel strategy and target for colitis and CAC therapy.
Disclosure of conflict of interest
None.
References
- 1.Bruckner M, Lenz P, Mucke MM, Gohar F, Willeke P, Domagk D, Bettenworth D. Diagnostic imaging advances in murine models of colitis. World J Gastroenterol. 2016;22:996–1007. doi: 10.3748/wjg.v22.i3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Xu L. Elevated IL-23R expression and Foxp3+Rorgt+ cells in intestinal mucosa during acute and chronic colitis. Med Sci Monit. 2016;22:2785–2792. doi: 10.12659/MSM.896827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trivedi PJ, Bruns T, Ward S, Mai M, Schmidt C, Hirschfield GM, Weston CJ, Adams DH. Intestinal CCL25 expression is increased in colitis and correlates with inflammatory activity. J Autoimmun. 2016;68:98–104. doi: 10.1016/j.jaut.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, Nakai T, Hasegawa A, Inoue N, Watanabe N, Akagawa KS, Hibi T. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach EC, Plevy SE. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm Bowel Dis. 2014;20:166–175. doi: 10.1097/MIB.0b013e3182a69dca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prantera C, Marconi S. Glucocorticosteroids in the treatment of inflammatory bowel disease and approaches to minimizing systemic activity. Therap Adv Gastroenterol. 2013;6:137–156. doi: 10.1177/1756283X12473675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prantera C, Pallone F, Brunetti G, Cottone M, Miglioli M. Oral 5-aminosalicylic acid (Asacol) in the maintenance treatment of Crohn’s disease. The Italian IBD Study Group. Gastroenterology. 1992;103:363–368. doi: 10.1016/0016-5085(92)90822-g. [DOI] [PubMed] [Google Scholar]
- 8.Sands BE. The risks and benefits of early immunosuppression and biological therapy. Dig Dis. 2012;30(Suppl 3):100–106. doi: 10.1159/000342731. [DOI] [PubMed] [Google Scholar]
- 9.Jo H, Luo HR. Exploiting effectors of Rac GTPase. Chem Biol. 2012;19:169–171. doi: 10.1016/j.chembiol.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babelova A, Jansen F, Sander K, Lohn M, Schafer L, Fork C, Ruetten H, Plettenburg O, Stark H, Daniel C, Amann K, Pavenstadt H, Jung O, Brandes RP. Activation of Rac-1 and RhoA contributes to podocyte injury in chronic kidney disease. PLoS One. 2013;8:e80328. doi: 10.1371/journal.pone.0080328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dooley JL, Abdel-Latif D, St Laurent CD, Puttagunta L, Befus D, Lacy P. Regulation of inflammation by Rac2 in immune complex-mediated acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1091–1102. doi: 10.1152/ajplung.90471.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Kunit T, Ciotkowska A, Rutz B, Schreiber A, Strittmatter F, Waidelich R, Liu C, Stief CG, Gratzke C, Hennenberg M. Inhibition of prostate smooth muscle contraction and prostate stromal cell growth by the inhibitors of Rac, NSC23766 and EHT1864. Br J Pharmacol. 2015;172:2905–2917. doi: 10.1111/bph.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblatt AE, Garcia MI, Lyons L, Xie Y, Maiorino C, Desire L, Slingerland J, Burnstein KL. Inhibition of the Rho GTPase, Rac1, decreases estrogen receptor levels and is a novel therapeutic strategy in breast cancer. Endocr Relat Cancer. 2011;18:207–219. doi: 10.1677/ERC-10-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 15.Dai L, Cui X, Zhang X, Cheng L, Liu Y, Yang Y, Fan P, Wang Q, Lin Y, Zhang J, Li C, Mao Y, Wang Q, Su X, Zhang S, Peng Y, Yang H, Hu X, Yang J, Huang M, Xiang R, Yu D, Zhou Z, Wei Y, Deng H. SARI inhibits angiogenesis and tumour growth of human colon cancer through directly targeting ceruloplasmin. Nat Commun. 2016;7:11996. doi: 10.1038/ncomms11996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 17.Tong L, Tergaonkar V. Rho protein GTPases and their interactions with NFkappaB: crossroads of inflammation and matrix biology. Biosci Rep. 2014:34. doi: 10.1042/BSR20140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan D, Amison RT, Riffo-Vasquez Y, Spina D, Cleary SJ, Wakelam MJ, Page CP, Pitchford SC, Welch HC. P-Rex and Vav Rac-GEFs in platelets control leukocyte recruitment to sites of inflammation. Blood. 2015;125:1146–1158. doi: 10.1182/blood-2014-07-591040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seinen ML, van Nieuw Amerongen GP, de Boer NK, van Bodegraven AA. Rac attack: modulation of the small GTPase rac in inflammatory bowel disease and thiopurine therapy. Mol Diagn Ther. 2016;20:551–557. doi: 10.1007/s40291-016-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R, Sun Q, Mihai G, Maiseyeu A, Rajagopalan S. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosco EE, Kumar S, Marchioni F, Biesiada J, Kordos M, Szczur K, Meller J, Seibel W, Mizrahi A, Pick E, Filippi MD, Zheng Y. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chem Biol. 2012;19:228–242. doi: 10.1016/j.chembiol.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agollah GD, Wu G, Peng HL, Kwon S. Dextran sulfate sodium-induced acute colitis impairs dermal lymphatic function in mice. World J Gastroenterol. 2015;21:12767–12777. doi: 10.3748/wjg.v21.i45.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Johnson RL, Snyder PW, DeSmet ML, Fleet JC. An inducible, large-intestine-specific transgenic mouse model for colitis and colitis-induced colon cancer research. Dig Dis Sci. 2016;61:1069–1079. doi: 10.1007/s10620-015-3971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N, Harpaz N, Itzkowitz S, Harris CC, Rotter V, Gorgoulis VG, Oren M. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–646. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonecchi R, Locati M, Mantovani A. Chemokines and cancer: a fatal attraction. Cancer Cell. 2011;19:434–435. doi: 10.1016/j.ccr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satpathy SR, Jala VR, Bodduluri SR, Krishnan E, Hegde B, Hoyle GW, Fraig M, Luster AD, Haribabu B. Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth. Nat Commun. 2015;6:7064. doi: 10.1038/ncomms8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arthur JC, Gharaibeh RZ, Muhlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA, Jobin C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun. 2014;5:4724. doi: 10.1038/ncomms5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]


