Abstract
Emerging evidence suggests that psychiatric disorders are associated with disturbances in structural brain networks. Little is known, however, about brain networks in those at high risk (HR) of bipolar disorder (BD), with such disturbances carrying substantial predictive and etiological value. Whole-brain tractography was performed on diffusion-weighted images acquired from 84 unaffected HR individuals with at least one first-degree relative with BD, 38 young patients with BD and 96 matched controls (CNs) with no family history of mental illness. We studied structural connectivity differences between these groups, with a focus on highly connected hubs and networks involving emotional centres. HR participants showed lower structural connectivity in two lateralised sub-networks centred on bilateral inferior frontal gyri and left insular cortex, as well as increased connectivity in a right lateralised limbic sub-network compared with CN subjects. BD was associated with weaker connectivity in a small right-sided sub-network involving connections between fronto-temporal and temporal areas. Although these sub-networks preferentially involved structural hubs, the integrity of the highly connected structural backbone was preserved in both groups. Weaker structural brain networks involving key emotional centres occur in young people at genetic risk of BD and those with established BD. In contrast to other psychiatric disorders such as schizophrenia, the structural core of the brain remains intact, despite the local involvement of network hubs. These results add to our understanding of the neurobiological correlates of BD and provide predictions for outcomes in young people at high genetic risk for BD.
Introduction
Large-scale brain networks arise from white matter (WM) tracts that link cortical regions and subcortical structures, following topologically complex,1, 2 geometrically constrained3 principles. Disturbances to these networks have been observed in a variety of neurological and psychiatric disorders, including schizophrenia,4 depression,5 attention-deficit hyperactivity disorder,6 mild cognitive impairment5 and epilepsy.7 Even subtle perturbations to brain networks can cause disturbances in cognitive and emotional processes,8 particularly if they target highly connected hubs in executive, emotional and association regions.9, 10
Bipolar disorder (BD) is a disabling psychiatric disorder characterised by episodic disturbances in emotion and cognition. Studies have inferred reduced WM integrity in BD from alterations to diffusion tensor imaging-derived indices, such as fractional anisotropy.11, 12, 13, 14 Although findings are somewhat inconsistent, there is a trend towards diffusion tensor imaging-derived alterations in BD patients compared with controls (CNs) in circuits linking prefrontal, striatal and limbic regions.14 Such findings suggest a link between the emotional and cognitive phenotype of BD and dysfunction in the networks supporting these functions.
Unaffected first-degree relatives of patients with BD have an odds ratio of ~7–14 of developing BD.15 Given the strong heritability of WM morphology,16, 17 studying individuals at familial risk of developing BD may help identify neurobiological factors that pre-empt the development of BD, as well as factors associated with resilience. This objective is particularly pertinent in young first-degree relatives who have not yet passed the peak age of illness onset.18 Studying unaffected relatives also mitigates the influence of illness-related confounds such as psychotropic medication.19 A number of studies have revealed evidence of WM alterations in unaffected first-degree relatives, although the spatial distribution and extent of these impairments remains uncertain.20, 21, 22, 23, 24, 25
Although diffusion tensor imaging-derived indices have shed light on disturbances in specific WM pathways, such methods are insensitive to complex interactions among multiple brain regions. Developments in the acquisition of diffusion-weighted imaging and fibre-bundle reconstruction have allowed structural brain networks to be mapped with increased precision. The application of graph theoretical techniques to these networks has shown that healthy brain networks demonstrate ‘small-world’ features (indicative of balanced integration and segregation), minimising total fibre length.2, 26, 27 Such organisational properties appear to be compromised in many disorders,26 exemplified by the burgeoning connectomic research elucidating network aberrations in schizophrenia.28, 29 Disturbances appear to involve highly connected hub regions,10, 30 in particular those hub-regions with dense wiring among themselves, known as the ‘rich club’.31 Intriguingly, this core architectural feature may also be compromised in unaffected relatives of schizophrenia patients.9
Although prior connectomic studies of BD show impairments in connectivity across the callosum and among limbic regions,32, 33 the rich club backbone may be preserved.33 A recent study of unaffected relatives of BD patients did not reveal any structural network differences in either patients or CNs.34 However, this study had a modest sample size (n=58) and the mean age of unaffected relatives was 42 years, suggesting that many would have already passed the typical onset age of BD illness (<30 years)35 and may represent a resilient group of HR individuals. Alterations of topographical network organisation in unaffected first-degree relatives of patients with BD, who have not passed the peak age of onset, remains to be elucidated.
We leveraged recent advances in tractography and complex network analyses, to investigate whole-brain structural networks in a large sample of young, unaffected HR first-degree relatives of patients with BD, patients with BD and CN subjects. Crucially, our study cohort is relatively young (<30 years), encompassing the peak age of illness onset. We studied specific sub-network differences in connectivity, as well as the topological properties of the highly connected ‘rich club’ and the global network architecture of the brain. We hypothesised that structural connectome disturbances in key regions involved in emotional regulation would be an early marker of vulnerability to BD.
Materials and methods
Participants
Two hundred and eighteen richly phenotyped participants aged 15–30 years comprised three age- and gender-matched groups as follows: (1) 84 participants at HR for BD, (2) 96 CNs without a family history of mental illness and (3) 38 BD participants. HR and BD participants were recruited from families who had previously participated in a BD pedigree molecular genetics study or a specialised BD research clinic, clinicians, mental health consumer organisations and other forms of publicity. CN subjects were recruited via print and electronic media, and noticeboards in universities and local communities. Sample ascertainment and separate clinical assessments for younger (15–21) and older (22–30) age categories are provided in Perich et al.36 and in Supplementary Information 1.1. We pooled data across both age cohorts unless there was a specific correlation between a brain network measure and an age-specific clinical variable in either group. Summary demographic and clinical data are presented in Table 1 and Supplementary Information 1.5.
Table 1. Demographic and clinical data for CN, HR and BD groups.
| CN (n=96) | HR (n=84) | BD (n=38) | Difference statistic | P-value | Post-hoc effects | |
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Females, n (%) | 53 (55.2) | 45 (53.6) | 23 (60.5) | χ2=0.52 | 0.77 | |
| Males, n (%) | 43 (44.8) | 39 (46.4) | 15 (39.5) | χ2=0.52 | 0.77 | |
| Intelligence quotient, mean (s.d.) | 117.7 (10.3) | 116.3 (10.7) | 117.3 (12.0) | F=0.36 | 0.69 | |
| Age, mean (s.d.) | 22.6 (3.8) | 22.4 (4.7) | 23.9 (3.4) | F=2.08 | 0.13 | |
| Lifetime DSM-IV diagnosis | ||||||
| Any diagnosis, n (%) | 24 (25.0) | 39 (46.4) | 38 (100.0) | χ2=61.59 | <0.001 | HR>CN** BD>CN*** |
| At least one MDE, n (%) | 9 (9.4) | 22 (26.2) | 36 (94.7) | χ2=94.51 | <0.001 | HR>CN** BD>CN*** BD>HR*** |
| Recurrent MDD, n (%) | 1 (1.0) | 7 (8.3) | − | χ2=5.61 | 0.01 | HR>CN* |
| Any anxiety disorder, n (%) | 9 (9.5) | 15 (18.3) | 15 (39.5) | χ2=16.45 | <0.001 | BD>CN*** BD>HR* |
| Any behavioural disorder, n (%) | 1 (1.1) | 6 (7.4) | 7 (18.9) | χ2=13.86 | <0.001 | HR>CN* BD>CN*** |
| Any substance disorder, n (%) | 6 (6.3) | 9 (10.7) | 6 (15.8) | χ2=3.03 | 0.220 | − |
| Symptom severity scales | ||||||
| 22–30 Years | n=51 | n=41 | n=25 | |||
| MADRS, mean (s.d.) | 1.9 (3.2) | 2.5 (3.7) | 10.1 (9.5) | F=22.10 | <0.001 | BD>CN*** BD>HR*** |
| 15–21 Years | n=34 | n=33 | n=9 | |||
| CDI, mean (s.d.) | 6.8 (3.7) | 9.0 (6.6) | 21.7 (8.7) | F=23.78 | <0.001 | BD>CN*** BD>HR*** |
| Clinical characteristics | ||||||
| Global functioning | ||||||
| GAF, mean (s.d.) | 91.7 (4.7) | 87.4 (8.5) | 78.6 (12.0) | F=38.49 | <0.001 | CN>HR*** CN>BD*** HR>BD*** |
| Age at first | ||||||
| MDE, mean (s.d.) | 19.1 (3.2) | 18.5 (4.5) | 15.4 (3.7) | F=6.63 | 0.002 | BD<CN** BD<HR** |
| Hypomanic episode, mean (s.d.) | − | − | 17.0 (4.3) | − | − | − |
| Manic episode, mean (s.d.) | − | − | 17.5 (3.2) | − | − | − |
| Elevated mood episode, mean (s.d.) | − | − | 17.0 (4.0) | − | − | − |
| Mood episode, mean (s.d.) | 19.1 (3.2) | 18.5 (4.5) | 14.9 (3.8) | F=8.68 | <0.001 | BD<CN** BD<HR** |
| Any anxiety disorder | 10.3 (6.7) | 13.5 (6.4) | 13.3 (7.0) | F=1.31 | 0.277 | |
| Number of episodes | ||||||
| MDE, mean (s.d.) | 1.4 (1.3) | 2.0 (2.1) | 12.0 (12.5) | F=12.89 | <0.001 | BD>CN*** BD>HR*** |
| Hypomanic episodes, mean (s.d.) | − | − | 10.2 (10.3) | − | − | − |
| Manic episode, mean (s.d.) | − | − | 2.8 (2.5) | − | − | − |
| Any elevated mood episode, mean (s.d.) | − | − | 9.9 (10.9) | − | − | |
| Any mood episode, mean (s.d.) | 1.4 (1.3) | 2.0 (2.1) | 21.7 (21.5) | F=16.93 | <0.001 | BD>CN*** BD>HR*** |
| Psychotropic medication | ||||||
| Anti-depressants, n (%) | − | − | 15 (39.5) | − | − | − |
| Mood stabilisers, n (%) | − | − | 26 (68.4) | − | − | − |
| Anti-psychotics, n (%) | − | − | 11 (28.9) | − | − | − |
| Benzodiazepines, n (%) | − | − | 0 (0.0) | − | − | − |
| Stimulants, n (%) | − | − | 0 (0.0) | − | − | − |
| Anti-convulsants, n (%) | − | − | 21 (65.6) | − | − | − |
Abbreviations: BD, bipolar disorder; CN, controls; GAF, Global Assessment of Functioning; HR, high risk; MDE, major depressive episode; MDD, major depressive disorder.
*P<0.05, **P<0.01, ***P<0.001.
Construction of structural networks
Diffusion-weighted imaging (DWI) data were acquired using a 3T Achieva X MRI scanner (Philips, Amsterdam, the Netherlands). The steps involved in the construction of whole-brain structural networks derived from these data are similar to those applied elsewhere3, 37 (see Supplementary Information 1.2). In brief, the diffusion-weighted imaging were first pre-processed. Constrained spherical deconvolution was then employed in conjunction with probabilistic tractography (iFOD2)38 to generate high-resolution whole-brain streamlines representing the most probable propagations of fibre tracts between brain regions (Supplementary Figure 1C).
The standard AAL template39 was subdivided into 512 cortical and sub-cortical parcellation regions (Supplementary Table 1) of approximately uniform size.40 Subject-specific parcellations (Supplementary Figure 1D) were combined with the individual’s whole brain tractography, to generate weighted structural networks (Supplementary Figure 1E), where each network edge corresponds to the total number of streamlines that intersect pairs of region, adjusted by the fibre length between those regions.41 All main analyses reported here are on structural networks thresholded with a connection density of 10% brain network investigations typically employ threshold levels centring around this value.2 We also checked the robustness of our main results at sparsity levels of 7.5 and 12.5%.
Network-based statistics
We tested for group differences in sub-networks of these structural connectomes. To achieve this, we used a General Linear Model in conjunction with the network-based statistics (NBS),42 a permutation-based method to CN for family-wise error over the large number of connectome edges tested (Supplementary Information 1.3.1). An omnibus F-test was first conducted, to test for the influence of group on sub-networks of connections, based on their topological extent, using a conservative height threshold of F=6.0 (corresponding to an uncorrected P=0.003). Two-sample one-tailed t-tests were then calculated to test for the presence and direction of any differences in sub-network connectivity between specific pairs of groups. A conservative test threshold of t=3.3 (corresponding to P=0.006) was chosen in order to focus on strong, focal subnetwork differences.43 Results corresponding to a relatively liberal height threshold of t=3.0 are provided in Supplementary Figure 3. This threshold, which is the default optimisation within NBS, identifies larger, more distributed subnetworks.
Complex network analyses
Hub nodes and connection classes
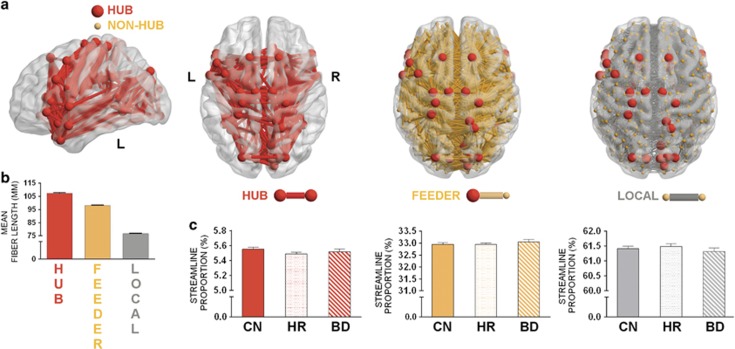
Each brain region’s ‘hubness’ was defined by virtue of its (binary) nodal degree; that is, the total number of edges connected to each region. The top 15% degree-ranking scores were used to identify hub-regions within each individual. The top 15% most consistent hubs across the CN group were then defined as hub-regions (Figure 1a). This cutoff threshold centres on values typically employed in brain network research.31, 37, 44 Classification of regions as either hubs (red) or non-hubs (grey) allowed network connections in each individual to be categorised into three classes as follows: (1) hub connections (red), linking hub nodes; (2) feeder connections (orange), linking hubs to non-hub nodes; and (3) local connections (grey), linking non-hub nodes.44
Figure 1.
Hub-regions and connection classes across the population groups. (a) Distribution of brain regions into hubs and non-hubs, with connections grouped into classes (hub, feeder or local). (b) Mean fibre length of each connection class across CNs. (c) Density of connections: left to right shows hub, feeder and local connections. Error bars indicate s.e.m. for each group. BD, bipolar disorder; CN, controls; HR, high risk; L, left; R, right.
Rich club organisation
High-degree hubs connect to other highly connected hubs more often than to peripheral nodes of low degree simply by virtue of their high degree. A rich club is said to exist when the connections among high-degree hubs are enriched above what would be predicted by their degree alone.31, 44 The recently reported rich club property of the brain has been attributed to the existence of a constellation of structural hubs, predominantly in heteromodal cortex, linked by long-range corticocortical connections.44 This organisation can be summarised by the rich club coefficient (RCC; see Supplementary Information 1.3.2). We calculated the RCC of the individual structural connectomes across a range of degrees. Significance testing of group differences in RCCs was assessed across all node degrees encompassing hub nodes using a false discovery rate correction.45
Network segregation and integration
We calculated two traditional graph-theoretical measures of global network topology as follows: a measure of integration (the shortest characteristic path length) and a measure of segregation46 (the clustering coefficient, CC; see Supplementary Information 1.3.3 and Supplementary Table 3). Biologically, the integrative properties of the brain (its relatively short path length) arises through long-range connections, in particular those linking heteromodal connections, whereas clustering arises from short-range circuit-like connections in local cortical motifs.2 For each region, we also calculated the nodal strength, namely the number of weighted connections that region shares with the network.
Statistical analyses
The 84 HR participants were drawn from 67 families. Generalised estimating equations47 were used to accommodate within-family correlations when assessing effects of diagnostic category, age-group interactions and when investigating whether group effects were influenced by depressive mood state. Post-hoc comparisons were carried out using Sidak’s adjustment for multiple comparisons. Corrections for multiple testing of the effects of diagnostic category were carried out with false discovery rate correction45 (see Supplementary Information 1.4.1).
Results
The structural connectomes of the young CN cohort exhibit a core-periphery hierarchy, consistent with that previously documented in healthy mid-life and elderly adults31, 37, 44 (Figure 1a). Densely connected hub regions form a bilateral structural core, including bilateral cortical regions located within dorsolateral and ventrolateral prefrontal cortices, anterior and middle cingulate, superior parietal and frontal, temporal poles, fronto-temporal, medial temporal, paracentral and precuneus areas, and subcortical structures (Supplementary Table 3). The topological distribution of hub-to-hub connections consist of long-range tracts, with mid-length feeder and short local connections (Figure 1b). There were no significant group differences in the relative proportion of hub, feeder and local edges between our three groups (Wald’s χ2 >1.1, P>0.20 and Wald’s χ2 >2.9, P>0.23), nor in the relative proportion of weighted streamlines across these classes (Wald’s χ2 >1.4, P>0.50; Figure 1c)
Sub-network connectivity differences: NBS
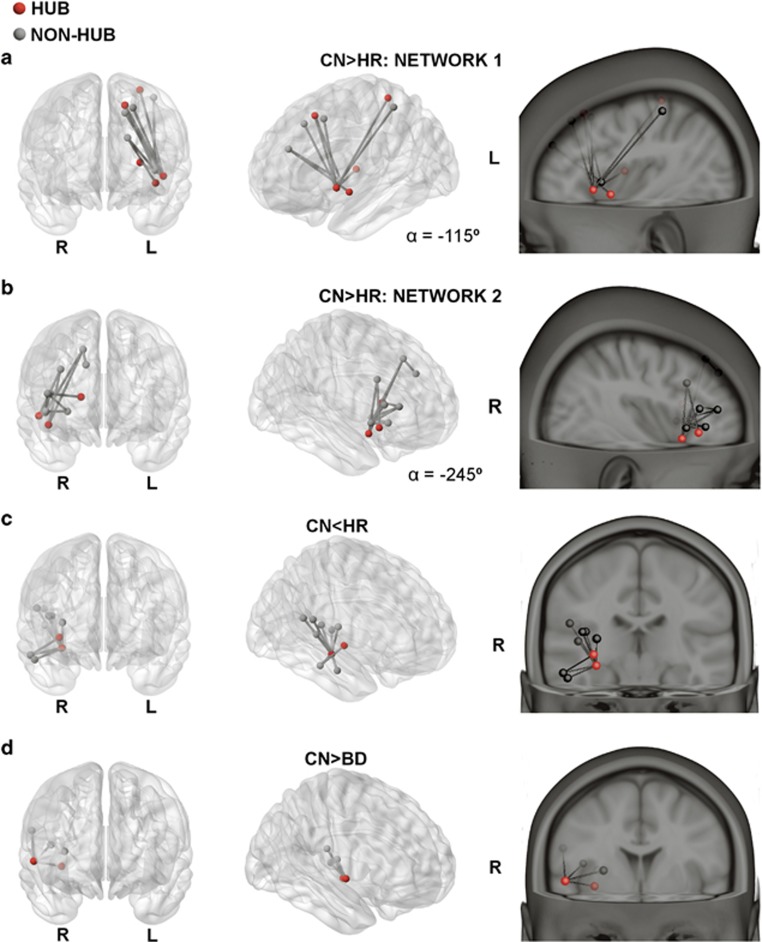
We applied NBS to study between-group sub-network differences. Application of an omnibus F-test revealed a strong and significant effect of group (F=6.0, pcorrected=0.018). Post-hoc t-tests revealed significant effects for CON>HR, HR>CON and CON>BD contrasts. The HR group show decreased connectivity in two lateralised structural networks compared with the CN group, both containing structural hubs. A left-lateralised network centres on the left inferior frontal gyrus (IFG) and insular cortex, with connections between superior frontal and postcentral areas (pcorrected=0.01, Hedge’s g 0.86; Figure 2a). A right-lateralised network largely encompasses connections from middle and superior frontal gyri to IFG and superior temporal poles (pcorrected=0.005, Hedge’s g=0.96; Figure 2b). Notably, 5 of the 10 nodes comprising the left sub-network are hubs (pars triangularis of the IFG, postcentral gyrus, insula and superior frontal gyrus), which is unlikely to occur by chance (P=0.009). Of the edges comprising this subnetwork, 33% are hub-to-hub and 44% are feeder (hub to local) connections, compared with much smaller proportions (5% and 32%, respectively) in the whole brain network (Supplementary Figure 2). The right sub-network includes three structural hubs (par orbitalis of the IFG, superior temporal pole, caudate). Although there are no hub-to-hub edges comprising this subnetwork, there are twice as many feeder edges (63%) compared with the whole brain (32%).
Figure 2.
Significant sub-networks of connections for group contrasts identified by the network-based statistic (NBS). Connections (lines) between nodes (circles) exhibiting significant (P<0.05, family-wise error-corrected; t=3.3) post-hoc group differences in streamline count. (a and b) CN>HR; (c) CN<HR; (d) CN>BD. Perspectives are from angular (middle panel), saggital (top right) and coronal views. BD, bipolar disorder; CN, controls; HR, high risk; L, left; R, right; α, azimuth.
A right-temporal network was more strongly connected in HR compared with CN participants (pcorrected<0.02, Hedge’s g=0.87; Figure 2c), with connections connecting the hippocampus (a hub-region) with the middle and superior temporal gyri. One small right-lateralised network showed weaker connectivity in BD compared with CN participants (pcorrected=0.027, Hedge’s g=0.93; Figure 2d). Notably, all edges connected a single hub region (the rolandic operculum) with neighbouring fronto-temporal areas.
Sub-networks derived from a more liberal test threshold (t=3.0) are provided in Supplementary Figure 3. For the CN>HR comparisons, this liberal threshold yields larger, but still lateralised sub-networks, which remain centred on IFG/insular regions of the corresponding hemispheres. These network differences are also expressed at sparsity levels of 7.5 and 12.5% (Supplementary Figure 4). Anatomical details and connectivity strengths for all nodes that constitute our NBS sub-networks are provided in Supplementary Table 4. No significant group effects were found for any of the other group contrasts (CN<BD, HR>BD, and HR<BD).
The distribution of node degree in our data is heavy tailed, showing an approximately log-normal distribution (Supplementary Figure 5). The classification of the top 15% of connected nodes as hub centres on thresholds previously employed in brain network research.31, 37, 44 In our data, this threshold is 1 s.d. above the mean, thus capturing the heavy right hand tail (Supplementary Figure 5A). We also identified hubs at more conservative (12.5%) and liberal (17.5%) cutoff points (Supplementary Figure 6). These supplementary analyses show that the relatively high proportion of hubs within the networks is robust to the exact choice of hub threshold.
Topological network analyses
Rich club organisation
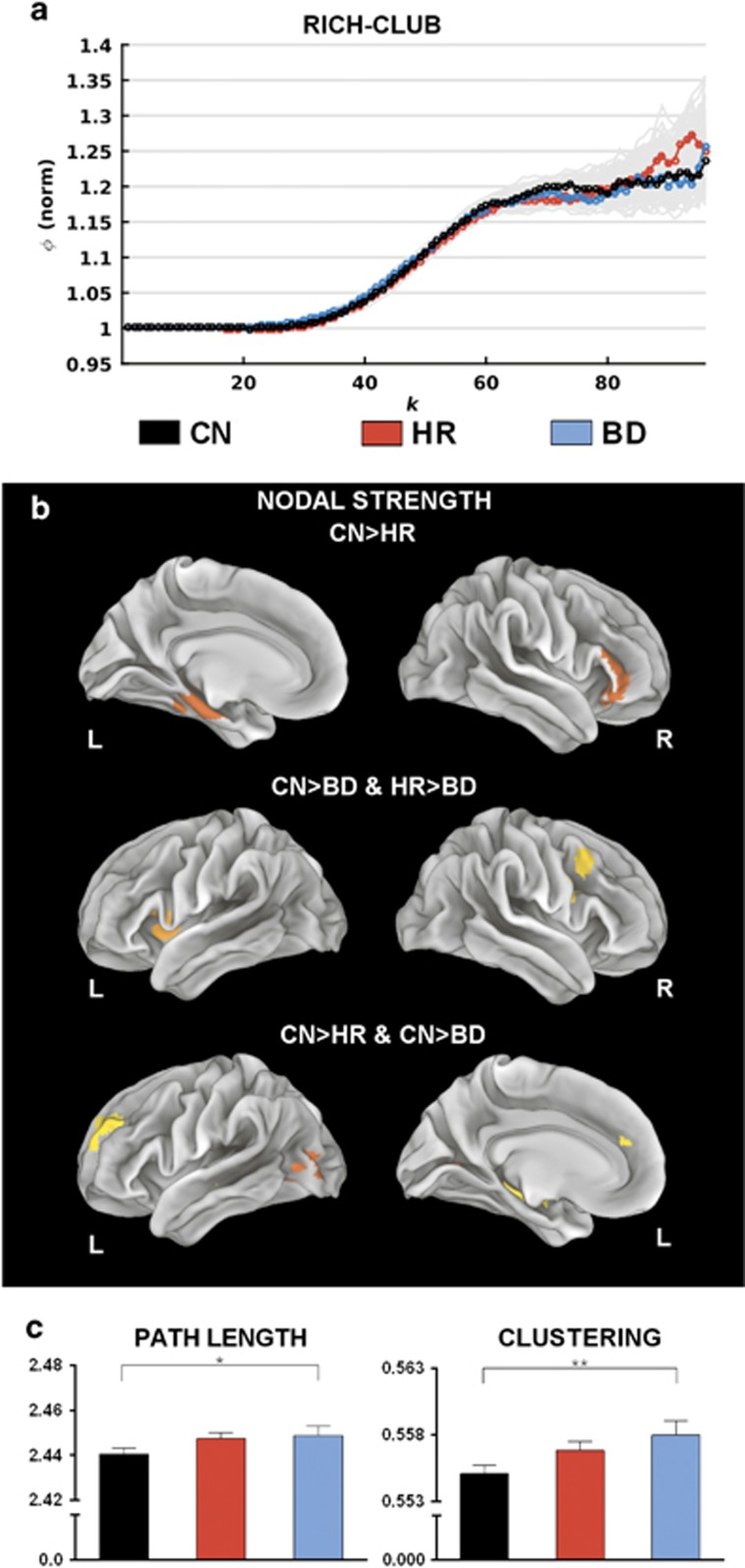
These NBS results suggest preferential involvement of hub nodes in group effects. To study whether these effects on hub nodes extend to involve hub-to-hub (rich) connections, we studied the RCCs. Data from all three groups show a highly enriched hub-to-hub (rich club) connectivity across a broad range of node degree (Figure 3a). However, there were no statistically significant differences (pcorrected>0.59) in the RCC between our three cohorts.
Figure 3.
Group contrasts of nodal, global and rich-club graph metrics. (a) Mean normalised rich-club coefficients across different k-levels for the population groups. Grey lines depict group uncertainty, obtained through permutation of group labels. (b) Regions exhibiting significant group (P<0.05, false discovery rate-corrected) differences in nodal strength. (c) Mean global graph metric values across population groups. Error bars indicate s.e.m. for each group. BD, bipolar disorder; CN, controls; HR, high risk; L, left; R, right. *P<0.05 and **P<0.01.
Nodal strength
Although the enrichment of the structural core is preserved, there remains the possibility that the localised connectivity of hubs differs between our three cohorts. This was tested by analysing the strength of all nodes (Figure 3b and Supplementary Table 5). Decreased strength of the HR group compared with the CN group occurred in the left parahippocampus and right IFG (top panel). Decreased nodal strength for the BD compared with both HR and CN groups was evident in the right precentral gyrus and left insula (middle). Decreased node strength for both HR and BD groups compared with CN was observed in the left SFG, left hippocampus and left middle occipital gyrus (bottom). Although these effects occur in cortical regions that contain hub nodes such as the IFG, insula and hippocampus, it is noteworthy that none of these eight nodes are themselves hubs.
Integration and segregation
A significant main effect of group was detected for both the CC (pcorrected=0.016) and characteristic path length (pcorrected=0.041), with a significantly higher CC (pcorrected=0.008, Hedge’s g=0.446) and longer characteristic path length (pcorrected=0.049, Hedge’s g=0.313) in BD subjects compared with the CN group (Figure 3c). Corresponding values in the HR group were intermediate to the BD and CN groups.
Auxiliary analyses
Current mood state was not significantly associated with total strength of NBS identified networks (P>0.07), or graph metrics (P>0.16), suggesting that these disturbances are not driven by mood but reflect an underlying trait disturbance. For nodal strengths, the precuneus demonstrated an effect of mood (P<0.001).
Given that a major depressive episode often precedes the onset of mania in those who will later develop BD,36 the occurrence of a major depressive episode in subjects at genetic risk for BD may represent a developmental stage of BD. We therefore undertook additional analyses to address this issue. We first subdivided our HR group into those with at least one lifetime depressive episode (n=22) or an anxiety disorder (n=15). For the HR>CN sub-network (Figure 2c), HR participants with a lifetime anxiety disorder show less connectivity compared with those without (P=0.037). No sub-group differences were evident for the remaining three NBS identified networks (P>0.12), nor for the graph metrics (P>0.60). We also removed subjects with a prior major depressive episode from within the HR group and re-analysed the between-group effects. Highly significant group differences (P<0.001) in structural connectivity remained for all NBS contrasts involving the HR cohort.
Within the BD group, current use of lithium, mood stabilisers or antidepressants were not associated with characteristic path length (P>0.32) or CC (P>0.34). Current antipsychotic use was significantly associated with lower CC (P=0.024). Measures of illness severity in the BD group (age of onset and total number of mood episodes) were not significantly associated with any connectivity measures.
Five of the 84 HR participants (6%) had a single relative within the BD group. We removed these 5 individuals from each group (10 in total) and repeated the corresponding analyses. As per the original contrasts, there were no significant differences in global graph metrics or NBS subnetworks (P>0.27) between the HR and BD groups. The greater nodal strength in HR compared with BD subjects in the left insula (Figure 3b, middle panel) remained significant when removing these subjects (P=0.007), although the effect in the right precentral gyrus drops below statistical threshold (P=0.054).
Age effects
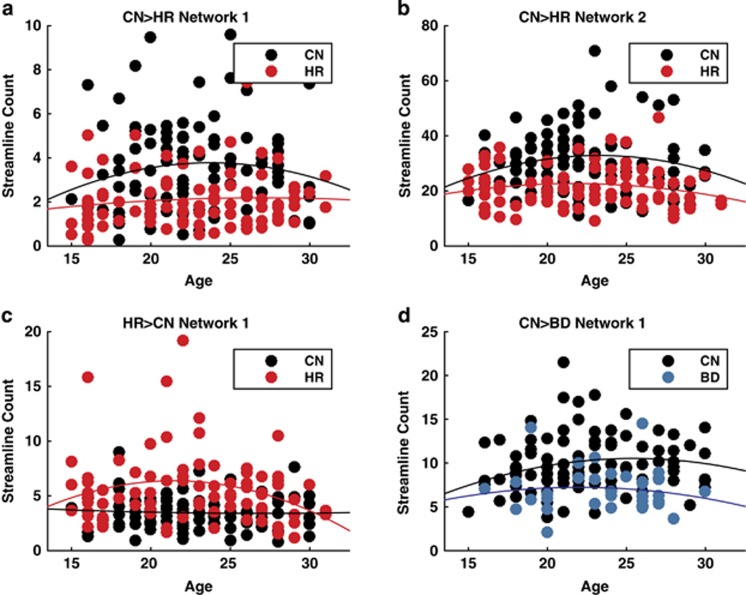
None of the NBS identified networks (P>0.33), nodal strengths (P>0.07), graph metrics (P>0.50) or mean RCC (P=0.36) revealed a significant age × group interaction. The age-range of 15–30 years encompasses a critical period in the development of cortical WM, which recent studies have suggested may show a nonlinear effect of age.48 To address this issue, we plotted the subject-specific strength of our four main NBS networks for each of the groups in the corresponding contrast. Of interest, consistent with prior work,48 all of the networks showed a broad inverted quadratic association with age (Figure 4). However, none of these effects reach statistical significance (P>0.19, false discovery rate corrected). Moreover, although the between-group effect for the HR>CN network appears to be accompanied by difference in the concavity of the quadratic fit, this difference does not exceed statistical significance (P>0.064, false discovery rate corrected).
Figure 4.
Scatter plots of streamline count as a function of age for the network-based statistic (NBS)-derived subnetworks. Each data point represents the total streamline counts for each subject within the significant NBS networks. (a) CN>HR network 1, (b) CN>HR network 2, (c) CN<HR network and (D) CN>BD network. Solid lines show the best-fitting nonlinear (quadratic) regression function of age overall across the groups. Although all four curves appear to show a negative (nonlinear) effect of age, the null hypothesis of a simpler linear model was not rejected for any of the networks (P>0.19, false discovery rate-corrected). BD, bipolar disorder; CN, controls; HR, high risk.
Discussion
In sum, our young HR cohort show weaker structural connections than the CN group in two lateralised sub-networks centring on fronto-temporal hubs and stronger connectivity in a right lateralised prefrontal network. The young BD group show reduced connection strengths in a single left fronto-temporal sub-network. Although the key structural hubs such as the IFG and insula are repeatedly involved in these sub-networks, the inter-connected structural ‘rich club’ backbone does not differ between groups. The perturbed sub-networks thus involve key emotional and cognitive circuitry, but ‘hang off’ a preserved structural core. Lack of substantial correlations with key clinical indices suggest that these effects represent a trait marker of increased risk for BD and not an effect of current mood state, prior major depressive episodes or medication.
The two lateralised networks that showed decreased connectivity in the HR group centre on bilateral prefrontal gyri, IFG and the left insular cortex. These regions recapitulate those reported to have reduced WM volume and density, and reductions in fractional anisotropy in prior studies of unaffected relatives of BD patients,21, 22, 39, 49, 50, 51 in addition to those with established BD.49, 52, 53, 54, 55, 56, 57, 58 The specific involvement of the IFG adds to a converging body of evidence from structural and functional studies of BD.59, 60, 61, 62, 63, 64 Likewise, the involvement of the insula adds to a growing number of reports of structural and functional differences in HR cohorts.23, 65, 66, 67 The involvement of a left-sided network is unique to the at-risk group (relative to CNs) and is not present in the bipolar group. Although the IFG is involved in a diverse range of tasks, meta-analysis of task functional magnetic resonance imaging suggests that the left (and not the right) IFG shows a selective involvement in empathic responses.68 The left IFG also shows a core involvement in disturbances in effective connectivity that are also unique to this at-risk group.60
Both the IFG and anterior insula are key areas for emotional, interoceptive and cognitive regulation.68, 69, 70, 71, 72, 73, 74 We extend prior research by suggesting that, rather than being focal abnormalities in HR individuals, the changes involving these regions occur in distributed structural networks that integrate interoception and emotional regulation with executive function and cognitive control. The presence of network disturbances involving both left and right fronto-insular circuits is of particular interest, given the putative left–right hemispheric asymmetry in control of the parasympathetic versus sympathetic system, respectively.75, 76, 77 Indeed, disturbances in this left–right balance may yield the pattern of autonomic disturbances often seen in frontotemporal dementia.78 In this light, bilateral disturbances speak to a ‘balanced’ interoceptive and autonomic dysregulation in those at HR of BD.
Although there were no cross-sectional correlates between these changes and clinical indices in our study, decreased structural connectivity between these important systems could underlie the decrease in general functioning that was observed in this group (Table 1). This functional impairment arises from a range of subtle but pernicious phenotypic changes in some (but not all) of those at HR.36, 79 Moving forward, such changes may underlie future BD illness expression in those who do convert to the disorder. This is to be the subject of future research using the broader longitudinal design in which the present study is nested.
The stronger structural connections in the HR cohort centre upon the right hippocampus (a hub-region) and connect neighbouring insular and superior temporal gyrus areas. Again, these regions reiterate those previously identified as having both altered fractional anisotropy24, 80 and decreased radial diffusivity20 in HR individuals. The presence of this sub-network of stronger connections could thus be a compensatory response to the weaker sub-networks elsewhere in our HR cohort, preserving adaptive emotional regulation. Such adaptive changes may be particularly pertinent in HR individuals who have passed the peak age of illness onset, thus reflecting resilience79, 81 However, brain network responses to a primary ‘disconnection’ can, depending on their network context, paradoxically have maladaptive consequences.26, 82 Accordingly, increased integration among this sub-network could confer risk in its own right, as cognitive function is thought to arise from a delicate balance of integration and segregation in structural networks.2, 82 The hippocampus is associated with memory and regulatory function during emotional processing,83 whereas the superior temporal gyrus is involved in social cognition processes such as facial emotion recognition.84 Therefore, strengthened connections between the hippocampus, other temporal regions and the insula could contribute to hypervigilance to emotional stimuli and social cues, which are seen in young HR individuals who have yet to pass the peak age of illness onset. Disambiguating these possibilities—and recalling that only a subset of our HR subjects will convert to BD—is to be the subject of future prospective study of this cohort.
Recent studies have reported a decrease in the enrichment of hub-to-hub connections in schizophrenia patients10 and their unaffected relatives.9 Despite involvement of key emotional and cognitive structural hubs in weakened sub-networks, we find preserved integrity of the rich club structure in young people with BD and our HR cohort, mirroring a recent finding in older BD patients.34 Although schizophrenia and BD show a substantial genetic overlap,85 this difference mirrors the relative preservation of cognitive function in BD86 and may be a key neurobiological difference between the disorders.
The BD group is smaller and is involved in fewer and less-extensive NBS contrasts than the HR cohort. However, several significant group effects, involving subnetwork and graph metric differences, did involve the BD participants but not the larger HR group. This suggests that contrasts involving BD were not underpowered, although more effects may have been observed had the group been larger. In BD, we observed a small right-sided sub-network of weaker connections between a rolandic opercular hub-region and neighbouring fronto-temporal areas (insula, Heschl’s gryus). This sub-network is distinct to and not an extension of the network of right-sided regions in the HR group. In addition, there were also subtle changes in measures of network integration and segregation specific to the BD group, with higher clustering and a longer path length in the BD compared with the CN group. Biologically, these putatively correspond to a subtle shift from long-range corticocortical connections to connections within local cortical motifs. We observed both shared and unique differences in node strength between groups. Hence, there are findings that are distinct to each of the HR and BD groups, as well as effects where the HR group falls between the CN and BD group. The former (group specific) changes may speak to the influence of mood-stabilising medication and/or compensatory responses to mood episodes in the BD group, as well as the heterogeneous nature of the HR group. Disambiguating these will again be the focus of future work as we follow this cohort longitudinally.
Acknowledgments
This study was funded by the Australian National Medical and Health Research Council (Program Grant 1037196), the Lansdowne Foundation and Brother’s Reid. MB acknowledges support from the Queensland Government Office of Health and Medical Research. MB and AP acknowledge the technical support from Kerstin Pannek.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors declare no conflict of interest.
Supplementary Material
References
- Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS Comput Biol 2005; 1: e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci 2013; 15: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Perry A, Lord AR, Roberts G, Mitchell PB, Smith RE et al. The contribution of geometry to the human connectome. NeuroImage 2016; 124(Part A): 379–393. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Seal ML, Cocchi L, Westin C-F, Bullmore ET et al. Disrupted axonal fiber connectivity in schizophrenia. Biological Psychiatry 2011; 69: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Shu N, Yuan Y, Shi Y, Yu H, Wu D et al. Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. Journal of Neuroscience 2012; 32: 4307–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Shu N, An L, Wang P, Sun L, Xia M-R et al. Probabilistic diffusion tractography and graph theory analysis reveal abnormal white matter structural connectivity networks in drug-naive boys with attention deficit/hyperactivity disorder. Journal of Neuroscience 2013; 33: 10676–10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja E, Zamyadi M, Raybaud C, Snead O, Doesburg S, Smith M. Disrupted global and regional structural networks and subnetworks in children with localization-related epilepsy. American Journal of Neuroradiology 2015; 36: 1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biological Psychiatry 2006; 59: 929–939. [DOI] [PubMed] [Google Scholar]
- Collin G, Kahn RS, de Reus MA, Cahn W, van den Heuvel MP. Impaired rich club connectivity in unaffected siblings of schizophrenia patients. Schizophrenia Bulletin 2014; 40: 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry 2013; 70: 783–792. [DOI] [PubMed] [Google Scholar]
- Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. Journal of Affective Disorders 2013; 150: 192–200. [DOI] [PubMed] [Google Scholar]
- Xekardaki A, Giannakopoulos P, Haller S. White matter changes in bipolar disorder, Alzheimer disease, and mild cognitive impairment: new insights from DTI. Journal of Aging Research 2011; 2011. [DOI] [PMC free article] [PubMed]
- Roberts G, Wen W, Frankland A, Perich T, Holmes-Preston E, Levy F et al. Interhemispheric white matter integrity in young people with bipolar disorder and at high genetic risk. Psychological Medicine 2016; 46: 2385–2396. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 2014; 171: 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen C, Melbye M, Mors O, Ewald H. Individual and familial risk factors for bipolar affective disorders in Denmark. Archives of General Psychiatry 2003; 60: 1209–1215. [DOI] [PubMed] [Google Scholar]
- Chiang M-C, Barysheva M, McMahon KL, de Zubicaray GI, Johnson K, Montgomery GW et al. Gene network effects on brain microstructure and intellectual performance identified in 472 twins. Journal of Neuroscience 2012; 32: 8732–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K-K, Rose S, Fripp J, McMahon KL, de Zubicaray GI, Martin NG et al. Investigating brain connectivity heritability in a twin study using diffusion imaging data. NeuroImage 2014; 100: 628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arat HE, Chouinard V-A, Cohen BM, Lewandowski KE, Öngür D. Diffusion tensor imaging in first degree relatives of schizophrenia and bipolar disorder patients. Schizophrenia Research 2015; 161: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HC, Sussmann JE, Chakirova G, Mukerjee P, Peel A, McKirdy J et al. The neural basis of familial risk and temperamental variation in individuals at high risk of bipolar disorder. Biological Psychiatry 2011; 70: 343–349. [DOI] [PubMed] [Google Scholar]
- Versace A, Ladouceur CD, Romero S, Birmaher B, Axelson DA, Kupfer DJ et al. Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry 2010; 49: 1249–1259, e1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E, Sussmann JE, Clugston A, Peel A, McKirdy J, Moorhead TWJ et al. White matter integrity in individuals at high genetic risk of bipolar disorder. Biological Psychiatry 2011; 70: 350–356. [DOI] [PubMed] [Google Scholar]
- Sprooten E, Brumbaugh MS, Knowles EE, McKay DR, Lewis J, Barrett J et al. Reduced white matter integrity in sibling pairs discordant for bipolar disorder. Am J Psychiatry 2013; 170: 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Kopecek M, Nicoletti M, Hatch J, Watanabe Y, Nery F et al. New structural brain imaging endophenotype in bipolar disorder. Molecular Psychiatry 2012; 17: 412–420. [DOI] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Ikuta T, Braga RJ, Gruner P, Malhotra AK et al. Abnormal temporal lobe white matter as a biomarker for genetic risk of bipolar disorder. Biological Psychiatry 2013; 73: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke J, King AV, Poupon C, Hennerici MG, Gass A, Wessa M. Impaired anatomical connectivity and related executive functions: differentiating vulnerability and disease marker in bipolar disorder. Biological Psychiatry 2013; 74: 908–916. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nature Reviews Neuroscience 2015; 16: 159–172. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. The Neuroscientist 2006; 12: 512–523. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. NeuroImage 2012; 62: 2296–2314. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. Journal of Neuroscience 2008; 28: 9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 2014; 137: 2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. The Journal of neuroscience 2011; 31: 15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow A, Ajilore O, Zhan L, Arienzo D, GadElkarim J, Zhang A et al. Impaired inter-hemispheric integration in bipolar disorder revealed with brain network analyses. Biological Psychiatry 2013; 73: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, van den Heuvel MP, Abramovic L, Vreeker A, de Reus MA, van Haren NE et al. Brain network analysis reveals affected connectome structure in bipolar disorder. Human Brain Mapping 2015; 37: 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde NJ, O'Donoghue S, Scanlon C, Emsell L, Chaddock C, Leemans A et al. Structural brain network analysis in families multiply affected with bipolar 1 disorder. Psychiatry Research: Neuroimaging 2015; 234: 44–51. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. Oxford University Press, New York, NY, USA, 2007. [Google Scholar]
- Perich T, Lau P, Hadzi-Pavlovic D, Roberts G, Frankland A, Wright A et al. What clinical features precede the onset of bipolar disorder? Journal of Psychiatric Research 2015; 62: 71–77. [DOI] [PubMed] [Google Scholar]
- Perry A, Wen W, Lord A, Thalamuthu A, Roberts G, Mitchell PB et al. The organisation of the elderly connectome. NeuroImage 2015; 114: 414–426. [DOI] [PubMed] [Google Scholar]
- Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proceedings of the 18th Annual Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM) 2010.
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002; 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Harding IH, Cocchi L, Yücel M, Pantelis C et al. Whole-brain anatomical networks: does the choice of nodes matter? NeuroImage 2010; 50: 970–983. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ et al. Mapping the structural core of human cerebral cortex. PloS Biology 2008; 6: e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. NeuroImage 2010; 53: 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Cocchi L, Fornito A, Murray MM, Bullmore E. Connectivity differences in brain networks. NeuroImage 2012; 60: 1055–1062. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends in Cognitive Sciences 2013; 17: 683–696. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995; 57: 289–300. [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage 2010; 52: 1059–1069. [DOI] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73: 13–22. [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat. Commun. 2014; 5: 4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. British Journal of Psychiatry 2009; 194: 527–534. [DOI] [PubMed] [Google Scholar]
- Kieseppä T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen V-P et al. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biological Psychiatry 2003; 54: 896–905. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TWJ, Harrison LK, Lawrie SM, Johnstone EC. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biological Psychiatry 2005; 58: 254–257. [DOI] [PubMed] [Google Scholar]
- Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry 2006; 163: 322–324. [DOI] [PubMed] [Google Scholar]
- Coffman JA, Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biological Psychiatry 1990; 27: 1188–1196. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Roversi F, Pallanti S, Baldini-Rossi N, Schnur DB, LiCalzi EM et al. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biological Psychiatry 2005; 57: 733–742. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. NeuroImage 2006; 30: 485–497. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Taylor WD, MacFall JR, Kuchibhatla M, Payne ME, Provenzale JM et al. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology 2005; 30: 2225–2229. [DOI] [PubMed] [Google Scholar]
- Bruno S, Cercignani M, Ron MA. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar disorders 2008; 10: 460–468. [DOI] [PubMed] [Google Scholar]
- Kafantaris V, Kingsley P, Ardekani B, Saito E, Lencz T, Lim K et al. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2009; 48: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papmeyer M, Giles S, Sussmann JE, Kielty S, Stewart T, Lawrie SM et al. Cortical thickness in individuals at high familial risk of mood disorders as they develop major depressive disorder. Biological Psychiatry 2015; 78: 58–66. [DOI] [PubMed] [Google Scholar]
- Breakspear M, Roberts G, Green MJ, Nguyen VT, Frankland A, Levy F et al. Network dysfunction of emotional and cognitive processes in those at genetic risk of bipolar disorder. Brain 2015; awv261. [DOI] [PubMed]
- Brotman MA, Deveney CM, Thomas LA, Hinton KE, Yi JY, Pine DS et al. Parametric modulation of neural activity during face emotion processing in unaffected youth at familial risk for bipolar disorder. Bipolar disorders 2014; 16: 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar disorders 2011; 13: 1–15. [DOI] [PubMed] [Google Scholar]
- Hajek T, Cullis J, Novak T, Kopecek M, Blagdon R, Propper L et al. Brain structural signature of familial predisposition for bipolar disorder: replicable evidence for involvement of the right inferior frontal gyrus. Biological Psychiatry 2013; 73: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G, Green MJ, Breakspear M, McCormack C, Frankland A, Wright A et al. Reduced inferior frontal gyrus activation during response inhibition to emotional stimuli in youth at high risk of bipolar disorder. Biological Psychiatry 2013; 74: 55–61. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Haldane M, Jogia J, Grasby PM, Collier D, Frangou S. Dissociable brain structural changes associated with predisposition, resilience, and disease expression in bipolar disorder. Journal of Neuroscience 2009; 29: 10863–10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepede G, De Berardis D, Campanella D, Perrucci MG, Ferretti A, Serroni N et al. Impaired sustained attention in euthymic bipolar disorder patients and non‐affected relatives: an fMRI study. Bipolar disorders 2012; 14: 764–779. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Goldstein JM, Milanovic SM, Whitfield‐Gabrieli S, Makris N, LaViolette P et al. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 2010; 153: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakakis G, Nickel J, Seitz RJ. Diversity of the inferior frontal gyrus—a meta-analysis of neuroimaging studies. Behavioural Brain Research 2011; 225: 341–347. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure & Function 2010; 214: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends in cognitive sciences 2013; 17: 565–573. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature reviews Neuroscience 2009; 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry 2008; 63: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function 2010; 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel U, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society of London Series B: Biological Sciences 1998; 265: 1927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci 2005; 9: 566–571. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clinical autonomic research: official journal of the Clinical Autonomic Research Society 1996; 6: 131–140. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience and biobehavioral reviews 2009; 33: 81–88. [DOI] [PubMed] [Google Scholar]
- Guo CC, Sturm VE, Zhou J, Gennatas ED, Trujillo AJ, Hua AY et al. Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proceedings of the National Academy of Sciences 2016; 113: E2430–E2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D, Roberts RE, Frangou S. Connectomic markers of disease expression, genetic risk and resilience in bipolar disorder. Transl Psychiatry 2016; 6: e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal DJ, Barnea-Goraly N, Kelley R, Bararpour L, Howe ME, Reiss AL et al. Widespread white matter tract aberrations in youth with familial risk for bipolar disorder. Psychiatry Research 2015; 232: 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei F, Dima D, Rubia K, Kumari V, Frangou S. Dissociable functional connectivity changes during the Stroop task relating to risk, resilience and disease expression in bipolar disorder. Neuroimage 2011; 57: 576–582. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch AF, Vértes PE, Stidd R, Lalonde F, Clasen L, Rapoport J et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cerebral Cortex 2012; 23: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature neuroscience 2004; 7: 278–285. [DOI] [PubMed] [Google Scholar]
- Neves MDCL, Albuquerque MR, Malloy-Diniz L, Nicolato R, Neves FS, de Souza-Duran FL et al. A voxel-based morphometry study of gray matter correlates of facial emotion recognition in bipolar disorder. Psychiatry Research: Neuroimaging 2015; 233: 158–164. [DOI] [PubMed] [Google Scholar]
- Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E et al. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Molecular Psychiatry 2009; 14: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohringer PA, Barroilhet SA, Amerio A, Reale ML, Alvear K, Vergne D et al. Cognitive impairment in bipolar disorder and schizophrenia: a systematic review. Frontiers in psychiatry 2013; 4: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.