Abstract
WeChat represents one of the most popular smartphone-based applications for communication. Although the application provides several useful features that simplify daily life, a growing number of users spend excessive amounts of time on the application. This may lead to interferences with everyday life and even to addictive patterns of use. In the context of the ongoing discussion on Internet Communication Disorder (ICD), the present study aimed to better characterize the addictive potential of communication applications, using WeChat as an example, by examining associations between individual variations in tendencies towards WeChat addiction and brain structural variations in fronto-striatal-limbic brain regions. To this end levels of addictive tendencies, frequency of use and structural MRI data were assessed in n = 61 healthy participants. Higher tendencies towards WeChat addiction were associated with smaller gray matter volumes of the subgenual anterior cingulate cortex, a key region for monitoring and regulatory control in neural networks underlying addictive behaviors. Moreover, a higher frequency of the paying function was associated with smaller nucleus accumbens volumes. Findings were robust after controlling for levels of anxiety and depression. The present results are in line with previous findings in substance and behavioral addictions, and suggest a similar neurobiological basis in ICD.
Introduction
In the first quarter of 2017 49.7% of the world population had access to the Internet. In the country with the largest population today, China, 52.7% of the population have been online in 20171. The Internet is increasingly accessed via mobile devices, particularly smartphones. Penetration rates of smartphones are growing at incredible speed with currently 663 million smartphone users alone in China2. Smartphone applications simplify daily life and promote social interactions via social network and messenger platforms, however, a number of users develop excessive, or even addictive, patterns of smartphone use that interfere with daily life and mental health3–6.
The importance of studying the neural basis of addictive Internet use has been recently emphasized by the inclusion of Internet Gaming Disorder (IGD) as an emerging mental disorder in the DSM-57. IGD represents a specific form of Internet addiction8, characterized by a loss of control of use, development of tolerance and loss of interest in alternative activities7,9. The symptoms strongly resemble dysregulations observed in other addictive disorders, including substance and behavioral addictions. During the last 20 years (e.g. early work by Young10,11) elaborate conceptualizations have been developed to describe mechanisms of Internet addiction that provide a research framework for specific forms of Internet addiction (e.g. IGD), as well as broader forms such as Internet Communication Disorder referring to addictive use of social network and messenger platforms12. With respect to the neurobiological basis of Internet addiction, converging evidence points to dysfunctions in the fronto-striatal-limbic circuitries accompanied by impaired executive functions, maladaptive reward processing and deficient emotion regulation12–14. This pattern partly converges with pathological alterations that have been documented in substance addiction and pathological gambling15–19, suggesting that disruptions in the fronto-striatal-limbic circuitries represent a common pathological denominator for addictive disorders that mirror exaggerated reward sensitivity and impulsivity in the amygdala-striatal systems in the context of impaired regulatory control via frontal regions.
The smartphone is a relatively new technical achievement and research has only recently begun to evaluate its addictive potential20–22 (for a direct comparison of Internet and smartphone addiction see the here cited articles21). A recent study that tracked smartphone use in everyday life revealed usage times in the range of about 2.5 h per day, suggesting that smartphones increasingly dominate daily life23. Moreover, this study demonstrated that in particular smartphone-based social communication applications such as WhatsApp (>one billion users worldwide24) represent driving forces of excessive smartphone usage. Initial findings suggest that escalating use of these applications may be rooted in acute and long term effects on the fronto-striatal-limbic circuitry, possibly reflecting their addictive potential. Accumulating evidence suggests that the use of these applications is accompanied by increased ventral striatal activity25–27 possibly reflecting the contribution of reward- and reinforcement-related mechanisms to the development of addictive patterns of use. Initial studies on the long term effects of excessive use suggest detrimental effects on emotional and cognitive functions that depend on the integrity of the fronto-striatal-limbic circuitry, including increased levels of depression and anxiety28, deficient inhibitory control29 and enhanced distraction via smartphones30–32. Notably, a recent prospective study provided initial evidence for a causal link between escalating smartphone use and increasing impulsivity as well as a declining cognitive and social functioning33. Studies employing structural brain imaging revealed initial evidence for an association between escalating social media use and decreased volumes of the nucleus accumbens (NAc)34,35 (however, see He et al.36), a key reward processing node in the ventral striatum, and the amygdala35,36 (however, see Montag et al.34). Smaller volumes of these regions have been previously associated with the development and maintenance of substance addiction19,37,38, with preclinical data emphasizing an important contribution of neuroplastic changes in these regions to pathological changes in motivational, impulsive and habitual behaviors that drive addiction39–42. Findings with regard to regulatory control regions, however, remained inconsistent, and one study even reported that (dorsal) anterior cingulate (ACC) volume increased as a function of social network addiction35. This finding contrasts with the important role of the ACC in implementing frontal control over limbic-striatal regions, and previous reports suggesting an association between decreased volumes of this region and inhibitory control deficits in substance and Internet gaming addicted populations43–46.
In summary, some of the here reviewed studies revealed initial evidence for ventral striatal and amygdala morphological pecularities in (excessive) social media users, possibly reflecting that the addictive potential of these applications is based on shared mechanisms with other addictions. In contrast, findings on the ACC remained inconsistent35,36,47 and might reflect a distinct characteristic of Internet Communication Disorder. However, conclusions are hampered by methodological limitations of the initial studies, particularly the small sample sizes and lack of control variables, as well as by the functional and anatomical heterogeneity of the ACC48–50, with converging evidence suggesting a division into dorsal (caudodorsal) and ventral (subgenual, pregenual) subregions48,51,52. In line with their distinct anatomical and functional connections48,53, dorsal regions are predominantly engaged in cognitive and motor functions whereas ventral regions contribute to emotion regulation and behavioral control52,54. Indeed, studies in patients with mental disorders emphasize the importance of subregion-specific anatomical changes of the ACC in disorders characterized by deficient cognitive and emotional functioning, such as depression and anxiety disorders55–57.
Within the context of a dimensional conceptualization of mental disorders58 the present study examined associations between levels of addictive social media/messenger use and morphological variations in the NAc, amygdala and ACC in a sample of healthy subjects (for a similar approach see Luo et al.59). To specifically clarify inconsistencies regarding the ACC, subregion-specific gray matter volumes of the major ACC subdivisions were assessed in a comparably large samples (n = 61). Given that elevated levels of depression and anxiety have been related to both, excessive social media use28 and fronto-striatal-limbic morphology60–62, levels of depression and anxiety were controlled for.
Whereas previous studies on brain structural alterations associated with excessive social media/messenger use in European or American populations and focused on Facebook/general social network usage34–36, the present study was conducted in China where WeChat ( ; Wēixìn, “micro message”, currently >900 million users63) represents one of the most popular social communication platforms. The literal translation of Wēixìn (“micro message”), refers to the initial development of WeChat as mobile messaging application that allows to send short text messages to individuals or groups and share photos or other files. Whereas the early versions of the Chinese WeChat were thus similar to Western messaging applications like WhatsApp, more recent versions integrate numerous additional functions and platforms including social sharing, paying, banking, and city services like paying traffic fines and booking transportation. Using this multi-purpose integration WeChat has penetrated several aspects of daily life and has become one of the largest social media platforms worldwide in terms of monthly active users.
; Wēixìn, “micro message”, currently >900 million users63) represents one of the most popular social communication platforms. The literal translation of Wēixìn (“micro message”), refers to the initial development of WeChat as mobile messaging application that allows to send short text messages to individuals or groups and share photos or other files. Whereas the early versions of the Chinese WeChat were thus similar to Western messaging applications like WhatsApp, more recent versions integrate numerous additional functions and platforms including social sharing, paying, banking, and city services like paying traffic fines and booking transportation. Using this multi-purpose integration WeChat has penetrated several aspects of daily life and has become one of the largest social media platforms worldwide in terms of monthly active users.
To determine individual levels of addictive behavior in the context of WeChat usage a validated digital addiction scale (short-Internet addiction scale; s-IAT64) that assesses key diagnostic criteria, namely loss of control/time management and craving/social problems, was adopted. Given that previous research has associated the frequency of online media use with both, levels of addictive symptoms6,65,66 and decreased NAc volumes34, the frequency of use of the most popular WeChat functions (texting, voice messaging, paying) was additionally assessed. To determine associations between individual variations in the level of addictive symptoms and the frequency of use with brain structural variations, T1-weigthed Magnetic Resonance Imaging (MRI) data was acquired from all subjects.
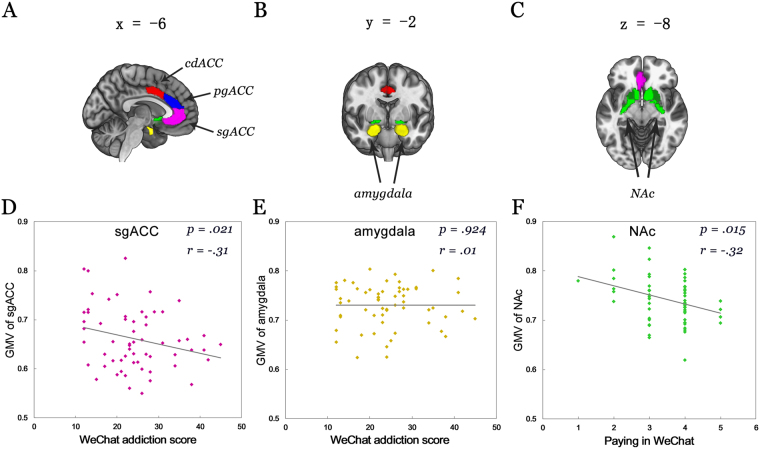
Based on previous studies34–36, we expected that higher levels of addictive symptoms associate with decreased gray matter volume (GMV) of the NAc and amygdala. In the context of the important role of deficient regulatory control in addictive disorders and initial behavioral evidence for associations between excessive smartphone use and deficits in this domain29,33, we expected a negative association between levels of addictive symptoms and GMV in ventral subregions of the ACC. Given inconsistent findings with regard to the involvement of the dorsal ACC (dACC) in social media addiction35,36 no directed hypothesis with respect to this region was specified. Finally, in line with our previous research34 we expected that a higher frequency of use would associate with decreased NAc volumes. An overview of the regions examined is provided in Fig. 1A–C.
Figure 1.
The regions of interest examined in the present study and associations between gray matter volume with WeChat addiction scores and use frequency. (A–C) Displays the regions examined in Montreal Neurological Institute (MNI) space. (D–E) Displays the correlations between brain volumes and WeChat addiction scores (D,E), and the frequency of use of the paying function (F) in the n = 61 participants. P and r values displayed correspond to correlation analysis (two-tailed) including age, gender, depression and anxiety included as control variables.
Results
Descriptive statistics
Table 1 provides descriptive statistics on the WeChat addiction scores and smartphone/WeChat usage variables (details on the scales are given in the Methods section).
Table 1.
Descriptive statistics of smartphone variables including WeChat usage.
| Investigated Variables | Mean and Standard Deviation; Actual Range |
|---|---|
| Smartphone use in hours each week for private/leisure | M = 24.28, SD = 18.48; actual range: 2–100 |
| Smartphone use in hours each week for business | M = 7.85, SD = 6.68; actual range: 0–30 |
| Smartphone usage in hours each week for both leisure and business (added from the previous two items) | M = 32.13, SD = 22.03; actual range: 3–120 |
| Smartphone Addiction (possible range: 10–60) | M = 34.43, SD = 9.01; actual range: 14–52 |
| WeChat use in hours each week for private/leisure | M = 9.95, SD = 10.30; actual range: 1–50 |
| WeChat use in hours each week for business | M = 3.85, SD = 6.63; actual range: 0–40 |
| WeChat usage in hours each week for both leisure and business (added from the previous two items) | M = 13.80, SD = 14.22; actual range: 1–70 |
| WeChat Addiction (possible range: 12–60) | M = 24.33, SD = 8.44; actual range: 12–45 |
| WeChat Addiction Loss of Control (possible range: 6–30) | M = 12.64, SD = 4.49; actual range: 6–23 |
| WeChat Addiction Social Problems (possible range: 6–30) | M = 11.69, SD = 4.21; actual range: 6–22 |
| WeChat: Texting (possible range: 1–5) | M = 3.97, SD = 0.97; actual range: 2–5 |
| WeChat: Voice Messaging (possible range: 1–5) | M = 2.67, SD = 0.96; actual range: 1–5 |
| WeChat: Paying in the store (possible range: 1–5) | M = 3.54, SD = 0.85; actual range: 1–5 |
WeChat addiction and distinct usage of WeChat channels
Table 2 presents the correlation between WeChat addiction scores and usage of its distinct functions. Given the significant correlation of WeChat addiction scores as well as sending text messages with age (e. g. r = 0.27, p = 0.035 for the total WeChat addiction scale; r = 0.31, p = 0.016 for sending text messages), Table 2 presents partial correlations (of note, as there were no gender differences in the WeChat addiction scale or the usage of distinct functions, gender was not included in these analyses). A hierarchical stepwise regression model with age and gender inserted in a first block and all distinct WeChat functions in a second step yielded a significant model with age (7.3% explained variance) and sending voice messages (22.3% additional explained variance) as the most important predictors for WeChat addiction scores. These variables together explain 29.6% in the variation of WeChat addiction scores (F(2, 58) = 12.17, p < 0.001).
Table 2.
Partial correlation between WeChat addiction and distinct usage of WeChat channels.
| WeChat LC | WeChat SP | Texting | Voice | Paying | ||
|---|---|---|---|---|---|---|
| r = 0.97, p < 0.001 | r = 0.97, p < 0.001 | r = 0.25, p = 0.054 | r = 0.49, p < 0.001 | r = 0.25, p = 0.058 | ||
| WeChat LC | r = 0.87, p < 0.001 | r = 0.23, p = 0.079 | r = 0.49, p < 0.001 | r = 0.25, p = 0.057 | ||
| WeChat SP | r = 0.26, p = 0.048 | r = 0.46, p < 0.001 | r = 0.23, p = 0.080 | |||
| Texting | r = 0.21, p = 0.112 | r = 0.41, p = 0.001 | ||||
| Voice | r = 0.16, p = 0.227 | |||||
| Paying |
WeChat LC: Loss of Control sub-scale; WeChat SP: Social Problems sub-scale. Partial correlations are calculated with age included as controlled variable.
Correlations between brain structure and levels of WeChat addiction
A negative correlation was observed between WeChat addiction scores and the GMV of the subgenual ACC (sgACC) while controlling for age and gender (r = −0.26, p = 0.043) (see Fig. 1D). Findings remained stable after controlling for depression and anxiety (r = −0.31, p = 0.021). No significant associations between WeChat addiction scores and the more dorsal regions of the ACC were observed (pregenual ACC, pgACC, r = 0.01, p = 0.942; caudodorsal, cdACC, r = 0.12, p = 0.381 controlling for age and gender; both p > 0.726 after additionally controlling for anxiety and depression). No associations were observed between WeChat addiction scores and GMV of the NAc (r = −0.07, p = 0.601 controlling for age and gender, r = −0.074, p = 0.587 after additionally controlling for anxiety and depression) or the amygdala (r = 0.05, p = 0.702 controlling for age and gender, p = 0.924 after additionally controlling for anxiety and depression; see also Fig. 1E).
Associations with WeChat usage parameters
Given that our previous study on Facebook usage specifically identified a negative association between GMV of the NAc and behavioral indices of excessive smartphone-based social media usage (daily frequency of Facebook checking/duration of Facebook usage on smartphone34), an additional analysis specifically examined associations between WeChat usage behavior and GMV of the NAc. In line with the previous results34 a negative correlation was observed between lower GMV of the NAc and higher usage of the application, although in this case specifically for paying functions (r = −0.29, p = 0.029, controlled for age and gender; r = −0.32, p = 0.015 with anxiety and depression additionally controlled for; non-parametric analysis using Spearman correlation; rho = −0.29, p = 0.022) (see Fig. 1F). Neither the total WeChat addiction scale, voice messaging nor texting showed significant associations with NAc GMV (all p > 0.183, two-tailed, controlled for age, gender; all p > 0.098, two-tailed, controlled for age, gender, anxiety and depression; non-parametric analyses using Spearman correlation; all p > 0.114).
Discussion
The present study aimed to investigate associations between individual variations in the levels of WeChat addiction with gray matter volumes of the amygdala, NAc and and subregions of the ACC. Based on previous findings associations between usage frequency and NAc volume was additionally examined. Higher levels of self-reported addictive symptoms and more frequent usage (of the paying service) were associated with lower gray matter volumes in the ventral (subgenual) ACC and NAc, respectively. A similar relationship between higher levels of addictive symptoms or more excessive patterns of use and volume reductions in these regions has been repeatedly reported in substance addiction44 as well as behavioral addictions including generalized Internet addiction47 and Internet gaming disorder67,68. Together with previous research on the brain structural correlates of excessive social media and messenger use34–36 the present findings suggest that structural alterations in the fronto-striatal-limbic circuitry represent a common denominator across different types of digital addiction, including Internet Communication Disorder.
Based on inconsistent findings with regard to structural changes of the ACC in Internet Communication Disorder, the present study specifically explored associations between levels of WeChat addiction symptoms and ACC volume on a subregional level. This approach revealed that higher symptom levels were associated with lower volume of the ventral ACC, specifically the sgACC. In contrast to a previous study reporting that dACC volumes increased as a function of higher social network addiction35, the present study did not observe significant associations with the dACC region. These inconsistencies may be due to a higher sensitivity of the subregional approach, differences in the levels of addictive symptoms in the samples examined (some participants scoring 40–45 points on the scale with a maximum of 60 points in the present sample), sample size (n = 61 in the present study vs. n = 20 in the He et al. study35), or the use of different scales to assess individual variations in social media addiction (with the present study focusing on WeChat addiction rather than general social media addiction).
The present findings of reduced sgACC volume in the context of higher addictive symptom scores converge with previous findings on reduced ACC volumes in other addictive disorders, including substance addiction44 and Internet Gaming Disorder67,68. In line with the regulatory role of the ACC, reduced volumes of this region in Internet addiction have been associated with deficient cognitive control68 and increased impulsivity46, and thus might contribute to the loss of behavioral and emotional control which represents a key symptom across addictive disorders. Given that convergent evidence from previous research using different methods including electroencephalography (EEG) and MRI indicates an important role of ACC dysfunctions in the development and maintenance of excessive and addictive digital media use (for an overview see Montag, Duke & Reuter69), the present findings argue for similar disruptions in frontal control regions across different forms of digital addiction.
On the subregional level the present analysis approach demonstrated a specific association with volumes of the sgACC. The sgACC projects to limbic and striatal regions including the NAc53 and plays a pivotal role in implicit emotion regulation54,70 and inhibitory control71. The present findings thus converge with previous conceptualizations discussing enhanced impulsivity and impaired inhibitory control as endophenotype markers across psychiatric diagnoses, that precede the development of the complete clinical picture of the disorder58.
Further support for the functional relevance of reduced sgACC volumes in this behavioral domain comes from previous morphological studies reporting associations between decreased sgACC volumes with deficient emotional and inhibitory control, including increased subjective experience of chronic stress72, reduced behavioral control during acute stress68, behavioral disinhibition in dementia patients73 as well as lower resilience in the normal population74. These functional characterizations of reduced sgACC volumes suggest that the present observations might either reflect that increasing levels of ICD symptoms are accompanied by a loss of regulatory control (as suggested by initial prospective data33), or represent a pre-existing vulnerability factor, such as a generally reduced inhibitory control or resilience, increasing the risk for the development of addictive behavior.
Moreover, in line with previous studies34,36 an association between a more frequent usage of a social media platform and smaller NAc volumes was observed, emphasizing the importance of alterations in the ventral striatal reward system in ICD. Alterations in ventral striatal morphology have been repeatedly observed in related disorders such as Internet Gaming Disorder possibly reflecting adaptations in the striatal reward system43. Although previous studies predominantly reported decreased ventral striatal volume in excessive Internet gaming43, increased volumes75,76 have also been demonstrated. As discussed in the context of the present findings on the ACC, the association between lower gray matter volume of the NAc and higher usage of WeChat’s paying function argues for similar neurobiological mechanisms across different addictive disorders. Mounting evidence from research on alcohol and nicotine addiction demonstrates similar associations between lower gray matter volume of the NAc and higher levels of addictive symptoms77,78. Reduced NAc volumes have additionally been observed in heroin addicts79. A noteworthy finding in our study is a significant association between gray matter volumes of the NAc and WeChat related variables specifically observed for paying, but not for functions such as texting or voice messaging (although associations with texting showed a similar trend for negative associations, see Table S3). In contrast, no associations were observed between NAc volume and the WeChat addiction scores. Together, this demonstrates the importance for a differential examination of excessive usage and problematic (addictive) behavior as well as of different functions of the applications to delineate a fine-grained picture of the underlying biological mechanisms that promote excessive and potentially addictive social media usage.
The present findings need to be interpreted in the context of some limitations. First, although the examination of different ACC subregions facilitated a more sensitive analysis of this heterogeneous structure, the approach increased the number of regions examined and the significance level was not adopted for the number of regions of interest. Second, the present study did not assess general tendencies towards social media overuse. Although such a scale might be highly correlated with WeChat addiction tendencies (see also correlations with smartphone addiction in the supplementary material), the brain structure associations might have been different in the present data set. Third, we relied on a rather young (Asian) student sample. Therefore, it is not clear if the present results can be generalized to the broader population of WeChat users going also beyond Asian populations. Moreover, future studies should consider implementation of smartphone tracking technologies from Psychoinformatics80 accompanying self-report assessment as used in previous research23,34,81,82. Given the overlap of the here reported WeChat-brain structure associations with other reported findings in the literature, we are still optimistic that this might be the case. Finally, the present data set only investigates structural associations in the human brain and provides no insights into functional aspects in the context of social media use. Future studies should aim to characterize alterations on the functional level.
In summary, these initial findings on the brain structural substrates of addictive behavior related to WeChat show that in line with previous reports and overarching neurobiological conceptualizations in substance-based and behavioral addictions including Internet addiction, lower ACC gray matter volumes are associated with higher addictive tendencies towards using this smartphone-based platform. Moreover, the present results provide further evidence for the important role of the NAc in excessive digital (over-)use.
Methods
Participants and Protocols
N = 67 healthy university students were recruited for the present study. Inclusion criteria were (1) no previous or current neurologic/mental disorder according to self-report (2) no contraindications for magnetic resonance imaging (MRI) (e.g. metal implants, pregnancy) (3) ownership of a smartphone and use of the WeChat application. In addition, depression and anxiety scores were collected from the participants and these resulted in non-obstrusive scores (depression score from BDI-II83, mean = 8.37, SD = 6.64; trait anxiety from STAI84, mean = 42.27, SD = 8.34; numbers are presented for the final sample of N = 61 participants). Based on the study inclusion criteria (n = 1 non using WeChat) and MRI data quality assessments (data from n = 5 participants excluded) 61 participants entered the final analyses (40 males and 21 females; mean-age: 22.34; SD = 2.29). WeChat addiction scores and usage behavior were assessed using self-report questionnaires (details see questionnaires). To determine associations between individual variations in the level of addictive symptoms and WeChat usage with brain structural variations, T1-weigthed MRI data was acquired from all subjects.
The study and protocols were approved by the local ethic committee of the University of Electronic Science and Technology (UESTC), Chengdu, China and participants provided informed consent before study inclusion. The study and procedures were in line with the most recent version of the Declaration of Helsinki.
Questionnaires
All participants were administered a modified version of the validated short-Internet addiction scale (s-IAT) as provided by Pawlikowski et al.64 that – in line with the proposed diagnostic criteria for Internet addiction - assesses loss of control/time management and craving/social problems as key characteristics of Internet addiction. In order to specifically assess levels of addictive WeChat use we carefully adopted the wordings with respect to WeChat usage. E. g. the original item called “How often do you find that you stay on-line longer than you intended?” has been changed to “How often do you find that you stay longer on WeChat than you intended?” (for a complete list of the items see Table 3). Participants answered the twelve items using a five point Likert scale ranging from never (1) to very often (5). Internal consistencies of the questionnaire were excellent (Cronbach’s α, complete scale, α = 0.93; loss of control/time management subscale (items 1, 2, 3, 6, 8, 9), α = 0.90; craving/social problems subscale (items 4, 5, 7, 10, 11, 12), α = 0.83). We report only associations with the total scale in the present work, given that results regarding our main hypothesis on the ventral ACC were equally strong for both subscales (association with sgACC: r = −0.29, p = 0.033 for loss of control and r = −0.31, p = 0.020 for social problems when controlling for age, gender, anxiety and depression).
Table 3.
Chinese and English version of WeChat addiction test ( -AT; WC-AT). Note that this is a modified version of the s-IAT as presented in Pawlikowski et al.64.
-AT; WC-AT). Note that this is a modified version of the s-IAT as presented in Pawlikowski et al.64.
1、 ? (LC) ? (LC) |
2、 ? (LC) ? (LC) |
3、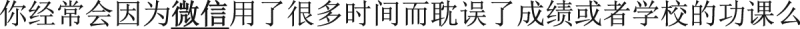 ? (LC) ? (LC) |
4、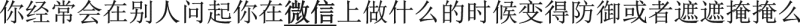 ? (SP) ? (SP) |
5、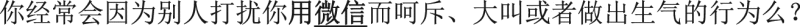 ? (SP) ? (SP) |
6、 ? (LC) ? (LC) |
7、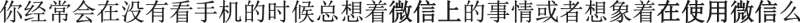 ? (SP) ? (SP) |
8、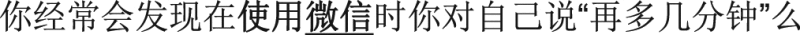 ? (LC) ? (LC) |
9、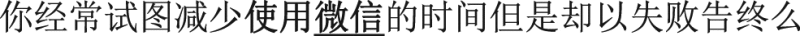 ? (LC) ? (LC) |
10、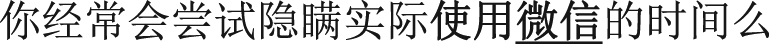 ? (SP) ? (SP) |
11、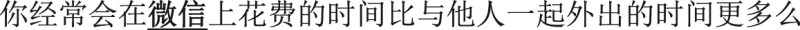 ? (SP) ? (SP) |
12、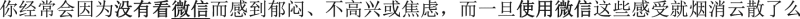 ? (SP) ? (SP) |
Answer options: (1)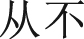 , (2) , (2) , (3) , (3) , (4) , (4)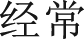 , (5) , (5)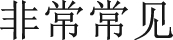 ; LC: loss of control/time management; SP: craving/social problems ; LC: loss of control/time management; SP: craving/social problems |
| 1. How often do you find that you stay on WeChat longer than you intended? (LC) |
| 2. How often do you neglect household chores to spend more time on WeChat? (LC) |
| 3. How often do your grades or school work suffer because of the amount of time you spend on WeChat? (LC) |
| 4. How often do you become defensive or secretive when anyone asks you what you do on WeChat? (SP) |
| 5. How often do you snap, yell, or act annoyed if someone bothers you while you are on WeChat? (SP) |
| 6. How often do you lose sleep due to being on WeChat late at night? (LC) |
| 7. How often do you feel preoccupied with WeChat when off-line, or fantasize about being on WeChat? (SP) |
| 8. How often do you find yourself saying “just a few more minutes” when on WeChat? (LC) |
| 9. How often do you try to cut down the amount of time you spend on WeChat and fail? (LC) |
| 10. How often do you try to hide how long you’ve been on WeChat? (SP) |
| 11. How often do you choose to spend more time on WeChat over going out with others? (SP) |
| 12. How often do you feel depressed, moody, or nervous when you are off-line, which goes away once you are back on WeChat? (SP) |
Answer options: (1) never, (2) rarely, (3) sometimes, (4) often, (5) very often; LC: loss of control/time management; SP: craving/social problems.
WeChat usage behavior was further characterized by administering a questionnaire that assessed hours per week for private and business matters, the frequency of use of texting, voice-messaging and paying with the application (details see Table 4).
Table 4.
Chinese and English version asking for amount of WeChat use and its distinct functions.
 : ________ : ________ |
 :________ :________ |
 : : |
|
|
|
|
|
|
Answer options: (1) , (2) , (2) , (3) , (3) , (4) , (4) , (5) , (5)
|
| WeChat use in hours each week for private/leisure: ______ |
| WeChat use in hours each week for business: ______ |
| Please indicate how often you use the following functions: |
| Texting |
| Voice-messaging |
| Paying in the store |
Answer options: (1) never, (2) rarely, (3) sometimes, (4) often, (5) very often.
Given that WeChat is nearly exclusively used on mobile devices, smartphone addiction was additionally assessed using a validated questionnaire provided by Kwon et al.20. This short questionnaire consisting of 10 items with answer option 1–6 (1 = strongly disagree to 6 = strongly agree) assesses individual differences in smartphone addiction including items targeting the main characteristics of addictive usage such as preoccupation with the smartphone and loss of control. Internal consistency of the smartphone addiction scale was excellent (α = 0.83). This questionnaire was not significantly associated with brain structure in the predefined regions of interest. Therefore, corresponding findings are presented in the supplementary material.
Based on accumulating evidence for associations between excessive smartphone use and increased levels of depression and anxiety28 and previous reports demonstrating associations between variations in levels of anxiety and depression and brain structure60–62, trait anxiety (TAI)84 and depressive symptoms (BDI-II)83 were assessed (Cronbach alpha was 0.64 for the Mandarin version of TAI85 and 0.86 for Mandarin version of BDI-II86). To control for potential confounding effects on associations between WeChat addiction and brain structure, analyses were recomputed including levels of anxiety and depression as additional covariates along with gender and age.
Brain Structure: data acquisition and analysis
All participants underwent a T1-weighted brain structural assessment on a 3-Tesla GE MR750 system (General Electric Medical System, Milwaukee, WI, USA). Data was acquired using an FSPGR sequence with the following imaging parameters: repetition time = 5.97 ms, echo time = 1.97 ms, flip angle = 9°, field of view = 256*256 mm2, slice thickness = 1 mm, slice (coronal) number = 128, matrix = 256*256.
The structural data was preprocessed using the VBM8 toolbox as implemented in SPM12 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm/). The brain volumes were first segmented into different brain tissues including gray matter, white matter and CSF using the unified segmentation87 as implemented in SPM12 (“New Segment”) and subsequently non-linearly normalized to MNI space using the DARTEL algorithm88 while controlling for variances within individual total brain volumes by modulating the voxel values of the gray matter images with the warping parameters generated during affine transformation, which is proportional to total intracranial volume (TIV)89.
Quality assessment procedures included VBM toolbox algorithms that examine the individual covariance of each brain volume. In line with the recommendations for VBM analyses, images with covariance below 2 standard deviations from the mean (0.78) of the total data were excluded from further analysis (n = 5 participants). To minimize individual differences in spatial registration and increase signal-to-noise ratio (SNR), gray matter volumes were finally spatially smoothed with an 8 mm FWHM (full width at half maximum) kernel.
Based on our regional a priori hypotheses and in line with previous studies on social network use addiction34–36 the brain structural analyses focused on the anterior cingulate cortex (ACC), nucleus accumbens (NAc) and amygdala as a priori regions of interest (ROIs). To account for the functional heterogeneity of the ACC (Etkin et al.52,54) and in line with previous studies specifically targeting subregion-specific ACC engagement in mental disorders55–57 the subgenual ACC (sgACC), pregenual ACC (pgACC), and caudodorsal ACC (cdACC) was assessed. To determine associations with levels of WeChat addiction and usage, gray matter volumes (GMV) were extracted from bilateral masks derived from the Brainnetome atlas90 (see Fig. 1A–C for the ROIs examined in the present study).
First, descriptive statistics as well as the relations between WeChat addiction scores and usage of distinct WeChat functions are presented. Associations between individual variations in WeChat addiction and variations in regional GMV were examined using partial correlations (controlling for age and gender as well as age, gender, anxiety and depression; see introduction part). The frequency variables texting, paying and sending voice messages were non-normally distributed. However, given that skewness and kurtosis of the variables was <1, parametric partial correlations (controlling for age and gender as well as age, gender, anxiety and depression) were used to examine associations between individual variations in these variables and variations in NAc volume. To further validate the robustness of the associations between the frequency variables and NAc volume, results from Spearman’s nonparametric rank correlation are additionally reported. All analyses were performed using a two-sided p < 0.05 as significance threshold.
Data Availability Statement
Upon reasonable request third parties will be granted access to the original data of this manuscript.
Electronic supplementary material
Acknowledgements
The study was supported by the National Natural Science Foundation of China (NSFC, 91632117, BB; 31530032, KK), the German Research Foundation (DFG, grant: BE5465/2-1, BB), and an Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning (BB). The position of CM is funded by a Heisenberg grant awarded to him by the German Research Foundation (MO 2363/3-2). CS is a stipend of the German Academic Scholarship Foundation (Studienstiftung des deutschen Volkes).
Author Contributions
C.M. and B.B. designed the present study. C.M. and B.B. drafted the main parts of the manuscript. Z.Z., B.B., C.M. and C.S. carried out the statistical analyses. L.X., M.F., J.L., X.Z. and J.D. scanned the participants and helped in the recruiting process, whereas C.S., Z.Z. and C.M. worked on the translation and administration process of the used inventories. All authors, in particular K.M.K. critically revised the manuscript. All authors approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Christian Montag and Zhiying Zhao contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19904-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christian Montag, Email: christian.montag@uni-ulm.de.
Benjamin Becker, Email: ben_becker@gmx.de.
References
- 1.Internetworldstats.com. Asia Marketing Research, Internet Usage, Population Statistics and Facebook Subscribers. Available at: http://internetworldstats.com/asia.htm#cn. (Accessed: 15th August 2017).
- 2.statista.com. Number of smartphone users in China from 2013 to 2022. Available at: https://www.statista.com/statistics/467160/forecast-of-smartphone-users-in-china/ (Accessed: 15th August 2017).
- 3.Clayton RB, Leshner G, Almond A. The extended iSelf: The impact of iPhone separation on cognition, emotion, and physiology. J Comput Commun. 2015;20:119–135. [Google Scholar]
- 4.Bian M, Leung L. Linking Loneliness, Shyness, Smartphone Addiction Symptoms, and Patterns of Smartphone Use to Social Capital. Soc Sci Comput Rev. 2015;33:61–79. doi: 10.1177/0894439314528779. [DOI] [Google Scholar]
- 5.Kushlev K, Dunn EW. Checking email less frequently reduces stress. Comput Human Behav. 2015;43:220–228. doi: 10.1016/j.chb.2014.11.005. [DOI] [Google Scholar]
- 6.Samaha M, Hawi NS. Relationships among smartphone addiction, stress, academic performance, and satisfaction with life. Comput Human Behav. 2016;57:321–325. doi: 10.1016/j.chb.2015.12.045. [DOI] [Google Scholar]
- 7.Petry NM, O’Brien CP. Internet gaming disorder and the DSM-5. Addiction. 2013;108:1186–1187. doi: 10.1111/add.12162. [DOI] [PubMed] [Google Scholar]
- 8.Montag C, et al. Is it meaningful to distinguish between generalized and specific Internet addiction? Evidence from a cross-cultural study from Germany, Sweden, Taiwan and China. Asia-Pacific Psychiatry. 2015;7:20–26. doi: 10.1111/appy.12122. [DOI] [PubMed] [Google Scholar]
- 9.Tao R, et al. Proposed diagnostic criteria for internet addiction. Addiction. 2010;105:556–564. doi: 10.1111/j.1360-0443.2009.02828.x. [DOI] [PubMed] [Google Scholar]
- 10.Young KS. Psychology of computer use: XL. Addictive use of the Internet: A case that breaks the stereotype. Psychol Rep. 1996;79:899–902. doi: 10.2466/pr0.1996.79.3.899. [DOI] [PubMed] [Google Scholar]
- 11.Young KS. InternetAddiction: The Emergence of a New Clinical Disorder. CyberPsychology Behav. 1998;1:237–244. doi: 10.1089/cpb.1998.1.237. [DOI] [Google Scholar]
- 12.Brand M, Young KS, Laier C, Wölfling K, Potenza MN. Integrating psychological and neurobiological considerations regarding the development and maintenance of specific Internet-use disorders: An Interaction of Person-Affect-Cognition-Execution (I-PACE) model. Neuroscience and Biobehavioral Reviews. 2016;71:252–266. doi: 10.1016/j.neubiorev.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Brand, M., Young, K. S. & Laier, C. Prefrontal Control and Internet Addiction: A Theoretical Model and Review of Neuropsychological and Neuroimaging Findings. Front Hum Neurosci8 (2014). [DOI] [PMC free article] [PubMed]
- 14.Sariyska R, Lachmann B, Markett S, Reuter M, Montag C. Individual differences in implicit learning abilities and impulsive behavior in the context of Internet addiction and Internet Gaming Disorder under the consideration of gender. Addict Behav Reports. 2017;5:19–28. doi: 10.1016/j.abrep.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goudriaan, A. E., Yücel, M. & van Holst, R. J. Getting a grip on problem gambling: what can neuroscience tell us? Front Behav Neurosci8 (2014). [DOI] [PMC free article] [PubMed]
- 19.Becker B, et al. Smaller amygdala and medial prefrontal cortex predict escalating stimulant use. Brain. 2015;138:2074–2086. doi: 10.1093/brain/awv113. [DOI] [PubMed] [Google Scholar]
- 20.Kwon, M., Kim, D. J., Cho, H., & Yang, S. The smartphone addiction scale: development and validation of a short version for adolescents. PLoS One, 8(12), e83558 (2013). [DOI] [PMC free article] [PubMed]
- 21.Duke, É. & Montag, C. In Internet Addiction 359–372 (Springer, 2017).
- 22.Montag, C. & Walla, P. Carpe diem instead of losing your social mind: Beyond digital addiction and why we all suffer from digital overuse. Cogent Psychol3 (2016).
- 23.Montag, C. et al. Smartphone usage in the 21st century: who is active on WhatsApp? BMC research notes8(1), 331 (2015). [DOI] [PMC free article] [PubMed]
- 24.Statista.com. Number of monthly active WhatsApp users worldwide from April 2013 toJuly 2017 (in millions).
- 25.Meshi, D., Morawetz, C. & Heekeren, H. R. Nucleus accumbens response to gains in reputation for the self relative to gains for others predicts social media use. Front Hum Neurosci7 (2013). [DOI] [PMC free article] [PubMed]
- 26.Sherman LE, Payton AA, Hernandez LM, Greenfield PM, Dapretto M. The Power of the Like in Adolescence: Effects of Peer Influence on Neural and Behavioral Responses to Social Media. Psychol Sci. 2016;27:1027–1035. doi: 10.1177/0956797616645673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turel O, He Q, Xue G, Xiao L, Bechara A. Examination of Neural Systems Sub-Serving Facebook ‘Addiction’. Psychol Rep. 2014;115:675–695. doi: 10.2466/18.PR0.115c31z8. [DOI] [PubMed] [Google Scholar]
- 28.Elhai JD, Dvorak RD, Levine JC, Hall BJ. Problematic smartphone use: A conceptual overview and systematic review of relations with anxiety and depression psychopathology. Journal of Affective Disorders. 2017;207:251–259. doi: 10.1016/j.jad.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 29.Chen, J., Liang, Y., Mai, C., Zhong, X. & Qu, C. General deficit in inhibitory control of excessive smartphone users: Evidence from an event-related potential study. Front Psychol7 (2016). [DOI] [PMC free article] [PubMed]
- 30.Thornton B, Faires A, Robbins M, Rollins E. The mere presence of a cell phone may be distracting implications for attention and task performance. Soc Psychol (Gott) 2014;45:479–488. doi: 10.1027/1864-9335/a000216. [DOI] [Google Scholar]
- 31.Ward AF, Duke K, Gneezy A, Bos MW. J Assoc Consum Res. 2017. Brain Drain: The Mere Presence of One?s Own Smartphone Reduces Available Cognitive Capacity; pp. 140–154. [Google Scholar]
- 32.Duke É, Montag C. Smartphone addiction, daily interruptions and self-reported productivity. Addict Behav Reports. 2017;6:90–95. doi: 10.1016/j.abrep.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadar, A. et al. Answering the missed call: Initial exploration of cognitive and electrophysiological changes associated with smartphone use and abuse. PLoS One12 (2017). [DOI] [PMC free article] [PubMed]
- 34.Montag C, et al. Behav Brain Res. 2017. Facebook usage on smartphones and gray matter volume of the nucleus accumbens; pp. 221–228. [DOI] [PubMed] [Google Scholar]
- 35.He Q, Turel O, Bechara A. Brain anatomy alterations associated with Social Networking Site (SNS) addiction. Sci Rep. 2017;7:45064. doi: 10.1038/srep45064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He, Q., Turel, O., Brevers, D. & Bechara, A. Excess Social Media Use in Normal Populations is Associated with Amygdala-Striatal but not with Prefrontal Morphology. Psychiatry Res Neuroimaging (2017). [DOI] [PubMed]
- 37.Wrase J, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- 38.Squeglia LM, Cservenka A. Adolescence and drug use vulnerability: findings from neuroimaging. Current Opinion in Behavioral Sciences. 2017;13:164–170. doi: 10.1016/j.cobeha.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neuroscience and Biobehavioral Reviews. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelloux Y, Murray JE, Everitt BJ. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur J Neurosci. 2013;38:3018–3026. doi: 10.1111/ejn.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scofield MD, et al. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev. 2016;68:816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein A, Livny A, Weizman A. New developments in brain research of internet and gaming disorder. Neuroscience and Biobehavioral Reviews. 2017;75:314–330. doi: 10.1016/j.neubiorev.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Daumann J, et al. Medial prefrontal gray matter volume reductions in users of amphetamine-type stimulants revealed by combined tract-based spatial statistics and voxel-based morphometry. Neuroimage. 2011;54:794–801. doi: 10.1016/j.neuroimage.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 45.Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Current Opinion in Neurobiology. 2013;23:615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Lee, D., Namkoong, K., Lee, J. & Jung, Y.-C. Abnormal gray matter volume and impulsivity in young adults with Internet gaming disorder. Addict Biol (2017). [DOI] [PubMed]
- 47.Zhou Y, et al. Gray matter abnormalities in Internet addiction: A voxel-based morphometry study. Eur J Radiol. 2011;79:92–95. doi: 10.1016/j.ejrad.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 48.Yu C, et al. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage. 2011;54:2571–2581. doi: 10.1016/j.neuroimage.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 49.McCormick LM, et al. Anterior cingulate cortex: An MRI-based parcellation method. Neuroimage. 2006;32:1167–1175. doi: 10.1016/j.neuroimage.2006.04.227. [DOI] [PubMed] [Google Scholar]
- 50.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 52.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 54.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- 55.Bijanki, K. R., Hodis, B., Brumm, M. C., Harlynn, E. L. & McCormick, L. M. Hippocampal and left subcallosal anterior cingulate atrophy in psychotic depression. PLoS One9 (2014). [DOI] [PMC free article] [PubMed]
- 56.Yucel K, et al. Anterior Cingulate Volumes in Never-Treated Patients with Major Depressive Disorder. Neuropsychopharmacology. 2008;33:3157–3163. doi: 10.1038/npp.2008.40. [DOI] [PubMed] [Google Scholar]
- 57.Asami T, et al. Anterior cingulate cortex volume reduction in patients with panic disorder. Psychiatry Clin Neurosci. 2008;62:322–330. doi: 10.1111/j.1440-1819.2008.01800.x. [DOI] [PubMed] [Google Scholar]
- 58.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends in Cognitive Sciences. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Luo, L. et al. A dimensional approach to determine common and specific neurofunctional markers for depression and social anxiety during emotional face processing. Human Brain Mapping, 10.1002/hbm.23880 (2017). [DOI] [PMC free article] [PubMed]
- 60.Mincic AM. Neuroanatomical correlates of negative emotionality-related traits: A systematic review and meta-analysis. Neuropsychologia. 2015;77:97–118. doi: 10.1016/j.neuropsychologia.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Hayakawa YK, et al. Structural brain abnormalities in women with subclinical depression, as revealed by voxel-based morphometry and diffusion tensor imaging. J Affect Disord. 2013;144:263–268. doi: 10.1016/j.jad.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 62.Hayakawa YK, et al. Depressive symptoms and neuroanatomical structures in community-dwelling women: A combined voxel-based morphometry and diffusion tensor imaging study with tract-based spatial statistics. NeuroImage Clin. 2014;4:481–487. doi: 10.1016/j.nicl.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WeChat. http://www.wechat.com/en/ (accessed on 15th January 2018).
- 64.Pawlikowski M, Altstötter-Gleich C, Brand M. Validation and psychometric properties of a short version of Young’s Internet Addiction Test. Comput Human Behav. 2013;29:1212–1223. doi: 10.1016/j.chb.2012.10.014. [DOI] [Google Scholar]
- 65.Kim SM, Huh HJ, Cho H, Kwon M, Choi JH. The effect of depression, impulsivity, and resilience on smartphone addiction in university students. J Korean Neuropsychiatr Assoc. 2014;53:214–20. doi: 10.4306/jknpa.2014.53.4.214. [DOI] [Google Scholar]
- 66.Mehroof M, Griffiths MD. Online Gaming Addiction: The Role of Sensation Seeking, Self-Control, Neuroticism, Aggression, State Anxiety, and Trait Anxiety. Cyberpsychology, Behav Soc Netw. 2010;13:313–316. doi: 10.1089/cyber.2009.0229. [DOI] [PubMed] [Google Scholar]
- 67.Choi J, et al. Structural alterations in the prefrontal cortex mediate the relationship between Internet gaming disorder and depressed mood. Sci Rep. 2017;7:1245. doi: 10.1038/s41598-017-01275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, et al. The alteration of gray matter volume and cognitive control in adolescents with internet gaming disorder. Front Behav Neurosci. 2015;9:64. doi: 10.3389/fnbeh.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montag, C., Duke, É. & Reuter, M. In Internet Addiction: Neuroscientific Approaches and Therapeutical Interventions 131–139 10.1007/978-3-319-07242-5_8 (2015).
- 70.Drevets WC, Savitz J, Trimble M. The Subgenual Anterior Cingulate Cortex in Mood Disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/S1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matthews S, et al. Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Res - Neuroimaging. 2009;172:1–6. doi: 10.1016/j.pscychresns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 72.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosen HJ, et al. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta A, et al. Morphological brain measures of cortico-limbic inhibition related to resilience. J Neurosci Res. 2017;95:1760–1775. doi: 10.1002/jnr.24007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai C, et al. Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain Imaging Behav. 2016;10:12–20. doi: 10.1007/s11682-015-9358-8. [DOI] [PubMed] [Google Scholar]
- 76.Yuan, K. et al. Frontostriatal circuits, resting state functional connectivity and cognitive control in internet gaming disorder. Addict Biol n/a-n/a, 10.1111/adb.12348 (2016). [DOI] [PubMed]
- 77.Das D, Cherbuin N, Anstey KJ, Sachdev PS, Easteal S. Lifetime cigarette smoking is associated with striatal volume measures. Addict Biol. 2012;17:817–825. doi: 10.1111/j.1369-1600.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- 78.Urošević, S. et al. Effects of reward sensitivity and regional brain volumes on substance use initiation in adolescence. Soc Cogn Affect Neurosci 106–113, 10.1093/scan/nsu022 (2014). [DOI] [PMC free article] [PubMed]
- 79.Seifert CL, et al. Reduced volume of the nucleus accumbens in heroin addiction. Eur Arch Psychiatry Clin Neurosci. 2015;265:637–645. doi: 10.1007/s00406-014-0564-y. [DOI] [PubMed] [Google Scholar]
- 80.Montag, C., Duke, É. & Markowetz, A. Toward Psychoinformatics: Computer Science MeetsPsychology. Computational and Mathematical Methods in Medicine 2016, (2016). [DOI] [PMC free article] [PubMed]
- 81.Montag C, et al. Correlating Personality and Actual Phone Usage. J Individ Differ. 2014;35:158–165. doi: 10.1027/1614-0001/a000139. [DOI] [Google Scholar]
- 82.Lin YH, et al. Time distortion associated with smartphone addiction: Identifying smartphone addiction via a mobile application (App) J Psychiatr Res. 2015;65:139–145. doi: 10.1016/j.jpsychires.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Beck, A., Steer, R. & Brown, G. Beck Depression Inventory-II. San Antonio 12–15, 10.1037/t00742-000(1996).
- 84.Spielberger, C. Manual for the State-Trait Anxiety Inventory (STAI). Consult Psychol Press 4–26 (1983).
- 85.Li, W. & Qian, M. Revision of the State-Trait Anxiety Inventory with Sample of Chinese College Students. Acta Sci. Natur. Univ. Pekinensis31(1), 108–114 (1995).
- 86.Yang, W. H. et al. Reliability and validity of Chinese version of the Beck Depression Inventory-II in Chinese adolescents. Chin. J. Clin. Psychol. 22 (2014).
- 87.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 88.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 89.Buckner RL, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 90.Fan L, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon reasonable request third parties will be granted access to the original data of this manuscript.