Abstract
Necrotizing enterocolitis (NEC) is one of the most severe and unpredictable complications of prematurity. There are two possible mechanisms involved in the pathogenesis of NEC: individual inflammatory response and impaired blood flow in mesenteric vessels with secondary ischemia of the intestine. The aim of this study was to evaluate the possible relationship between polymorphisms: Il-1β 3953C>T, Il-6 −174G>C and −596G>A, TNFα −308G>A, and 86 bp variable number tandem repeat polymorphism of interleukin-1 receptor antagonist (Il-1RN VNTR 86 bp) and three polymorphisms that may participate in arteries tension regulation and in consequence in intestine blood flow impairment: eNOS (894G>T and −786T>C) and END-1 (5665G>T) and NEC in 100 infants born from singleton pregnancy, before 32 + 0 weeks of gestation, exposed to antenatal steroids therapy, and without congenital abnormalities. In study population, 22 (22%) newborns developed NEC. Surgery-requiring NEC was present in 7 children. Statistical analysis showed 20-fold higher prevalence of NEC in infants with the genotype TT [OR 20 (3.71–208.7); p = 0.0004] of eNOS 894G>T gene polymorphism. There was a higher prevalence of allele C carriers of eNOS 786T>C in patients with surgery-requiring NEC [OR 4.881 (1.33–21.99); p = 0.013]. Our investigation did not confirm any significant prevalence for NEC development in another studied genotypes/alleles. This study confirms the significant role of polymorphisms that play role in intestine blood flow. Identifying gene variants that increase the risk for NEC development may be useful in screening infants with inherent vulnerability and creating strategies for individualized care.
Electronic supplementary material
The online version of this article (doi:10.1007/s11010-017-3135-5) contains supplementary material, which is available to authorized users.
Keywords: Gene, Polymorphism, Necrotizing enterocolitis, Preterm newborn
Background
Necrotizing enterocolitis (NEC) is one of the most severe and unpredictable complications of prematurity. 7% of neonates born with very low birth weight (VLBW) will develop NEC [1]. Despite the efforts, up to 1/3 of the cases will be fatal [2] and the rest remain threatened by chronic gastrointestinal complications and poor neurodevelopmental outcome [3]. Several risk factors for developing NEC have been reported, although the exact pathogenesis of this disease remains unclear. Onset of clinical symptoms might be abrupt and the severity of the disease may lead to surgical intervention. Thereby the morbidity and mortality tends to be higher in patients requiring laparotomy [2, 4].
According to the present consensus, NEC develops in the premature intestine in the setting of improper bacterial colonization, often after enteral feedings with formula instead of breast milk. A baseline increased reactivity of the premature intestinal mucosa to microbial ligands leads to increased and flawed inflammatory response [5, 6]. The proof of this theory seems to be the fact that plasma concentrations and tissue expression of the proinflammatory cytokines interleukin 1β (Il-1β) and interleukin 6 (Il-6), as well as tumor necrosis factor alpha (TNF-α) [7], are elevated in neonates with NEC [8, 9].
Another somewhat controversial hypothesis involves hypoxia/ischemia injury [10]. Nitric oxide (NO), a product of endothelial (eNOS) and inducible NO (iNOS) synthases [11], has a crucial role in gut physiology. Not only is NO responsible for the tension of vascular wall, but it also promotes damage to the intestinal barrier by inducing apoptosis of enterocytes and inhibiting their regeneration [12]. NO might be produced through the activity of commensal intestinal bacteria [13]. Breast milk, with its rich levels of nitrate, may have a protective role against NEC due to its vasodilatory effect [14]. In state of chronic inflammation and oxidative stress, which characterizes late NEC, mesenteric production of NO decreases and contributes to further vasoconstriction and intestinal ischemia [15]. Nitric oxide synthase is crucial for intestinal microcirculation and therefore, it may play a key role in the pathogenesis of NEC [16]. On the contrary, endothelin 1 (END-1) is the strongest known vasoconstrictor and plays a major role in maintaining hemodynamic homeostasis by changing the distribution of blood in the system [17].
We propose that the recognition of Single Nucleotide Polymorphisms (SNP) for inflammatory response markers: Il-1β, Il-6, TNF-α, Il-1RN, as well as vasoactive agents eNOS and END-1, might add insight to the pathogenesis of NEC. SNPs, single base pair changes, occur naturally in the human genome and can alter the translation of related proteins, influencing various processes. Since studies on animal models have proposed that modulating the inflammatory cytokine cascade could be profitable for treating preterm neonates with NEC [18, 19], undoubtedly better understanding of the diversity of NEC patients’ biology could lead to enhancement of individualized treatment options.
Materials and methods
Population
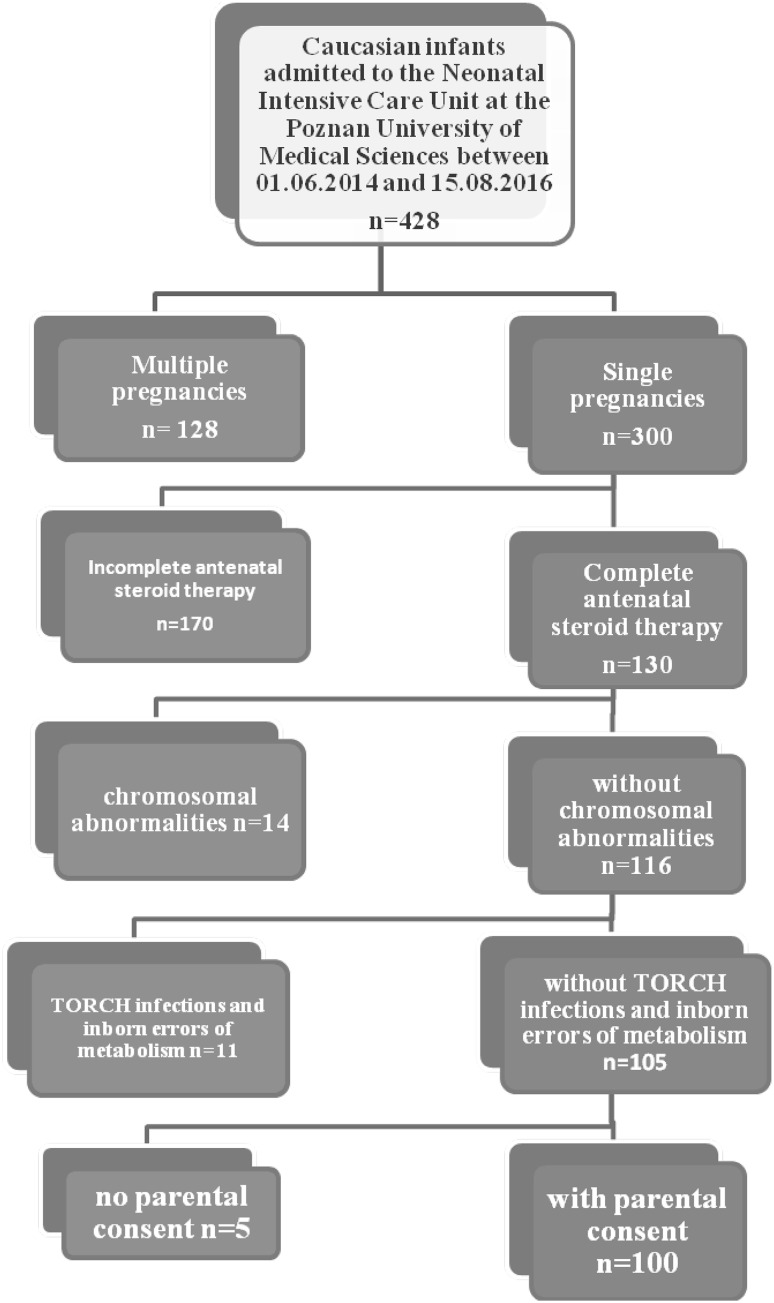
In order to guarantee a homogenous group of patients, we used the following inclusion criteria: Caucasian origin, neonates born between 24 + 0 and 32 + 0 weeks of gestation, single pregnancy neonates, pregnancies without death of one of the fetuses, and newborns with antenatal steroid therapy (AST). Newborns with chromosomal abnormalities, TORCH infections (toxoplasmosis, other, rubella, cytomegalovirus, herpes), and inborn errors of metabolism were excluded from this study—Fig. 1. We enrolled 100 (23.3%) out of the 428 infants admitted to the Neonatal Intensive Care Unit at the Poznan University of Medical Sciences between June 1st, 2014 and August 15th, 2016.
Fig. 1.
Inclusion and exclusion criteria for study
Additional data
We collected the following information: gender, gestational age (GA; weeks), birth weight (BW, g), birth weight under 3rd percentile, the type of delivery (vaginal vs. cesarean section), birth asphyxia (defined as APGAR score <6 at 10 min and pH <7.0 or blood base excess (BE) <−12 mmol/l in cord blood), intrauterine infection (defined as positive blood culture accompanied by clinical symptoms or inborn pneumonia diagnosed in the first 48 h after birth), surfactant replacement therapy (indications for the treatment based on European guidelines [20]), ventilatory support (conventional vs. non-invasive), intraventricular hemorrhage (IVH, definition based on Papile classification [21]), and the presence of bronchopulmonary dysplasia (definition by the National Institute of Child Health and Human Development [22]). Our patients were fed according to a protocol proposed by ESPHGAN [23].
Diagnosis of NEC
The diagnosis was based on Modified Bell’s staging criteria for NEC [24]. Auxiliary examination in the diagnosis of NEC was used as an abdomen ultrasound (5–8 and 13 MHz transducer, Prosound α7 Premier, Aloka) and X-ray of the abdomen. Abdomen ultrasounds were performed by a single trained neonatologist.
Surgical treatment
Every patient developing the symptoms of mild, moderate, or severe forms of NEC was consulted by the pediatric surgeon. Qualification for surgical treatment was based on past history, symptoms, and results of laboratory and imaging studies. Surgical procedures included laparotomy, resection of necrotic intestine, and intestinal anastomosis with decompressing temporary enterostomy using T-tube (TTES) [25]. In case of multilocal or extensive necrosis found during laparotomy, the proximal enterostomy with Hartmann procedure was performed. In critically ill and unstable premature children, the peritoneal drainage was used as a primary and definitive procedure or as the first step in surgical treatment.
Ethical considerations
Informed consent was obtained from the parents of all infants enrolled in the study.
The study followed the tenets of the Declaration of Helsinki and was approved by the Bioethics Committee of Poznan University of Medical Sciences (66/14 and 799/16).
Laboratory method
Our selection of candidate genes is based on two possible mechanisms involved in the pathogenesis of NEC: individual inflammatory response and impaired blood flow in mesenteric vessels with secondary ischemia of the intestine. Accordingly, we analyzed the following polymorphisms: Il-1β 3953C>T, Il-6 −174G>C and −596G>A, TNF-α −308G>A, and 86 bp variable number tandem repeat polymorphism of interleukin-1 receptor antagonist (Il-1RN VNTR 86 bp) and three polymorphisms that may participate in arterial tension regulation and in consequence in intestinal blood flow impairment: eNOS (894G>T and −786T>C) and END-1 (5665G>T).
A blood sample (0.5 ml) was taken directly post-delivery and banked. Genomic DNA was extracted from blood leukocytes using QIAamp DNA Blood Mini Kit (QIAGEN inc; Germany) according to the manufacturer’s recommendations. Genotyping was performed using polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) procedures. Primer sequences and conditions for PCR–RFLP analyses and restriction fragment length are presented in Table 1. Products were analyzed by electrophoresis on 2% agarose gel with Midori Green Advanced DNA Stain (Nippon Genetics, Europe GmbH).
Table 1.
Description of the studied polymorphisms
| Gene symbol | Polymorphism | Sequence of primers | Restriction enzyme | Products |
|---|---|---|---|---|
| Il-1β | +3953C>T (rs1143634) | F 5′-gTTgTC ATC Aga CTT TgA CC-3′ R 5′-TTC AgT TCA TAT ggA CCA gA-3′ |
TaqI |
CC 137, 114 bp CT 251, 137, 114 bp TT 251 bp |
| Il-1RN | 86 bp VNTR (rs2234663) | F 5′-CTC AgC AAC ACT CCT AT-3′ R 5′-TCC Tgg TCT gCAggT AA-3′ |
IL1RN*0 154 bp IL1RN*1 410 bp IL1RN*2 240 bp IL1RN*3 500 bp IL1RN*4 325 bp IL1RN*5 595 bp |
|
| Il-6 | −174G>C (rs1800795) | F 5′-ACA TgC CAA gTgCTgAgT CA-3′ R 5′-AAT CTT TgTTggAgggTg Ag-3′ |
LweI |
GG 114, 100 bp GC 214, 114, 100 bp CC 214 bp |
| Il-6 | −596G>A (rs1800797) | F 5′-ggAgTC ACA CAC TCC ACC Tg-3′ R 5′-AAgCAg AAC CAC TCT TCC TTT ACT T-3′ |
BseGI (BtsCI) |
GG 420 bp GA 420, 354, 66 bp AA 354, 66 bp |
| TNF-α | −308G>A (rs1800629) | 5′-AAA TggAgg CAA Tag gTTTTgAggggCTTg-3′ 5′-TAC CCC TCA CAC TCC CCA TCC TCCCTg ATC-3′ |
FaqI (BsmFI) |
GG 86, 45 bp GA 131, 86, 45 bp AA 131 bp |
| eNOS | 894G>T (rs1799983) | F 5′-AAggCAggAgACAgTggATgg A-3′ R 5′-CCC AgT CAA TCC CTT TggTgC TCA-3′ |
MboI |
GG 248 bp GT 248, 158, 90 bp TT 158, 90 bp |
| eNOS | −786T>C (rs2070744) | F 5′-CCA CCC TgT CAT TCA gTg AC-3′ R 5′-TCT CTgAgg TCT CgA AAT CA-3′ |
PdiI |
TT 296 bp TC 296, 220, 76 bp CC 220, 76 bp |
| EDN1 | 5665G>T (rs5370) | F 5′-TCA TgA TCC CAA gCTgAAAgg CTA-3′ R 5′-ACC TTT CTT ggAATg TTT TgA AC-3′ |
NheI |
GG 203, 25 bp GT 228, 203, 25 bp TT 228 bp |
Il-1β interleukin-1β, Il-6 interleukin 6, TNFα tumor necrosis factor alpha, Il1 RN 86 bp variable number tandem repeat polymorphism of interleukin-1 receptor antagonist, END-1 endothelin-1, eNOS endothelial nitric oxide synthase
Statistical analysis
The normality of variable distribution was assessed by Shapiro–Wilk test. Accordingly, the results are presented as percentage for categorical variables, or median (range) for non-normally distributed continuous variables. p value <0.05 indicates statistical significance. The following tests were used to evaluate the association between NEC and categorical variables: the Fisher‘s exact probability test, the Chi-square test, Fisher Freeman Halton, and Chi-squared test with Yates's correction. Differences in non-normally distributed continuous variables were compared with the U Mann–Whitney test. Logistic regression analysis was used to compute ORs and their 95% confidence intervals (CI) for patients without NEC and with NEC combined with different genotypes and alleles. The odds ratio (OR) and 95% confidence intervals (95% CI) were calculated in surgically and non-surgically treated NEC in different genotype distributions of the studied polymorphisms. Statistical analysis was performed using CytelStudio version 10.0, created January 16, 2013 (CytelStudio Software Corporation, Cambridge, Massachusetts, United States), and Statistica version 10, 2011 (Stat Soft, Inc., Tulsa, Oklahoma, United States).
Results
The median gestational age of enrolled infants was 28 + 4 (range 24 + 0–32 + 0). The median birth weight was 1124.4 ± 378.7 grams. In our study population, we found that 22 (22%) preterm newborns developed NEC. Surgery-requiring NEC was present in 7 children (7% of total, 31.8% of NEC cases). The primary peritoneal drainage was performed in 4 cases. Laparotomy was done in 6 patients: 3 of them as primary operation and in 3 others secondary to peritoneal drainage. In one patient, three surgical procedures were performed: primary peritoneal drainage (PPD), laparotomy with intestinal resection and TTES formation, and relaparotomy with resection and TTES. Finally, in 7 patients, 11 surgeries were done.
The incidence of NEC was comparable in female (11; 50%) and male (11; 50%) neonates with no significance. The incidence of NEC was significantly higher in children born from 24 + 0 to 28 + 6 weeks of gestation than born from 29 + 0 to 32 + 0 weeks of gestation (72.73 vs. 27.27%; p = 0.046). Moreover, we confirmed that the lower the gestational age, the greater the lower birth weight (p = 0.013); the higher the incidence of NEC. The disease was more prevalent in children ventilated conventionally (68.18 vs. 21.82%; p = 0.031).
Ten of 100 (10%) cases were fatal, including 5 patients treated due to NEC. In our Department, the mortality rate of infants with NEC was 22%. In 2 out of 5 deceased neonates (40%), NEC was surgically treated which provides the surgical NEC mortality rate of 28.5%. Table 2 shows clinical and demographic data of enrolled infants.
Table 2.
Demographic and clinical characteristic of enrolled infants
| Group without NEC N = 78 (%) |
Group with NEC N = 22 (%) |
p value | |
|---|---|---|---|
| Gender | 0.670a | ||
| Male | 43 (55.13) | 11 (50.00) | |
| Female | 35 (44.87) | 11 (50.00) | |
| Gestational age (week) | 0.046a | ||
| 24–28 | 38 (48.72) | 16 (72.73) | |
| 29–32 | 40 (51.28) | 6 (27.27) | |
| Birth weight (g) | 0.013a | ||
| < 750 | 9 (11.54) | 5 (22.73) | |
| 750–1000 | 17 (21.79) | 10 (45.45) | |
| >1000 | 52 (66.67) | 7 (31.82) | |
| Birth weight < 3rd percentile | 0.165b | ||
| Yes | 67 (85.90) | 16 (72.73) | |
| No | 11 (14.10) | 6 (27.27) | |
| Apgar score (median and range) | 0.334d
0.396d |
||
| 1st minute | 6 (1–10) | 5 (1–9) | |
| 5th minute | 7 (1–10) | 7 (5–9) | |
| Mode of delivery | 0.052b | ||
| Vaginal | 28 (35.90) | 13 (59.09) | |
| Cesarean section | 50 (64.10) | 9 (40.91) | |
| Asphyxia (ph lower than 7.0 or BE lower than −12) | 0.458c | ||
| Yes | 3 (4.00) | 0 (0.00) | |
| No | 72 (96.00) | 22 (100.0) | |
| Surfactant therapy | 0.068a | ||
| Yes | 36 (46.15) | 15 (68.18) | |
| No | 42 (53.85) | 7 (31.82) | |
| Ventilation support | 0.031a | ||
| Non-invasive | 45 (57.69) | 7 (31.82) | |
| Conventional | 33 (42.31) | 15 (68.18) | |
| IVH II–IV | 0.036b | ||
| Yes | 20 (25.64) | 11 (50.00) | |
| No | 58 (74.36) | 11 (50.00) | |
| BPD | 0.043b | ||
| Yes | 24 (30.77) | 12 (54.55) | |
| No | 54 (69.23) | 10 (45.45) | |
| Deaths | 0.040b | ||
| Yes | 5 (6.49) | 5 (22.73) | |
| No | 72 (93.51) | 17 (77.27) |
Results are expressed as absolute number of patients (percentage) and median (interquartile range)
NEC necrotizing enterocolitis, IVH intraventricular hemorrhage, BPD bronchopulmonary dysplasia
aChi-square test
bFisher Freeman Halton test
cChi-square test with Yate’s correction
dMann–Whitney test
Statistical analysis showed 20-fold higher prevalence of NEC in infants with the genotype TT (OR 20 (3.71–208.7); p = 0.0004) of eNOS 894G>T gene polymorphism. There was a higher prevalence of allele C carriers of eNOS 786T>C in patients with surgery-requiring NEC (OR 4.881 (1.33–21.99); p = 0.013). Our investigation did not confirm any significant prevalence for NEC development in other studied genotypes/alleles such as END-1 5665G>T; Il-1β 3953C>T, Il-6 −174G>C and −596G>A, TNF-α −308G>A; and Il-1RN VNTR 86 bp. Genotype distribution of the polymorphisms involved in inflammation pathways in infants with/without NEC and with/without surgery-requiring NEC is presented in Table 3. Genotype distribution of eNOS (894G>T and −786T>C) and END-1 (5665G>T) in infants without/with NEC and with/without surgery-requiring NEC is presented in Table 4.
Table 3.
Genotype distribution of the five polymorphisms involved in inflammation pathway in infants without and with NEC and without/with NEC surgery-requiring
| Gene symbol db SNP |
Genotypes/allele | Group without NEC N (%) |
Group with NEC N (%) |
p | OR 95% CI p |
Group with NEC without surgery N (%) |
Group with NEC with surgery N (%) |
p | OR 95% CI p |
|---|---|---|---|---|---|---|---|---|---|
| Il-1β +3953C>T (rs1143634) |
CC | 47 (60.26) | 15 (68.18) | – | References | 57 (61.29) | 5 (71.43) | – | References |
| CT | 26 (33.33) | 5 (22.73) | 0.540 | 0.603 (0.154–2.016) | 29 (31.18) | 2 (28.57) | 1.000 | 0.786 (0.071–5.182) | |
| TT | 5 (6.41) | 2 (9.09) | 1.000 | 1.253 (0.108–8.656) | 7 (7.53) | 0 (0.00) | 1.000 | 0.000 (0.000–10.83) | |
| C | 120 (76.92) | 35 (79.55) | – | References | 143 (76.88) | 12 (85.71) | – | References | |
| T | 36 (23.08) | 9 (20.45) | 0.887 | 0.857 (0.331–2.042) | 43 (23.12) | 2 (14.29) | 0.702 | 0.554 (0.058–2.646) | |
|
IL1RN
86 BP VNTR (rs2234663) |
1/1 | 40 (51.28) | 10 (45.45) | – | References | 47 (50.54) | 3 (42.86) | – | References |
| 1/2 | 26 (33.33) | 7 (31.28) | 1.000 | 1.077 (0.306–3.603) | 31 (33.33) | 2 (28.57) | 1.000 | 1.011 (0.080–9.358) | |
| 1/3 | 2 (2.56) | 0 (0.00) | 1.000 | 0..000 (0.000–23.05) | 2 (2.15) | 0 (0.00) | 1.000 | 0.000 (0.000–101.6) | |
| 2/2 | 9 (11.54) | 5 (22.73) | 0.379 | 2.222 (0.470–9.455) | 12 (12.90) | 2 (28.57) | 0.599 | 2.611 (0.194–25.15) | |
| 2/3 | 1 (1.28) | 0 (0.00) | 1.000 | 0.000 (0.000–159.9) | 1 (1.08) | 0 (0.00) | 1.000 | 0.000 (0.000–624) | |
| 1 | 108 (69.23) | 27 (61.36) | – | References | 127 (68.28) | 8 (57.14) | – | References | |
| 2 | 45 (28.85) | 17 (38.64) | 0.328 | 1.511 (0.699–3.198) | 56 (30.11) | 6 (42.86) | 0.503 | 1.701 (0.462–5.874) | |
| 3 | 3 (1.92) | 0 (0.00) | 1.000 | 0.000 (0.000–10.10) | 3 (1.61) | 0 (0.00) | 1.000 | 0.000 (0.000–42.83) | |
| Il-6 −174G>C (rs1800795) |
GG | 14 (17.95) | 7 (31.82) | – | References | 19 (20.43) | 2 (28.57) | – | References |
| GC | 49 (62.82) | 12 (54.55) | 0.326 | 0.489 (0.144–1.776) | 57 (61.29) | 4 (57.14) | 0.969 | 0.667 (0.088–7.963) | |
| CC | 15 (19.23) | 3 (13.64) | 0.414 | 0.400 (0.057–2.250) | 17 (18.28) | 1 (14.29) | 1.000 | 0.559 (0.009–11.81) | |
| G | 77 (49.36) | 26 (59.09) | – | References | 95 (51.08) | 8 (57.14) | – | References | |
| C | 79 (50.64) | 18 (40.91) | 0.332 | 0.675 (0.321–1.399) | 91 (48.92) | 6 (42.86) | 0.875 | 0.783 (0.215–2.691) | |
| Il-6 −596G>A (rs1800797) |
GG | 16 (20.51) | 7 (31.82) | – | References | 21 (22.58) | 2 (28.57) | – | References |
| GA | 48 (61.54) | 12 (54.55) | 0.465 | 0.571 (0.171–2.036) | 56 (60.22) | 4 (57.14) | 1.000 | 0.750 (0.099–8.901) | |
| AA | 14 (17.95) | 3 (13.64) | 0.586 | 1.489 (0.069–2.729) | 16 (17.20) | 1 (14.29) | 1.000 | 0.656 (0.010–13.80) | |
| G | 80 (51.28) | 26 (59.09) | – | References | 98 (52.69) | 8 (57.14) | – | References | |
| A | 76 (48.72) | 18 (40.91) | 0.457 | 0.729 (0.347–1.510) | 88 (47.31) | 6 (42.86) | 0.969 | 0.835 (0.229–2.870) | |
| TNF-α −308G>A (rs1800629) |
GG | 60 (76.92) | 17 (77.27) | – | References | 72 (77.42) | 5 (71.43) | – | References |
| GA | 18 (23.08) | 5 (22.73) | 1.000 | 0.980 (0.248–3.304) | 21 (22.58) | 2 (28.57) | 1.000 | 1.371 (0.122–9.117) | |
| AA | 0 (0.00) | 0 (0.00) | – | – | 0 (0.00) | 0 (0.00) | – | – | |
| G | 138 (88.46) | 39 (88.64) | – | References | 165 (88.71) | 12 (85.71) | – | References | |
| A | 18 (11.54) | 5 (11.36) | 1.000 | 0.983 (0.268–2.983) | 21 (11.29) | 2 (14.29) | 0.993 | 1.310 (0.133–6.525) |
Results are expressed as absolute number of patients (percentage). The odds ratio (OR) and 95% confidence intervals (95% CI)
Il-1β interleukin-1β, Il-6 interleukin 6, TNF-α tumor necrosis factor alpha, Il1 RN 86 bp variable number tandem repeat polymorphism of interleukin-1 receptor antagonist, NEC necrotizing enterocolitis
Table 4.
Genotype distribution of the three polymorphisms involved in regulation of arteries tension in infants without and with NEC and without/with NEC surgery-requiring
| Gene symbol db SNP |
Genotypes/allele | Group without NEC N (%) |
Group with NEC N (%) |
p | OR 95% CI p |
Group with NEC without surgery N (%) |
Group with NEC with surgery N (%) |
p | OR 95% CI p |
|---|---|---|---|---|---|---|---|---|---|
| eNOS 894G>T (rs1799983) |
GG | 45 (57.69) | 9 (40.91) | – | References | 50 (53.76) | 4 (57.14) | – | References |
| GT | 31 (39.74) | 5 (22.73) | 0.964 | 0.807 (0.193–3.00) | 35 (37.63) | 1 (14.29) | 0.662 | 0.357 (0.007–3.850) | |
| TT | 2 (2.56) | 8 (36.36) | 0.0004 | 20 (3.71–208.7) | 8 (8.60) | 2 (28.57) | 0.467 | 3.125 (0.239–25.81) | |
| G | 121 (77.56) | 23 (52.27) | – | References | 135 (72.58) | 9 (64.29) | – | References | |
| T | 35 (22.44) | 21 (47.73) | 0.003 | 3.157 (1.466–6.726) | 51 (27.42) | 5 (35.71) | 0.696 | 1.471 (0.368–5.156) | |
| eNOS −786T>C (rs2070744) |
TT | 32 (41.03) | 6 (27.27) | – | References | 38 (40.86) | 0 (0.00) | – | References |
| TC | 40 (51.28) | 11 (50.00) | 0.685 | 1.467 (0.437–5.362) | 47 (50.54) | 4 (57.14) | – | – | |
| CC | 6 (7.69) | 5 (22.73) | 0.105 | 4.444 (0.773–24.27) | 8 (8.60) | 3 (42.86) | – | – | |
| T | 104 (66.67) | 23 (52.27) | – | References | 123 (66.13) | 4 (40.00) | – | References | |
| C | 52 (33.33) | 21 (47.73) | 0.118 | 1.826 (0.871–3.799) | 63 (33.87) | 10 (60.00) | 0.013 | 4.881 (1.330–21.99) | |
| END-1 5665G>T (rs5370) |
GG | 52 (66.67) | 14 (63.64) | – | References | 63 (67.74) | 3 (42.86) | – | References |
| GT | 24 (30.77) | 7 (31.82) | 1.000 | 1.083 (0.326–3.329) | 28 (30.11) | 3 (42.86) | 0.577 | 2.250 (0.281–17.70) | |
| TT | 2 (2.56) | 1 (4.55) | 1.000 | 1.857 (0.029–37.76) | 2 (2.15) | 1 (14.29) | 0.333 | 10.5 (0.134–243.2) | |
| G | 128 (82.05) | 35 (79.55) | – | References | 154 (82.80) | 9 (64.29) | – | References | |
| T | 28 (17.95) | 9 (20.45) | 0.854 | 1.176 (0.445–2.863) | 32 (17.20) | 5 (35.71) | 0.184 | 2.674 (0.655–9.549) |
Results are expressed as absolute number of patients (percentage). The odds ratio (OR) and 95% confidence intervals (95% CI)
END-1 endothelin-1, eNOS endothelial nitric oxide synthase, NEC necrotizing enterocolitis
Discussion
The aim of our study was to have a homogenous tested probe which is representative for a population of extremely premature infants. The mean gestational age in our population of infants diagnosed with NEC was comparable to the one found in literature (28 + 4 vs. 27 + 3) [26]. In our Department, however, the prevalence of NEC (22%) among newborns under 33 GA was higher than the 6,3% incidence of NEC stage II/III reported by the EPICE research group [27]. These data reflect the statistics for around 300 neonates treated in the Wielkopolska region of Poland where our department is located. US Neonatal Research Network [28] reported a 7% NEC morbidity. According to other American data, NEC prevalence varied from 11 to 30% [11, 26].
The significant variation in data from different NICUs may be related to the disparity in understanding the definition and diagnosing criteria of NEC. Signs and symptoms of the disease tend to be non-specific and involve a high index of suspicion. Recently, this issue has been raised by a group of researchers on the subject [29]. The exclusion of Bell’s stage I—“suspected NEC”—in the analyses has been the proposed [30], similar to the EPICE research. In our study, stage I qualified as a diagnosis of NEC which could have led to the overestimation of exact numbers of NEC cases. High incidence of NEC in our ward might be also a result of statistically significant lower gestational age of patients in Polish NICUs [27] and the fact that our department is of the highest reference in neonatal medicine in our region.
Moreover, in our study, the percentage of surgical NEC (around 30% of NEC cases and 7% of our probe) is higher than in EPICE (2,7% of population) and lower than in the American data (50% VLBW NEC cases) [31]. Again, the 22% mortality rate of our NEC patients is lower than the one generally reported 30% [18]. The mortality in surgical NEC among our patients is lower (28 vs. 45%). These data may reflect the influence of proper decision making concerning timing and the choice of surgical procedures. Rest of the demographic features of our probe seem consistent with the well-documented NEC characteristics and co-morbidities of prematurity, proving that our group was homogenous and representative for the given population.
To date, there have been no studies assessing the association of eNOS: 894G>T and −786 T>C polymorphism genes with NEC. The eNOS gene polymorphism in which guanine (G) is replaced with thymine (T) at nucleotide 894 (exon 7), results in a change of the amino acid sequence Glu298Asp. The −786T>C polymorphism of the eNOS gene replaces thymine with cytosine in the eNOS gene promoter at position 786. It is thought that in the presence of 894G>T and −786T>C polymorphic variants, eNOS enzymatic activity is impaired [32]. Our analysis showed a 20-fold increased prevalence of NEC in infants with the genotype TT (OR 20 (3.71-208.7); p = 0.0004) of eNOS 894G>T gene polymorphism. Additionally, there was a higher prevalence of allele C carriers of eNOS 786T>C in patients with surgery-requiring NEC. These novel findings suggest that the role of NO in pathogenesis of NEC is significant. Decreased levels of NO in homozygous TT of eNOS 894G>T gene polymorphisms and allele C carriers of eNOS −786T>C may predispose to NEC due to ischemia. Franklin et al. analyzed the 6797G>A (rs1800779) eNOS polymorphism. The role of this polymorphism in NEC pathogenesis, however, still remains unclear [33].
Polymorphism 5665G>T of endothelin-1 gene consists of replacing guanine for thymine on position 5665, which affects the amino acid sequence (Lys198Asn). Abnormal production of END-1 in individuals with genotype GT and TT 5665G>T polymorphism may lead to impaired regulation of vascular smooth muscle tension and ischemia [34]. To our knowledge, there have been no studies assessing the association of NEC and 5665G>T END-1 polymorphism. We could not, however, confirm any role of the END-1 5665G>T polymorphism in pathogenesis of this disease.
We analyzed the following polymorphisms involved in inflammation pathway: Il-1β 3953C>T, Il-6 −174G>C and −596G>A, TNFα −308G>A, and Il-1RN VNTR 86 bp. Polymorphism Il-1β 3953C>T consists of replacing cytosine with thymine. It leads to appearance of a rarer allele 2 which is connected with higher production of Il-1β [35]. Il-6 −174G>C is connected with replacing guanine with cytosine at position −174 and Il-6 −596G>A consists of replacing of guanine with adenosine at position −596. Both homozygotes CC of polymorphism Il-6 −174G>C and AA of polymorphism Il-6 −596G>A result in decreased production of Il-6 [36]. Polymorphism TNF −308G>A is caused by replacement of guanine by adenosine, resulting in loss of binding site for transcription factor AP-2 [37]. Treszl et al. found that the prevalence of alleles with guanine–adenine transition in the −308 and −238 positions was the same in NEC and control subjects [38]. Interleukin-1 receptor antagonists competitively inhibit Il-1-induced proinflammatory activity. Its encoding gene is polymorphic, resulting in quantitative differences in both Il-1 receptor antagonist and Il-1β production. The VNTR polymorphism occurs within intron 2 of the human Il-1RN gene, consisting of repeats of 86 bp sequence. The number of repeats is of functional significance as these repeats contain binding sites for transcription factors. It was proven that the occurrence of IL1RN*2 is connected with a more severe and prolonged inflammatory response [39]. To our knowledge, the polymorphism of this gene has not been studied in accordance to neonates with NEC. In our analysis, we did not find any significant association.
Our study confirms the finding of Treszl et al. that no significant differences were present in the allelic frequencies of Il-1 and Il-6 genes between NEC and control infants [40]. Large 2009 meta-analysis by Henderson et al. [41] also confirmed that none of the candidate cytokine polymorphisms were significantly associated with NEC. However, Henderson showed that (by the upper limit of the 95% CI of the OR) the common Il-6 −174G>C had a modest protective effect towards NEC, reducing the odds of developing the disease by half. Previously, this polymorphism has also been linked to an increased Il-6 production in neonatal lymphocytes [42]. Exclusively in neonates, it is thought to alter the promotor region that increases the transcription of Il-6 protein [43]. Decreased susceptibility of invasive infection in preterm infants might also be connected with Il-6 −174G>C [44] presumably due to a strong immune response to a pathogen. A recent 2015 paper by Franklin et al. showed that 59 Caucasian neonates with Il-6 −174G>C were over 6 times more likely to have NEC (p = 0.013) and over 7 times more likely to have Stage III disease (p = 0.011) [33]. This association was not observed in Black neonates. These conflicting data reflect the complexity of immunologic findings in NEC—the observations can suggest rather concomitant than causal role of all the polymorphisms we tested [45].
Conclusions
Homozygous TT of eNOS 894G>T gene polymorphism are 20 times more likely to develop NEC. In children with surgery-requiring NEC, a higher prevalence of allele C carriers of eNOS 786T>C was found. This study confirms the significant role of NO and ischemia in the pathogenesis of NEC. Identifying gene variants that increase the risk for NEC development may be useful in screening infants with inherent vulnerability and creating strategies for individualized care. New information about NEC pathogenesis may create new opportunities in preventive medicine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Funding
This study was funded by Poznan University of Medical Sciences (Grant No. 502-14-02215338-09691).
Author contributions
D.S. designed research. D.S., N.N., M.B., collected and analyzed data and wrote the manuscript. D.S., J.G., A.S.-M., G.K., K.D., M.S. performed research. G.K. was responsible for PCR procedure. All authors commented on the manuscript at all stages.
Compliance with ethical standards
Conflict of interest
All authors of this manuscript declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of Poznan University of Medical Sciences (Nos. 66/14 and 799/16).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11010-017-3135-5) contains supplementary material, which is available to authorized users.
References
- 1.Henry MCW, Moss RL. Neonatal necrotizing enterocolits. Semin Pediatr Surg. 2008;17:98–109. doi: 10.1053/j.sempedsurg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Holman RC, Stoll BJ, Curns AT, et al. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 3.Hintz SR. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 4.Lim JC, Golden JM, Ford HR. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr Surg Int. 2015;31:509–518. doi: 10.1007/s00383-015-3697-9. [DOI] [PubMed] [Google Scholar]
- 5.Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. 2016;13:590–600. doi: 10.1038/nrgastro.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian J, Liu Y, Jiang Y, et al. Association of single nucleotide polymorphisms of IL23R and IL17 with necrotizing enterocolitis in premature infants. Mol Cell Biochem. 2017 doi: 10.1007/s11010-017-2972-6. [DOI] [PubMed] [Google Scholar]
- 7.Yurttutan S, Ozdemir R, Canpolat FE, et al. Beneficial effects of Etanercept on experimental necrotizing enterocolitis. Pediatr Surg Int. 2014;30:71–77. doi: 10.1007/s00383-013-3415-4. [DOI] [PubMed] [Google Scholar]
- 8.Harris MC, Costarino AT, Sullivan JS, et al. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. J Pediatr. 1994;124:105–111. doi: 10.1016/S0022-3476(94)70264-0. [DOI] [PubMed] [Google Scholar]
- 9.Viscardi RM, Lyon NH, Sun CC, et al. Inflammatory cytokine mRNAs in surgical specimens of necrotizing enterocolitis and normal newborn intestine. Pediatr Pathol Lab Med. 1997;17:547–559. doi: 10.1080/15513819709168731. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Chang KTE, Lian DWQ, et al. The role of ischemia in necrotizing enterocolitis. J Pediatr Surg. 2016;51:1255–1261. doi: 10.1016/j.jpedsurg.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Galluccio E, Cassina L, Russo I, et al. A novel truncated form of eNOS associates with altered vascular function. Cardiovasc Res. 2014;101:492–502. doi: 10.1093/cvr/cvt267. [DOI] [PubMed] [Google Scholar]
- 12.Grishin A, Bowling J, Bell B, et al. Roles of nitric oxide and intestinal microbiota in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 2016;51:13–17. doi: 10.1016/j.jpedsurg.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladwin MT, Tejero J. Nitrite-NO bailout for a NOS complex too big to fail. Nat Med. 2011;17:1556–1557. doi: 10.1038/nm.2591. [DOI] [PubMed] [Google Scholar]
- 14.Yazji I, Sodhi CP, Lee EK, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS–NO–nitrite signaling. Proc Natl Acad Sci USA. 2013;110:9451–9456. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitehouse JS, Xu H, Shi Y, et al. Mesenteric nitric oxide and superoxide production in experimental necrotizing enterocolitis. J Surg Res. 2010;161:1–8. doi: 10.1016/j.jss.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang Q, Bao L, Guo H, et al. Contribution of glutaredoxin-1 to S-glutathionylation of endothelial nitric oxide synthase for mesenteric nitric oxide generation in experimental necrotizing enterocolitis. Transl Res. 2016 doi: 10.1016/j.trsl.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Kawanabe Y, Nauli SM. Endothelin. Cell Mol Life Sci. 2011;68:195–203. doi: 10.1007/s00018-010-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travadi J, Patole S, Charles A, et al. Pentoxifylline reduces the incidence and severity of necrotizing enterocolitis in a neonatal rat model. Pediatr Res. 2006;60:185–189. doi: 10.1203/01.pdr.0000228325.24945.ac. [DOI] [PubMed] [Google Scholar]
- 19.Halpern MD. Reduction of experimental necrotizing enterocolitis with anti-TNF- AJP Gastrointest Liver Physiol. 2006;290:G757–G764. doi: 10.1152/ajpgi.00408.2005. [DOI] [PubMed] [Google Scholar]
- 20.Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants-2013 update. Neonatology. 2013;103:353–368. doi: 10.1159/000349928. [DOI] [PubMed] [Google Scholar]
- 21.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 22.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 23.Agostoni C, Buonocore G, Carnielli V, et al. Enteral nutrient supply for preterm infants: commentary from the european society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91. doi: 10.1097/MPG.0b013e3181adaee0. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003;8:449–459. doi: 10.1016/S1084-2756(03)00123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Błaszczyński M, Porzucek W, Becela P, Gadzinowski J. T-tube enterostomy in surgical management of emergency cases in neonate. Arch Perinat Med. 2011;17:93–96. [Google Scholar]
- 26.Yee WH, Soraisham AS, Shah VS, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–e304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 27.Zeitlin J, Manktelow BN, Piedvache A, et al. Use of evidence based practices to improve survival without severe morbidity for very preterm infants: results from the EPICE population based cohort. BMJ. 2016;354:i2976. doi: 10.1136/bmj.i2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon PV, Swanson JR, MacQueen BC, Christensen RD. A critical question for NEC researchers: can we create a consensus definition of NEC that facilitates research progress? Semin Perinatol. 2016 doi: 10.1053/j.semperi.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Gordon PV, Swanson JR. A critical question for NEC researchers: Can we create a consensus definition of NEC that facilitates research progress? Semin Perinatol. 2016;41(1):7–14. doi: 10.1053/j.semperi.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Hull MA, Fisher JG, Gutierrez IM, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective Cohort study. J Am Coll Surg. 2014;218:1148–1155. doi: 10.1016/j.jamcollsurg.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Veldman BA, Spiering W, Doevendans PA, et al. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. J Hypertens. 2002;20:2023–2027. doi: 10.1097/00004872-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Franklin AL, Said M, Cappiello CD, et al. Are immune modulating single nucleotide polymorphisms associated with necrotizing enterocolitis? Sci Rep. 2015;5:18369. doi: 10.1038/srep18369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei M, Cheng G, Sun B, et al. EDN1 gene variant is associated with neonatal persistent pulmonary hypertension. Sci Rep. 2016;6:29877. doi: 10.1038/srep29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serafin M, Kalinka J. The role of chosen polymorphism of gens coding cytokines IL-1s, IL1ra, IL-6 and TNFalpha in the pathogenesis of the preterm delivery. Ginekol i Poloznictwo. 2014;33:9–23. [Google Scholar]
- 36.Drews-Piasecka E, Seremak-Mrozikiewicz A, Barlik M, et al. The significance of TNF-α gene polymorphisms in preterm delivery. Ginekol Pol. 2014;85:428–434. doi: 10.17772/gp/1748. [DOI] [PubMed] [Google Scholar]
- 37.Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391–399. doi: 10.1016/S0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 38.Treszl A, Kocsis I, Szathmári M, et al. Genetic variants of the tumour necrosis factor-alpha promoter gene do not influence the development of necrotizing enterocolitis. Acta Paediatr. 2001;90:1182–1185. doi: 10.1111/j.1651-2227.2001.tb03251.x. [DOI] [PubMed] [Google Scholar]
- 39.Witkin SS, Gerber S, Ledger WJ. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clin Infect Dis. 2002;34:204–209. doi: 10.1086/338261. [DOI] [PubMed] [Google Scholar]
- 40.Treszl A, Hèninger E, Kálmán A, et al. Lower prevalence of IL-4 receptor α-chain gene 1902G variant in very-low-birth-weight infants with necrotizing enterocolitis. J Pediatr Surg. 2003;38:1374–1378. doi: 10.1016/S0022-3468(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 41.Henderson G, Craig S, Baier RJ, et al. Cytokine gene polymorphisms in preterm infants with necrotising enterocolitis: genetic association study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F124–F128. doi: 10.1136/adc.2007.119933. [DOI] [PubMed] [Google Scholar]
- 42.Chen H. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;15:519–529. doi: 10.1093/hmg/ddi469. [DOI] [PubMed] [Google Scholar]
- 43.Kilpinen S, Hulkkonen J, Wang XY, Hurme M. The promoter polymorphism of the interleukin-6 gene regulates interleukin-6 production in neonates but not in adults. Eur Cytokine Netw. 2001;12:62–68. [PubMed] [Google Scholar]
- 44.Harding D, Dhamrait S, Millar A, et al. Is interleukin-6 -174 genotype associated with the development of septicemia in preterm infants? Pediatrics. 2003;112:800–803. doi: 10.1542/peds.112.4.800. [DOI] [PubMed] [Google Scholar]
- 45.Treszl A, Kocsis I, Szathmári M, et al. Genetic variants of TNF-[FC12]a, IL-1beta, IL-4 receptor [FC12]a-chain, IL-6 and IL-10 genes are not risk factors for sepsis in low-birth-weight infants. Biol Neonate. 2003;83:241–245. doi: 10.1159/000069484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.