Abstract
Autism spectrum disorders (ASD) and attention-deficit/hyperactivity disorder (ADHD) frequently co-occur. The presence of a genetic link between ASD and ADHD symptoms is supported by twin studies, but the genetic overlap between clinically ascertained ASD and ADHD remains largely unclear. We therefore investigated how ASD and ADHD co-aggregate in individuals and in families to test for the presence of a shared genetic liability and examined potential differences between low- and high-functioning ASD in the link with ADHD. We studied 1 899 654 individuals born in Sweden between 1987 and 2006. Logistic regression was used to estimate the association between clinically ascertained ASD and ADHD in individuals and in families. Stratified estimates were obtained for ASD with (low-functioning) and without (high-functioning) intellectual disability. Individuals with ASD were at higher risk of having ADHD compared with individuals who did not have ASD (odds ratio (OR)=22.33, 95% confidence interval (CI): 21.77–22.92). The association was stronger for high-functioning than for low-functioning ASD. Relatives of individuals with ASD were at higher risk of ADHD compared with relatives of individuals without ASD. The association was stronger in monozygotic twins (OR=17.77, 95% CI: 9.80–32.22) than in dizygotic twins (OR=4.33, 95% CI: 3.21–5.85) and full siblings (OR=4.59, 95% CI: 4.39–4.80). Individuals with ASD and their relatives are at increased risk of ADHD. The pattern of association across different types of relatives supports the existence of genetic overlap between clinically ascertained ASD and ADHD, suggesting that genomic studies might have underestimated this overlap.
Introduction
Autism spectrum disorders (ASD) and attention-deficit/hyperactivity disorder (ADHD) are highly heritable childhood-onset chronic disorders.1, 2 Evidence has accumulated to suggest substantial overlap between ASD and ADHD traits and symptoms.3, 4 Twin studies suggest that the observed overlap is mainly attributable to correlated genetic risk factors, both in children and in adults.5, 6, 7, 8, 9, 10, 11 ASD and ADHD also seem to share rare genetic risk variants (copy number variants).12, 13, 14 In contrast, recent cross-disorder analyses from the Psychiatric Genomics Consortium failed to detect sharing of common single-nucleotide polymorphisms by ASD and ADHD.15, 16
The molecular genetic studies are therefore still inconclusive, and twin studies have investigated the genetic overlap of ASD and ADHD trait measures in general population samples, rather than in clinically defined cases. Large genetically informative studies with data on clinical diagnoses are therefore needed to investigate sharing of genetic liability between ASD and ADHD. Thus far, only two studies have investigated the familial transmission of the disorders in population-based samples using clinical diagnoses. One study, which included >35 000 children and their mothers, found that offspring of mothers with clinically diagnosed ADHD were at increased risk of ASD.17 However, maternal transmission comprises genetic as well as environmental prenatal or perinatal risk factors, and thus this study was unable to disentangle genetic from non-genetic influences on the familial overlap between the disorders. A recent study including 6022 full siblings of 3578 ASD cases and 22 127 full siblings of 11 775 matched controls found an increased risk of ADHD in siblings of ASD cases and no difference in risk between ASD with or without intellectual disability (ID).18 In contrast, other studies have reported differences in the genetic architecture of ASD related to intelligence. Spontaneously arising (de novo) genetic mutations have been implicated in ASD cases with intelligence quotient <100,19 and the rate of these mutations has been found to be negatively associated with intelligence quotient in ASD.20
The aim of this study was to investigate the extent to which ASD and ADHD co-aggregate in individuals and in families, considering several types of relatives with varying degree of genetic and environmental relatedness. In addition, we examined potential heterogeneity in the association between the disorders by stratifying high-functioning ASD (that is, with normal intellectual ability) and low-functioning ASD (that is, with ID).
Materials and methods
The study was approved by the regional ethics review board in Stockholm, Sweden. The requirement for informed consent was waived, because the study was register based, and the included individuals were not personally identifiable at any time.
Study population
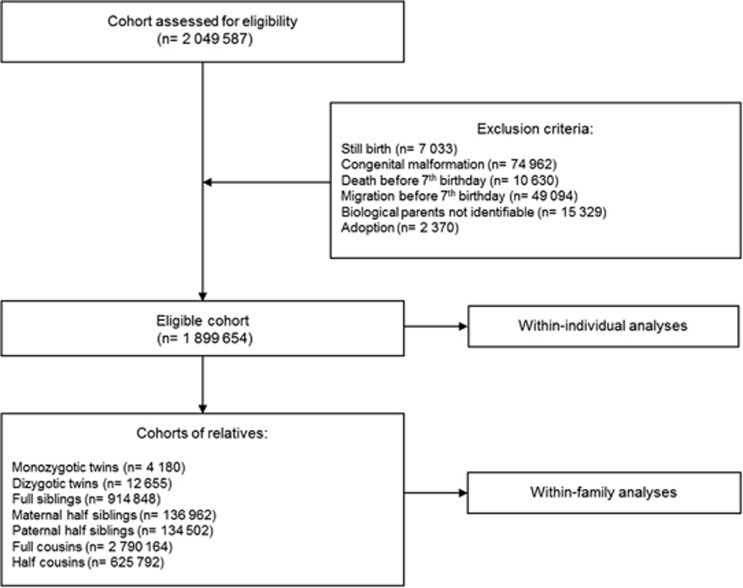
We used data from the linkage of several nationwide Swedish registers using the unique personal identification number.21 From the Medical Birth Register,22 we identified all individuals born in Sweden between 1987 and 2006 (Figure 1). We linked this information to the Total Population Register,23 Cause of Death Register and the Multi-Generation Register.24 After having excluded still births, individuals with serious congenital malformations, those who died or migrated before their seventh birthday, those whose biological parents were unidentifiable and those who were adopted away, the eligible population included 1 899 654 individuals (Figure 1). Using information from the Multi-Generation Register, we identified seven cohorts of different types of relatives (Figure 1). For each cohort a data set was created, where each individual occurred at least twice, once as index person and once as relative.
Figure 1.
Flow diagram of the study population. The flow diagram illustrates how the study population was defined. In each cohort of relatives, the reported numbers refer to the number of relative pairs.
ASD and ADHD case definition
We used information from the National Patient Register (NPR),25 in which diagnoses were recorded according to the International Classification of Diseases, Ninth Revision (ICD-9; 1987–1996) and ICD-10 (1997–2013) and from the Prescribed Drug Register (PDR; June 2005–2014).26 A recorded diagnosis of ASD in the NPR (ICD-9 code 299; ICD-10 code F84) at age ⩾1 year was used to define ASD cases.27, 28 A recorded diagnosis of ID at age ⩾3 years (ICD-9 codes 317–319; ICD-10 codes F70–F79) in ASD cases was used to define low- vs high-functioning ASD cases.29 According to ICD-9 and ICD-10 criteria, an ASD diagnosis explicitly precludes an ADHD diagnosis. Hence, we used additional information from the PDR for the definition of ADHD cases, as these are almost exclusively prescribed for ADHD in children and adolescents in Sweden.30 A recorded diagnosis of ADHD in the NPR (ICD-9 code 314; ICD-10 code F90) or a recorded prescription of an ADHD medication in the PDR (Methylphenidate, Amphetamine, Dexamphetamine, Atomoxetine, Lisdexamfetamine) at age ⩾3 years was used to define ADHD cases.
Two additional definitions of cases were used in sensitivity analyses to assess the robustness of our results. First, ADHD case definition was based only on information from the NPR, without using information from the PDR, to ensure that the possible overlap reflects clinically diagnosed cases. Then ASD and ADHD cases were defined as individuals having at least two recorded diagnoses17 in the NPR, without using information from the PDR, to test whether a stricter definition of cases would lead to similar results.
Covariates
As the administrative prevalence of ASD and ADHD differs in males and females and has changed over the study period,31 we included sex, obtained from the Total Population Register, and birth year, obtained from the Medical Birth Register, as covariates in the analysis.
Statistical analysis
We tested the association between ASD and ADHD (defined as binary variables) in individuals and in families using logistic regression. Results are presented as odds ratios (ORs) with two-sided 95% confidence intervals (CIs). We used cluster-robust variance estimation to adjust the precision of the estimates (that is, the CIs) for non-independence of data within family clusters in all the analyses.
Within-individual association
We estimated the ORs of having an ADHD diagnosis in individuals with a diagnosis of ASD compared with those without a diagnosis of ASD. Estimates were adjusted for birth year and sex. We also performed the analyses stratified by high- and low-functioning ASD diagnosis.
Within-family association
Within-family association was estimated in several types of relatives (Figure 1) in order to test the existence of a shared familial liability to the disorders. In this set of analyses, we estimated the ORs of having ADHD in individuals whose relative(s) had ASD compared with individuals whose relative(s) did not have ASD. Given that ADHD was the outcome measure, we refer to individuals in whom ADHD status was evaluated as the ‘outcome person’ and to their relatives as the ‘exposure person’. Estimates were adjusted for birth year and sex of both the outcome and the exposure person. We also stratified the estimates by high- and low-functioning ASD. This analysis was carried out only in full siblings and full cousins to ensure sufficient sample size, considering that ID has a low prevalence, around 1% in our sample.
An increased risk of ADHD in individuals whose relatives had ASD suggests that aetiological factors shared among relatives (that is, genetic and environmental risk factors) contribute to the observed association. Monozygotic twins are genetically identical. Dizygotic twins and full siblings share on average 50% of their segregating alleles, while half siblings share on average 25%. Full cousins share on average 12.5% of their segregating alleles, while half cousins share on average 6.25%. Higher ORs in full siblings and dizygotic twins than in half siblings and cousins provide support for the role of genetic influences. Moreover, as children continue to live predominantly with their mothers after parental separation,32 maternal half siblings are likely to share family environment to a similar extent as full siblings and to a larger extent than paternal half siblings. Maternal half siblings also share pregnancy-related environmental influences constant within the same mother. Hence, higher ORs in maternal half siblings than in paternal half siblings provide support for the role of shared environmental influences.
Sensitivity analysis
We obtained unadjusted estimates of the association between ASD and ADHD (crude ORs) and conducted several additional analyses.
First, we used different definitions of case, that is, ADHD case definition based only on the NPR (Model 1) and ASD and ADHD case definitions based on two recorded diagnoses in the NPR (Model 2)17 to test the robustness of our results to different case definitions. In these analyses, information from the PDR was excluded.
Then we tested the alternative hypothesis that a possible familial association between the two conditions might be better explained by the presence of the two disorders in the same person, that is, by potential effects of one disorder on the other, rather than by the presence of a shared familial liability for the two disorders. For this purpose, we tested both directions, in which the two disorders may be associated in individuals. First, we estimated ORs of ADHD in individuals whose relatives had ASD, compared with those whose relatives did not have ASD, including ASD in the outcome person as a covariate (Model 3). Then changing the definition of outcome and exposure person, we estimated ORs of ASD in individuals whose relatives had ADHD, compared with those whose relatives did not have ADHD, including ADHD in the outcome person as a covariate (Model 4). If the within-family association was purely driven by direct effects of one disorder on the other, rather than by familial factors common to the two disorders, the estimates of ORs would be expected to attenuate to the null or even be reversed in this analysis.33
In addition, estimates stratified by sex of the outcome and of the exposure person were obtained for the within-individual association and the within-family association in full siblings to test possible differences between males and females in the role of familial liability.
Data management was performed using SAS, version 9.3 (SAS Institute, Cary, NC, USA); analyses were performed using Stata, version 14.0 (StataCorp., College Station, TX, USA).
Code availability
Computer codes are available upon request from the corresponding author.
Results
Among the 1 899 654 individuals included in the study, we identified 28 468 ASD cases (prevalence 1.50%: 1.20% for high-functioning ASD, 0.3% for low-functioning ASD) and 82 398 ADHD cases (4.34% Table 1), with 13 793 individuals (0.73%) being comorbid cases (Table 1). Despite precluded from ICD-9 and ICD-10 criteria, we did identify individuals with both diagnoses from the NPR (n=11 837), with the majority of them having both the diagnoses on the same day at least once during the follow-up period (n=10 512; Supplementary Table S1). Prevalence estimates were consistently above 1% for ASD and around 3% for ADHD, regardless of the case definition (Supplementary Table S1). The prevalence of both the disorders was higher in males (2.03% for ASD and 5.63% for ADHD) than in females (0.94% for ASD and 2.98% for ADHD; Table 1). Similarly, the prevalence of ASD–ADHD comorbidity was higher in males (1.01%) than in females (0.43% Table 1). Almost half of the individuals with ASD (48%) also had ADHD, and around 17% of individuals with ADHD also had ASD.
Table 1. Overall and sex-specific prevalence of ASD, ADHD and comorbid cases in the population under study (n=1 899 654).
| Overall | Males | Females | |
|---|---|---|---|
| ASD | 28 468 (1.50%) | 19 734 (2.03%) | 8 734 (0.94%) |
| ADHD | 82 398 (4.34%) | 54 759 (5.63%) | 27 639 (2.98%) |
| Comorbid cases | 13 793 (0.73%) | 9 805 (1.01%) | 3 988 (0.43%) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorders.
Within-individual association
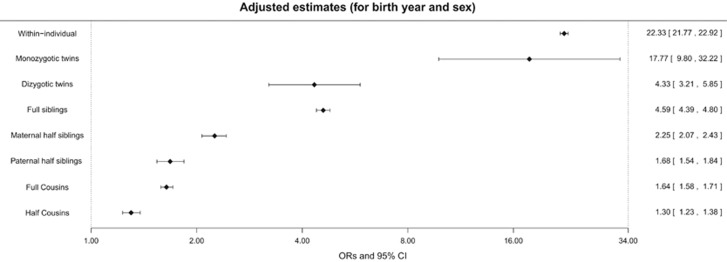
Individuals with ASD were at higher risk of having ADHD, compared with those who did not have ASD (OR=22.33, 95% CI: 21.77–22.92; Figure 2).
Figure 2.
Within-individual and within-family associations between autism spectrum disorders (ASD) and attention-deficit/hyperactivity disorder (ADHD). Forest plot illustrating odds ratios (ORs, diamonds) and 95% confidence interval (CI, bars) expressing the association between ASD and ADHD obtained from the within-individual analysis performed in the whole sample and from the within-family analyses performed in the cohorts of different relatives. All the estimates were adjusted for birth year and sex. The numbers of observations for each cohort are: 1 899 654 for the whole sample (that is, within-individual estimates); 8360 for monozygotic twins; 25 310 for dizygotic twins; 1 829 696 for full siblings; 273 924 for maternal half siblings; 269 004 for maternal half siblings; 5 580 328 for full cousins; and 1 251 584 for half cousins.
Within-family association
Within-family associations (Figure 2) showed that monozygotic twins of ASD cases had an increased risk of having ADHD (OR=17.77, 95% CI: 9.80–32.22). The association estimated in monozygotic twins was significantly higher (P-value<0.001) than the ones estimated in dizygotic twins (OR=4.33, 95% CI: 3.21–5.85) and in full siblings (OR=4.59, 95% CI: 4.39–4.80; Figure 2). Further, maternal half siblings of ASD cases had an increased risk of having ADHD (OR=2.25, 95% CI: 2.07–2.43) (Figure 2). The magnitude of this association was significantly lower (P-value<0.001) than the one found in full siblings and significantly higher (P-value<0.001) than the one found in paternal half siblings (OR=1.68, 95% CI: 1.54–1.84; Figure 2). The risk of having ADHD was also increased in full (OR=1.64, 95% CI: 1.58–1.71) and half cousins (OR=1.30, 95% CI: 1.23–1.38) of ASD cases (Figure 2). The association was significantly stronger in full cousins than in half cousins (P-value<0.001).
The magnitude of the association was larger (P-value<0.001) in high-functioning ASD cases (that is, with no recorded diagnosis of ID; OR=26.08, 95% CI: 25.35–26.83) than in low-functioning ASD cases (that is, with a recorded diagnosis of ID; OR=1.25, 95% CI: 1.16–1.34; Table 2). A similar pattern of results was observed in full siblings and full cousins (Table 2).
Table 2. Within-individual and within-family associations between low- and high-functioning ASD and ADHD.
| Cohorts | Low-functioning ASD | High-functioning ASD |
|---|---|---|
|
OR (95% CI) |
||
| Within individual (N Obs=1 899 654) | 1.25 (1.16–1.34) | 26.08 (25.35–26.83) |
| Full siblings (N Obs=1 829 696) | 1.17 (1.03–1.32) | 4.93 (4.70–5.17) |
| Full cousins (N Obs=5 580 328) | 0.93 (0.85–1.02) | 1.68 (1.62–1.75) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorders; CI, confidence interval; OR, odds ratio. ORs and 95% CI expressing the association between low- and high-functioning ASD (that is, with or without comorbid intellectual disability) and ADHD, obtained from the within-individual analysis performed in the whole sample and from the within-family analyses performed in the cohorts of full siblings and full cousins. All the estimates are adjusted for birth year and sex.
Sensitivity analysis
Crude ORs did not differ from those estimated from the adjusted model (for birth year and sex, Figure 1), with the exception of within-individual associations, which showed slightly higher ORs, when no potential confounder was added to the model (Supplementary Table S2, crude estimates).
Results did not change when the definition of ADHD was based only on information from the NPR (Supplementary Table S2 and Model 1) or when ASD and ADHD cases were identified using stricter criteria, that is, at least two diagnoses in the NPR (Supplementary Table S2 and Model 2), excluding information from the PDR. Adjustments for comorbidity in the outcome person, when analysing family co-aggregation of the conditions, led to a decrease of the ORs, with the largest change observed for estimates obtained in the cohort of monozygotic twins (Supplementary Table S2, Models 3 and 4). However, all ORs remained positive and statistically significant, confirming that the results were not mainly attributable to the effect of within-individual association.33
Estimates stratified by sex are presented in Supplementary Table S3. The magnitude of the within-individual association was larger (P-value<0.001) in females (OR=29.79, 95% CI: 28.46–31.18) than in males (OR=19.47, 95% CI: 8.90–20.07). No sex differences in the magnitude of within-family association were detected (Supplementary Table S3).
Discussion
To the best of our knowledge, this is the first study investigating the familial liability to clinically ascertained ASD and ADHD using a genetically informative design, comparing the association between the disorders across different types of relatives. We found evidence of a strong association between ASD and ADHD within individuals and among individuals belonging to the same family.
Previous studies have found similar increased risk in first-degree relatives of ASD and ADHD cases. A 2.5-fold increase in the risk of ASD without comorbid ADHD was found in the offspring of mothers with ADHD compared with those of mothers without ADHD.17 A 3.7-fold increase in the risk of ADHD was found in siblings of ASD cases.18 Our study extends these findings by estimating the familial association between ASD and ADHD in several types of relatives. The pattern of within-family associations between ASD and ADHD across different types of relatives reflected the decreasing genetic resemblance among them, confirming findings from twin studies on ASD and ADHD traits5, 6, 7, 8, 9, 10, 11 and providing strong evidence that genetic factors have a role in the co-occurrence of clinically ascertained ASD and ADHD.
The association between ASD and ADHD was weaker among paternal half siblings than among maternal half siblings. This may indicate that environmental factors shared by relatives contribute to the co-occurrence of these conditions, as maternal and paternal half siblings share the same amount of co-segregating genes, but maternal half siblings share familial environment to a larger extent. This interpretation is based on the observation that, after parental separation, the majority of children tend to live predominantly with their mothers.32 Although this might change with time, with children spending more time with both parents,34 other shared environmental factors, such as prenatal factors constant across pregnancies within the same mother, may contribute to more similarity among maternal rather than paternal half siblings. Previous twin studies have found little evidence for the role of shared environment in the overlap between ASD and ADHD traits,9, 11 but methodological differences such as the use of ICD-based diagnoses and the comparison of several types of relatives might explain the inconsistency.
Greater within-individual and within-family associations were detected for high-functioning ASD compared with low-functioning ASD. A plausible explanation is that ASD with ID may represent a more severe ASD phenotype showing less aetiological overlap with other neurodevelopmental disorders. Heterogeneity in the aetiological architecture of ASD has been suggested by the observation that family history of neuropsychiatric disease is more strongly associated with high-functioning ASD than with low-functioning ASD.20 In this group, other types of genetic mechanisms, such as de novo mutations, may be of relatively greater importance.19, 20 An alternative explanation is that ASD patients with ID are less likely to receive an additional diagnosis, even when displaying ADHD symptoms. If this was the case, the association found here would be an underestimation of the real overlap in this subgroup. Further research, that combines information on psychometric measures, may address this issue.
Our results should be considered within the context of the study strengths and limitations. Using a genetically informative design and register-based data, we tested the familial association of ASD and ADHD in the largest population-representative sample analysed so far. On the other hand, register-based data on diagnoses and medication prescriptions do not include information about symptom profiles. Furthermore, ADHD diagnosis from ICD only captures the combined type of ADHD, which is more strongly associated with autistic traits.35, 36 This might explain the higher co-occurrence of ASD and ADHD observed in our study compared with what reported in other population-derived samples.37, 38 In addition, only treatment-seeking individuals were identified. As higher psychopathology and poorer functioning have been observed in ADHD cases displaying ASD traits,39, 40 these cases may have higher probability of seeking help and being clinically reported, compared with those not displaying such traits. If this was the case, the estimate of the within-individual association might be inflated. Similarly, if relatives of cases are more likely to seek help compared with relatives of non-cases, within-family association might be an overestimation of the familial liability shared by the disorders.
In sensitivity analyses, all the ORs remained statistically significant after adjusting for comorbidity in the outcome person, confirming that the results were not mainly attributable to the effect of within-individual association (that is, a direct effect of one disorder on the other). Although we cannot completely rule out the action of this type of mechanism, this further supports our main hypothesis that the observed co-occurrence of ASD and ADHD in individuals and families is (at least partly) due to shared liability between the disorders. The large decrease observed in monozygotic twins might reflect unstable estimates due to power issues, given the smaller size of this cohort compared with the others.
Our results were not dependent on either source of information or case definition. When considering only information from the NPR, we obtained similar prevalence and association estimates, confirming the validity of the use of information about ADHD medication from PDR to identify ADHD cases30 and suggesting that the observed overlap most likely reflects true comorbidity. Similarly, a more stringent definition of ADHD and ASD cases, requiring at least two recorded diagnoses17 in the NPR, did not lead to different results.
Additional analyses showed a stronger within-individual association in females than in males, in accordance with a previous study of children with ADHD, in which the percentage of females having clinically relevant autistic traits was higher than the percentage of males.41 However, we did not find support for sex differences in shared familial liability between ASD and ADHD, as within-family association estimated in full siblings did not differ depending on the sex of the exposing sibling. According to the female protection hypothesis, that is, that females clinically referred with neurodevelopmental disorders present a higher ‘mutational burden’ compared with males,42 we were expecting to find higher rates of ADHD in siblings of female ASD cases compared with siblings of male ASD cases. Such an effect was not supported by another study conducted in a population-based sample, which investigated familial association between ASD and other neuropsychiatric disorders.18
In conclusion, our study provides robust and representative estimates of the association between ASD and ADHD in individuals and in families, supporting the importance of genetic and family-shared environmental influences. These results suggest that genomic studies might have underestimated the genetic link between ASD and ADHD.15, 16 Such studies may have suffered from insufficient sample size to detect genetic overlap. Another potential explanation for the low genetic link between ASD and ADHD in previous genomic studies is the use of strict exclusion criteria for comorbidity. Future molecular genetic studies with a focus on within-disorder heterogeneity and cross-disorder similarities may thus benefit not only from larger sample size but also from less stringent exclusion criteria about comorbidities;43, 44 such a study design could be used to disentangle disorder-specific from cross-diagnostic genetic effects.44 In addition, either genetic imprinting or shared environmental effects have to be assumed owing to the higher risk in maternal half siblings compared with paternal half siblings. Thus our results emphasise the importance of assessing environmental exposures in future research on the aetiology of neurodevelopmental disorders.
Acknowledgments
We acknowledge financial support from the Swedish Research Council (2014-3831). The project leading to this publication has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 643051. This report reflects only the authors’ views and the European Union is not responsible for any use that may be made of the information it contains. Professor Freitag is supported by the European Union (FemNAT-CD, grant no. 602407). Professor Asherson is supported by NIHR Biomedical Research Centre for mental health, NIHR/MRC (14/23/17), Action Medical Research (GN 2315) and European Union (643051, 602805 and 667303).
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
BF has received educational speaking fees from Merz and Shire. PL has served as a speaker for Medice. HL has received educational speaking fees from Eli-Lilly and Shire and has received a research grant from Shire, all outside the submitted work. King’s College London research support account for Asherson received honoraria for consultancy to Shire, Eli-Lilly and Novartis; received educational/research awards from Shire, Lilly, Novartis, Vifor Pharma, GW Pharma and QbTech; and speaker’s fees at sponsored events for Shire, Lilly and Novartis. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 2005; 57: 1313–1323. [DOI] [PubMed] [Google Scholar]
- Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry 2007; 12: 2–22. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 2010; 19: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner Y. The co-occurrence of autism and attention deficit hyperactivity disorder in children - what do we know? Front Hum Neurosci 2014; 8: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Grimmer M, Martin NG, Todd RD. Evidence for shared genetic influences on self-reported ADHD and autistic symptoms in young adult Australian twins. Twin Res Hum Genet 2008; 11: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman TJ, Hoekstra RA, Posthuma D, Larsson H. The co-occurrence of autistic and ADHD dimensions in adults: an etiological study in 17,770 twins. Transl Psychiatry 2014; 4: e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Edelson LR, Asherson P, Saudino KJ. Exploring the relationship between autistic-like traits and ADHD behaviors in early childhood: findings from a community twin study of 2-year-olds. J Abnorm Child Psychol 2010; 38: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Larsson H, Anckarsater H, Lichtenstein P. Symptoms of autism and ADHD: a Swedish Twin Study examining their overlap. J Abnorm Psychol 2014; 123: 440–451. [DOI] [PubMed] [Google Scholar]
- Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J Child Psychol Psychiatry 2008; 49: 535–542. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Charman T, Robinson EB, Plomin R, Happe F, Asherson P et al. Developmental associations between traits of autism spectrum disorder and attention deficit hyperactivity disorder: a genetically informative, longitudinal twin study. Psychol Med 2013; 43: 1735–1746. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Charman T, Ronald A. Where are the strongest associations between autistic traits and traits of ADHD? Evidence from a community-based twin study. Eur Child Adolesc Psychiatry 2015; 24: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Cooper M, Hamshere ML, Pocklington A, Scherer SW, Kent L et al. Biological overlap of attention-deficit/hyperactivity disorder and autism spectrum disorder: evidence from copy number variants. J Am Acad Child Adolesc Psychiatry 2014; 53: 761–770, e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NM, Franke B, Mick E, Anney RJ, Freitag CM, Gill M et al. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: the role of rare variants and duplications at 15q13. 3. Am J Psychiatry 2012; 169: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NM, Zaharieva I, Martin A, Langley K, Mantripragada K, Fossdal R et al. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet 2010; 376: 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 2013; 45: 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 2013; 381: 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Hawkey E, Kachan-Liu SS, Lees P, Roullet JB, Goddard K et al. Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. J Child Psychol Psychiatry 2014; 55: 819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiranta-Olkoniemi E, Cheslack-Postava K, Sucksdorff D et al. Risk of psychiatric and neurodevelopmental disorders among siblings of probands with autism spectrum disorders. JAMA Psychiatry 2016; 73: 622–629. [DOI] [PubMed] [Google Scholar]
- Samocha KE, Robinson EB, Sanders SJ, Stevens C, Sabo A, McGrath LM et al. A framework for the interpretation of de novo mutation in human disease. Nat Genet 2014; 46: 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH et al. Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proc Natl Acad Sci 2014; 111: 15161–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Board of Health and Welfare. The Swedish medical birth register: a summary of content and quality. Stockholm, Sweden: National Board of Health and Welfare, 2003.
- Ludvigsson JF, Almqvist C, Bonamy A-KE, Ljung R, Michaëlsson K, Neovius M et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016; 31: 125–136. [DOI] [PubMed] [Google Scholar]
- Ekbom A. The Swedish multi-generation register. Methods Mol Biol 2011; 675: 215–220. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettermark B, Hammar N, MichaelFored C, Leimanis A, Otterblad Olausson P, Bergman U et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–735. [DOI] [PubMed] [Google Scholar]
- van Daalen E, Kemner C, Dietz C, Swinkels SHN, Buitelaar JK, van Engeland H. Inter-rater reliability and stability of diagnoses of autism spectrum disorder in children identified through screening at a very young age. Eur Child Adolesc Psychiatry 2009; 18: 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. J Child Psychol Psychiatry 2013; 54: 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idring S, Rai D, Dal H, Dalman C, Sturm H, Zander E et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS ONE 2012; 7: e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterqvist J, Asherson P, Halldner L, Långström N, Larsson H. Stimulant and non‐stimulant attention deficit/hyperactivity disorder drug use: total population study of trends and discontinuation patterns 2006–2009. Acta Psychiatr Scand 2013; 128: 70–77. [DOI] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal InvestigatorsCenters for Disease Control and Prevention. Prevalence of autism spectrum disorders: Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 2012; 61: 1–19. [PubMed] [Google Scholar]
- Statistiska Centralbyrån. Fakta om den Svenska Familjen Demografiska Rapporter 2. Statistiska Centralbyrån: Stockholm, Sweden, 1994. [Google Scholar]
- Yao S, Kuja-Halkola R, Thornton LM, Runfola CD, D’Onofrio BM, Almqvist C et al. Familial liability for eating disorders and suicide attempts: evidence from a Population Registry in Sweden. JAMA Psychiatry 2016; 73: 284–291. [DOI] [PubMed] [Google Scholar]
- Bergström M, Modin B, Fransson E, Rajmil L, Berlin M, Gustafsson PA et al. Living in two homes-a Swedish national survey of wellbeing in 12 and 15 year olds with joint physical custody. BMC Public Health 2013; 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. J Autism Dev Disord 2006; 36: 271–283. [DOI] [PubMed] [Google Scholar]
- Grzadzinski R, Di Martino A, Brady E, Mairena MA, O'Neale M, Petkova E et al. Examining autistic traits in children with ADHD: does the autism spectrum extend to ADHD? J Autism Dev Disord 2011; 41: 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47: 921–929. [DOI] [PubMed] [Google Scholar]
- Rao PA, Landa RJ. Association between severity of behavioral phenotype and comorbid attention deficit hyperactivity disorder symptoms in children with autism spectrum disorders. Autism 2014; 18: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Martin J, Langley K, Hamshere M, Thapar A. Autistic traits in children with ADHD index clinical and cognitive problems. Eur Child Adolesc Psychiatry 2014; 23: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotte A, Joshi G, Fried R, Uchida M, Spencer A, Woodworth KY et al. Autistic traits in children with and without ADHD. Pediatrics 2013; 132: e612–e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry 2007; 48: 464–472. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet 2014; 94: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R. Genomics and the classification of mental illness: focus on broader categories. Genome Med 2013; 5: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey M, Avenevoli S, Anderson K. The National Institute of Mental Health Research Domain Criteria and Clinical Research in Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry 2016; 55: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.