Abstract
A single nucleotide polymorphism, rs17070145, in the KIdney and BRAin expressed protein (KIBRA) gene has been associated with cognition and hippocampal volume in cognitively normal (CN) individuals. However, the impact of rs17070145 on longitudinal cognitive decline and hippocampal atrophy in CN adults at greatest risk of developing Alzheimer’s disease is unknown. We investigated the impact rs17070145 has on the rate of cognitive decline and hippocampal atrophy over six years in 602 CN adults, with known brain Aβ-amyloid levels and whether there is an interactive effect with APOE genotype. We reveal that whilst limited independent effects of KIBRA genotype were observed, there was an interaction with APOE in CN adults who presented with high Aβ-amyloid levels across study duration. In comparison to APOE ε4-ve individuals carrying the rs17070145-T allele, significantly faster rates of cognitive decline (global, p = 0.006; verbal episodic memory, p = 0.004), and hippocampal atrophy (p = 0.04) were observed in individuals who were APOE ε4 + ve and did not carry the rs17070145-T allele. The observation of APOE effects in only non-carriers of the rs17070145-T allele, in the presence of high Aβ-amyloid suggest that carriers of the rs17070145-T allele are conferred a level of resilience to the detrimental effects of high Aβ-amyloid and APOE ε4.
Introduction
In cognitively normal older individuals, high levels of neocortical amyloid-β (Aβ-amyloid) are associated with subtle but detectable cognitive decline1 and hippocampal atrophy2. This observation is consistent with models of Alzheimer’s disease (AD) which propose a protracted preclinical phase that could take up to 20 years3. This provides a period of opportunity for understanding, and even interfering with, AD pathogenesis and thus the identification of biological factors, or trait characteristics, that themselves can influence AD progression has become of increased importance.
Several genes have been associated with cognitive performance, particularly episodic memory, and hippocampal atrophy. Previous studies have associated genetic polymorphisms, in particular apolipoprotein E (APOE) ε2/ε3/ε4 genotype (see review4,5) and the non-synonymous rs6265 (Val66Met) SNP in brain derived neurotropic factor (BDNF)6–9, with altered rates of episodic memory decline and hippocampal atrophy. Decline in measures of episodic memory, modified by genetic variation, have been reported in both the healthy elderly10 and those predicted to be in the early stages of AD based on neocortical Aβ-amyloid imaging6,7,11. These findings raise the potential that other genetic factors may also moderate the toxic effects of Aβ-amyloid early in AD and contribute to altered rates of cognitive decline and hippocampal atrophy.
One such candidate is the gene encoding the KIdney and BRAin expressed protein (KIBRA; sometimes referred to as WW domain-containing protein 1 (WWC1))12. KIBRA is a cytoplasmic, signal transducer protein expressed mainly in the kidney and brain13 and in vitro experiments suggest that, through reduction in postsynaptic levels, it mediates tau induced memory loss and disruption of synaptic plasticity14. This in vitro data is supported through genetic studies that report the association of allelic variation in the KIBRA gene with memory performance, hippocampal atrophy and measurable differences in brain activation. Specifically, a substitution of C for T in the 9th intron (rs17070145), was initially identified through a GWAS of verbal episodic memory performance and replicated in two additional independent cohorts12. Episodic memory is one of the earliest cognitive domains to decline, with previous studies observing decline 4–8 years prior to executive function and up to 7–10 years prior to other cognitive domains15–17.
However, there is a lack of consensus in subsequent studies that attempted to replicate these genetic associations with memory performance. Cross-sectional studies of cognitively normal (CN) older adults, carriage of the rs17070145-T allele has been associated with better performance in episodic memory18–22, delayed recall23–25 and spatial learning26 and increased hippocampal volume20 and activity19,24. Conversely, several studies have either associated the absence of rs17070145-T with better semantic27 and long-term28 memory, executive function29 and overall cognitive performance30 or were unable to show any association of the SNP with cross sectional episodic memory29,31–33 and hippocampal volume31 or longitudinal decline in episodic memory and hippocampal volume31. However, common to all these studies is the lack of inclusion of Aβ-amyloid imaging, which may contribute to the lack of consensus due to the impact of underlying Aβ-amyloid burden on cognition not being considered1,6,7,11.
To address this conjecture requires the availability of comprehensive longitudinal data from the prospective cohort studies of AD, such as the Australian Imaging, Biomarkers and Lifestyle (AIBL) Study, which offers the opportunity to retrospectively evaluate candidate biological factors (e.g. genetic variation) to determine the impact on progression of AD related phenotypes, such as cognitive decline and hippocampal atrophy. The AIBL Study has now more than six years of serial cognitive and neuroimaging assessments, including Aβ-amyloid and structural imaging, in a group of CN adults collected at 18-month intervals. Therefore, the aim of this study was to characterize, through reporting on 6-years of longitudinal data, the role of KIBRA rs17070145 allelic variation in this highly characterised CN adult sample and examine the extent to which this allelic variation is associated with Aβ-amyloid related cognitive decline and atrophy of the hippocampus. The hypothesis was that CN adults who carry the rs17070145-T allele would show a slower rate of memory decline and hippocampal atrophy than those not carrying this allele, though this relationship would be dependent on the presence of a high brain Aβ-amyloid burden and interact with APOE genotype.
Results
The effect of KIBRA on cognition and hippocampal atrophy in cognitively normal adults
A total of 602 CN older adults, defined through the AIBL battery of clinical and neuropsychological assessments34 were included in this study. As shown in Table 1 there were no significant differences or trends between rs17070145 (henceforth referred to simply as KIBRA) T carriers and non-T carriers at baseline with respect to demographic variables, premorbid intellect, depressive symptoms, or genotype. In the initial analysis, co-varied for APOE ε4 carrier and Aβ-amyloid status (classified by being above (Αβhigh) or below (Αβlow) Positron Emission Tomography (PET) Aβ-amyloid tracer-specific thresholds) there were no significant differences in the trajectories between T carriers and non-carriers for measures of global cognition or episodic memory amongst CN adults (Supplementary Figure 1, Supplementary Table 1). However, there was a trend towards T-carriers having a mild improvement (0.028 standard deviations (SD)/year) in both global cognition (non-T carriers, −0.025 SD/year; p = 0.051) and verbal episodic memory (non-T carriers, −0.019 SD/year; p = 0.085), likely due to a practice effect. When evaluating the effect of KIBRA on hippocampal atrophy in all cases, and co-varying for APOE ε4 carrier and Aβ-amyloid status, no significant difference (p = 0.242) was observed between T carriers (−0.017 cm3/year), and non-T carriers (−0.026 cm3/year) over six years (Supplementary Figure 1, Supplementary Table 1). Further, no significant differences were observed at baseline in any measures of cognition or hippocampal volume.
Table 1.
Demographic Information.
| Overall n = 602 | KIBRA T carrier n = 335 | KIBRA non-T carrier n = 267 | p | ||
|---|---|---|---|---|---|
| Age (years) | 70.79 (6.55) | 70.73 (6.49) | 70.72 (6.41) | 0.9788 | |
| Female (%) | 334 (55.48) | 188 (56.12) | 146 (54.68) | 0.7871 | |
| Years of Education | 0–8 | 48 (8.00) | 27 (8.08) | 21 (7.89) | 0.9419 |
| 9–12 | 222 (37.00) | 127 (38.02) | 95 (35.71) | ||
| 13–15 | 126 (21.00) | 69 (20.66) | 57 (21.43) | ||
| 15+ | 204 (34.00) | 111 (33.23) | 93 (34.96) | ||
| Premorbid IQ (FSIQ) | 107.86 (7.23) | 107.66 (7.28) | 108.14 (7.30) | 0.4311 | |
| Depressive Symptoms (GDS) | 1.05 (1.28) | 1.05 (1.35) | 1.04 (1.18) | 0.9156 | |
| APOE ε4 carriage (%) | 165 (27.97) | 84 (25.53) | 81 (31.03) | 0.1655 | |
| High Aβ-amyloid burden (%) | 145 (24.09) | 76 (22.69) | 69 (25.84) | 0.4215 | |
| MRI (n) | 548 | 301 | 247 | NA |
Baseline demographic and clinical characteristics of all imaged cognitively normal adults in the AIBL study, and based on KIBRA rs17070145 T carriage (T_T and C_T) and non-carriage (C_C). p values represent statistical significance when comparing T carriage and non-carriage. GDS, Geriatric Depression Scale; FSIQ, Wechsler Adult Intelligence Scale 3rd Edition (WAIS-III) Full Scale Intelligence Quotient.
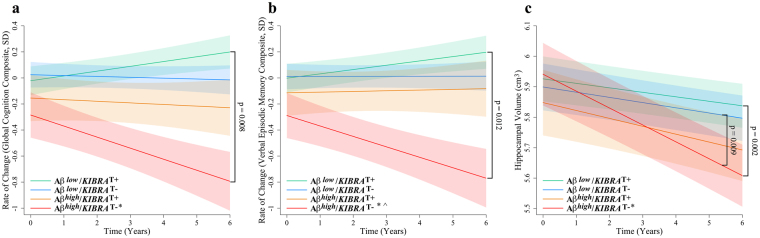
No significant differences were observed at baseline in either measure of cognition or hippocampal volume when investigating the Aβ × KIBRA × Time interaction. Relative to Αβlow/KIBRA T carriers, the Αβhigh/KIBRA non-T carrier group showed a significantly greater rate of decline in global cognition (0.037 SD/year; −0.085 SD/year; p = 0.008, q = 0.036), and the verbal episodic memory (0.033 SD/year; −0.080 SD/year; p = 0.012, q = 0.042) (Fig. 1, Table 2). However, no statistical difference was seen between Αβhigh/KIBRA T carriers and Αβlow/KIBRA non-T carriers. Analysis of hippocampal atrophy revealed that relative to Αβlow/KIBRA T carriers (−0.015 cm3/year), the Αβhigh/KIBRA non-T carrier group (−0.055 cm3/year) showed a significantly greater rate of hippocampal atrophy (p = 0.002, q = 0.034) over six years (Fig. 1, Table 2). Likewise, this trajectory of hippocampal atrophy was also significantly different (p = 0.009, q = 0.034) relative to Αβlow/KIBRA non-T carriers (−0.017 cm3/year). In contrast, Αβhigh/KIBRA T carriers’ rate of atrophy did not differ from the Αβlow groups.
Figure 1.
Rates of change in cognitively normal adults based on KIBRA T carriage and Aβ-amyloid status. Rates of change are presented for (a) a statistically driven global composite, (b) a verbal episodic memory composite, and (c) hippocampal atrophy (n = 548) in cognitively normal adults (n = 602 unless otherwise stated). Αβlow, low Αβ-amyloid burden; Αβhigh, high Αβ-amyloid burden. Αβlow/KIBRA T carriers (green), Αβlow/KIBRA non-T carriers (blue), Αβhigh/KIBRA T carriers (orange), Αβhigh/KIBRA non-T carriers (red), controlling for APOE ε4 carrier status. Hippocampal atrophy analysis also controlled for gender (shading represents time dependent standard error, *p < 0.05 when comparing to the Αβlow/KIBRA T carrier group, ^p < 0.05 when comparing to the Αβlow/KIBRA non-T carrier group, φp < 0.05 when comparing to the Αβhigh/KIBRA T carrier).
Table 2.
Group slopes for cognitive composites and hippocampal atrophy in all imaged cognitively normal participants by KIBRA carrier and Αβ-amyloid status.
| Αβlow KIBRA T carrier n = 259 | Αβlow KIBRA non-T carrier n = 198 | Αβhigh KIBRA T carrier n = 76 | Αβhigh KIBRA non-T carrier n = 69 | |
|---|---|---|---|---|
| β | β | β | β | |
| Global | 0.037 | −0.006 | −0.012 | −0.085* |
| Verbal Episodic Memory | 0.033 | 0.0004 | 0.005 | −0.080* |
| Hippocampal Atrophy | −0.015 | −0.017 | −0.026 | −0.055*^ |
Group slopes for cognitive composites (presented in SD/year; n = 602) and hippocampal atrophy (presented in cm3/year; n = 548) in all imaged cognitively normal participants, controlling for APOE ε4 carrier status. Αβlow, low Αβ-amyloid burden; Αβhigh, high Αβ-amyloid burden. *p < 0.05 when comparing to the Αβlow/KIBRA T carrier (T_T and C_T) group, ^p < 0.05 when comparing to the Αβlow/KIBRA non-T carrier group, φp < 0.05 when comparing to the Αβhigh/KIBRA T carrier.
The effect of KIBRA on cognition and hippocampal atrophy in cognitively normal adults with high Aβ-amyloid
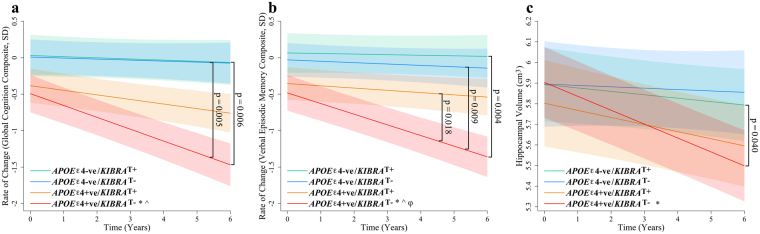
No significant differences were observed in Αβhigh CN adults at baseline in either measure of cognition or hippocampal volume when investigating the APOE × KIBRA × Time interaction. Relative to APOE ε4-ve/KIBRA T carriers, the APOE ε4 + ve/KIBRA non-T carrier group showed a significantly greater rate of decline in global cognition (p = 0.006, q = 0.034) and verbal episodic memory (p = 0.004, q = 0.034) over six years (Fig. 2, Table 3). Further, relative to APOE ε4 + ve/KIBRA T carriers, the APOE ε4 + ve/KIBRA non-T carrier group showed a nominally significantly greater rate of decline on the verbal episodic memory composite, however after FDR correction this remained only a strong trend (p = 0.018, q = 0.055) over six years (Fig. 2, Table 3). Hippocampal atrophy analysis revealed that relative to APOE ε4-ve/KIBRA T carriers (−0.016 cm3/year), the APOE ε4 + ve/KIBRA non-T carrier group (−0.067 cm3/year) had nominally significantly different rates of hippocampal atrophy however did not survive correction for multiple testing (p = 0.040, q = 0.107) over six years (Fig. 2, Table 3). This trajectory of hippocampal atrophy was suggestive of being different to APOE ε4-ve/KIBRA non-T carriers (−0.006 cm3/year), however this did not reach significance (p = 0.125), even though this trajectory showed negligible difference to APOE ε4-ve/KIBRA T carriers. APOE ε4 + ve/KIBRA T carriers’ rate of atrophy did not differ from the APOE ε4-ve groups. To ascertain that these differences in rates of decline were not due to disproportionate rates of clinical conversion, the frequency of individuals who converted to Mild Cognitive Impairment (MCI) or AD over the course of the study was investigated. Within the APOE ε4 + ve group there was no significant difference (p = 0.43) between KIBRA non-T carriers (0.294, 15 out of 41) and KIBRA T carriers (0.294, 10 out of 34) in terms of clinical conversion.
Figure 2.
Rates of change in cognitively normal adults with high Aβ-amyloid burden. Rates of change are presented for (a) a statistically driven global composite, (b) a verbal episodic memory composite, (c) hippocampal atrophy in cognitively normal adults with high Aβ-amyloid (n = 145). APOE ε4-negative/KIBRA T carriers (green), APOE ε4-ve/KIBRA non-T carriers (blue), APOE ε4 + ve/KIBRA T carriers (orange), APOE ε4 + ve/KIBRA non-T carriers (red). Hippocampal atrophy analysis controlled for gender (shading represents time dependent standard error, *p < 0.05 when comparing to the APOE ε4-ve/KIBRA T carrier group, ^p < 0.05 when comparing to the APOE ε4-ve/KIBRA non-T carrier group, φp < 0.05 when comparing to the APOE ε4 + ve/KIBRA T carrier).
Table 3.
Group slopes for cognitive composites and hippocampal atrophy in imaged cognitively normal adults with high Aβ-amyloid.
| APOE ε4-ve KIBRA T carrier n = 38 | APOE ε4-ve KIBRA non-T carrier n = 27 | APOE ε4 + ve KIBRA T carrier n = 34 | APOE ε4 + ve KIBRA non-T carrier n = 40 | |
|---|---|---|---|---|
| β | β | β | β | |
| Global | −0.016 | −0.014 | −0.063 | −0.163*^† |
| Verbal Episodic Memory | −0.008 | −0.019 | −0.031 | −0.146*^φ† |
| Hippocampal Atrophy | −0.016 | −0.006 | −0.034 | −0.067* |
Group slopes for cognitive composites (presented in SD/year) and hippocampal atrophy (presented in cm3/year) in imaged cognitively normal adults with high Aβ-amyloid (n = 145). *p < 0.05 when comparing to the APOE ε4-ve/KIBRA T carrier group, ^p < 0.05 when comparing to the APOE ε4-ve/KIBRA non-T carrier group, φ p < 0.05 when comparing to the APOE ε4 + ve/KIBRA T carrier. †q < 0.05 for those reporting nominal significance at p < 0.05.
Discussion
The data reported here support the hypothesis that KIBRA genotype, in combination with APOE ε4 and Aβ-amyloid, affects rates of memory decline and hippocampal atrophy in cognitively normal adults. In those CN adults with high Aβ-amyloid burden at baseline, KIBRA non-T carriers showed significantly faster decline in the statistically driven global composite, and verbal episodic memory when compared to T carriers with low Aβ-amyloid burden. Within the subset of CN adults with high Aβ-amyloid burden, we showed that those who are APOE ε4 + ve and KIBRA non-T carriers had significantly faster rates of decline in verbal episodic memory over 6 years, compared to APOE ε4 + ve/KIBRA T carrier and both APOE ε4-ve groups. Importantly, minimal decline was also observed in the APOE ε4 + ve/KIBRA T carrier group, suggesting that carriage of the KIBRA T allele imparts a level of resilience to negative effects of APOE ε4 and Aβ-amyloid on memory performance. Further, between group comparisons of the rates of clinical conversion (CN > MCI/AD) over the course of the study revealed no significant differences, suggesting that the faster rates of decline were not due to a higher rate of clinical conversion.
This is further supported by the observations that rates of hippocampal atrophy in this study also differ based on KIBRA genotype. In CN adults Aβ-amyloid has been previously reported to be associated with increased hippocampal atrophy2,35,36, however in this study this was only observed in those individuals who did not possess the KIBRA T-allele, whilst in contrast KIBRA T-carriers’ rate of atrophy did not significantly differ from the Αβlow groups. In a meta-analysis of APOE neuroimaging studies, hippocampal atrophy has been shown to be increased in APOE ε4 carriers5. Here we report that this association, in a group of Αβhigh CN individuals, was again only observed in those individuals who did not possess the KIBRA T-allele, whilst in contrast APOE ε4 + ve/KIBRA T-carriers’ rate of atrophy did not differ from the APOE ε4-ve groups. Taken together, we propose that the KIBRA T allele affords carriers a level of resilience to the detrimental effects of Aβ-amyloid and APOE ε4 allele on neurodegeneration, specifically hippocampal atrophy.
The findings presented herein are in line with the original study12 and subsequent reports linking the KIBRA T allele with resilience in episodic memory performance18–21,24. The absence of replication by other studies27–29,31–33 may be in part due to the lack of consistency in the measures of memory decline, whereby varying single neuropsychological tests, aiming to measure a certain feature of memory or cognition, were used. The use in this current study of a combination of global and episodic memory composite scores, which encompass several different tests best associated with a cognitive construct, could also have contributed to the ability to detect associations with the KIBRA genotype. However, the lack of inclusion of an assessment of underlying Aβ-amyloid burden in the previous studies may in fact be the more telling contributor to the lack of consensus on the association of KIBRA with cognitive performance. The level of neocortical Aβ-amyloid is associated with differential rates of cognitive decline1,37, and this is further altered by genetic factors, in particular APOE10,11 and BDNF6,7. Accounting for the underlying Aβ-amyloid burden in the current study may have further contributed to the detection of differences in rates of cognitive decline and hippocampal atrophy reported with APOE ε4 and KIBRA.
Whilst the incorporation of cognitive composites and accounting for underlying Aβ-amyloid burden is considered a strength of this study, the following limitations of the study are acknowledged. Firstly, the use of different cognitive tests individually or in combination for the calculation of domain composites, then those specifically described in this study and using the methodology described herein, may yield different results. Second, this study included 6-years of longitudinal follow-up and validation in other longitudinal cohorts, not undertaken herein, over longer durations of follow-up, may result in different findings. Third, the cognitively normal participants in this study were volunteers and not selected at random from the community, they were generally well educated and performed well on cognitive assessments and as such the findings presented herein may be applicable only to similar cohorts. Fourth, there is an overlap between those who are Aβhigh and those who are APOE ε4 + ve, which could confound the results when looking at their interaction. Finally, the KIBRA T-allele’s previously reported association with altered brain activation using fMRI12,19 could not be tested due to the lack of fMRI data, under a non-resting state, in the AIBL Study.
Studies have previously demonstrated the main areas of KIBRA expression in the brain are those also that are implicated in memory function, the hippocampus and temporal cortex12,38. Furthermore, increased KIBRA gene expression in the temporal cortex39 and hippocampus22 has been associated with late onset AD. However, in a recent post-mortem brain transcriptomic study in neuropathogically normal individuals by Piras and colleagues a trend towards increased KIBRA gene expression was observed in KIBRA T homozygotes40. Further quantitative PCR analysis reported an over-expression in T-homozygotes compared to C-homozygotes in the hippocampus40. Further, the transcriptomic analysis revealed differential activation of the MAPK pathway40, a pathway important in learning and memory processes, suggesting a potential mechanism underpinning a decline in memory performance reported in this study. It has also been shown that there is increased hippocampal activity in episodic memory performance tasks in KIBRA T carriers when compared with non-T carriers19, consistent with the notion of protection from memory decline. KIBRA T allele carriers have also been shown to have a decreased levels of brain activation compared to non-T allele carriers in several hippocampal regions activated during memory retrieval12. The authors hypothesised that individuals who do not carry the T allele require a greater level of hippocampal activation for memory retrieval12.
In addition to the association studies described above, recent in vivo evidence provides molecular insights into mechanisms by which KIBRA is involved in memory performance. Synaptic plasticity, which is altered in AD, is modulated by dendrin, which in turn binds to the protein that KIBRA encodes (KIBRA; see review41). Further, KIBRA protein contains a protein kinase C (isoform ζ; PKCζ) binding domain42 and has been reported to co-localise with protein kinase M (isoform ζ; PKMζ)43, a brain specific variant of PKCζ, which plays important roles in memory formation and long-term potentiation. Johannsen et al. have shown the function of the KIBRA protein to be regulated by its C2 domain38, which is required for Ca2+ binding and is therefore involved in signal transduction in the neurons. This regulation is hypothesised to mediate the effect of the KIBRA protein on memory formation38. In a recent study, Tracy and colleagues have proposed a novel mechanism by which acetylated tau associated memory loss and disruption of synaptic plasticity is mediated by a reduction in postsynaptic KIBRA protein14. This finding links the previous reports of reduced KIBRA gene expression in AD with a biological mechanism mediated by acetylated tau. Whether the KIBRA T allele affords a level of resilience to this loss of synaptic plasticity remains to be determined.
Our findings indicate that KIBRA rs17070145 genotype, when combined with high brain Aβ-amyloid burden and APOE ε4 carriage, modifies longitudinal rates of decline in verbal episodic memory, a global cognitive composite and hippocampal volume. We propose that early in the disease process of AD, carriers of the KIBRA T-allele are conferred a level of resilience to Aβ-amyloid and APOE ε4 driven decline. The potential mechanisms by which KIBRA contributes to synaptic plasticity, and AD progression warrant further investigation, including the potential impact on Aβ-amyloid accumulation, and may reveal novel pathways contributing to neuroprotection/neurodegeneration. Our results also highlight the potential application of genetics for risk stratification when designing clinical trials, particularly those that employ Aβ-amyloid imaging for screening. The nature of the effects of genetic variations, specifically assessing the combined effect(s) of additional genes affecting cognitive performance would have merit in such settings and requires further investigation.
Methods
Participants
This study included 602 CN Caucasian adults enrolled in the AIBL Study, a prospective longitudinal study of ageing. Information regarding the AIBL Study’s design, enrolment process, neuropsychological assessments, and diagnostic criteria has been previously described34. The clinical classification of CN, MCI or AD was determined, after clinical review, by a panel of old age psychiatrists, geriatricians, neurologists, and neuropsychologists who were blinded to Aβ-amyloid status. Individuals were classified as CN if they did not meet the clinical criteria for diagnosis of MCI44 or dementia45, as described previously34. Approval of the AIBL Study has been granted by each of the ethics committees of each of the member institutions; Austin Health, St Vincent’s Health, Hollywood Private Hospital, and Edith Cowan University, and informed written consent was given by all volunteers. All clinical investigations were conducted in accord with the principles expressed in the Declaration of Helsinki 1975. All participants were assessed every 18-months. Cognitive, neuroimaging and laboratory assessment were acquired within 3-months of each other.
Cognitive Measures
The neuropsychological test battery administered in the AIBL study has been described in detail previously34. Briefly, it incorporates at each 18-month follow-up, the Mini-Mental State Examination (MMSE), Clock Drawing Test, California Verbal Learning Test-Second edition (CVLT-II), Logical Memory I and II (LMI; LMII; Story A only), D-KEFS verbal fluency, a 30-item version of the Boston Naming Test (BNT), Wechsler Test of Adult Reading (WTAR) for premorbid IQ, Digit Span and Digit Symbol-Coding subtests of the Wechsler Adult Intelligence Scale-Third edition (WAIS-III), the Stroop task (Victoria version), and the Rey Complex Figure Test (RCFT). Resultant data from this battery, in addition to the Clinical Dementia Rating (CDR), have been previously used to statistically derive cognitive composites as previously described46. In this study, a verbal episodic memory composite (CDR sum of boxes (CDRSB), LMII, CVLT false positives (CVLTFP) and long delay free recall (CVLTLDFR)), and a statistically driven global composite (CDRSB, MMSE, LMII, CVLTFP and Clock), aimed as a sensitive measure for longitudinal decline in individuals predisposed to AD46, were investigated across five study time points: baseline, 18, 36, 54 and 72 months. A correction for age, gender, years of education, WTAR-estimated premorbid IQ (WAIS-III Full Scale Intelligence Quotient (FSIQ)) and depressive symptoms (Geriatric Depression Scale (GDS)) was incorporated in the calculation of the cognitive composites47.
Brain Imaging
The 602 CN adults included in this study had undergone Aβ-amyloid imaging, at varying time points, with PET using 11C-Pittsburgh Compound B (PiB), 18F-florbetapir or 18F-flutemetamol as previously described48–50. PET standardized uptake value (SUV) ratio (SUVR) data was determined for all tracers using using CapAIBL, a web based freely availably MR-less methodology51. Briefly, SUVs were summed and normalized to either the cerebellar cortex SUV (PiB), whole cerebellum SUV (florbetapir) or pons SUV (flutemetamol) to yield the target-region to reference-region SUVR. These SUVRs were then classified as either low (Αβlow) or high (Αβhigh) Aβ-amyloid burden, based on a tracer-specific SUVR threshold; ≥ 1.5, ≥ 1.10 and ≥ 0.62 for PiB, florbetapir and flutemetamol, respectively, as previously described52. Of these 602 participants, 548 also underwent clinical magnetic resonance imaging (MRI) for clinical screening and co-registration with PET images. MRI parameters have been described in detail previously53. Briefly, a 3 T T1-weighted MRI was performed using the ADNI magnetization-prepared rapid gradient echo protocol, with an in-plane resolution of 1 × 1 mm and a slice thickness of 1.2 mm. Hippocampal volume was calculated after correcting for age in years and intracranial volume, defined as the sum of grey matter, white matter and cerebrospinal fluid volumes, as previously described35.
Genotyping
DNA extraction from 5 mL of whole blood was performed using QIAamp DNA Blood Maxi Kits (Qiagen, Hilden, Germany) according to manufacturer’s instructions. TaqMan® genotyping assays were used to determine APOE (rs7412, assay ID: C____904973_10; rs429358, assay ID: C___3084793_20) and KIBRA (rs17070145, assay ID: C__33286269_10) genotypes (Life Technologies, Carlsbad, CA). All TaqMan® genotyping assays were performed on a QuantStudio 12 K Flex™ Real-Time-PCR systems (Applied Biosystems, Foster City, CA) using the TaqMan® GTXpress™ Master Mix (Life Technologies) methodology as per manufacturer instructions. KIBRA genotype was observed not depart from Hardy-Weinberg equilibrium. For the purpose of this study APOE carrier status is defined by the presence (1 or 2 copies) or absence (0 copies) of the APOE ε4 allele, henceforth referred to as APOE ε4 + ve or APOE ε4-ve, respectively.
Statistical Analyses
All statistical analyses were performed using Rstudio (Rstudio Team 2015) Version 0.98.1103 for Macintosh54. All analyses were performed based on a dominant model for the KIBRA rs17070145-T (minor) allele, i.e. T carrier (i.e. C_T and T_T) compared with non-T carrier (i.e. C_C), as per previous studies12,18–21,24. Baseline demographic data analyses provided means, standard deviations, and percentages across the entire PET imaged cognitively normal sample and stratified by KIBRA rs17070145-T allele carrier (KIBRA-T) and non-carrier (KIBRA non-T) status. ANOVA (age, premorbid IQ, depressive symptoms) and chi-squared tests (gender, years of education, APOE ε4 + ve, high Aβ-amyloid burden) were used to determine the significance of differences between allelic groups. To determine differences in rates of cognitive change and hippocampal atrophy random intercepts linear mixed-effects (LME) models were performed using the “nlme” package in R. LMEs were performed due to their ability to model fixed and random effects, and their robustness when dealing with missing data55.
After the inclusion of main effects within the model, i.e. KIBRA genotype, interaction terms and covariates were included and modelled as described here. Specifically, to investigate the effect of KIBRA on the rate of cognitive decline and hippocampal atrophy, initially a KIBRA × Time interaction was modelled across the entire sample, covarying for APOE ε4 carrier and Aβ-amyloid status, with the cognitive composites and hippocampal volume as the dependent variables. The effect of Aβ status in combination with KIBRA was then investigated by separately modelling an Aβ × KIBRA × Time interaction, co-varying for APOE ε4 carrier status. The third analysis focused on only Αβhigh participants, with APOE included within an APOE × KIBRA × Time interaction. In addition, all analyses for hippocampal atrophy co-varied for gender. Graphical representations of all models are presented with time dependent standard error. Further, for all analyses correction for the False Discovery Rate (FDR) using Q-Value (bootstrap method) was performed56. Finally, chi-squared analyses were performed between groups to ascertain that group differences in rates of decline were not due to disproportionate rates of clinical conversion over the course of the study.
Data availability
All data and samples used in this study are derived from the Australian Imaging, Biomarkers and Lifestyle (AIBL) Study of Ageing. All AIBL data, and that specific to this study, is publically accessible to all interested parties through an Expression of Interest procedure and is governed by the AIBL Data Use Agreement, for more information please see https://aibl.csiro.au/awd/.
Electronic supplementary material
Acknowledgements
Funding for the AIBL study was provided in part by the study partners [Commonwealth Scientific Industrial and research Organization (CSIRO), Edith Cowan University (ECU), Mental Health Research institute (MHRI), National Ageing Research Institute (NARI), Austin Health, CogState Ltd.]. The AIBL study has also received support from the National Health and Medical Research Council (NHMRC) and the Dementia Collaborative Research Centres program (DCRC2), as well as funding from the Science and Industry Endowment Fund (SIEF) and the Cooperative Research Centre (CRC) for Mental Health – funded through the CRC Program (Grant ID:20100104), an Australian Government Initiative. We thank all those who took part as subjects in the study for their commitment and dedication to helping advance research into the early detection and causation of AD. We kindly thank all AIBL Research Group members (http://aibl.csiro.au/about/aibl-research-team/).
Author Contributions
T.P. contributed to acquisition of genetic data, statistical analysis, interpretation of findings, drafting the manuscript. S.C.B. contributed to specific study concept and design, study supervision, statistical analysis, interpretation of findings, and drafting of the manuscript. V.D., P.B. contributed to acquisition and analysis of imaging data and revising the manuscript. G.S. contributed to A.I.B.L. study design, obtaining funding, interpretation of findings. K.B., L.M. contributed to acquisition of genetic data. D.A., A.I.B., C.L.M., C.C.R., R.N.M. contributed to A.I.B.L. study design, obtaining funding and revising the manuscript. P.M. contributed to A.I.B.L. study design, obtaining funding, interpretation of findings, revising the manuscript. SRS contributed to revising the manuscript. D.G., G.V. contributed to study supervision and revising the manuscript. V.L.V. contributed to current study concept and design, obtaining funding, study supervision, acquisition of data, interpretation of findings and drafting of the manuscript. S.M.L. contributed to current study concept and design, obtaining funding, study supervision, acquisition of data, interpretation of findings and drafting of the manuscript. All authors read and approved the final manuscript.
Competing Interests
CLM is an advisor to Prana Biotechnology Ltd and a consultant to Eli Lilly. PM is a full-time employee of Cogstate Ltd. DA has served on scientific advisory boards for Novartis, Eli Lilly, Janssen, and Pfizer Inc. RNM is a consultant to Alzhyme. SML has previously been a paid consultant to Alzhyme. CCR has served on scientific advisory boards for Bayer Pharma, Elan Corporation, GE Healthcare and AstraZeneca; has received speaker honoraria from Bayer Pharma and GE Healthcare; and has received research support from Bayer Pharma, GE Healthcare, Piramal Lifesciences and Avid Radiopharmaceuticals. VLV served as a consultant for Bayer Pharma; and received research support from a NEDO grant from Japan. All other authors have nothing to disclose.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20513-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Villemagne VL, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Annals of neurology. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews KA, et al. Atrophy rates in asymptomatic amyloidosis: implications for Alzheimer prevention trials. PLoS One. 2013;8:e58816. doi: 10.1371/journal.pone.0058816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villemagne VL, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 4.El Haj M, et al. Apolipoprotein E (APOE) epsilon4 and episodic memory decline in Alzheimer’s disease: A review. Ageing research reviews. 2016;27:15–22. doi: 10.1016/j.arr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, et al. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86:127–134. doi: 10.1136/jnnp-2014-307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim YY, et al. BDNF Val66Met, Abeta amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiology of aging. 2013;34:2457–2464. doi: 10.1016/j.neurobiolaging.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Lim YY, et al. APOE and BDNF polymorphisms moderate amyloid beta-related cognitive decline in preclinical Alzheimer’s disease. Molecular psychiatry. 2015;20:1322–1328. doi: 10.1038/mp.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy KM, et al. BDNF val66met polymorphism affects aging of multiple types of memory. Brain research. 2015;1612:104–117. doi: 10.1016/j.brainres.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cathomas F, Vogler C, Euler-Sigmund JC, de Quervain DJ, Papassotiropoulos A. Fine-mapping of the brain-derived neurotrophic factor (BDNF) gene supports an association of the Val66Met polymorphism with episodic memory. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2010;13:975–980. doi: 10.1017/S1461145710000519. [DOI] [PubMed] [Google Scholar]
- 10.Lim YY, et al. Abeta amyloid, cognition, and APOE genotype in healthy older adults. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013;9:538–545. doi: 10.1016/j.jalz.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Lim YY, et al. APOE epsilon4 moderates amyloid-related memory decline in preclinical Alzheimer’s disease. Neurobiology of aging. 2015;36:1239–1244. doi: 10.1016/j.neurobiolaging.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Papassotiropoulos A, et al. Common Kibra alleles are associated with human memory performance. Science. 2006;314:475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- 13.Kremerskothen J, et al. Characterization of KIBRA, a novel WW domain-containing protein. Biochemical and biophysical research communications. 2003;300:862–867. doi: 10.1016/S0006-291X(02)02945-5. [DOI] [PubMed] [Google Scholar]
- 14.Tracy, T. E. et al. Acetylated Tau Obstructs KIBRA-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss. Neuron, 10.1016/j.neuron.2016.03.005 (2016). [DOI] [PMC free article] [PubMed]
- 15.Elias MF, et al. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Archives of neurology. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 16.Grober E, et al. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society. 2008;14:266. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derby CA, et al. Screening for predementia AD Time-dependent operating characteristics of episodic memory tests. Neurology. 2013;80:1307–1314. doi: 10.1212/WNL.0b013e31828ab2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida OP, et al. KIBRA genetic polymorphism influences episodic memory in later life, but does not increase the risk of mild cognitive impairment. Journal of cellular and molecular medicine. 2008;12:1672–1676. doi: 10.1111/j.1582-4934.2008.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kauppi K, Nilsson LG, Adolfsson R, Eriksson E, Nyberg L. KIBRA polymorphism is related to enhanced memory and elevated hippocampal processing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:14218–14222. doi: 10.1523/JNEUROSCI.3292-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witte AV, Kobe T, Kerti L, Rujescu D, Floel A. Impact of KIBRA Polymorphism on Memory Function and the Hippocampus in Older Adults. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2016;41:781–790. doi: 10.1038/npp.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasuda Y, et al. Association study of KIBRA gene with memory performance in a Japanese population. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2010;11:852–857. doi: 10.3109/15622971003797258. [DOI] [PubMed] [Google Scholar]
- 22.Corneveaux JJ, et al. Evidence for an association between KIBRA and late-onset Alzheimer’s disease. Neurobiology of aging. 2010;31:901–909. doi: 10.1016/j.neurobiolaging.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bates TC, et al. Association of KIBRA and memory. Neuroscience letters. 2009;458:140–143. doi: 10.1016/j.neulet.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 24.Muse J, et al. WWC1 genotype modulates age-related decline in episodic memory function across the adult life span. Biological psychiatry. 2014;75:693–700. doi: 10.1016/j.biopsych.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaper K, Kolsch H, Popp J, Wagner M, Jessen F. KIBRA gene variants are associated with episodic memory in healthy elderly. Neurobiology of aging. 2008;29:1123–1125. doi: 10.1016/j.neurobiolaging.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Schuck NW, et al. Aging and KIBRA/WWC1 genotype affect spatial memory processes in a virtual navigation task. Hippocampus. 2013;23:919–930. doi: 10.1002/hipo.22148. [DOI] [PubMed] [Google Scholar]
- 27.Laukka EJ, et al. Genetic effects on old-age cognitive functioning: a population-based study. Psychology and aging. 2013;28:262–274. doi: 10.1037/a0030829. [DOI] [PubMed] [Google Scholar]
- 28.Nacmias B, et al. KIBRA gene variants are associated with episodic memory performance in subjective memory complaints. Neuroscience letters. 2008;436:145–147. doi: 10.1016/j.neulet.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Wersching H, et al. Impact of common KIBRA allele on human cognitive functions. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:1296–1304. doi: 10.1038/npp.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JJ, Lavebratt C, Lou F, Forsell Y. KIBRA genetic polymorphism and cognitive dysfunction in depression. Psychiatry research. 2015;226:405–406. doi: 10.1016/j.psychres.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Boraxbekk CJ, et al. Investigating the influence of KIBRA and CLSTN2 genetic polymorphisms on cross-sectional and longitudinal measures of memory performance and hippocampal volume in older individuals. Neuropsychologia. 2015;78:10–17. doi: 10.1016/j.neuropsychologia.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Franks KH, Summers MJ, Vickers JC. KIBRA gene polymorphism has no association with verbal or visual episodic memory performance. Frontiers in aging neuroscience. 2014;6:270. doi: 10.3389/fnagi.2014.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Need AC, et al. Failure to replicate effect of Kibra on human memory in two large cohorts of European origin. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2008;147B:667–668. doi: 10.1002/ajmg.b.30658. [DOI] [PubMed] [Google Scholar]
- 34.Ellis KA, et al. TheAustralian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. International psychogeriatrics / IPA. 2009;21:672–687. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 35.Dore V, et al. Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA neurology. 2013;70:903–911. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- 36.Andrews KA, et al. Acceleration of hippocampal atrophy rates in asymptomatic amyloidosis. Neurobiology of aging. 2016;39:99–107. doi: 10.1016/j.neurobiolaging.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Lim YY, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79:1645–1652. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- 38.Johannsen S, Duning K, Pavenstadt H, Kremerskothen J, Boeckers TM. Temporal-spatial expression and novel biochemical properties of the memory-related protein KIBRA. Neuroscience. 2008;155:1165–1173. doi: 10.1016/j.neuroscience.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Burgess JD, et al. Association of common KIBRA variants with episodic memory and AD risk. Neurobiology of aging. 2011;32:557 e551–559. doi: 10.1016/j.neurobiolaging.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piras IS, et al. Whole transcriptome profiling of the human hippocampus suggests an involvement of the KIBRA rs17070145 polymorphism in differential activation of the MAPK signaling pathway. Hippocampus. 2017;27:784–793. doi: 10.1002/hipo.22731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwab LC, Luo V, Clarke CL, Nathan PJ. Effects of the KIBRA Single Nucleotide Polymorphism on Synaptic Plasticity and Memory: A Review of the Literature. Current neuropharmacology. 2014;12:281–288. doi: 10.2174/1570159X11666140104001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buther K, Plaas C, Barnekow A, Kremerskothen J. KIBRA is a novel substrate for protein kinase Czeta. Biochemical and biophysical research communications. 2004;317:703–707. doi: 10.1016/j.bbrc.2004.03.107. [DOI] [PubMed] [Google Scholar]
- 43.Yoshihama Y, Hirai T, Ohtsuka T, Chida K. KIBRA Co-localizes with protein kinase Mzeta (PKMzeta) in the mouse hippocampus. Biosci Biotechnol Biochem. 2009;73:147–151. doi: 10.1271/bbb.80564. [DOI] [PubMed] [Google Scholar]
- 44.Winblad B, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 45.McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 46.Burnham SC, et al. Novel Statistically-Derived Composite Measures for Assessing the Efficacy of Disease-Modifying Therapies in Prodromal Alzheimer’s DiseaseTrials: An AIBL Study. Journal of Alzheimer’s disease: JAD. 2015;46:1079–1089. doi: 10.3233/JAD-143015. [DOI] [PubMed] [Google Scholar]
- 47.Burnham SC, et al. Comparision of three normative data correction approaches: A cross-sectional evaluation in the AIBL study. Alzheimer’s & Dementia. 2014;10:P4–293. doi: 10.1016/S1552-5260(14)02425-X. [DOI] [Google Scholar]
- 48.Rowe CC, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Clark CM, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandenberghe R, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Annals of neurology. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 51.Bourgeat P, et al. Comparison of MR-less PiB SUVR quantification methods. Neurobiology of aging. 2015;36(Suppl 1):S159–166. doi: 10.1016/j.neurobiolaging.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 52.Rowe CC, et al. Predicting Alzheimer disease with beta-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Annals of neurology. 2013;74:905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 53.Bourgeat P, et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- 54.RStudio: Integrated Development for R v. 0.98.1103 (Boston, MA, 2015).
- 55.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of general psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 56.Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64:479–498. doi: 10.1111/1467-9868.00346. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and samples used in this study are derived from the Australian Imaging, Biomarkers and Lifestyle (AIBL) Study of Ageing. All AIBL data, and that specific to this study, is publically accessible to all interested parties through an Expression of Interest procedure and is governed by the AIBL Data Use Agreement, for more information please see https://aibl.csiro.au/awd/.