Abstract
A safe and effective colorectal cancer (CRC) chemoprevention agent remains to be discovered. We aim to evaluate the association between the use of glucosamine and/or chondroitin sulphate and risk of colorectal cancer (CRC) in the MCC-Spain study, a case-control study performed in Spain that included 2140 cases of CRC and 3950 population controls. Subjects were interviewed on sociodemographic factors, lifestyle, family and medical history and regular drug use. Adjusted odds ratios and their 95% confidence intervals were estimated. The reported frequency of chondroitin and/or glucosamine use was 2.03% in controls and 0.89% in cases. Users had a reduced risk of CRC (OR: 0.47; 95% CI: 0.28–0.79), but it was no longer significant when adjusted for NSAID (nonsteroidal anti-inflammatory drugs) use (OR: 0.82; 95% CI: 0.47–1.40). A meta-analysis with previous studies suggested a protective effect, overall and stratified by NSAID use (OR: 0.77; 95% CI: 0.62–0.97). We have not found strong evidence of an independent preventive effect of CG on CRC in our population because the observed effects of our study could be attributed to NSAIDs concurrent use. These results merit further research due to the safety profile of these drugs.
Introduction
The high incidence of colorectal cancer (CRC), the known colorectal adenoma-to carcinoma sequence and the poor survival rate of advanced CRC has prompted the emphasis on its prevention. Faecal occult blood test has demonstrated a reduction of CRC mortality1. This strategy is based on early detection, and requires repeated testing to increase sensitivity. Although lifestyle risk factors have been described in CRC aetiology, randomized trials have failed to show a reduction of adenomas recurrence with special diets2. Moreover, a safe and effective CRC chemoprevention agent has not been found to date in order to reduce the incidence of polyps and/or CRC. Acetylsalicylic acid (ASA) is the agent with more evidence and, indeed, the United States Preventive Services Task Force3 has recently stated that there is adequate evidence4 that aspirin can be used to reduce risk for CRC in adults ages 50 to 69 years who are at increased risk for cardiovascular diseases. However, a general use of this drug in younger people or without cardiovascular disease is not recommended because of its adverse events such as gastrointestinal and intra-cerebral haemorrhage5. Other drugs and supplements have also been studied as candidate chemoprevention agents for CRC such as other non-steroidal anti-inflammatory drugs (NSAIDs)6–8, folic acid9,10, calcium10,11, and diverse vitamins10,12. None of them have shown enough evidence to be implemented as chemoprevention agents for general population.
Recent evidence suggests that glucosamine and chondroitin sulphate supplements could reduce CRC risk13–15. These drugs are widely used in osteoarthritis due to their immunomodulatory effect, which reduces the nuclear factor κβ (NF-kβ) translocation. NF-kβ has an established role in the coordination of innate and adaptive immune responses and cell-cycle regulation and it has a role in tumorigenesis16. In the VITamins And Lifestyle (VITAL)13,14 study and in two prospective cohorts, Kantor et al.15 reported that the use of these drugs had a protective effect of CRC risk. Moreover, the good tolerability of these drugs has been proven in trials that aimed to study the efficacy and safety of chondroitin sulphate plus glucosamine in osteoarthritis17–19. For this reason, we wanted to explore the association between glucosamine and chondroitin sulphate and CRC in the MCC-Spain case-control study.
Methods
Study population
A Multi Case-control (MCC-Spain) study was performed between 2008 and 2013, in which 10183 total subjects aged 20–85 years were enrolled in 12 Spanish provinces. A detailed description has been previously published20. The recruitment included incident cases of CRC (C18, C19, C20, D01.0, D01.1, D01.2) which were identified through an active search in the participating hospitals. Both cases and controls were free of personal CRC history. Controls, selected from the general population, were frequency-matched to cases, by age, sex, and region. In this study, we included 2140 cases of CRC and 3950 controls.
All procedures were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The protocol of MCC-Spain was approved by each of the ethics committees of the participating institutions. The specific study reported here was approved by the Bellvitge Hospital Ethics Committee with reference PR149/08. Written informed consent was obtained from all individual participants included in the study.
Data collection
A structured computerised epidemiological questionnaire was administered by trained personnel in a face-to-face interview. This questionnaire included information of sociodemographic factors, personal and family medical history, anthropometric data, lifestyle and medication. Also, subjects filled in a semi-quantitative Food Frequency Questionnaire (FFQ).
Complete history of regular drug use was recorded, obtained by personal interview, but only chondroitin sulphate (ATC code: M01AX25), glucosamine (ATC code: M01AX05) and NSAIDs including non-ASA NSAIDs (ATC code: M01A) and ASA (ATC code: B01AC06, N02BA01, NA02BA51) were considered for this study. For each drug, the brand name, dose and duration of exposure were recorded. Unless specified, we will refer to NSAIDs as the combination of ASA and other NSAIDs. Regular use NSAIDs was defined as consuming ≥1 times/day for at least one year. However, the low frequency of use of glucosamine or chondroitin sulphate only allowed dividing patients into users (either “regular” or “sporadic”) and nonusers. Given that chondroitin sulphate and glucosamine are frequently consumed combined and there were few individuals using these drugs, their combined use was analysed.
Statistical analysis
A study design adjustment score (SDAS) was built to reduce bias related to differences in case and control selection frequencies. This SDAS was derived as the prediction of a logistic regression model on case-control status that included age, sex, recruiting centre and level of education and it also included the interactions between age and sex and centre and sex. All analyses were adjusted by the SDAS, and multivariable-adjusted analyses also included non-ASA NSAIDs and ASA use, family history of CRC, tobacco use, alcohol consumption, BMI (estimated at age 45 years), physical activity, red meat intake and vegetables intake. Stratified analyses were also performed to assess the association of chondroitin/glucosamine with CRC according to NSAIDs use and BMI, since previous studies suggested a possible interaction with these factors. Logistic regression models were used to test for adjusted effects and interactions. Results are reported as odds ratios (OR) and 95% confidence intervals (CI). All reported p-values are two-tailed. A fixed-effects meta-analysis was performed, combining the estimates from this study with previous published results14,15. Statistical analysis was carried out using R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Overall, 99 participants (1.63%) reported use of chondroitin sulphate (n = 60) and/or glucosamine (n = 45). Table 1 shows the characteristics of chondroitin sulphate and glucosamine users versus non-users among controls. The use of these drugs was only associated with no-ASA NSAIDs consumption (p < 0.001). In contrast, its use was independent of sex, age, tobacco use, alcohol consumption, BMI, physical activity, intake of vegetables or red meat, and ASA prescription. Regarding other drugs used to treat osteoarthritis, we observed that concurrent use of chondroitin sulphate and glucosamine with non-ASA NSAIDs was around 84.9% but with ASA was only 14.1%. In fact, 98% participants consumed non-ASA NSAIDs as an analgesic drug (30.7% for joint pain and 67.8% for pain in other locations); 91.7% and 87.5% consumed chondroitin sulphate and glucosamine for a joint disease, respectively; and 62.9% subjects were prescribed ASA for primary or secondary prevention of cardiovascular events.
Table 1.
Characteristics of the chondroitin sulphate and glucosamine users in controls.
| Characteristic | Nonusersa | Users | P-valueb | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Age (years) | |||||
| 26–65 | 2066 | (53.4) | 39 | (48.8) | |
| 65–85 | 1804 | (46.6) | 41 | (51.3) | 0.41 |
| Sex | |||||
| Female | 1886 | (48.7) | 46 | (57.5) | |
| Male | 1984 | (51.3) | 34 | (42.5) | 0.12 |
| Smoking | |||||
| Non-smoker | 1721 | (44.5) | 42 | (52.5) | |
| Former/Current smoker | 2149 | (55.5) | 38 | (47.5) | 0.15 |
| Alcohol | |||||
| Low consumption | 3315 | (85.7) | 66 | (82.5) | |
| High consumption | 555 | (14.3) | 14 | (17.5) | 0.43 |
| BMI at 45-year age | |||||
| <25kg/m2 | 2225 | (57.5) | 54 | (67.5) | |
| ≥25kg/m2 | 1645 | (42.5) | 26 | (32.5) | 0.80 |
| Physical activity in leisure time | |||||
| No | 1623 | (41.9) | 26 | (32.5) | |
| Yes | 2247 | (58.1) | 54 | (67.5) | 0.09 |
| Vegetables | |||||
| ≤200g/day | 2564 | (66.3) | 54 | (67.5) | |
| >200g/day | 1306 | (33.8) | 26 | (32.5) | 0.81 |
| Red meat | |||||
| ≤65g/day | 2269 | (58.6) | 52 | (65.0) | |
| >65g/day | 1601 | (41.4) | 28 | (35.0) | 0.25 |
| ASA | |||||
| Nonuser/sporadically use | 3386 | (87.5) | 66 | (57.5) | |
| Regular use in the last year | 484 | (12.5) | 14 | (17.5) | 0.19 |
| Non-ASA NSAIDs | |||||
| Nonuser/sporadically use | 3200 | (82.7) | 11 | (13.8) | |
| Regular use in the last year | 670 | (17.3) | 69 | (86.3) | <0.0001 |
aUser includes sporadically use and regular use.
bP-values derived from a chi-square test.
ASA: acetylsalicylic acid; BMI: body mass index; CRC: colorectal cancer; NSAID: Nonsteroidal anti-inflammatory drugs.
In the crude analysis (only adjusted for the SDAS), chondroitin sulphate and/or glucosamine (CG) use was associated with a 53% reduced risk of CRC (OR: 0.47; 95% CI: 0.28–0.79). Both the use of chondroitin sulphate alone (OR: 0.42; 95% CI: 0.21–0.84) and glucosamine alone (OR: 0.47, 95% CI: 0.22–1.01) were protective for CRC.
In the multivariate-adjusted analysis (Table 2), the use of CG was not significantly associated with CRC (adjusted OR: 0.82; 95% CI: 0.47–1.40), probably due to the small number of exposed that limited power, and the concurrent use of NSAIDs. Regular use of ASA and non-ASA NSAIDs significantly reduced CRC risk by 25–43% in the MCC-Spain study (adjusted OR 0.75; 95% CI: 0.63–0.90, and adjusted OR: 0.54; 95% CI: 0.46–0.65, respectively). Table 3 shows how the protective effect was no longer significant when adjusted for NSAIDs use.
Table 2.
Multivariate-adjusted risk factors associated with CRC.
| Characteristic | Controls | Cases | Adjusted | 95% CI | P-Value | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ORa | |||
| Family history of CRC | |||||||
| No | 3483 | (88.2) | 1663 | (77.7) | 1.00 | ||
| Yes | 467 | (11.8) | 477 | (22.3) | 2.43 | 2.09–2.83 | <0.0001 |
| Smoking | |||||||
| Non-smoker | 1763 | (44.6) | 893 | (41.7) | 1.00 | ||
| Former/Current smoker | 2187 | (55.8) | 1247 | (58.3) | 1.05 | 0.93–1.84 | 0.43 |
| Alcohol | |||||||
| Low consumption | 3381 | (85.6) | 1685 | (78.7) | 1.00 | ||
| High consumption | 569 | (14.4) | 455 | (21.3) | 1.35 | 1.16–1.57 | <0.0001 |
| BMI at 45-year age | |||||||
| <25kg/m2 | 2279 | (57.7) | 982 | (45.9) | 1.00 | ||
| ≥25kg/m2 | 1671 | (42.3) | 1158 | (54.1) | 1.16 | 1.03–1.30 | 0.01 |
| Physical activity in leisure time | |||||||
| No | 1649 | (41.8) | 1101 | (51.5) | 1.00 | ||
| Yes | 2301 | (58.3) | 1039 | (48.6) | 0.70 | 0.63–0.78 | <0.0001 |
| Vegetables | |||||||
| ≤200g/day | 2618 | (66.3) | 1526 | (71.3) | 1.00 | ||
| >200g/day | 1332 | (33.7) | 614 | (28.7) | 0.75 | 0.66–0.85 | <0.0001 |
| Red meat | |||||||
| ≤65g/day | 2321 | (58.8) | 1070 | (50.0) | 1.00 | ||
| >65g/day | 1629 | (41.2) | 1070 | (50.0) | 1.22 | 1.09–1.38 | 0.0008 |
| ASA | |||||||
| Non-user/sporadically use | 3452 | (87.4) | 1894 | (88.5) | 1.00 | ||
| Regular use in the last year | 498 | (12.6v | 246 | (11.5) | 0.75 | 0.63–0.90 | 0.0013 |
| Non-ASA NSAIDs | |||||||
| Non-user/sporadically use | 3181 | (80.5) | 1912 | (89.4) | 1.00 | ||
| Regular use in the last year | 769 | (19.5v | 228 | (10.7) | 0.54 | 0.46–0.65 | <0.0001 |
| Chondroitin and/or glucosamine | |||||||
| Non-user | 3870 | (98.0) | 2121 | (99.1) | 1.00 | ||
| Userb | 80 | (2.0v | 19 | (0.9) | 0.82 | 0.47–1.40 | 0.37 |
aAdjusted by the study design adjustment and the variables shown in this table.
ASA: acetylsalicylic acid; BMI: Body mass index; CRC: colorectal cancer; NSAID: Nonsteroidal anti-inflammatory drugs.
Table 3.
Chondroitin sulphate and glucosamine association to risk of CRC.
| OR | 95% CI | P-value | |
|---|---|---|---|
| Crude effecta | 0.47 | 0.28–0.79 | 0.0023 |
| Adjusted for ASA use | 0.47 | 0.28–0.79 | 0.0045 |
| Adjusted for non-ASA NSAIDs use | 0.72 | 0.43–1.23 | 0.23 |
| Adjusted for NSAIDs use | 0.62 | 0.37–1.05 | 0.077 |
| Adjusted for multivariateb without NSAIDs use | 0.52 | 0.31–0.88 | 0.017 |
| Adjusted for multivariateb | 0.82 | 0.47–1.40 | 0.37 |
aAdjusted by the study design variables (age, gender, region and education).
bAdjusted by the study design variables plus alcohol consumption, BMI, physical activity, vegetables and red meat intake, family history and NSAIDs use (see Table 3).
ASA: acetylsalicylic acid; NSAID: Nonsteroidal anti-inflammatory drugs (includes ASA except when indicated).
The combined analysis (Table 4) showed that the protective effect of CG on CRC was only among subjects that were NSAIDs users. An increased protective effect with concurrent use of CG and NSAIDs was found, suggesting a possible additive action.
Table 4.
Analysis of chondroitin and/or glucosamine protective effect of CRC according to NSAID use
| Control | Case | ||||||
|---|---|---|---|---|---|---|---|
| Interaction analysis between chondroitin and/or glucosamine use and NSAIDs | |||||||
| n | % | n | % | Adjusted ORa | 95% CI | ||
| CG nonuserb - NSAIDs nonuserc | 2776 | 70.28 | 1697 | 79.30 | 1.00 | ||
| CG user - NSAIDs nonuser | 10 | 0.250 | 4 | 0.19 | 1.04 | 0.31–3.55 | |
| CG nonuser - NSAIDs user | 1094 | 27.70 | 414 | 19.81 | 0.62 | 0.53–0.71 | |
| CG user - NSAIDs user | 70 | 1.77 | 15 | 0.70 | 0.40 | 0.22–0.72 | |
| Stratified analysis of chondroitin and/or glucosamine protective effect of CRC according to NSAID use | |||||||
| n | % | n | % | Adjusted OR a | 95% CI | p-interaction | |
| NSAIDs nonuser | 0.50 | ||||||
| CG nonuser | 2803 | 99.64 | 1715 | 99.77 | 1 | ||
| CG user | 10 | 0.36 | 4 | 0.23 | 1.04 | 0.30–3.54 | |
| NSAIDs user c | |||||||
| CG nonuser | 1067 | 93.84 | 406 | 96.44 | |||
| CG user | 70 | 6.16 | 15 | 3.56 | 0.66 | 0.37–1.21 | |
aAdjusted by the study design variables (age, gender, region and education) plus alcohol consumption, BMI, physical activity, vegetables and red meat intake, family history and NSAIDs.
bUser includes sporadically use and regular use.
cUser includes only regular use of NSAIDs.
CG: chondroitin and/or glucosamine. NSAID: Nonsteroidal anti-inflammatory drugs (including ASA).
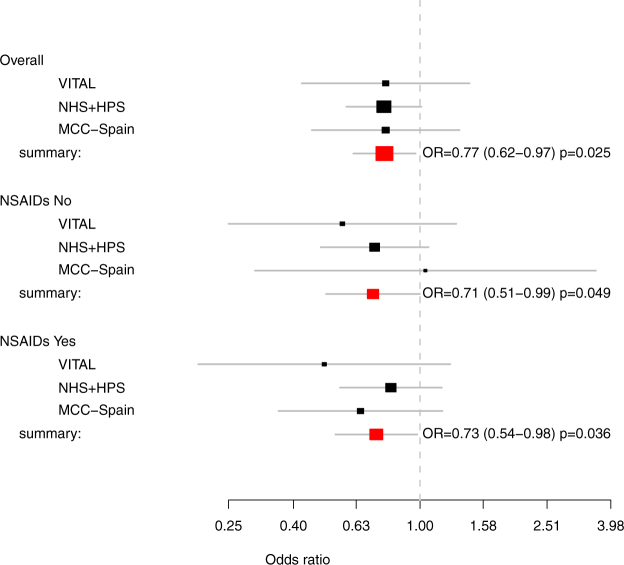
The fixed-effects meta-analysis of the multivariate-adjusted estimates, both overall (OR 0.77; 95%CI: 0.62–0.97; p = 0.025) and stratified by NSAID use (OR for NSAID users 0.73; 95%CI: 0.54–0.98; p = 0.036. OR for non-NSAID users 0.71; 95%CI: 0.51–0.99; p = 0.049) confirmed a significant protective effect of CG in CRC (Fig. 1). No evidence of heterogeneity was observed (estimated heterogeneity variance = 0.01, p = 0.99; test for funnel plot asymmetry: z = 0.052, p = 0.96).
Figure 1.
Meta-analysis of studies of chondroitin sulphate and glucosamine and the risk of CRC. Estimated heterogeneity variance = 0.01, P = 0.99.
Discussion
The results of this case-control study did not show clear evidence of a preventive effect of CG on CRC because, though in the univariate analysis CG had a significant association, this effect was no longer significant when adjusted for NSAID use. The number of subjects exposed to CG was low, and this reduced the power to detect a protective effect. The analysis stratified by NSAIDs use indicated that the effect of CG was additive to the concurrent use of these drugs (Table 4).
The OR for CG was no longer significant when adjusted for NSAIDs use but the magnitude of the adjusted effect was similar to that reported recently by Kantor et al.15 in two prospective cohorts in North America (RR: 0.77; 95% CI: 0.58–0.99). Previously, Satia et al.13 already observed in an exploratory analysis within the VITamins And Lifestyle (VITAL) study that use of glucosamine (HR: 0.72; 95% CI, 0.54–0.98) and chondroitin sulphate (HR, 0.65; 95% CI, 0.45–0.93) supplements were associated with reduced risk of CRC after 5 years of follow-up. These results were not statistically significant when adding two years of follow-up (HR: 0.55; 95 % CI 0.30–1.01)14. Despite the fact that we could not find an association of CG with CRC risk that was independent of NSAID use, all the above-mentioned studies13,15 did control for ASA and NSAIDs and they did find an independent effect. In fact, our meta-analysis of the VITAL study14, the Nurses’ Health Study and Health Professionals follow-up study15 and the MCC-Spain showed a significant overall effect, multivariate-adjusted, which was also significant both for concurrent non-NSAID users and for NSAID users (Fig. 1). The lack of heterogeneity among the studies reinforced the observed protective effect.
Though glucosamine and chondroitin sulphate have anti-inflammatory effect, its mechanism is independent of cyclooxygenase-2 inhibition and the anti-inflammatory mechanism is thought to be independent of NSAIDs. However, our stratified analysis by NSAID use does not seem to support this, as the inverse association with chondroitin sulphate and glucosamine was seen only among NSAID users. Because of the low number of CG users, we could not analyse duration and time exposure, so we could not differentiate concomitant and sequential exposure of CG and NSAIDs. We do not know if the increased protection of CG and non-ASA NSAIDs or ASA use was because of CG itself or because this subgroup used a higher dose or longer period use of NSAIDs.
In vitro and animal studies14,15,21–24 suggest that this protective effect might be caused through reduction in inflammation25–27 by the suppression of the NF-kβ pathway16, this alternative mechanism is relevant and explains the better toxicity profile of glucosamine and chondroitin sulphate observed in multiple clinical trials28. We should highlight that glucosamine increases insulin resistance in skeletal muscle and diabetics should take caution when taking it, however, alteration of glucose homoeostasis was not found in a 3-year randomised controlled study in patients without diabetes29. Moreover, some preparations that contain glucosamine extracted from seafood could increase the risk of hypersensitivity reactions among people with an allergy to shellfish30,31. A 2006 Cochrane systematic review32 concluded that glucosamine is as safe as placebo and Matheson et al.33 reported less gastrointestinal symptoms, skin reactions or fatigue with glucosamine than ibuprofen. As for chondroitin sulphate, it is considered to be safe, with rare incidence of adverse reactions which suggests its long term safety28,31,34. Only mild gastrointestinal side effects such as nausea, diarrhoea or constipation, stomach pain, and heart burn have been reported34.
Previous studies13,14 had controversial results of the association of chondroitin sulphate and glucosamine to CRC according to BMI. We did not find any evidence of an effect modification of CG by BMI, though we had limited power to detect this interaction. The OR for CG was 0.67 (95%CI: 0.29–1.56) for BMI ≥25kg/m2 and 0.76 (95%CI: 0.37–1.48) for BMI <25kg/m2.
This study has several limitations that might explain the low prevalence of use of chondroitin sulphate and glucosamine (1.63%). First, drug consumption was self-reported, which could introduce a recall bias and attenuate the association observed. Although we wanted to recollect detailed information of dosages and prescription duration, we could not analyse the dose-effect relationship because patients did not provide enough detailed data regarding drugs consumption. The reported prevalence of use in the USA was 13%15, a country in which these drugs can be self-purchased as nutritional supplements, while in Spain a prescription is required. Also, the mean age of our population (64.6 years, range 22–85) is a few years younger and with less women (44.4%) than previous studies14,15, which reduces the prevalence of subjects with osteoarthritis. In fact, it is reported that the highest prevalence of knee pain is amongst women aged 7535. There could be also surveillance bias as patients with osteoarthritis are in the same age range as CRC. Patients with osteoarthritis have regular medical visits that could result in increased screening for CRC, early intervention for polyp removal and prevention of actual CRC. Another concern is the confounding by association with NSAIDs as chondroitin sulphate and glucosamine are used essentially for osteoarthritis, and generally with NSAIDs, so the univariate analysis could essentially represent the effect of concomitant NSAIDs that have already demonstrated a protective effect7,36. The fact is that in the very few patients without NSAIDs there is no effect of CG on CRC. The increased effect when CG is associated with NSAIDs could simply be a dose effect of NSAIDs, if CG use selected patients with more severe osteoarthritis (a higher dose or longer period use of NSAIDs). To address these limitations, larger studies, preferably with prospective design and with exposure assessment based on registered data, should be performed.
In conclusion, we have not found clear evidence of an independent preventive effect of CG on CRC because the observed effects of our study could be attributed to NSAIDs concurrent use. However, the good toxicity profile merits further research to examine their effect and a potential role as chemopreventive agents.
Acknowledgements
This work was supported by the ‘Acción Transversal del Cancer’, approved by the Spanish Ministry Council on the 11th October 2007, by the Instituto de Salud Carlos III, co-founded by FEDER funds –‘a way to build Europe’ (grants PI08/1770, PI08/0533, PI08/1359, PI09/00773, PI09/01286, PI09/01903, PI09/02078, PI09/01662, PI11/01403, PI11/01889, PI11/00226, PI11/01810, PI11/02213, PI12/00488, PI12/00265, PI12/01270, PI12/00715, PI12/00150, PI14/01219, PI14/00613, and PI15/00069). Support was also provided by the Fundación Marqués de Valdecilla (grant API 10/09); the Junta de Castilla y León (grant LE22A10–2); the Consejería de Salud of the Junta de Andalucía (2009-S0143); the Conselleria de Sanitat of the Generalitat Valenciana (grant AP 061/10); the Recercaixa (grant 2010ACUP 00310); the Regional Government of the Basque Country; the Consejería de Sanidad de la Región de Murcia; European Commission grants FOOD-CT-2006-036224-HIWATE; the Fundación Científica Asociación Española Contra el Cáncer (AECC); the Catalan Government DURSI (grant 2014SGR647); the Fundación Caja de Ahorros de Asturias; the University of Oviedo; Societat Catalana de Digestologia; and COST action BM1206 Eucolongene.
Author Contributions
Study design: Victor Moreno and Gemma Ibáñez-Sanz; literature review: Gemma Ibáñez-Sanz; analysis: Anna Díez-Villanueva and Elisabet Guinó; draft of the manuscript: Gemma Ibáñez-Sanz, Anna Díez-Villanueva and Victor Moreno; acquisition of data and revision of the manuscript: Gemma Ibáñez-Sanz, Anna Díez-Villanueva, Laura Vilorio-Marqués, Esther Gracia, Nuria Aragonés, Rocío Olmedo-Requena, Javier Llorca Juana Vidán, Pilar Amiano, Pilar Nos, Guillermo Fernández-Tardon, Ricardo Rada, María Dolores Chirlaque, Elisabet Guinó, Verónica Dávila-Batista, Gemma Castaño-Vinyals, Beatriz Pérez-Gómez, Benito Mirón-Pozo, Trinidad Dierssen-Sotos, Jaione Etxeberria, Amaia Molinuevo, Begoña Álvarez-Cuenllas, Manolis Kogevinas, Marina Pollán, and Victor Moreno.
Competing Interests
Victor Moreno has received consulting fees for Bioiberica, S.A., Barcelona. Bioiberica S.A. has not been involved in the preparation of the manuscript.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 2.Alberts DS, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 3.Force, U. S. P. S. T. Draft Recommendation Statement: Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Preventive Medication, http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/aspirin-toprevent-cardiovascular-disease-and-cancer (2015).
- 4.Chubak, J., Kamineni, A., Buist, D. S. M., Anderson, M. L. & Whitlock, E. P. Aspirin Use for the Prevention of Colorectal Cancer: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force (Agency for Healthcare Research and Quality, Rockville 2015). [PubMed]
- 5.Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5–18. doi: 10.1007/s10654-014-9971-7. [DOI] [PubMed] [Google Scholar]
- 6.Rostom A, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Zhang FC, Wang YJ. The efficacy and safety of non-steroidal anti-inflammatory drugs in preventing the recurrence of colorectal adenoma: a meta-analysis and systematic review of randomized trials. Colorectal Dis. 2015;17:188–196. doi: 10.1111/codi.12838. [DOI] [PubMed] [Google Scholar]
- 8.Thompson, P. A. et al. Celecoxib for the Prevention of Colorectal Adenomas: Results of a Suspended Randomized Controlled Trial. J Natl Cancer Inst108 (2016). [DOI] [PMC free article] [PubMed]
- 9.Qin T, et al. Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials. Sci Rep. 2015;5:12044. doi: 10.1038/srep12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heine-Broring RC, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer. 2015;136:2388–2401. doi: 10.1002/ijc.29277. [DOI] [PubMed] [Google Scholar]
- 11.Bonovas S, Fiorino G, Lytras T, Malesci A, Danese S. Calcium supplementation for the prevention of colorectal adenomas: A systematic review and meta-analysis of randomized controlled trials. World J Gastroenterol. 2016;22:4594–4603. doi: 10.3748/wjg.v22.i18.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, et al. Vitamin and multiple-vitamin supplement intake and incidence of colorectal cancer: a meta-analysis of cohort studies. Med Oncol. 2015;32:434. doi: 10.1007/s12032-014-0434-5. [DOI] [PubMed] [Google Scholar]
- 13.Satia JA, Littman A, Slatore CG, Galanko JA, White E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol Biomarkers Prev. 2009;18:1419–1428. doi: 10.1158/1055-9965.EPI-09-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantor ED, et al. Use of glucosamine and chondroitin supplements and risk of colorectal cancer. Cancer Causes Control. 2013;24:1137–1146. doi: 10.1007/s10552-013-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantor, E. D. et al. Use of glucosamine and chondroitin supplements in relation to risk of colorectal cancer: Results from the Nurses' Health Study and Health Professionals follow-up study. Int J Cancer, (2016). [DOI] [PMC free article] [PubMed]
- 16.Vaiopoulos AG, Papachroni KK, Papavassiliou AG. Colon carcinogenesis: Learning from NF-kappaB and AP-1. Int J Biochem Cell Biol. 2010;42:1061–1065. doi: 10.1016/j.biocel.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75:37–44. doi: 10.1136/annrheumdis-2014-206792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier JP, et al. Chondroitin sulfate efficacy versus celecoxib on knee osteoarthritis structural changes using magnetic resonance imaging: a 2-year multicentre exploratory study. Arthritis Res Ther. 2016;18:256. doi: 10.1186/s13075-016-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawitzke AD, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459–1464. doi: 10.1136/ard.2009.120469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castano-Vinyals G, et al. Population-based multicase-control study in common tumors in Spain (MCC-Spain): rationale and study design. Gac Sanit. 2015;29:308–315. doi: 10.1016/j.gaceta.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Navarro SL, et al. Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PLoS One. 2015;10:e0117534. doi: 10.1371/journal.pone.0117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalirfardouei R, Karimi G, Jamialahmadi K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016;152:21–29. doi: 10.1016/j.lfs.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Hori Y, et al. Effects of chondroitin sulfate on colitis induced by dextran sulfate sodium in rats. Jpn J Pharmacol. 2001;85:155–160. doi: 10.1254/jjp.85.155. [DOI] [PubMed] [Google Scholar]
- 24.du Souich P. Absorption, distribution and mechanism of action of SYSADOAS. Pharmacol Ther. 2014;142:362–374. doi: 10.1016/j.pharmthera.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Kantor ED, et al. Association between use of specialty dietary supplements and C-reactive protein concentrations. Am J Epidemiol. 2012;176:1002–1013. doi: 10.1093/aje/kws186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantor ED, et al. Associations between glucosamine and chondroitin supplement use and biomarkers of systemic inflammation. J Altern Complement Med. 2014;20:479–485. doi: 10.1089/acm.2013.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantor ED, et al. Specialty supplement use and biologic measures of oxidative stress and DNA damage. Cancer Epidemiol Biomarkers Prev. 2013;22:2312–2322. doi: 10.1158/1055-9965.EPI-13-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun, L. & Cohen, M. Herbs and Natural Supplements: An evidence-based guide. Fourth edn, Vol. 2 1379 (Elsevier, 2015).
- 29.Reginster JY, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 30.Committee, A. D. R. A. in Aust Adverse Drug React Bull Vol. 24 (2005).
- 31.Hathcock JN, Shao A. Risk assessment for glucosamine and chondroitin sulfate. Regul Toxicol Pharmacol. 2007;47:78–83. doi: 10.1016/j.yrtph.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Towheed, T. E. et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev, CD002946 (2005). [DOI] [PMC free article] [PubMed]
- 33.Matheson AJ, Perry CM. Glucosamine: a review of its use in the management of osteoarthritis. Drugs Aging. 2003;20:1041–1060. doi: 10.2165/00002512-200320140-00004. [DOI] [PubMed] [Google Scholar]
- 34.Bishnoi M, Jain A, Hurkat P, Jain SK. Chondroitin sulphate: a focus on osteoarthritis. Glycoconj J. 2016;33:693–705. doi: 10.1007/s10719-016-9665-3. [DOI] [PubMed] [Google Scholar]
- 35.Urwin M, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis. 1998;57:649–655. doi: 10.1136/ard.57.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]