Abstract
Genotyping-by-sequencing (GBS) or restriction-site associated DNA marker sequencing (RAD-seq) is a practical and cost-effective method for analysing large genomes from high diversity species. This method of sequencing, coupled with methylation-sensitive enzymes (often referred to as methylation-sensitive restriction enzyme sequencing or MRE-seq), is an effective tool to study DNA methylation in parts of the genome that are inaccessible in other sequencing techniques or are not annotated in microarray technologies. Current software tools do not fulfil all methylation-sensitive restriction sequencing assays for determining differences in DNA methylation between samples. To fill this computational need, we present msgbsR, an R package that contains tools for the analysis of methylation-sensitive restriction enzyme sequencing experiments. msgbsR can be used to identify and quantify read counts at methylated sites directly from alignment files (BAM files) and enables verification of restriction enzyme cut sites with the correct recognition sequence of the individual enzyme. In addition, msgbsR assesses DNA methylation based on read coverage, similar to RNA sequencing experiments, rather than methylation proportion and is a useful tool in analysing differential methylation on large populations. The package is fully documented and available freely online as a Bioconductor package (https://bioconductor.org/packages/release/bioc/html/msgbsR.html).
Introduction
Methylation-sensitive restriction enzyme sequencing (MRE-seq), often referred to as methylation-sensitive genotype-by-sequencing (msGBS), is a cost effective next-generation sequencing method to analyse DNA methylation in large genome species. Reducing genome complexity with restriction enzymes (REs) can be advantageous as it may reach parts of the genome inaccessible to sequence capture approaches1. However, current MRE-seq data analysis tools do not satisfy all experimental designs. For example, using methylation-sensitive restriction enzymes in a MRE-seq experiment2,3 is an effective way to identify differentially methylated sites that may not be annotated or accessible in other technologies, such as microarrays. While other packages, such as Stacks4 and TASSEL5 exist to analyse restriction-site associated DNA marker sequencing (RAD-seq) data, these focus on identifying sequence variants and carrying out association mapping, and do not enable the analysis of methylation sites.
With the cost of NGS declining dramatically in recent years due to the increased throughput of current sequencing machines such as Illumina HiSeq X Ten and NovaSeq platforms, it is now feasible to determine DNA methylation on a large population. However, it is often difficult to adequately assess a large number of samples across a genome in one sequencing assay. The advantage of MRE-seq is that sequencing libraries are simple to produce, with only an enzyme digestion, adapter ligation and library amplification step needed, enabling an unbiased analysis of methylation sites across the genome. Conversely, while quite accurate, the most popular approach for analysing large numbers of human methylation samples, Illumina HumanMethylation450 BeadChip array6 relies on prior knowledge of individual probes sites (the majority being CpGs), restricting its application to only well-studied and annotated genomes.
Compared to other sequencing approaches designed to identify DNA methylation, such as whole-genome bisulfite sequencing (WGBS) or methylation-capture techniques, MRE-seq infers methylation via read coverage and does not require additional sample treatment to convert methylated cytosines (i.e. sodium bisulfite treatment). WGBS, a more high-resolution approach, is used in a more diverse set of biological systems (human, mouse, plants, fungi etc) yet is costly to produce due to the large amount of sequencing data required to accurately quantify methylation states on each bisulfite converted strand7. Determining read coverage, as opposed to WGBS, reduced representation bisulfite sequencing (RRBS) and array methods, enables a library preparation step that avoids treatment with sodium bisulfite, a process that damages and fragments input DNA8.
Many existing software already exist that can be used to analyse DNA methylation data (Table 1), however no particular software package can be used exclusively for large-scale MRE-seq experiments, creating a gap in the current computational methods for analysing this DNA methylation data. For example, to analyse methylation array data, packages such as ChAMP and minfi contain functions that analyse probe intensity to derive methylation states. Packages such as BiSeq9 and bsseq10 work by enabling downstream analysis of samples after bisulfite alignment procedures, and while these packages analyse sequencing data, the tools available to WGBS are not compatible with MRE-seq sequencing data. While WGBS packages aim to identify converted and unconverted cytosine’s to calculate DNA methylation, most software tools are unable to extract the total number of reads that mapped to a given RE recognition site. The extraction of sequencing coverage at genomic locations however, is a well-used application in other genomic apporaches and hence, computational functions are able to be leveraged from analysis packages used to analyse transcriptome sequencing (RNA-seq) and chromatin immunoprecipitation with massively parallel DNA sequencing (ChIP-seq) data.
Table 1.
Comparison between msgbsR and existing DNA methylation pipeline software tools.
| Package | Type of DNA methylation data | Format of files for importing | Filtering outliers and poor quality probes/sequences | Differential methylation analysis |
|---|---|---|---|---|
| msgbsR | MRE-seq | BAM | Yes | Yes |
| ChAMP 39 | Array | IDAT | Yes | Yes |
| minfi 40 | Array | IDAT | Yes | Yes |
| charm 41 | Array | XYS | Yes | Yes |
| methylpipe 42 | WGBS | BAM | Yes | Yes |
| BSmooth 10 | WGBS | BAM | Yes | Yes |
| BiSeq 9 | RRBS/WGBS | bismarkbed2graph output43 | Yes | Yes |
RRBS: reduced representation bisulfite sequencing, MRE-seq: methylation sensitive restriction enzyme sequencing, WGBS: whole genome bisulfite sequencing.
In this study, we developed msgbsR, an R package for the analysis of data obtained from MRE-seq experiments. Our analysis pipeline allows researchers to conduct analyses of MRE-seq experiments, in order to identify differentially methylated sites. msgbsR includes tools which assesses read counts from a sorted and indexed genome alignments or BAM file(s) directly into the R environment, checking that the cut sites match the RE sequence, identifying differentially methylated sites, and seamless annotation using available reference genomes in the R/Bioconductor framework. To demonstrate the utility of the msgbsR analysis package, we analysed a population of rats (control vs treatment) for differential DNA methylation (Rattus norvegicus), and two publicly available agricultural crop datasets from barley (Hordeum vulgare) and maize (Zea mays) to show the extensive potential applications in epigenetic research.
Results
Overview of msgbsR pipeline
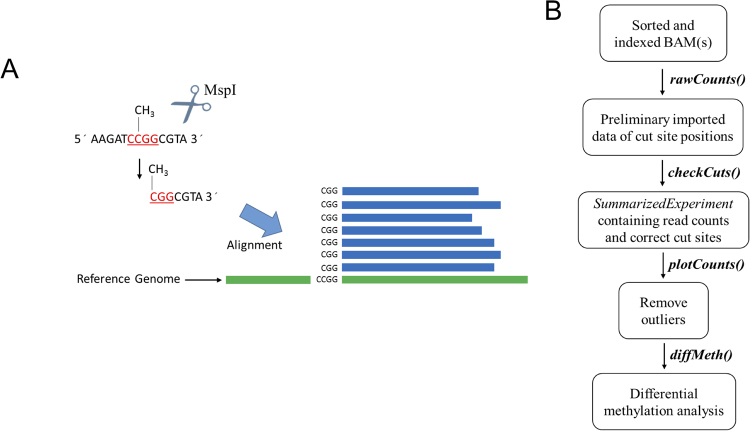
The msgbsR package is a collection of functions that automate the process of identifying differentially methylated sites from a MRE-seq experiment, and enable visualisation of methylation-senstive sites within the genome. The analysis package works initially by analysing samples (restriction-digested Illumina sequencing libraries) that have been aligned to their target genome. The set of alignment (BAM) files are then read by the package to create a preliminary table of read counts containing the locations of the reads that were mapped to the genome. To correctly identify true positive RE sites, the table is then filtered to remove reads that did not map to the correct RE recognition site. Since restriction-digested counts are similarly distributed to other count data types, differential methylation analyses can be implemented from existing Bioconductor packages that were developed in RNA-seq studies. The msgbsR package contains wrapper functions to test for differential methylation using edgeR, however flexibility exists to enable additional packages to be used once the final table of read counts is obtained. In addition, msgbsR uses the Bioconductor package SummarizedExperiment to enable fast integration of raw count data with phenotypic/metadata and genomic information in a single data object.
Generating the table of read counts
Using a reference genome, alignment of the sequencing data results in reads that begin at RE cut sites. As a result, reads will begin at a defined RE cut site, producing a pileup of reads at those genomic positions (Fig. 1A). Thus, it is possible to count the total number of reads that mapped to these RE cut site positions. The msgbsR analysis pipeline firstly starts with functions allowing the import alignments from indexed and sorted BAM file(s) (Fig. 1B), and verification of raw read counts at the RE cut sites. These sorted and indexed BAM files can be the output from an alignment tool such as Bowtie211 or Burrows-Wheeler Alignment (BWA)12 and sorted with SAMtools13. The rawCounts function takes a list of sorted and indexed BAM files and imports the raw read counts into the R environment. The rawCounts function internally takes advantage of Rsamtools14 and GenomicFeatures15. After alignment, the beginning of each mapped read starts within the cut site of the recognition sequence of the RE. Rsamtools is used to extract the genomic location of the start of the read which then becomes the location of the cut site for each given read. The msgbsR package utilises other Bioconductor packages to ease data analysis, with rawCounts being directly applicable to the data format of a RangedSummarizedExperiment from the Bioconductor package SummarizedExperiment16. The resulted RangedSummarizedExperiment data object contains a table of read counts of potential cut site locations with their genomic coordinates, such as the chromosome, position and strand information. The genomic locations within the table of read counts correspond to the beginning of each mapped read from the BAM files(s) which are now located within a GRanges format enabling the data to be utilised by other Bioconductor packages.
Figure 1.
A simplified schematic of methylation-sensitive restriction enzyme sequencing approach and the msgbsR pipeline. (A) An example of MRE-seq/msGBS using the restriction enzyme, MspI, which cleaves DNA at the recognition sequence C^CGG if the internal cytosine is methylated. However, MspI does not cut at the recognition site if both cytosines are methylated or the external cytosine is methylated. (B) The data analysis pipeline represented by a flowchart which highlights the names of the main functions in the msgbsR package.
The output of the rawCounts function uses the start position of all mapped reads in a BAM file. However, there may be incorrectly mapped reads that do not correspond to a specified RE recognition sequence. Incorrectly mapped reads can be filtered out of the analysis prior to any downstream analyses using the checkCuts function, which takes a GRanges data object that contains the positioning of the potential cut sites and the recognition sequence of the RE. The checkCuts function then uses a reference genome in the format of a BSgenome which is obtainable from Bioconductor. However, if a BSgenome is unavailable, a user-defined FASTA file can also be used to determine where the recognition sequence matches the reference genome. This function makes use of GenomicFeatures and can be used to extract the sequence from a BSgenome or FASTA file given a set of genomic coordinates. The user must firstly take the genomic coordinates of the mapped reads from the table of read counts and adjust the locations such that the positioning fits over the recognition site of the RE. It then uses these coordinates to extract the sequence from the reference genome and compare it with the supplied RE recognition site. A GRanges object with the correct positions of the cut site that matched the input sequence is then returned. Incorrectly mapped reads can then be filtered out of the RangedSummarizedExperiment by removing locations that do not match with the output from checkCuts.
Package validation
We performed the msgbsR analysis pipeline on our own MRE-seq dataset consisting of DNA from prostate tissue from the offspring of rats who were either fed a control (n = 26) or experiment high fat maternal diet (n = 18). This experiment focused on using the methylation sensitive RE, MspI, which cleaves at the recognition site C^CGG (Fig. 1A). Initially, after mapping there were a potential 1,616,611 MspI sites. However, after running checkCuts, this was reduced to 1,252,042 MspI sites. The incorrectly mapped reads were unique to an individual sample. In other words, the same incorrect site did not occur in multiple samples. We therefore found it advantageous having the function checkCuts, as it can remove incorrectly mapped reads which may have been introduced in an earlier step prior to running the msgbsR pipeline. By running the checkCuts function ensures there are no incorrect mapped reads within any downstream analyses. This is important as incorrect sites can impact downstream analyses such as returning sites that are differentially methylated but are in fact false positives.
We also used msgbsR on a publicly available MRE-seq experiment focusing on barley and maize (SRP004282.1) leaf samples2 (The script on how this was downloaded and analysed from NCBI SRA is supplied in Supplementary Data 1 and 2). This experiment used ApeKI, a methylation-sensitive endonuclease that recognizes the 5 bp sequence GCWGC (W = A or T). Firstly, we mapped the barley and maize samples to their respective available reference genomes (see methods) and used the rawCounts function on the resulted sorted and indexed BAM files to determine count numbers. Initially, this resulted in a total of 4,081,975 and 1,155,762 potential ApeKI sites for the maize and barley data set respectively. However, after running the checkCuts function this was reduced to 3,791,316 and 1,032,360 cut sites for the maize and barley data set respectively. This was potentially due to potentially incorrectly mapped reads and ensured all downstream analyses were performed using sites that were correctly mapped.
Visualisation
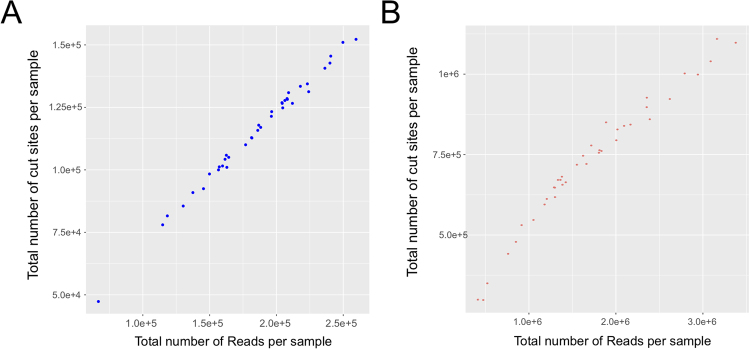
MRE-seq experiments can produce varying numbers of cut sites and reads depending on the DNA methylation state and the efficiency of the library preparation step for each individual sample. A way to overcome false positives associated with differences in read numbers between samples is to remove samples that produced a low number of reads and/or cut sites. This can be done before performing differential DNA methylation analysis using the plotCounts function incorporated in the msgbsR package. The plotCounts function calculates the library size of each sample by calculating the total number of reads per sample. It also determines the total number of cut sites produced per sample by calculating the total number of cut sites that contained at least one read in each sample. The plot shown in Fig. 2 was generated using the plotCounts function, showing the total number of reads compared to the total number of cut sites produced for each individual sample from the publicly available data set described above2 for the Barley (Fig. 2A) or the Maize (Fig. 2B) data set. We also performed this function on our own data set focusing on prostates from rat offspring from either a control or experimental high fat maternal diet. The total number of cut sites for each individual sample before and after running the checkCuts function is supplied in Supplementary Data 3. Ideally, MRE-seq should be performed multiple times to produce technical replicates enabling us to determine if outliers were introduced as a result during sequencing. To identify additional outliers, an unsupervised clustering analysis such as principle component analysis (PCA), can also be performed (as shown with our sample data in Supplementary Data 4), however for demonstration purposes all sample were used in downstream analyses.
Figure 2.
The output of the plotCounts function showing the distribution of the library size compared to the total number of ApeKI cut sites produced for each sample from either the (A) barley or (B) maize data set. Each individual point represents a unique sample.
Differential methylation analysis
One of the advantages of MRE-seq experiments is the ability to sequence hundreds of samples from different groups or conditions, without the need of additional sequencing applications (such as MeDIP-seq within the MethylMnM analysis example), and thus increase statistical power in differential methylation analyses. The msgbsR package contains a function that automates normalisation and determines differentially methylated sites between groups, enabling the analysis of complex experimental designs. Since the data generated from a MRE-seq experiment is in the form of read counts, we can take advantage of tools typically used in RNA-seq analyses17. The diffMeth function uses edgeR18 tools to automate splitting the data, perform normalisation and identify differentially methylated sites. diffMeth is a wrapper function which automatically normalises based on library size. However, if the user wants to use other differential expression tools such as limma19 or DEseq220, they can directly take the raw read counts from msgbsR and use them combination with those packages.
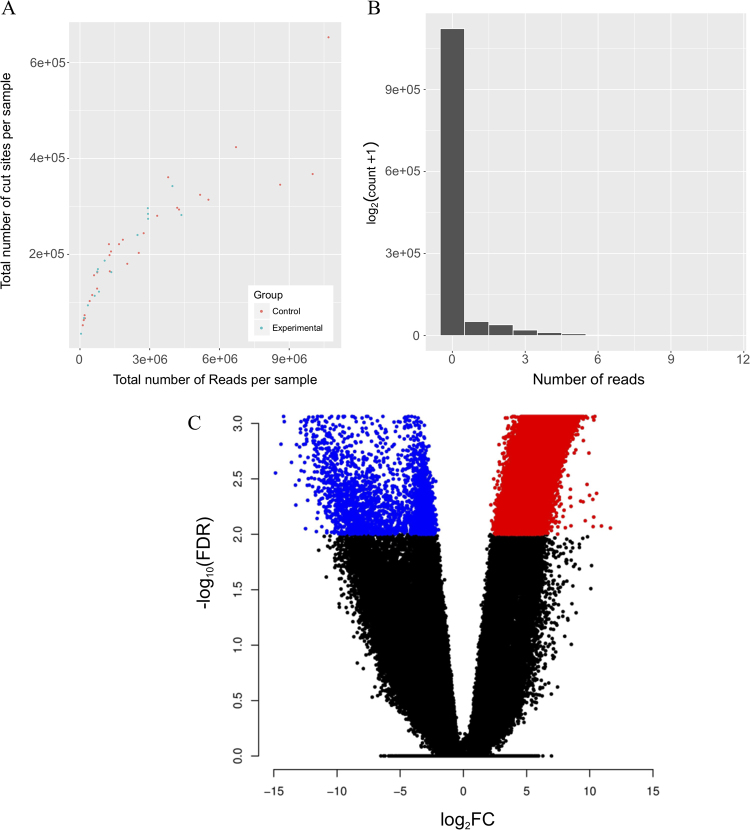
Gene expression count packages such as edgeR18, developed initially through gene expression microarrays, work on negative bionomically distributed data. The read counts from MRE-seq have a negative binomial distribution (demonstrated in Fig. 3B using a random control sample from our own MRE-seq data), ensuring that RNA analysing packages can be leveraged for analysis. We tested if the data using all samples was negative binomial using the goodfit function from the vcd R package21 which returns a p-value after fitting data to a negative binomial distribution. Using our data presented here in this manuscript, we found the data to fit a negative binomial distrubtion (p < 2.2e–16), which ensured us that existing methods could be used to test for differential methylation.
Figure 3.
The msgbsR pipeline on our rat prostate MRE-seq data. (A) Output of the plotCounts function showing the distribution of the total number of reads and cut sites per sample. Samples are coloured depending on their diet group. (B) A histogram of reads for a control sample showing a negative distribution. (C) A volcano plot showing differentially methylated sites (FDR < 0.01) between the control diet (blue dots) and the experimental diet (red dots).
We performed differential DNA methylation analysis using the diffMeth function, and found 31,768 sites to be significantly differentially methylated (FDR < 0.05) between control and experimental sample groups (Fig. 3C). We further explored the differentially methylated sites by annotating the sites to the nearest gene (see methods) and using these genes to perform a gene ontology (GO) analysis. We found GO terms such as cell development (GO:0048468) and cell differentiation (GO:0030154) to be significantly associated with the annotated genes suggesting that the methylation differences between the control and experimental groups are associated with genes that may be altered in prostate tissue that has been exposed to a high fat diet environment during its development.
Comparisons to existing packages
As explained above, msgbsR is unique in its analysis of MRE-seq data, and while most packages do not implement the same approach, we compared our rat analysis to an existing package called MethylMnM22. MRE-seq data is primarily analysed in MethylMnM as a companion with methylated DNA immunoprecipitation (MeDIP) sequencing data to accurately identify differentially methylated regions (DMRs) between sample groups3. Using MethylMnM package we aimed to firstly identify whether CpG sites were accurately defined in rat genome bins that compared with our package, and whether we could compare our differentially methylation results. Using a 10 kb bin size, we found 88% of rat genome bins contain at least one MspI cut site.
Unfortunately, given that MethylMnM determines differential methylation using additional MeDIP data to determine a p-value within its MnM.test function, we were unable to compare the results of differential methylation. Therefore, the comparison with MethylMnM not only demonstrates that MRE-seq can reach good coverage throughout the genome, but also that given a population-level project, such as the high-fat rat diet study or barley and maize leaf samples analysed above, msgbsR can be used as a complete analysis pipeine to analyse sequence data and determine differential methylated sites without the need for additional sequencing technologies.
Discussion
The advancement of high throughput technologies has enabled varying sequencing techniques. However, there is a limited number of bioinformatics tools available for the analysis of all the available sequencing protocols. MRE-seq is a reduced representation of whole genome sequencing which can be used to study DNA methylation and parts of the genome that are normally inaccessible in other sequencing technologies1. However, there is a current lack of bioinformatics tools that are tailor made for the analysis of MRE-seq experiments within the literature. Here in this study we outline msgbsR, an R package which can be used in part of the pipeline in analysing large-scale MRE-seq experiments. Our package works by identifying methylated sites and read counts directly from sorted and indexed BAM files into the R environment and can verify if the reads have mapped correctly to the recognition site of the RE by using a reference genome in the format of either a BSgenome or FASTA file.
To our knowledge, this is the first software package that is available that can work with an entire MRE-seq project directly and specifically, and create a table of reads based on REs cut sites. This fills a significant computational analysis gap in the current DNA methylation analysis approaches, especially given the increased use of MRE-seq data in agricultural and ecological analysis settings. Similar analysis pipelines and R/Bioconductor packages are available for methylation data which share significant features of msgbsR yet differ in input data. For example, the ChAMP package, which works solely with Illumina HumanMethylation450 BeadChip, is a pipeline package that takes data generated outside of the R environment (IDAT files) to create a matrix of DNA methylation values (beta values). Both have options to filter the data such as removing probes or cut sites, as well as the ability to perform differential methylation analyses.
Reduced representation sequencing conducted in MRE-seq enables a larger number of samples to be sequenced, making this a more suitable methylation analysis platform compared to high-resolution protocols such WGBS. For example, in an agricultural setting it may be used to assess both genetic and epigenetic variation over mapping populations23 or for assessing the epigenetic impact of breeding populations in new environments24. In a medical setting the DNA methylation data can be used to make group comparisons25. Furthermore, single nucleotide polymorphisms (SNPs) can also be obtained from MRE-seq data, thereby making this approach essential for genome-wide and epigenome-wide associations studies at the same time. msgbsR can also be used with non-methylation sensitive data to verify reads have been mapped correctly and to determine if there are any differences in read counts between groups, allowing it to be used in conjunction with other Bioconductor packages for assessing genetic variation, such as GWAStools26.
Differential DNA methylation can be performed using msgbsR which contains a wrapper function using edgeR18. We choose to make a wrapper function of edgeR since MRE-seq experiments typically contain samples from multiple groups. Performing differential methylation analyses can become time consuming especially when there are multiple comparisons to consider. Our wrapper function uses the recommend trimmed mean of M-values (TMM) normalisation method suggested by edgeR27. However, we do acknowledge users may wish to use other bioinformatics tools to perform differential methylation analyses. Users may want to perform other normalisation methods or use other downstream packages such as methylSig28, BiSeq29 or DSS30, packages designed to identify differentially methylated sites and regions. However, these tools have been primarily designed to work with whole genome bisulphite (WGBS) sequencing whereby methylation is determined through the proportion of methylated and un-methylated reads, and may not necessarily fulfil the user requirements when working with MRE-seq data.
The msgbsR package is fully documented, contains a tutorial data set and is freely available from Bioconductor. With its extended use with large-scale DNA methylation projects, we hope to further develop additional analysis features, such as the ability to input different DNA methylation data types and DMR identification functions.
Methods
Library preparation and sequencing of rat MRE-seq
DNA was extracted from prostates and then digested with EcoRI and MspI using the MSAP technique31,32. EcoRI is a RE and recognises the sequence G^AATTC and is not methylation sensitive. Illumina sequencing primer adapters were ligated to the digested genomic DNA. Using a technique as previously described33,34, cycling was performed using a BioRad 100 thermocycler at 37 °C for 2 hours followed by enzyme inactivation for 20 min at 65 °C. Barcoded adapters were designed with an MspI overhang and a common Y adapter with an EcoRI overhang using the script by Thomas P. van Gurp (www.deenabio.com/services/gbs-adapters) and were ligated as previously described33. T4 ligase (200U) and T4 ligase buffer (NEB T4 DNA Ligase #M0202) along with 0.1 ρmol and 15 ρmol of the barcoded MspI adapter and EcoRI adapter respectively. The reaction mixture was incubated at 22 °C for 2 hours and then 65 °C for 20 mins for enzyme inactivation. 5 µL from each ligation reaction were pooled together and then divided into equal volumes for column clean-up using the PureLink PCR Purification Kit (Life Technologies). Samples were then pooled back together for a total of 60 µL in molecular biology grade water. PCR reactions were performed in a 25 µL volume with 10 µL of digested DNA, 5 µL of 5× NEB MasterMix, 2 µL of 10 µM Forward and Reverse primers at 10 µM. PCR cycle reactions (Solexa) were performed at 98 °C for 30 seconds, followed by 16 cycles of 98 °C for 30 seconds, 62 °C for 20 seconds and 72 °C for 30 seconds and finally 72 °C for 5 min. Size selection of fragments was performed using Ampure XP magnetic beads (Beckman). Fragments were captured and eluted into 30 µL of water. Samples were sequenced using an Illumina HiSeq2500 (Illumina Inc., San Diego, CA, USA) at the Queensland Brain Institute (QBI).
Publicly available data set
The publicly available data set (SRP004282.1) used to demonstrate several functions of msgbsR was firstly obtained from the Sequence Read Archive (SRA)35. SRA files were then converted to FASTQ files using the SRA tool kit version 2.2.2a36. This study contained two data sets containing samples from either barley or maize leaves2. Both data sets were demultiplexed using specific barcodes provided within the study2 and GBSX37. This resulted in each individual sample from each data set in a FASTQ format.
Processing of sequencing data
Alignment of reads was performed using bowtie2 v2.2.311 to each respective reference genome. We used the latest barley reference genome (ASM32608v1) which was obtained from the plant Ensembl website (plants.ensembl.org/Hordeum_vulgare/). For maize, we used the Ensembl release (AGPv4) which we obtained from the Illumina iGenomes website. For the Rat data we used UCSC latest release (rn6) which was obtained from the Illumina iGenomes website. Alignment with bowtie2 resulted in BAM files which were then sorted and indexed using SAMtools v1.213. The sorted and indexed BAM files were then directly read into the R environment using the rawCounts function within the msgbsR package enabling downstream analyses with msgbsR. Since the offspring were from some of the same mothers, differential methylation was performed using the mother as a blocking factor.
Gene Ontology Analysis
Differentially methylated sites were annotated to the nearest gene using the nearest function within the GenomicRanges Bioconductor package15. These genes were then used for GO analysis which was performed using g:Profiler38 where term with an adjusted p-value < 0.05 were considered significant.
Electronic supplementary material
Acknowledgements
The authors would like to thank all the individuals who were involved in the creation of the publicly available data set (SRP004282.1) that was used as an example in this manuscript. We would also like to thank Bioconductor for their support, code reviews and development of our package. This project was funded in part by a National Health and Medical Research Council of Australia (NHMRC) Project Grant (GNT1059120) awarded to CTR and TB-M, Waite Research Institute Research Capacity Development Funding awarded to TB-M and CRL, and Cancer Council of South Australia/SAHMRI Beat Cancer Project Grant (APP1030945) awarded to TB-M. CTR is supported by a NHMRC Senior Research Fellowship GNT1020749. SB is supported by an NHMRC-ARC Dementia Research Development Fellowship Grant (APP1111206). BTM is supported by an Australian Postgraduate Award.
Author Contributions
B.T.M. designed and created the R package, analysed, interpreted the data and wrote the manuscript with input from J.B. C.R.L. and S.B. conceived the analysis method, and along with J.B. and S.L. were involved in study design provided critical discussion and intellectual input into the manuscript. T.B.-M. and C.R.L. designed, conducted the MRE-seq experiment on the rat prostates and provided critical discussion and intellectual input into the manuscript. C.T.R. provided critical discussion and intellectual input into the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19655-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benjamin T. Mayne, Email: benjamin.mayne@adelaide.edu.au
James Breen, Email: jimmy.breen@adelaide.edu.au.
References
- 1.He J, et al. Genotyping-by-sequencing (GBS), an ultimate marker-assisted selection (MAS) tool to accelerate plant breeding. Frontiers in Plant Science. 2014;5:484. doi: 10.3389/fpls.2014.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elshire, R. J. et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One6, 10.1371/journal.pone.0019379 (2011). [DOI] [PMC free article] [PubMed]
- 3.Li D, Zhang B, Xing X, Wang T. Combining MeDIP-seq and MRE-seq to investigate genome-wide CpG methylation. Methods. 2015;72:29–40. doi: 10.1016/j.ymeth.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catchen, J., Hohenlohe, P. A., Bassham, S., Amores, A. & Cresko, W. A. Stacks: an analysis tool set for population genomics. Mol Ecol. 22, 10.1111/mec.12354 (2013). [DOI] [PMC free article] [PubMed]
- 5.Bradbury PJ, et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23:2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- 6.Pidsley R, et al. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016;17:208. doi: 10.1186/s13059-016-1066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, et al. Detection of differentially methylated regions from whole-genome bisulfite sequencing data without replicates. Nucleic acids research. 2015;43:e141. doi: 10.1093/nar/gkv715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plongthongkum N, Diep DH, Zhang K. Advances in the profiling of DNA modifications: cytosine methylation and beyond. Nat Rev Genet. 2014;15:647–661. doi: 10.1038/nrg3772. [DOI] [PubMed] [Google Scholar]
- 9.H, H. K. a. K. BiSeq: Processing and analyzing bisulfite sequencing data. R package version 1.16.0 (2015).
- 10.Hansen KD, Langmead B, Irizarry RA. BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol. 2012;13:R83. doi: 10.1186/gb-2012-13-10-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan M, P. H., Obenchain V and Hayden N. Rsamtools: Binary alignment (BAM), FASTA, variant call (BCF), and tabix file import. R package version 1.24.0, http://bioconductor.org/packages/release/bioc/html/Rsamtools.html (2016).
- 15.Lawrence M, et al. Software for computing and annotating genomic ranges. PLoS computational biology. 2013;9:e1003118. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan M, O. V., Hester J & Pagès H. SummarizedExperiment: SummarizedExperiment container. R package version 1.6.0 (2017).
- 17.Conesa A, et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:13. doi: 10.1186/s13059-016-0881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson MD, McCarthy DJ, Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res., 10.1093/nar/gkv007 (2015). [DOI] [PMC free article] [PubMed]
- 20.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. 2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer D, Zeileis A, Hornik K. The Strucplot Framework: Visualizing Multi-way Contingency Tables with vcd. 2006. 2006;17:48. [Google Scholar]
- 22.Zhou Y, Z. B., Lin N, Zhang B and Wang T. methylMnM: detect different methylation level (DMR). R package version 1.16.0. (2013).
- 23.Xiong W, et al. DNA Methylation Alterations at 5′-CCGG Sites in the Interspecific and Intraspecific Hybridizations Derived from Brassica rapa and B. napus. PLoS One. 2013;8:e65946. doi: 10.1371/journal.pone.0065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarquín D, et al. Genotyping by sequencing for genomic prediction in a soybean breeding population. BMC Genomics. 2014;15:740. doi: 10.1186/1471-2164-15-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazarenko MS, et al. A Comparison of Genome-Wide DNA Methylation Patterns between Different Vascular Tissues from Patients with Coronary Heart Disease. PLoS One. 2015;10:e0122601. doi: 10.1371/journal.pone.0122601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gogarten SM, et al. GWASTools: an R/Bioconductor package for quality control and analysis of genome-wide association studies. Bioinformatics. 2012;28:3329–3331. doi: 10.1093/bioinformatics/bts610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park Y, Figueroa ME, Rozek LS, Sartor MA. MethylSig: a whole genome DNA methylation analysis pipeline. Bioinformatics. 2014;30:2414–2422. doi: 10.1093/bioinformatics/btu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hebestreit K, Dugas M, Klein HU. Detection of significantly differentially methylated regions in targeted bisulfite sequencing data. Bioinformatics. 2013;29:1647–1653. doi: 10.1093/bioinformatics/btt263. [DOI] [PubMed] [Google Scholar]
- 30.Feng H, Conneely KN, Wu H. A Bayesian hierarchical model to detect differentially methylated loci from single nucleotide resolution sequencing data. Nucleic Acids Res. 2014;42:e69. doi: 10.1093/nar/gku154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyna-Lopez GE, Simpson J, Ruiz-Herrera J. Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphisms. Mol. Gen. Genet. 1997;253:703–710. doi: 10.1007/s004380050374. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez López CM, et al. Detection and quantification of tissue of origin in salmon and veal products using methylation sensitive AFLPs. Food Chemistry. 2012;131:1493–1498. doi: 10.1016/j.foodchem.2011.09.120. [DOI] [Google Scholar]
- 33.Poland JA, Brown PJ, Sorrells ME, Jannink JL. Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS One. 2012;7:e32253. doi: 10.1371/journal.pone.0032253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia Z, Zou M, Zhang S, Feng B, Wang W. AFSM sequencing approach: a simple and rapid method for genome-wide SNP and methylation site discovery and genetic mapping. Sci. Rep. 2014;4:7300. doi: 10.1038/srep07300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leinonen R, Sugawara H, Shumway M. On behalf of the International Nucleotide Sequence Database, C. The Sequence Read Archive. Nucleic Acids Res. 2011;39:D19–D21. doi: 10.1093/nar/gkq1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SRA Knowledge Base [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); Available from: https://www.ncbi.nlm.nih.gov/books/NBK56551/ (2011).
- 37.Herten K, Hestand MS, Vermeesch JR, Van Houdt JK. GBSX: a toolkit for experimental design and demultiplexing genotyping by sequencing experiments. BMC Bioinformatics. 2015;16:1–6. doi: 10.1186/s12859-015-0514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimand J, et al. g:Profiler-a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–89. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris TJ, et al. ChAMP: 450k Chip Analysis Methylation Pipeline. Bioinformatics. 2014;30:428–430. doi: 10.1093/bioinformatics/btt684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aryee, M. J. et al. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA Methylation microarrays. Bioinformatics30, 10.1093/bioinformatics/btu049 (2014). [DOI] [PMC free article] [PubMed]
- 41.Aryee MJ, et al. Accurate genome-scale percentage DNA methylation estimates from microarray data. Biostatistics. 2011;12:197–210. doi: 10.1093/biostatistics/kxq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kishore K, et al. methylPipe and compEpiTools: a suite of R packages for the integrative analysis of epigenomics data. BMC Bioinformatics. 2015;16:313. doi: 10.1186/s12859-015-0742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.