Abstract
Inflammatory cytokines are commonly elevated in acute depression and are associated with resistance to monoaminergic treatment. To examine the potential role of cytokines in the pathogenesis and treatment of depression, we carried out a systematic review and meta-analysis of antidepressant activity of anti-cytokine treatment using clinical trials of chronic inflammatory conditions where depressive symptoms were measured as a secondary outcome. Systematic search of the PubMed, EMBASE, PsycINFO and Cochrane databases, search of reference lists and conference abstracts, followed by study selection process yielded 20 clinical trials. Random effect meta-analysis of seven randomised controlled trials (RCTs) involving 2370 participants showed a significant antidepressant effect of anti-cytokine treatment compared with placebo (standardised mean difference (SMD)=0.40, 95% confidence interval (CI), 0.22–0.59). Anti-tumour necrosis factor drugs were most commonly studied (five RCTs); SMD=0.33 (95% CI; 0.06–0.60). Separate meta-analyses of two RCTs of adjunctive treatment with anti-cytokine therapy and eight non-randomised and/or non-placebo studies yielded similar small-to-medium effect estimates favouring anti-cytokine therapy; SMD=0.19 (95% CI, 0.00–0.37) and 0.51 (95% CI, 0.34–0.67), respectively. Adalimumab, etanercept, infliximab and tocilizumab all showed statistically significant improvements in depressive symptoms. Meta-regression exploring predictors of response found that the antidepressant effect was associated with baseline symptom severity (P=0.018) but not with improvement in primary physical illness, sex, age or study duration. The findings indicate a potentially causal role for cytokines in depression and that cytokine modulators may be novel drugs for depression in chronically inflamed subjects. The field now requires RCTs of cytokine modulators using depression as the primary outcome in subjects with high inflammation who are free of other physical illnesses.
Introduction
The association between the immune system and the brain may offer new mechanistic understanding and insights for novel therapies for depression. Cytokine-mediated communication between the immune system and the brain has been implicated in the pathogenesis of depression.1, 2, 3 Major depression is common (one in four) after interferon treatment, a potent inducer of cytokines, in patients affected by hepatitis C virus.4 Experimental immuno-activation in healthy volunteers leads to depressive symptoms and reduced cognitive performance.5, 6 Meta-analyses of cross-sectional studies confirm elevated levels of circulating inflammatory cytokines in depressed patients,7, 8, 9, 10 and longitudinal studies have demonstrated that elevated serum cytokine levels precede, so potentially cause depressive symptoms.11, 12 A dose–response relationship between serum concentration of interleukin 6 (IL-6) in childhood at 9 years and subsequent depressive symptoms in early-adulthood at 18 years has been reported in the Avon Longitudinal Study of Parents and Children birth cohort.11 A longitudinal association between circulating inflammatory markers and subsequent depressive symptoms has also been reported from the Whitehall II cohort.12 Furthermore, activation of the inflammatory system is thought to underlie antidepressant resistance highlighting an involvement in treatment response.13, 14 Therefore, whether targeting inflammation, particularly inflammatory cytokines, could provide therapeutic benefit for patients with depression is a key question.
A meta-analysis of randomised controlled trials (RCTs)15 of non-steroidal anti-inflammatory drugs (NSAIDs), given as sole treatment or as adjunct to antidepressants, indicates that they may be more effective than placebo in treating depression (Cohen’s d=0.27; 95% confidence interval (CI), 0.08–0.45),16 although there are limitations of individual studies included such as attrition. Although these results point to an inflammatory component to depression, further examination of the evidence regarding cytokine-modulating drugs may help to elucidate the relevance of inflammation for pathogenesis and treatment of depression specifically. NSAIDs are broad-spectrum anti-inflammatory agents that also act on other targets, such as glucocorticoid receptors.17 These receptors are, themselves, relevant for depression pathophysiology,18 so the extent to which the antidepressant effect of NSAIDs is due solely to anti-inflammatory action is unclear. Some NSAIDs such as cyclooxygenase-2 inhibitors increase risk of cardiovascular disease,19 a known comorbidity for depression, so their use in depression may be problematic. Besides, many trials of anti-inflammatory drugs for depression are based on people with chronic physical illness16 but it is unclear whether the improvement in depression is due to the improvement in physical illness.
Cytokine modulators, which include monoclonal antibodies and cytokine inhibitors, constitute a more pure anti-inflammatory class because they target-specific cytokine pathways. Recently, a proof-of-concept RCT of infliximab, a tumour necrosis factor alpha (TNF-α) specific monoclonal antibody, has reported improvements in patients with treatment-resistant depression characterised by high inflammation at baseline.20 Supported by a large number of RCTs, cytokine modulators are well established treatments for chronic inflammatory conditions such as rheumatoid arthritis21 and psoriasis.22 Many of these clinical trials have also reported on secondary psychosocial outcome measures including depressive symptoms, which could be utilised to address important questions regarding potential usefulness of anti-cytokine treatment for depression. In addition to quantifying the antidepressant effect of anti-cytokine treatment, examination of the relationship between the improvement in depressive symptoms and that in physical symptoms could elucidate the role of inflammation in depression specifically. Previous RCTs indicate baseline severity of depression moderates the antidepressant effects of monoaminergic drugs23 and psychotherapy;24 it not clear whether this is also the case for anti-cytokine treatment.
We report a systematic review and meta-analysis of secondary data from clinical trials of anti-cytokine treatment in chronic inflammatory conditions to address the following key outstanding questions: (1) does blocking-specific inflammatory cytokine pathways lead to improvement in depressive symptoms; (2) what is the relationship between the antidepressant effect and the improvement in physical illness; (3) is the antidepressant effect related to baseline severity of depressive symptoms; (4) is there any sex or age difference of the antidepressant effect. Answers to these questions would provide important clues on whether inflammatory cytokines have a causal role in depression, and whether cytokine modulators may be useful for treating depression.
Materials and methods
Search strategy and study selection
A systematic search of the PubMed, Embase, PsycINFO and Cochrane databases was carried out for all clinical trials of cytokine modulators involving human subjects published in the English language until 5th April 2016, where depression or depressive symptoms were reported as an outcome. The search terms included indexing terms as well as wildcards to maximise return: ‘(monoclonal antibod* OR cytokine inhibitor OR tocilizumab OR infliximab OR adalimumab OR ustekinumab OR etanercept OR dupilumab) AND (depression OR depressive symptom)’. We also searched reference lists of retrieved articles and conference abstracts, and wrote to key authors in the field for unpublished data.
Clinical trials of specific cytokine inhibitors or monoclonal antibodies against specific inflammatory cytokines that measured depressive symptoms using a recognised tool were included. In order to obtain a comprehensive overview of the literature, we included all clinical trial designs, that is, RCTs, double-blind or open-label, as well as non-randomised studies (analysed separately). Studies that examined cytokine modulators as an adjunct to interferon therapy, used monoclonal antibodies as a method for detecting leukocyte subtypes, or examined monoclonal antibodies against targets other than an inflammatory cytokine (for example, vascular growth factor, adhesion molecule) were excluded.25 The literature search and study selection were carried out by NK and GMK; any differences were resolved by discussion.
Data extraction and statistical analysis
Data were extracted from published articles or obtained by directly contacting the authors if relevant data were not reported. When authors could not be reached but data were available in the paper in graph format (two RCTs),26, 27 values were extracted using software that has been shown to be a reliable method of data extraction for meta-analyses.28 RCTs were quality assessed using the CONSORT criteria;29 scores reflect the percentage of CONSORT items the study adhered to. We aligned depression scales so higher scores reflect higher symptom severity by multiplying scores from reversed scales by -1. For studies that included multiple intervention arms based on different dosages of the same drug, we pooled means and s.d.s of these groups to calculate a single outcome measure. Here, the pooled s.d. (sdpooled) equalled the square root of (sd1^2 × (n1−1)+sd2^2 × (n2−1))/((n1−1)+(n2−1)). Effect sizes were calculated as standardised mean difference (SMD) for continuous outcomes according to the formula 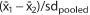 . For binary outcomes odds ratios were created according to the formula (Outcome1Group1 × Outcome2Group2)/(Outcome2Group1 × Outcome1Group2). Then odds ratios were transformed to SMD by dividing the natural logarithm of the odds ratios by 1.81.30
. For binary outcomes odds ratios were created according to the formula (Outcome1Group1 × Outcome2Group2)/(Outcome2Group1 × Outcome1Group2). Then odds ratios were transformed to SMD by dividing the natural logarithm of the odds ratios by 1.81.30
Based on study methodology, three types of studies were combined separately using random effect meta-analysis: RCTs of anti-cytokine drug vs placebo; RCTs of adjunctive treatment with anti-cytokine therapy (anti-cytokine drug plus active treatment vs active treatment); and other trials (non-randomised and/or non-placebo studies). For RCTs effect estimates were calculated as SMD using depression severity scores in treatment and placebo groups at the end of trial. SMD is an appropriate measure of effect estimate when studies assess the same variable (for example, depressive symptoms) but measure it in a variety of ways.31 In addition, we calculated SMD using change in depression severity score from baseline to end of trial in each group to examine the robustness of original results (sensitivity analysis). Sensitivity analyses were also carried out after excluding specific RCTs from meta-analysis. For other trials, SMD was calculated as difference in depression severity score from baseline to end of trial, as no control group was present. All studies were weighted using an inverse-variance method so studies with larger samples were given higher weight. When possible, pooled effect estimates were calculated for specific drugs. Heterogeneity between studies was investigated by calculating the Cochrane’s heterogeneity statistic Q and the I2 statistic that represents the fraction of variation between studies attributable to heterogeneity.32 Publication bias was assessed for each group of studies by visual inspection of funnel plots including Egger’s test,33 and the trim and fill method.34 We analysed the RCTs of adjunctive treatment with anti-cytokine therapy separately because these studies compared combination of anti-cytokine drug and a disease-modifying anti-rheumatic drug (DMARD) with DMARD alone. One RCT35 administered adalimumab to all participants for 4 weeks before randomising them to placebo or adalimumab for subsequent 52 weeks, so we also included data from the first 4 weeks in the meta-analysis of ‘other trials’. Data from two studies36, 37 that randomised individuals to different dosage regimens of the same drug but lacked a placebo group, and a non-randomised study38 were also included in this category.
All secondary analyses were based on placebo-controlled RCTs only as they represent the highest quality of scientific evidence of efficacy. Meta-regressions were performed to examine the relationship between the effect estimates for depression and (1) the effect estimates for primary physical outcome; (2) baseline severity of depressive symptoms; (3) sex (percentage of male participants in each sample); and (4) mean age of sample. We quantified baseline depression symptom severity in a standardised manner rather than simply using mean scores because the studies used different scales to measure depression. We identified general population distributions for each scale39, 40, 41, 42, 43 that were used to calculate effect sizes (SMD) of baseline symptom severity for the sample compared with the general population. All analyses were performed in R using the metaphor package for meta-analysis and meta-regression;44 analysis codes are available on request.
Results
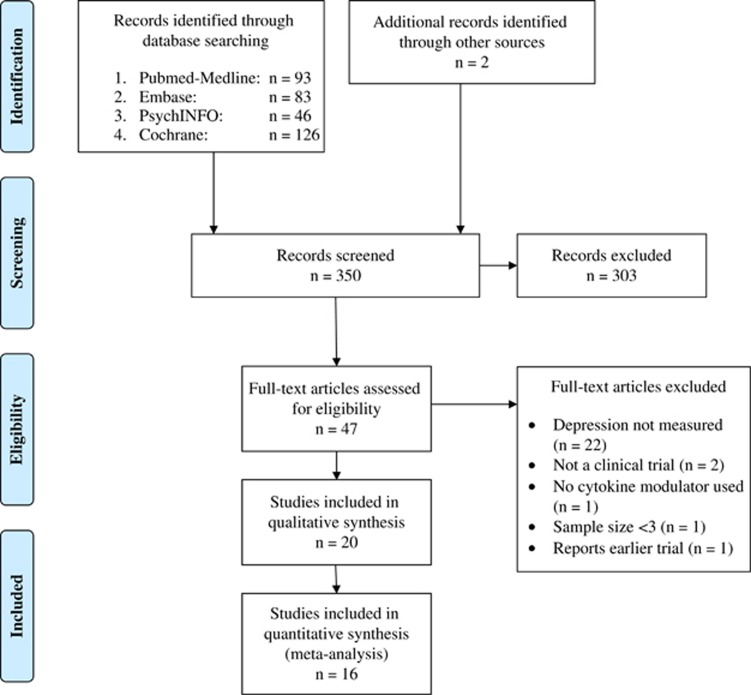
The literature search retrieved 350 potentially relevant articles of which 20 studies were finally included in the review (see Figure 1 for a PRISMA diagram of literature search). Of the included studies, seven were RCTs of anti-cytokine drug vs placebo, three were RCTs of adjunctive treatment with anti-cytokine therapy, and 10 were other study designs such as non-randomised and/or non-placebo studies (Table 1).
Figure 1.
PRISMA Flow diagram of study selection for systematic review.
Table 1. Clinical trials included in the systematic review of antidepressant activity of anti-cytokine treatment.
| Study | Design | Drug (n) | Placebo/comparison group (n) | Main outcome | Drug target | Dosage | Study duration | Assessment of depressive symptoms |
Change in mean depression score from baselinea |
CONSORT score (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Placebo/comparison group | ||||||||||
| RCTs of anti-cytokine drug vs placebo | |||||||||||
| Tyring et al. (2006)27 | Double-blind RCT | Etanercept (305) | Placebo (292) | Psoriasis | TNF-α | 50 mg twice weekly | 12 weeks | BDI | −3.9 | −2.1 | 94% |
| Loftus et al. (2008)35 (weeks 4–56) | Double-blind RCT | Adalimumab (324) | Placebo (168) | Crohn’s Disease | TNF-α | 40 mg weekly or every other week | 52 weeks | ZDS | −1.1 | +1.8 | 53% |
| Langley et al. (2010)47 | Double-blind RCT | Ustekinumab (800) | Placebo (398) | Psoriasis | IL-12, 23 | 45/90 mg at weeks 0,4; then every 12 weeks | 12 weeks | HADS-D | −1.9 | +0.2 | 52% |
| Menter et al. (2010)46 | Double-blind RCT | Adalimumab (44) | Placebo (52) | Psoriasis | TNF-α | 40 mg every other week | 12 weeks | ZDS | −6.7 | −1.6 | 56% |
| Tyring et al. (2013)26 | Double-blind RCT | Etanercept (59) | Placebo (62) | Psoriasis | TNF-α | 50 mg twice weekly | 12 weeks | PROMIS depression score | −5.5 | −2.7 | b |
| Raison et al. (2013)20 | Double-blind RCT | Infliximab (27) | Placebo (28) | Treatment-resistant depression | TNF-α | 5 mg kg−1 at weeks 0, 2 and 6 | 12 weeks | HAM-D | −7.5 | −9.6 | 91% |
| Simpson et al. (2015)45 | Double-blind RCT | Dupilumab (318) | Placebo (61) | Atopic dermatitis | IL-4 R-α | Variable (100 to 600 mgs every 1–4 weeks) | 16 weeks | HADS | −4.2 | +0.1 | b |
| RCTs of anti-cytokine drugs as adjuncts to an active treatment | |||||||||||
| Kekow et al. (2010)50 | Double-blind RCT | Etanercept+DMARD (265) | DMARD (263) | Rheumatoid arthritis | TNF-α | 50 mg weekly | 52 weeks | HADS-D | −2.4 | −2.0 | 48% |
| Bae et al. (2013)48 | Randomised open-label | Etanercept+DMARD (192) | DMARD (100) | Rheumatoid arthritis | TNF-α | 25 mg twice weekly | 16 weeks | HADS-D | −2.2 | −1.3 | 63% |
| Machado et al. (2014)49 | Randomised open-label | Etanercept+DMARD (279) | DMARD (142) | Rheumatoid arthritis | TNF-α | 50 weekly | 24 weeks | HADS-D | −2.8 | −1.9 | 77% |
| Non-randomised and/or non-placebo trials | |||||||||||
| Minderhoud et al. (2007)38 | Single-blind | Infliximab (14) | — | Crohn’s Disease | TNF-α | 5 mg kg−1 at baseline | 4 weeks | CES-D | −5.7 | — | — |
| Loftus et al. (2008)35 (weeks 0–4) | Open-label | Adalimumab (499) | — | — | TNF-α | 80 mg at baseline; 40 mg at week 2 | 4 weeks | ZDS | −9.1 | — | — |
| Gelfand et al. (2008)56 | Open-label | Etanercept (2546) | — | — | TNF-α | 50 mg twice weekly | 12 weeks | BDI | Not reported | — | — |
| Dauden et al. (2009)37 | Randomised (to dosage regimen), open-label | Etanercept (703) | — | — | TNF-α | 25 or 50 mg twice weekly | 54 weeks | HADS-D | −1.6 | — | — |
| Guh et al. (2010)51 | Open-label | Adalimumab (174) | — | — | TNF-α | 80 mg at baseline; 40 mg every other week | 24 weeks | BDI | −4.4 | — | — |
| Ertenli et al. (2012)53 | Open-label | Infliximab (16) | — | — | TNF-α | 5 mg kg−1 at weeks 0, 2 and 6 | 6 weeks | HADS-D | −3.0 | — | — |
| Gniadecki et al. (2012)36 | Randomised (to dosage regimen) double-blind | Etanercept (752) | — | — | TNF-α | 50 mg weekly; 50 mg twice weekly | 12 weeks | HADS-D | −1.5 | — | — |
| Bhutani et al. (2013)52 | Open-label | Adalimumab (32) | — | — | TNF-α | 80–40 mg every other week | 24 weeks | PGWB depression | −1.9 | — | — |
| Eisenberg et al. (2013)57 | Open-label | Eisenberg (9) | — | Complex regional pain syndrome type 1 | TNF-α | 40 mg twice every other week | 4 weeks | BDI | Not reported | — | — |
| Traki et al. (2013)54 | Open-label | Tocilizumab (26) | — | Rheumatoid arthritis | IL-6 R | 8 mg kg−1 monthly | 26 weeks | HADS-D | −1.0 | — | — |
| Gossec et al. (2015)55 | Open-label | Tocilizumab (610) | — | Rheumatoid arthritis | IL-6 R | – | – | HADS-D | −1.3 | — | — |
Abbreviations: BDI, Beck’s Depression Inventory; CES-D, Centre for Epidemiological Studies Depression Scale; DMARD, disease-modifying anti-rheumatic drug; HADS(-D), Hospital Anxiety and Depression Scale (Depression); HAM-D, Hamilton Depression Rating Scale; IL, interleukin; PGWB depression, Psychological General Well-Being depression; RCT, randomised controlled trial; TNF-α, tumor necrosis factor alpha; ZDS, Zung Depression Inventory.
(−)ve=decrease in depression score from baseline, (+)ve=increase in depression score from baseline.
Could not assess fully because articles published as a letter or as a conference abstract (data from Tyring et al. was extracted using software and from Simpson et al. by contacting authors directly).
Meta-analysis of RCTs of anti-cytokine drug vs placebo
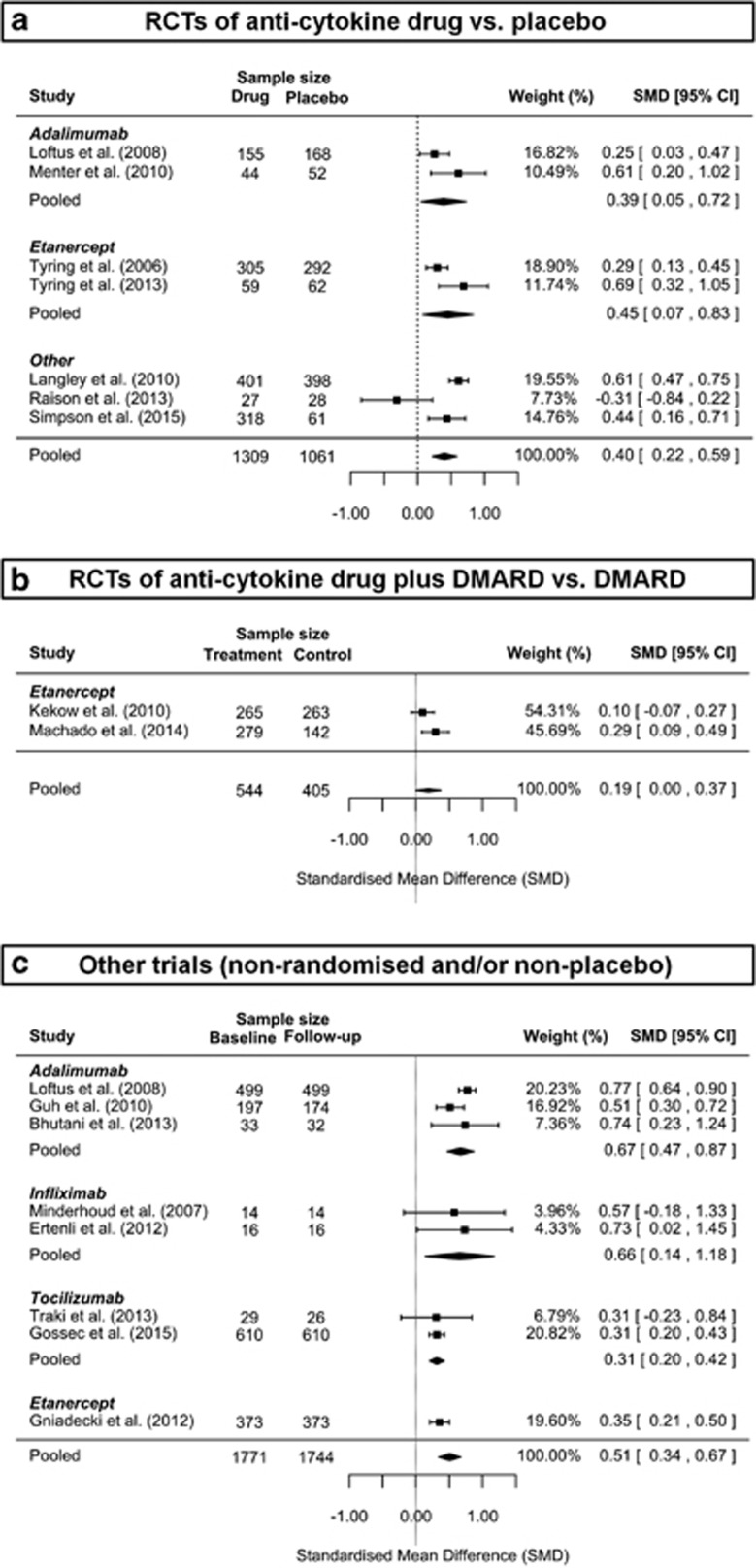
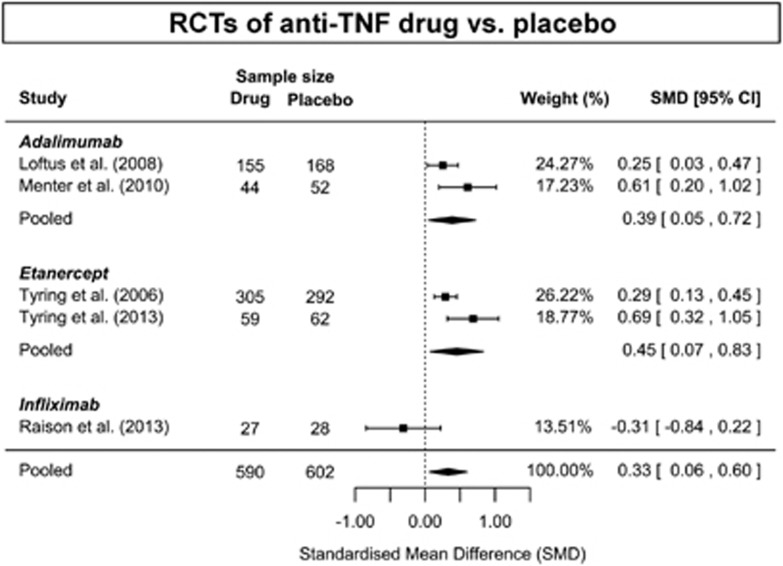
Meta-analysis of seven randomised, double-blind, placebo-controlled trials,20, 26, 27, 35, 45, 46, 47 involving 1309 subjects treated with anti-cytokine drugs and 1061 subjects treated with placebo, showed significant improvement in depressive symptoms with anti-cytokine treatment compared to placebo; SMD=0.40 (95% CI, 0.22–0.59) (Figure 2a). There was evidence of significant heterogeneity among studies (P<0.001; I2=73%). Anti-TNF drugs were most commonly studied (five RCTs),20, 26, 27, 35, 46 which as a group showed significant antidepressant effect; SMD=0.33; 95% CI, 0.06–0.60 (Figure 3). There was also evidence of significant heterogeneity among these studies (P=0.02; I2=75%). Regarding specific drugs, based on two trials each, the effect estimate for adalimumab35, 46 (SMD=0.39; 95% CI, 0.05–0.72) and that for etanercept26, 27 (SMD=0.45; 95% CI, 0.07–0.83) were similar (Figure 2a). There was no evidence of significant heterogeneity between the adalimumab trials (P=0.134; I2=55%) but there was evidence of heterogeneity among the etanercept trials (P=0.05, I2=73%).
Figure 2.
Meta-analysis of antidepressant activity of anti-cytokine treatment. (a) Meta-analysis of RCTs of anti-cytokine drug vs. placebo. (b) Meta-analysis of RCTs of anti-cytokine drug plus DMARD vs. DMARD. (c) Meta-analysis of other trials (non-randomised and/or non-placebo). CI, confidence interval; DMARD, Disease Modifying Anti-rheumatoid Drug; RCT, randomised controlled trial.
Figure 3.
Antidepressant activity of anti-TNF treatment: meta-analysis of RCTs. CI, confidence interval; RCT, randomised controlled trial; TNF, tumor necrosis factor.
Sensitivity analysis of seven RCTs,20, 26, 27, 35, 45, 46, 47 of anti-cytokine drugs vs placebo based on change in depression severity scores from baseline to end of trial yielded very similar results to that of the original analysis; SMD=0.37 (95% CI; 0.18–0.57) (see online Supplementary Figure 1). However, evidence for heterogeneity remained (P<0.001; I2=76%).
Meta-analysis of RCTs of adjunctive treatment with anti-cytokine therapy
Of three trials48, 49, 50 that used anti-cytokine drugs as adjunctive treatment, two provided enough data for meta-analysis (Figure 2b).49, 50 Both of these studies compared combination of etanercept and a DMARD (n=544) with DMARD alone (n=405). Meta-analysis showed a small effect of etanercept plus DMARD on depressive symptoms compared with DMARD alone (SMD=0.19; 95% CI, 0.00–0.37). There was no evidence of heterogeneity between studies (P=0.158; I2=49%).
Meta-analysis of other trials
Out of 10 non-randomised and/or non-placebo studies,36, 37, 38, 51, 52, 53, 54, 55, 56, 57 eight provided enough data for meta-analysis involving 1744 patients with data at baseline and follow-up (Figure 2c).35, 36, 38, 51, 52, 53, 54, 55 Meta-analysis of these studies suggested significant improvement in depressive symptoms following anti-cytokine treatment (SMD=0.51; 95% CI, 0.34–0.67). There was evidence of significant heterogeneity among these studies (P<0.001; I2=73%). Anti-TNF drugs were most commonly studied (six studies), which as a group showed significant antidepressant effect (SMD=0.58; 95% CI, 0.39–0.77) (see online Supplementary Figure 2). However, there was evidence for significant heterogeneity among these studies (P=0.002; I2=67%). Regarding specific drugs, adalimumab, infliximab and tocilizumab all showed statistically significant improvements in depressive symptoms. Meta-analytic effect estimates for adalimumab: SMD=0.67 (95% CI, 0.47–0.87); infliximab: SMD=0.66 (95% CI, 0.14–1.18); and tocilizumab: SMD=0.31 (95% CI, 0.20–0.42). There was no evidence of significant heterogeneity in any of these meta-analyses (all P>0.05).
Association with improvement in physical illness
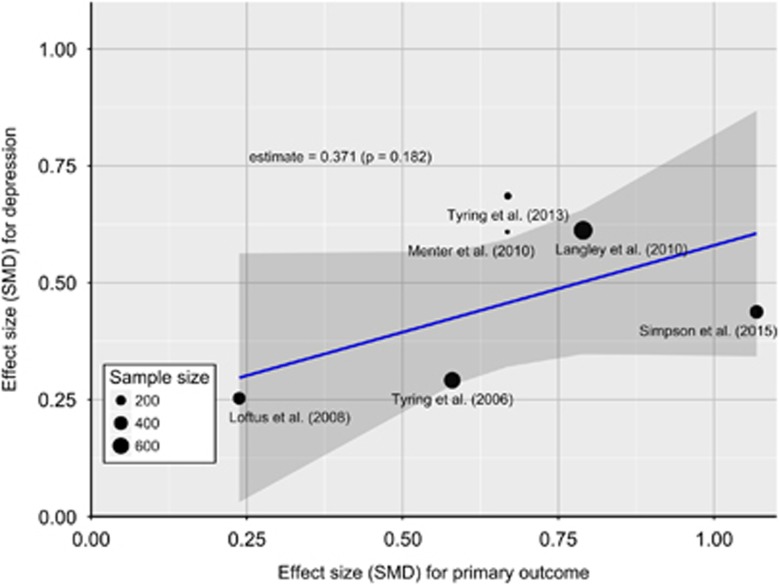
Meta-regression of six RCTs26, 27, 35, 45, 46, 47 found no evidence for an association between improvement in depressive symptoms and that in primary physical illness outcome measure at the end of trial (slope=0.37; SE=0.28; P=0.182) (Figure 4). The study by Raison et al.20 was excluded from this analysis because in this trial depression was the primary outcome so no physical illness was studied.
Figure 4.
Meta-regression of the association between antidepressant effects and improvements in physical illness. P-value for meta-regression slope indicates no statistically significant association between antidepressant effect of anti-cytokine treatment and improvement in physical illness. The Figure shows that the antidepressant effect does not change significantly (that is, increase or decrease) across the range of effect estimates for physical illness. SMD, standardised mean difference.
Association with depression symptom severity at baseline
Meta-regression of seven RCTs20, 26, 27, 35, 45, 46, 47 showed an association between antidepressant effect of anti-cytokine treatment and baseline severity of depressive symptoms (slope=−0.12; SE=0.05; P=0.018). However, the association became non-significant after excluding the RCT by Raison et al.20 (slope=−0.05; SE=0.12; P=0.662). We explored the association by excluding the RCT by Raison et al.20 because baseline depression severity was much higher in this study (SMD of baseline depression 6.36) compared with the other RCTs (0.08–1.91).
Association with sex and age
There was no strong evidence for a sex difference in the antidepressant effect of anti-cytokine treatment as the association between improvements in depressive symptoms and the percentage of male subjects in the sample, based on meta-regression of seven RCTs,20, 26, 27, 35, 45, 46, 47 did not reach significance (slope=1.29; SE=0.66; P=0.051). Re-analysis after excluding the study by Raison et al.,20 an outlier in which only 33% of subjects were male, attenuated the association further (slope=0.66; SE=0.71; P=0.355). Similarly, there was no association between SMD for depressive symptoms and mean age of sample (slope=0.008; SE=0.028; P=0.788).
Association with study duration
Duration of follow-up in the RCTs of anti-cytokine drug vs placebo was similar (12–16 weeks) except the study by Loftus et al. (Table 1). Meta-regression of seven RCTs20, 26, 27, 35, 45, 46, 47 showed no association between the antidepressant effect of anti-cytokine treatment and study duration (slope=−0.004; SE=0.007; P=0.52).
Sensitivity analyses
Sensitivity analysis of RCTs20, 35, 45, 46, 47 excluding two studies by Tyring et al.26, 27 from which data were extracted using software did not change results substantially; SMD=0.37 (95% CI; 0.11–0.63) (see online Supplementary Figure 3). However, evidence for significant heterogeneity remained (P=0.003; I2=79%). Unlike the other RCTs, the trial by Raison et al.20 was based on cases of treatment-resistant depression without any significant physical comorbidity. Meta-analysis of RCTs26, 27, 35, 45, 46, 47 excluding this trial yielded a slightly larger effect estimate; SMD=0.46 (95% CI; 0.30–0.61) (see online Supplementary Figure 4). However, there was evidence for significant heterogeneity among these studies (P=0.016; I2=61%). The RCT by Loftus et al.35 was an outlier in terms of follow-up length (see Table 1). Meta-analysis excluding this RCT20, 26, 27, 45, 46, 47 did not alter results greatly; SMD=0.43 (95% CI; 0.21–0.65) (see online Supplementary Figure 5). However, evidence for significant heterogeneity remained (P=0.002; I2=76%).
Assessment of publication bias
Visual inspection of funnel plot of effect estimates from seven RCTs suggested that there was no evidence of publication bias (see online Supplementary Figure 6), which was in line with a non-significant Egger’s test for funnel plot asymmetry (P>0.05). Similarly, trim and fill analyses did not show any evidence of funnel plot asymmetry for RCTs. There were too few studies to allow assessment of publication bias among RCTs of adjunctive treatment with anti-cytokine therapy. For the other trials (non-randomised, non-placebo) Egger’s test for funnel plot asymmetry (P>0.05) and visual inspection of the funnel plot did not suggest any evidence for publication bias (see online Supplementary Figure 7). However, the trim and fill method indicated presence of funnel plot asymmetry, and that additional four studies would be needed to reach symmetry leading to slight attenuation of effect size (trim and fill SMD=0.36; 95% CI, 0.18–0.55). It is known that the trim and fill method can underestimate effect estimates when there is significant heterogeneity among studies;58 indeed, after restricting the analysis to anti-TNF studies heterogeneity decreased and there was no longer any evidence of funnel plot asymmetry (P=0.50).
Discussion
Findings from this large systematic review of 20 studies including meta-analyses of 16 studies totalling 5063 participants indicate that anti-cytokine treatment improves depressive symptoms. We observed significant results favouring cytokine modulators over respective control groups with effect estimates of 0.40 for RCTs of anti-cytokine treatment vs placebo, 0.19 for RCTs of adjunctive treatment with anti-cytokine therapy, and 0.51 for other (non-randomised and/or non-placebo) studies. Regarding predictors of response, additional analyses based on RCTs indicated that the antidepressant effect was associated with severity of depressive symptoms at baseline, but not with improvement in physical illness (primary outcome under investigation in all but one RCT), sex and age of participants, or study duration. Sensitivity analyses using a different method to calculate SMD or exclusion of specific RCTs did not alter results substantially suggesting that the findings are robust. The results provide important clues regarding the role of inflammatory cytokines in depression and the potential for cytokine modulators as treatments for depression. Anti-cytokine drugs seem to offer treatment effects in the range of small-to-moderate effect sizes, which is comparable to estimates observed for common antidepressants.23 The results are in line with a previous meta-analysis of NSAIDs, which included three RCTs of cytokine inhibitors.16 The results are also consistent with a recent meta-analysis of anti-TNF treatment in people with chronic physical illness that reported improvements in depression and anxiety symptoms.59 However, based on a meta-analysis of seven RCTs the current study provides a robust, statistically significant effect estimate favouring anti-cytokine treatment for depression. In addition to the randomised, double-blind, placebo-controlled clinical trials (gold standard), we have examined RCTs of adjunctive treatment with anti-cytokine therapy, and non-randomised studies offering a comprehensive update of the literature.
Studies included in this review except one by Raison et al.20 report continuous measures of depression, that is, symptom severity scores, not a categorical diagnosis of depression. To calculate effect size we have used depression severity scores for treatment and placebo groups at the end of trial for all RCTs including one by Raison et al. We also used change in depression scores from baseline to end of trial. The lack of an association between the improvement in depressive symptoms and that in primary physical illness points to a causal role for inflammatory cytokines in depression, suggesting that the mood improvement is not simply an artefact of feeling physically better after anti-cytokine treatment. Examination of the time course of effect in individual studies may provide some clues regarding the underlying mechanism of antidepressant effect of anti-cytokine treatment. The RCT by Tyring et al.27 reported scores for fatigue and depression during the course of 12-week treatment with etanercept vs placebo for psoriasis. Compared with placebo, etanercept led to improvements in fatigue by 2 weeks into the study and in depressive symptoms by week 4. In this study improvement in depressive symptoms was associated with improvement in fatigue but not (strongly) with improvement in psoriasis, which is in line with our meta-regression finding. Neurovegetative symptoms such as fatigue, which develop rapidly following immune activation in humans are attributed to actions of inflammatory cytokines on the brain.60, 61 Therefore, it is possible that the antidepressant effect of anti-cytokine treatment is mediated by improvements in cytokine-induced neurovegetative symptoms. This hypothesis needs testing in future studies.
In future, RCTs of anti-cytokine treatment using depression as the primary outcome in subjects with high inflammation who are free of other physical illnesses are needed. Although further studies are currently underway (for example, NCT02363738 and NCT02473289), results from the RCT by Raison et al.20 included in this meta-analysis showed beneficial effects of infliximab for treatment-resistant depression cases only in those with elevated serum CRP levels at baseline. Treatment-resistant depression cases with elevated inflammatory marker levels may be ideal candidates for RCTs of cytokine modulators for a number of reasons. About a third of all depressed patients are antidepressant resistant62 and about a third of all depressed patients have elevated serum CRP (>3 mg l−1).63 This may not be a coincidence. Indeed, activation of the inflammatory system as reflected by elevated serum inflammatory marker concentrations predicts poor antidepressant response,13, 14 and treatment-resistant patients continue to show elevated cytokine levels.64, 65 Inflammation and consequent activation of the tryptophan-metabolising enzyme indoleamine 2,3-dioxygenase is thought to underlie persistent symptoms despite antidepressant therapy in depressed patients who are ‘inflamed’.66 Inflammatory cytokines such as interferon-γ, TNF-α and IL-6 can induce indoleamine 2,3-dioxygenase.67, 68 In mice, blocking TNF-α with etanercept67 or IL-6 with a monoclonal antibody69 have been reported to prevent depression-like behaviour following exposures to an inflammatory stimulus or stress. Therefore, cytokine-modulating therapy is likely to be beneficial for a subset of depressed patients, specifically those with evidence of inflammation.
Regarding specific types of studies, meta-analysis of two RCTs of anti-cytokine drugs as adjuncts to DMARD treatment showed a smaller effect size than that of the placebo-controlled randomised trials, which might be due to potential antidepressant effects of drugs used as comparison treatment. On the other hand, the pooled effect size for the non-randomised and/or non-placebo studies was larger than that for RCTs probably because of the lack of a comparison group to account for placebo effect. The association between baseline severity of depressive symptoms and response to anti-cytokine treatment was markedly reduced after excluding an outlier although an association between initial severity and antidepressant response is well known.23 This is possibly because depression severity was relatively low in all of the RCTs except the one by Raison et al.,20 which included cases of treatment-resistant depression. It is well known that baseline symptom severity is positively correlated with antidepressant treatment effect, so demonstration of a beneficial effect of anti-cytokine treatment in samples with relatively low depression scores indicates strong antidepressant properties of these drugs. There was no evidence for an association between antidepressant effect of cytokine modulators and sex or age of participants. Similar to depression, autoimmune chronic inflammatory conditions such as rheumatoid arthritis are more common in women than men (3:1),70 but sex does not influence response to anti-TNF therapy in patients with established rheumatoid arthritis,71 which is in line with our findings. A possible explanation for the lack of an association with age could be limited variability in age among the included studies (<10 years).
Strengths of the systematic review presented here include a relatively large number of studies including RCTs, which were quality assessed. The literature search was comprehensive, as supported by a lack of evidence for publication bias for most analyses. Inclusion of different trial methodologies allowed meta-analyses of two types of RCTs and non-randomised studies. The focus on a specific type of anti-inflammatory drug (that is, cytokine modulators) helped to demonstrate the relevance of inflammation, particularly inflammatory cytokines, for the pathogenesis and treatment of depression. A potential limitation is the use of data extracted from published graphs due to lack of response from authors of two studies.26, 27 However, the software used to extract data has been reported to be a reliable method for data extraction for meta-analysis,28 so any variation from original data would be too small to have any meaningful impact on the pooled effect estimate. Because a physical illness was the primary outcome in all but one studies included in this review, we were not able to comment on potential side-effects of cytokine-modulating treatment in depressed individuals specifically. Administration of monoclonal antibodies carries the risk of immune reactions such as acute anaphylaxis as well as various target-specific adverse effects including increased risk of infections, cancer and autoimmune disease.72 Infliximab and placebo groups in the study by Raison et al.20 were similar in terms of adverse events (except increased urinary leukocyte esterase in the placebo group) and no serious side-effects were observed in either group. Nevertheless, further studies regarding safety and tolerability of cytokine modulators in depressed individuals are needed. There was evidence for significant heterogeneity in the meta-analysis of RCTs and that of other trials. Heterogeneity is likely to be driven by heterogeneity of effect size because all studies included in these analyses except one showed effect in the same direction, that is, anti-cytokine treatment improves depressive symptoms (see Figures 2a and c). Heterogeneity was reduced when studies were grouped by specific monoclonal antibodies. For example, there was no evidence of heterogeneity among studies of adalimumab, infliximab and tocilizumab. However, sensitivity analyses after excluding specific RCTs did not reduce heterogeneity significantly.
In conclusion, this systematic review and meta-analysis provides an up-to-date summary of the existing literature on antidepressant effect of anti-cytokine treatment. The findings show robust improvements in depressive symptoms after anti-cytokine therapy (monoclonal antibody or cytokine inhibitor) with a small-to-moderate size effect. These results suggest inflammatory cytokines may have a key role in the pathogenesis of depression and that anti-cytokine drugs may be effective for some patients with depression, particularly treatment-resistant cases characterised by increased inflammation. The field now needs RCTs of anti-cytokine treatment using such patients, which would pave the way for novel, effective and personalised treatment for depression and could reduce the burden presented by such a serious and multifactorial illness.
Acknowledgments
GMK is supported by an Intermediate Clinical Fellowship from the Wellcome Trust (201486/Z/16/Z), a Clinical Lecturer Starter Grant from the Academy of Medical Sciences, UK (grant no. 80354) and a Gosling Fellowship from the Royal College of Psychiatrists, UK (2015). PBJ acknowledges grant support from the Wellcome Trust (095844/Z/11/Z and 088869/Z/09/Z) and NIHR (RP-PG-0606-1335, Cambridge Biomedical Research Centre and CLAHRC East of England). RD has received grants from the National Institute of Neurological Diseases and Stroke of the National Institutes of Health (grants R01 NS073939; R01 NS074999). We would like to thank the following individuals for supplying additional data for meta-analysis: Dr Daphne Guh, Centre for Health Evaluation and Outcome Sciences, Canada; Dr Sara Berkö, BiogenIdec Sweden AB, Sweden; Dr Anders Svenningsson, Umeå University and University Hospital of Northern Sweden, Sweden; Dr Eric Simpson, University of Rochester Medical Centre, USA; Dr Catherine Au, Regeneron Pharmaceuticals, USA; Professor Laure Gossec, Pierre and Marie Curie University, France; Dr Tina Bhutani, University of California San Francisco, CA, USA. We would also like to thank Dr Valter Silva, Federal University of São Paulo, Brazil for his correspondence regarding data extraction methodology, and Dr Jan Stochl, University of Cambridge, UK for helpful comments on an earlier version of this manuscript.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors have no competing financial interests in relation to the work described. PBJ received an honorarium that he donated to his department, from Roche (UK) for taking part in an advisory board to advise on education about schizophrenia for psychiatrists. RD received consulting fee and honorarium from Ironwood Pharma (USA) and Pfizer (France).
Supplementary Material
References
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuro-Psychopharmacol Biol Psychiatry 1995; 19: 11–38. [DOI] [PubMed] [Google Scholar]
- Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, Forns X et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry 2012; 73: 1128–1138. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001; 58: 445–452. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009; 66: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 2016; doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immunity 2015; 49: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 2014; 71: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med 2009; 39: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Torre JP, Papadopoulos AS, Poon L, Juruena MF, Markopoulou K et al. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord 2013; 148: 136–140. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Ueda N, Nakamura J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog Neuro-Psychopharmacol Biol Psychiatry 2009; 33: 722–726. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 2006; 11: 680–684. [DOI] [PubMed] [Google Scholar]
- Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014; 71: 1381–1391. [DOI] [PubMed] [Google Scholar]
- Hu F, Wang X, Pace TW, Wu H, Miller AH. Inhibition of COX-2 by celecoxib enhances glucocorticoid receptor function. Mol Psychiatry 2005; 10: 426–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 2001; 49: 391–404. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001; 286: 954–959. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaltonen KJ, Virkki LM, Malmivaara A, Konttinen YT, Nordstrom DC, Blom M. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PloS One 2012; 7: e30275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. Br J Dermatol 2012; 166: 179–188. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S et al. Therapist-delivered Internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009; 374: 628–634. [DOI] [PubMed] [Google Scholar]
- Svenningsson A, Falk E, Celius EG, Fuchs S, Schreiber K, Berko S et al. Natalizumab treatment reduces fatigue in multiple sclerosis. Results from the TYNERGY trial; a study in the real life setting. PloS One 2013; 8: e58643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Bagel J, Lynde C, Klekotka P, Thompson EH, Gandra SR et al. Patient-reported outcomes in moderate-to-severe plaque psoriasis with scalp involvement: results from a randomized, double-blind, placebo-controlled study of etanercept. J Eur Acad Dermatol Venereol 2013; 27: 125–128. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006; 367: 29–35. [DOI] [PubMed] [Google Scholar]
- Jelicic Kadic A, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J Clin Epidemiol 2016; 74: 119–123. [DOI] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 2000; 19: 3127–3131. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith DG, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ: London, UK, 2001. [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455–463. [DOI] [PubMed] [Google Scholar]
- Loftus EV, Feagan BG, Colombel JF, Rubin DT, Wu EQ, Yu AP et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn's disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol 2008; 103: 3132–3141. [DOI] [PubMed] [Google Scholar]
- Gniadecki R, Robertson D, Molta CT, Freundlich B, Pedersen R, Li W et al. Self-reported health outcomes in patients with psoriasis and psoriatic arthritis randomized to two etanercept regimens. J Eur Acad Dermatol Venereol 2012; 26: 1436–1443. [DOI] [PubMed] [Google Scholar]
- Dauden E, Griffiths CE, Ortonne JP, Kragballe K, Molta CT, Robertson D et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol 2009; 23: 1374–1382. [DOI] [PubMed] [Google Scholar]
- Minderhoud IM, Samsom M, Oldenburg B. Crohn's disease, fatigue, and infliximab: is there a role for cytokines in the pathogenesis of fatigue? World J Gastroenterol 2007; 13: 2089–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz A, Brahler E. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J Psychosom Res 2011; 71: 74–78. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Posternak M. A review of studies of the Hamilton depression rating scale in healthy controls: implications for the definition of remission in treatment studies of depression. J Nerv Ment Dis 2004; 192: 595–601. [DOI] [PubMed] [Google Scholar]
- Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the State—Trait Anxiety Inventory and the Zung Self-Rating Depression scale. Br J Clin Psychol 1983; 22: 245–249. [DOI] [PubMed] [Google Scholar]
- Jylha P, Isometsa E. Temperament, character and symptoms of anxiety and depression in the general population. Eur Psychiatry 2006; 21: 389–395. [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010; 63: 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analysis in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- Simpson E, Worm M, Soong W, Blauvelt A, Eckert L, Wu R et al. Dupilumab improves patient-reported outcomes (PROs) in a phase 2 study in adults with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2015; 135: AB167. [Google Scholar]
- Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol 2010; 62: 812–818. [DOI] [PubMed] [Google Scholar]
- Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu MC et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol 2010; 63: 457–465. [DOI] [PubMed] [Google Scholar]
- Bae SC, Gun SC, Mok CC, Khandker R, Nab HW, Koenig AS et al. Improved health outcomes with etanercept versus usual DMARD therapy in an Asian population with established rheumatoid arthritis. BMC Musculoskelet Disord 2013; 14: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado DA, Guzman RM, Xavier RM, Simon JA, Mele L, Pedersen R et al. Open-label observation of addition of etanercept versus a conventional disease-modifying antirheumatic drug in subjects with active rheumatoid arthritis despite methotrexate therapy in the Latin American region. J Clin Rheumatol 2014; 20: 25–33. [DOI] [PubMed] [Google Scholar]
- Kekow J, Moots RJ, Emery P, Durez P, Koenig A, Singh A et al. Patient-reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann Rheum Dis 2010; 69: 222–225. [DOI] [PubMed] [Google Scholar]
- Guh D, Papp K, Lynde C, Bansback N, Zhang W, Qian H et al. Impact of adalimumab on quality of life and depression in psoriasis patients: results from PRIDE. Value Health 2010; 132010: A148. [Google Scholar]
- Bhutani T, Patel T, Koo B, Nguyen T, Hong J, Koo J. A prospective, interventional assessment of psoriasis quality of life using a nonskin-specific validated instrument that allows comparison with other major medical conditions. J Am Acad Dermatol 2013; 69: e79–e88. [DOI] [PubMed] [Google Scholar]
- Ertenli I, Ozer S, Kiraz S, Apras SB, Akdogan A, Karadag O et al. Infliximab, a TNF-alpha antagonist treatment in patients with ankylosing spondylitis: the impact on depression, anxiety and quality of life level. Rheumatol Int 2012; 32: 323–330. [DOI] [PubMed] [Google Scholar]
- Traki L, Rostom S, Tahiri L, Bahiri R, Harzy T, Abouqal R et al. Responsiveness of the euroQol EQ-5D and hospital anxiety and depression scale (HADS) in rheumatoid arthritis patients receiving tocilizumab. Clin Rheumatol 2014; 33: 1055–1060. [DOI] [PubMed] [Google Scholar]
- Gossec L, Steinberg G, Rouanet S, Combe B. Fatigue in rheumatoid arthritis: quantitative findings on the efficacy of tocilizumab and on factors associated with fatigue. The French multicentre prospective PEPS Study. Clin Exp Rheumatol 2015; 33: 664–670. [PubMed] [Google Scholar]
- Gelfand JM, Kimball AB, Mostow EN, Chiou CF, Patel V, Xia HA et al. Patient-reported outcomes and health-care resource utilization in patients with psoriasis treated with etanercept: continuous versus interrupted treatment. Value Health 2008; 11: 400–407. [DOI] [PubMed] [Google Scholar]
- Eisenberg E, Sandler I, Treister R, Suzan E, Haddad M. Anti tumor necrosis factor - alpha adalimumab for complex regional pain syndrome type 1 (CRPS-I): a case series. Pain Pract 2013; 13: 649–656. [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between‐study heterogeneity. Stat Med 2007; 26: 4544–4562. [DOI] [PubMed] [Google Scholar]
- Abbott R, Whear R, Nikolaou V, Bethel A, Coon JT, Stein K et al. Tumour necrosis factor-alpha inhibitor therapy in chronic physical illness: A systematic review and meta-analysis of the effect on depression and anxiety. J Psychosom Res 2015; 79: 175–184. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord 2009; 119: 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002; 26: 643–652. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry 2007; 68: 17–25. [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry 2013; 70: 176–184. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997; 9: 853–858. [DOI] [PubMed] [Google Scholar]
- O'Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res 2007; 41: 326–331. [DOI] [PubMed] [Google Scholar]
- Christmas DM, Potokar J, Davies SJ. A biological pathway linking inflammation and depression: activation of indoleamine 2,3-dioxygenase. Neuropsychiatr Dis Treat 2011; 7: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neuroscience 2009; 29: 4200–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 2014; 5: 1038–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA 2014; 111: 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol 2008; 173: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen LE, Kapetanovic MC, Gulfe A, Soderlin M, Saxne T, Geborek P. Predictors of response to anti-TNF therapy according to ACR and EULAR criteria in patients with established RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology 2008; 47: 495–499. [DOI] [PubMed] [Google Scholar]
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug discov 2010; 9: 325–338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.