ABSTRACT
DNT (2,4-dinitrotoluene), a volatile impurity in military-grade 2,4,6-trinitrotoluene (TNT)-based explosives, is a potential tracer for the detection of buried landmines and other explosive devices. We have previously described an Escherichia coli bioreporter strain engineered to detect traces of DNT and have demonstrated that the yqjF gene promoter, the sensing element of this bioreporter, is induced not by DNT but by at least one of its transformation products. In the present study, we have characterized the initial stages of DNT biotransformation in E. coli, have identified the key metabolic products in this reductive pathway, and demonstrate that the main DNT metabolite that induces yqjF is 2,4,5-trihydroxytoluene. We further show that E. coli cannot utilize DNT as a sole carbon or nitrogen source and propose that this compound is metabolized in order to neutralize its toxicity to the cells.
IMPORTANCE The information provided in this article sheds new light both on the microbial biodegradability of nitroaromatic compounds and on the metabolic capabilities of E. coli. By doing so, it also clarifies the pathway leading to the previously unexplained induction of the E. coli yqjF gene by 2,4-dinitrotoluene, an impurity that accompanies 2,4,6-trinitrotoluene (TNT)-based explosives. Our improved understanding of these processes will serve to molecularly enhance the performance of a previously described microbial bioreporter of buried landmines and other explosive devices, in which the yqjF gene promoter serves as the sensing element.
KEYWORDS: biosensors; bioreporters; biotransformation; biodegradation; 2,4-dinitrotoluene; landmines; Escherichia coli
INTRODUCTION
DNT (2,4-dinitrotoluene) is a common impurity in 2,4,6-trinitrotoluene (TNT)-based explosives, which are present in ca. 80% of all buried landmines (1). Although DNT accounts for less than 1% of the explosive material, its vapor pressure is much higher than that of TNT, resulting in higher concentrations in the soil above buried explosive devices (2). This attribute, along with better stability than other TNT impurities such as 1,3-dinitrobenzene (3), renders DNT a preferred tracer for detecting buried landmines.
Yagur-Kroll et al. (4, 5) have reported the construction and characterization of an Escherichia coli-based bioreporter for the detection of TNT and DNT harboring a fusion of the promoter region of the yqjF gene (GenBank accession no. AAC76136.1), encoding a predicted quinol oxidase subunit, to either the green fluorescent protein GFPmut2 gene or to the Photorhabdus luminescens bioluminescence luxCDABE genes. Remote detection of buried antipersonnel landmines by the fluorescent variant was recently demonstrated (6). Yagur-Kroll et al. (4) have also shown that the yqjF gene promoter was induced neither by DNT nor by any growth medium component, but rather by one or more transformation products of DNT and TNT; these compounds, however, have not been identified.
While TNT biotransformation by E. coli has been characterized (7), to our knowledge the metabolic fate of DNT by this bacterium has not been previously described. Two main aerobic pathways for bacterial biotransformation of DNT have been reported in other microorganisms (Fig. 1). Reductive transformation of DNT was observed in several species (8–12), in a pathway (Fig. 1A) that includes the reduction of the nitro groups to amines by nitroreductases, with possible hydroxylamino and nitroso intermediates (not shown), yielding 2-amino-4-nitrotoluene (2A4NT) and 4-amino-2-nitrotoluene (4A2NT). Both of these compounds are then reduced to 2,4-diaminotoluene (DAT). A few studies also suggested the subsequent acetylation of the amino products (8, 10). To our knowledge, full mineralization of DNT by a single strain via a reductive pathway has not been reported. The nitroso intermediates mentioned above are highly unstable, and their detection is extremely challenging (13). They are also very rapidly transformed in vivo by nitroreductases, the affinity of which to nitroso derivatives is approximately 10,000-fold higher than the affinity to nitro species (14).
FIG 1.
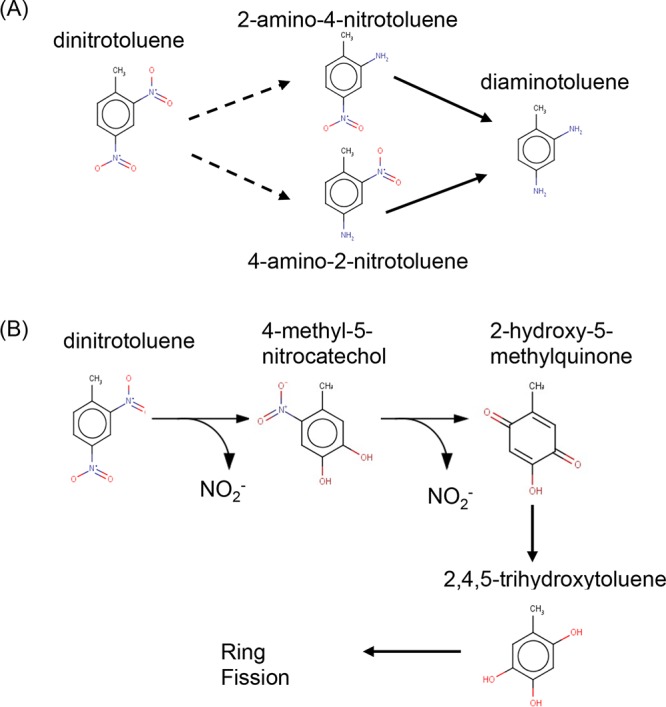
Reductive (A) and oxidative (B) pathways for the initial stages in DNT biotransformation. Dashed arrows represent possible intermediates. Panel A is a simplification of the pathway shown in a study by McCormick et al. (8), while panel B is adapted from a paper by Suen and Spain (15).
An oxidative pathway for DNT degradation (Fig. 1B), described first in a Pseudomonas sp. strain by Suen and Spain (15), is initiated by a dioxygenase attack on DNT, yielding 4-methyl-5-nitrocatechol (4M5NC), with a subsequent release of nitrite. This is followed by monooxygenase activity yielding 2-hydroxy-5-methylquinone (2H5MQ), leading to the release of an additional nitrite. This compound is then reduced to 2,4,5-trihydroxytoluene (THT), followed by ring fission. Dioxygenation of the aromatic ring during oxidative degradation of polynitroaromatics leaves it electron deficient, which hinders subsequent electrophilic dioxygenations. Therefore, most polynitroaromatic compounds appear to be metabolized via reductive mechanisms (16).
Several microbial strains able to use DNT as the sole carbon (17, 18) or nitrogen (19, 20) source were reported. DNT can serve as a potential nitrogen source through the release of either nitrite or ammonium during oxidative or reductive biotransformation, respectively (16).
We report here the characterization of an aerobic biotransformation pathway of DNT in E. coli that includes both reductive and oxidative steps. Furthermore, we show that the DNT metabolite THT is the main inducer of the yqjF-based DNT bioreporter.
RESULTS
As previously reported (4), the induction of the yqjF gene promoter, which drives the bioluminescent response of a DNT reporter strain harboring a plasmid-borne yqjF::luxCDABE fusion, is not caused directly by DNT but rather by one or more of its metabolites. This is demonstrated by the results shown in Fig. 2, which displays the response of this reporter to DNT and to its transformation products. It is shown in Fig. 2A that while the induction by freshly supplied DNT is delayed and peaks only after ca. 500 min, there is an immediate response to its transformation products present in spent growth medium (removed from a culture after 300 min of growth). It may be observed in Fig. 2B that the delayed response diminishes with time whereas the immediate one increases in strength for the first 5 h and reaches much higher intensities. The complete lack of induction after over 20 h of incubation probably indicates the full transformation of both DNT and the inducing metabolite(s) to downstream transformation products.
FIG 2.
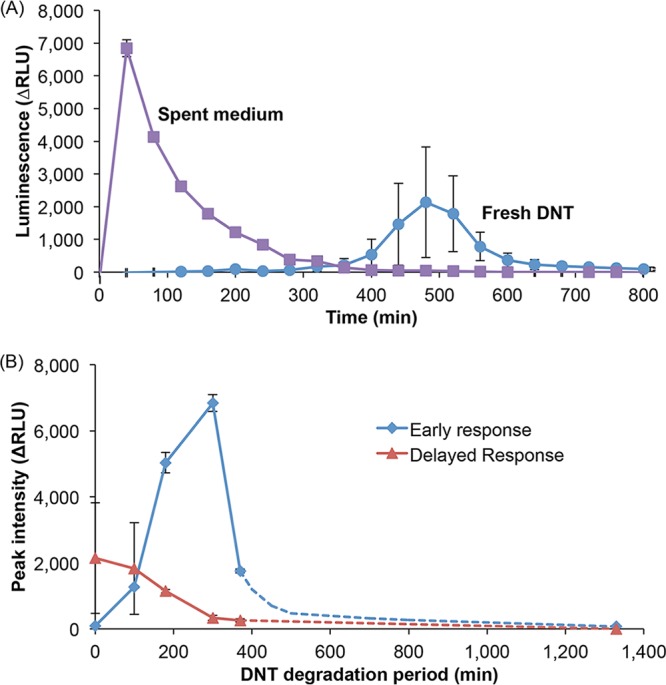
Induction of yqjF by DNT and its transformation products. A WT E. coli culture was incubated with DNT (100 mg/liter) in minimal M9 medium; medium samples were withdrawn at the indicated time points and tested for their ability to induce bioluminescence in an E. coli strain harboring the plasmid-borne yqjF::luxCDABE fusion. (A) Response of yqjF::luxCDABE to medium samples withdrawn at time zero (fresh DNT) and following 300 min of incubation (spent medium). (B) Immediate (response to spent medium) and delayed (response to fresh medium) peak intensities as a function of DNT biodegradation time. Presented values are averages and standard deviations from at least two independent duplicate experiments.
Characterization of DNT metabolites and roles of selected genes.
The biotransformation products of DNT metabolism in E. coli were identified and quantified by liquid chromatography/mass spectrometry (LC/MS) and LC/UV (see Fig. S1 in the supplemental material) and tested for their ability to induce yqjF (Table 1). The accumulation kinetics of the two main metabolites found, 4A2NT and 4-hydroxylamino-2-nitrotoluene (4HA2NT) (Fig. 3), suggest a reductive transformation mechanism. 4M5NC, a potential metabolite that is the result of oxidative biotransformation of DNT, was not detected in the medium, nor did it induce yqjF when introduced to it.
TABLE 1.
DNT metabolites identified in this study
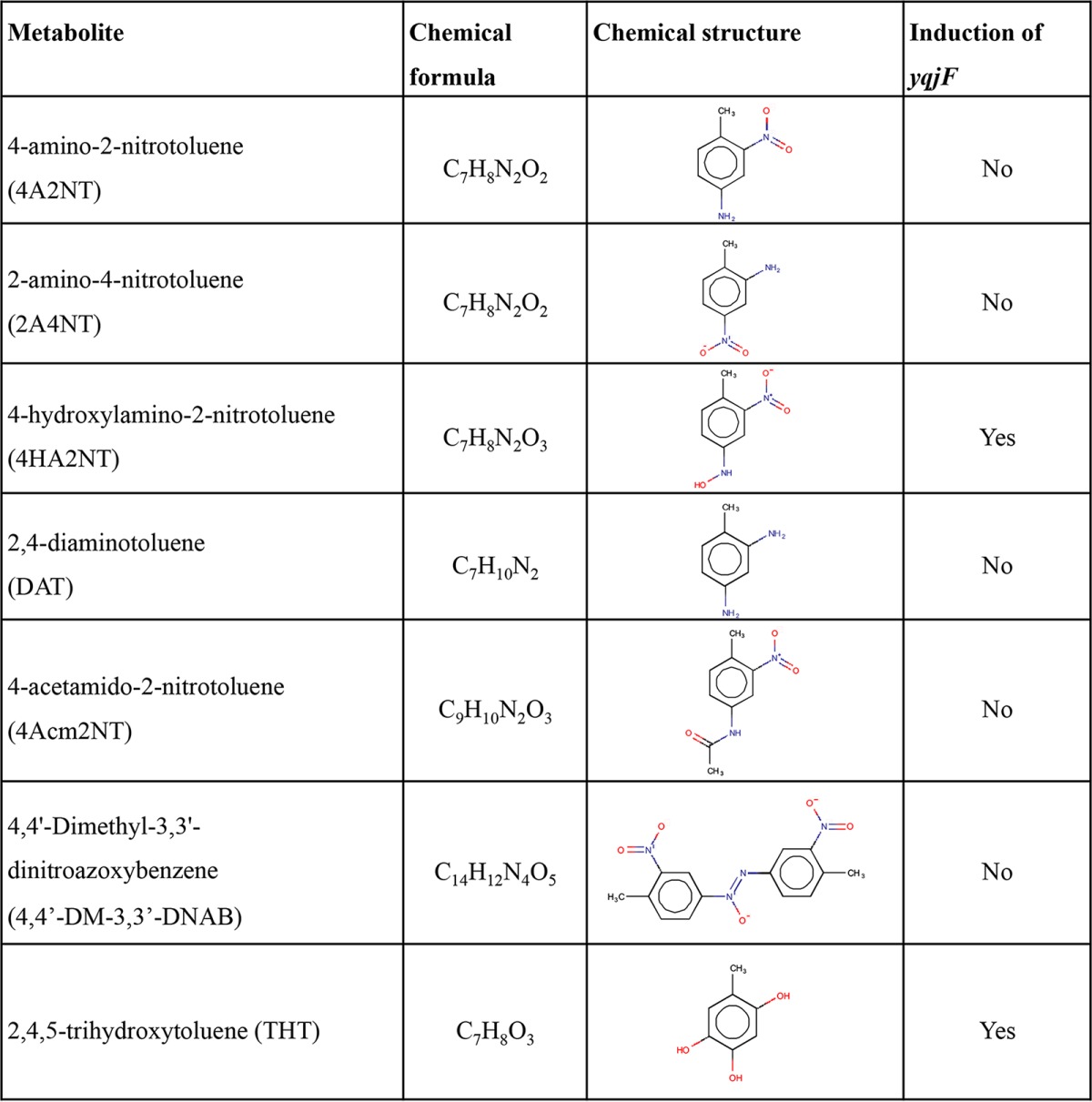
FIG 3.
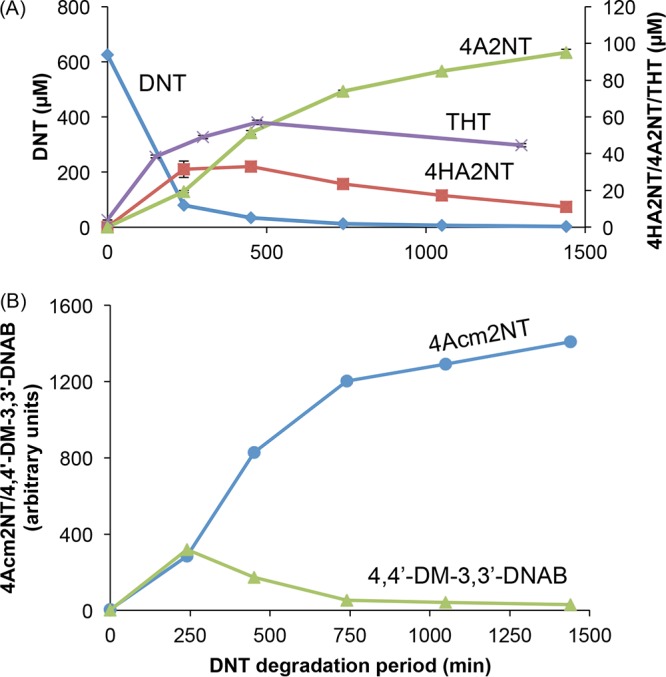
DNT degradation and metabolite accumulation. (A) DNT removal and accumulation kinetics of 4HA2NT, 4A2NT, and THT; (B) accumulation of 4Acm2NT and 4,4′-DM-3,3′-DNAB. Concentrations of the last two compounds are presented in the LC/MS-derived arbitrary units.
The main metabolic product found in the medium was 4A2NT, with very limited accumulation of the equivalent isomer, 2A4NT. This compound continued to accumulate for at least 25 h (Fig. 3). Two potential transformation products of 4A2NT, DAT and 4-acetamido-2-nitrotoluene (4Acm2NT), were also observed, but at much lower concentrations (DAT concentrations were below the quantification limit, and this compound is therefore absent from Fig. 3). The former product could be detected only when the cells were grown in lysogeny broth (LB) but not in M9 medium (not shown), probably due to slower metabolic rates in the latter medium. The other major metabolite identified, 4HA2NT, accumulated for the first 500 to 600 min, and then its concentration slowly decreased. It is therefore suggested that DNT is reduced to 4HA2NT, via a possible nitroso intermediate, followed by additional reduction of the nitro/hydroxylamino groups to 4A2NT, which is then very slowly transformed to DAT and 4Acm2NT.
Another compound shown to accumulate and then to be depleted was 4,4-dimethyl-3,3′-dinitroazoxybenzene (4,4′-DM-3,3′-DNAB), a condensation product of 4HA2NT. A possible isomer of this molecule was also detected, exhibiting the same m/z ratio and a similar fragmentation pattern, but a different retention time.
A different compound identified as a DNT transformation product, the appearance of which cannot be explained by a reductive biotransformation pathway, was 2,4,5-trihydroxytoluene (THT). When fragmented with tandem mass spectrometry (MS/MS), a typical fragment with m/z of 109.029 was identically detected both in a sample containing DNT biotransformation products and in the THT standard (see Fig. S2 in the supplemental material). This metabolite accumulates in the medium for ca. 200 to 300 min (Fig. 3A) and then slowly disappears. It was also detected in a sample containing 4HA2NT biotransformation products (see Fig. S3 in the supplemental material). A hypothetical pathway for the transformation of 4HA2NT to THT involves the monooxygenation of 4HA2NT on the ortho position, followed by dioxygenation of the aromatic ring on the para position. Dioxygenation of the carbon atom bearing the hydroxylamino group on the para position before the monooxygenation of the carbon atom bearing the second nitro group is unlikely, as this would yield 4M5NC as an intermediate metabolite, a compound that did not induce the yqjF-based bioreporter (not shown). Furthermore, no 4M5NC was detected in the growth medium, leading to the conclusion that DNT is not transformed to this compound. It should be noted that a compound with a mass corresponding to that of 2H5MQ, the quinone derivative of THT, was also identified. This compound may account for some of the missing mass that may be inferred from Fig. 3A data.
It was previously reported that a mutation in both nitroreductase genes nfsA and nfsB inhibits the induction of yqjF by DNT (4), implying that the direct inducer of yqjF is produced downstream of the activity of the enzymes encoded by these genes. To assess the nature of this inhibition, E. coli strains lacking these genes were incubated with DNT, and the composition of the growth medium was analyzed by LC/MS as a function of incubation time. As displayed in Fig. 4A, DNT removal by the ΔnfsA mutant was not significantly different from that of the wild-type (WT) strain, and the ΔnfsB mutant exhibited only a minor reduction of the DNT transformation rate. However, a deletion of both genes resulted in a very significant inhibition of DNT elimination (only ca. 30% removal in 24 h). Figure 4B presents 4HA2NT accumulation data in the same experimental systems. As expected, synthesis of this compound was almost completely abolished in the double mutant; in the single mutants, its accumulation was partially inhibited, indicating that while DNT reduction is only marginally affected, there may be a more obvious impact of the individual mutations in the downstream process.
FIG 4.
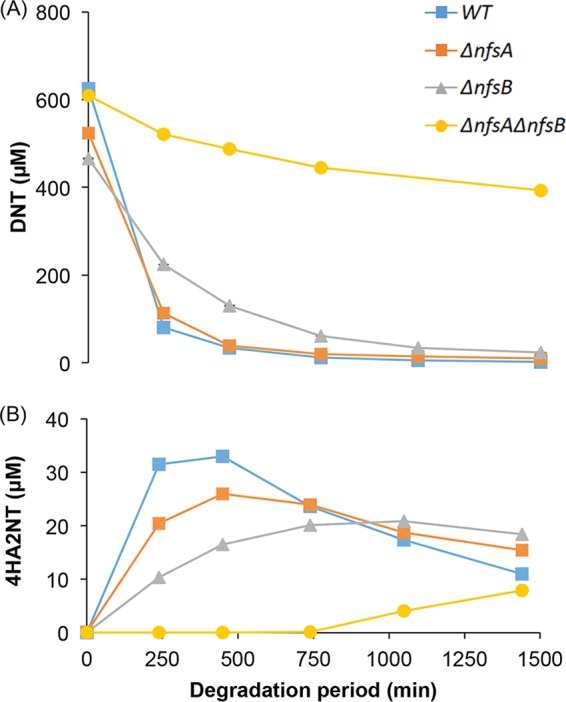
Elimination of DNT (A) and accumulation of 4HA2NT (B) in WT and in nitroreductase-deficient strains.
DNT as a sole carbon/nitrogen source for E. coli.
To assess the biochemical/metabolic rationale for DNT biotransformation by E. coli, we have attempted to grow it with DNT as either the only carbon or the only nitrogen source, but no significant growth was observed under any of the experimental conditions tested.
Nevertheless, following prolonged incubation with glucose as a carbon source and DNT as the sole potential N source, a partial depletion (by ca. 13% in 48 h) of DNT was reproducibly observed, with the concomitant accumulation of metabolites characteristic of reductive DNT biotransformation. This was not observed in the absence of glucose (see Fig. S4 in the supplemental material). Thus, while DNT by itself cannot support growth, it is nevertheless at least partially metabolized by the cells even under nongrowing conditions. A possible rationale for this phenomenon may be provided by the data in Table 2, which show that DNT is toxic to E. coli cells in the absence of glucose, but not in its presence. It thus appears that DNT exerts a negative effect on the cells' viability, which is eliminated when it is partially metabolized in the presence of a utilizable carbon source such as glucose. It is therefore possible that DNT biotransformation may act as a detoxification defense mechanism against nitroaromatics toxicity.
TABLE 2.
DNT exerts an inhibitory effect on E. coli growth in the absence of glucose but not in its presence
| Presence or absence of: |
E. coli CFU log differencea | ||
|---|---|---|---|
| DNT | Glucose (2 g/liter) | NH4Cl (1 g/liter) | |
| + | − | − | −1.24 ± 0.2 |
| + | + | − | −0.07 ± 0.1 |
| + | − | + | −0.84 ± 0.1 |
| + | + | + | 1.83 ± 0.3 |
| − | + | + | 1.74 ± 0.04 |
| − | − | + | −0.05 ± 0.2 |
| − | + | − | −0.17 ± 0.1 |
Difference in CFU counts after 21 h of incubation of cultures grown with DNT (50 mg/liter), in the absence or presence of either glucose, ammonium chloride, or both. A sample containing glucose and NH4Cl but no DNT served as a positive control. Initial cell concentration in all cultures was 1.73 × 107/ml.
Induction of yqjF by DNT and its metabolic by-products.
The effects of the nsfA and nsfB mutations on yqjF induction by DNT, previously reported by Yagur-Kroll et al. (4), were reinvestigated along with a study of the effects of these two mutations on the induction by its downstream metabolite 4HA2NT. The bioluminescent response to DNT of the yqjF::luxCDABE fusion in the double nitroreductase-deficient host was practically abolished (a negative mutation effect of over 99.9% [Table 3]), as was that in the nsfB mutant (−98.8%). A knockout of nfsA only did not significantly change the luminescent response of the yqjF reporter. When spiked with 4HA2NT, the inhibition in the mutants missing nfsB or both nitroreductases was not as dramatic (ca. −70%). Interestingly, an increase in response was observed when only nfsA was missing (Table 3). When the same experiment was performed with externally supplied THT, the mutation effects demonstrated by these mutated strains were insignificant.
TABLE 3.
Effects of nfs mutations on the response of yqjF::luxCDABE to either DNT, 4HA2NT, or THTa
| Mutation(s) | Mutation effect (%) |
||
|---|---|---|---|
| DNT (67.5 mg/liter) | 4HA2NT (50 mg/liter) | THT (25 mg/liter) | |
| ΔnfsA | −13.26 ± 6.8 | 137.7 ± 17.9 | −38.6 ± 3.2 |
| ΔnfsB | −98.83 ± 1.1 | −70.3 ± 7.1 | −26.5 ± 5.5 |
| ΔnfsA ΔnfsB | −99.93 ± 0.04 | −66 ± 19.8 | 15.9 ± 10.0 |
Mutation effect values were calculated as described in Materials and Methods. Presented values are averages and standard deviations from at least 3 independent duplicate experiments.
Identification of the yqjF inducer.
As previously shown (reference 4 and Fig. 2), a used medium containing DNT biotransformation products caused a practically immediate induction of yqjF, indicating that this gene is induced by a DNT metabolite rather than directly by DNT. The direct yqjF inducer would therefore have to display two properties: (i) a lag-free activation of yqjF and (ii) accumulation in the growth medium in a pattern that correlates with both the degree of yqjF induction and that of DNT depletion. As shown in Fig. 3 above, 4HA2NT accumulated during the first 350 to 500 min of DNT biotransformation and then slowly disappeared from the medium. Correspondingly, the strongest response of yqjF to DNT biotransformation products was achieved with a used medium after 300 min of biotransformation (Fig. 2).
An nfsB mutant exhibited both a very limited 4HA2NT accumulation (Fig. 4B) and induction of yqjF (Table 3), while a mutant strain in which both nfsA and nfsB were missing exhibited practically no 4HA2NT accumulation or induction of yqjF, suggesting that both enzymes are active in the initial reduction of DNT to 4HA2NT. Since the two reductive biotransformation products of 4HA2NT, 4A2NT and DAT, as well as its condensation product, 4,4′-DM-3,3′-DNAB, did not induce the yqjF reporter (Table 1), it was hypothesized that either 4HA2NT or a downstream metabolite in a potentially nonreductive pathway of this molecule is the inducing metabolite.
Indeed, as may be observed in Fig. 5A, induction of yqjF by 4HA2NT is, as expected, immediate. However, ca. 100 min after exposure to 4HA2NT, a second and much larger induction peak is observed, suggesting that 4HA2NT is further metabolized to a more potent inducer. To confirm this hypothesis, the yqjF::lux bioreporter was incubated with 4HA2NT biotransformation products, obtained from a used medium in which strain BW25113 was incubated with 50 mg/liter 4HA2NT for 120 min. The induction kinetics clearly show the existence of a metabolic product of 4HA2NT in the spent medium that acts as a potent and direct inducer of yqjF. The transformation product that best fits this role is THT; indeed, a strong and direct response was observed when a culture bearing the yqjF::luxCDABE fusion was exposed to this compound (Fig. 5B). The accumulation of this metabolite in the growth medium, as shown above in Fig. 3, follows the response kinetics of the yqjF-based bioreporter to DNT.
FIG 5.
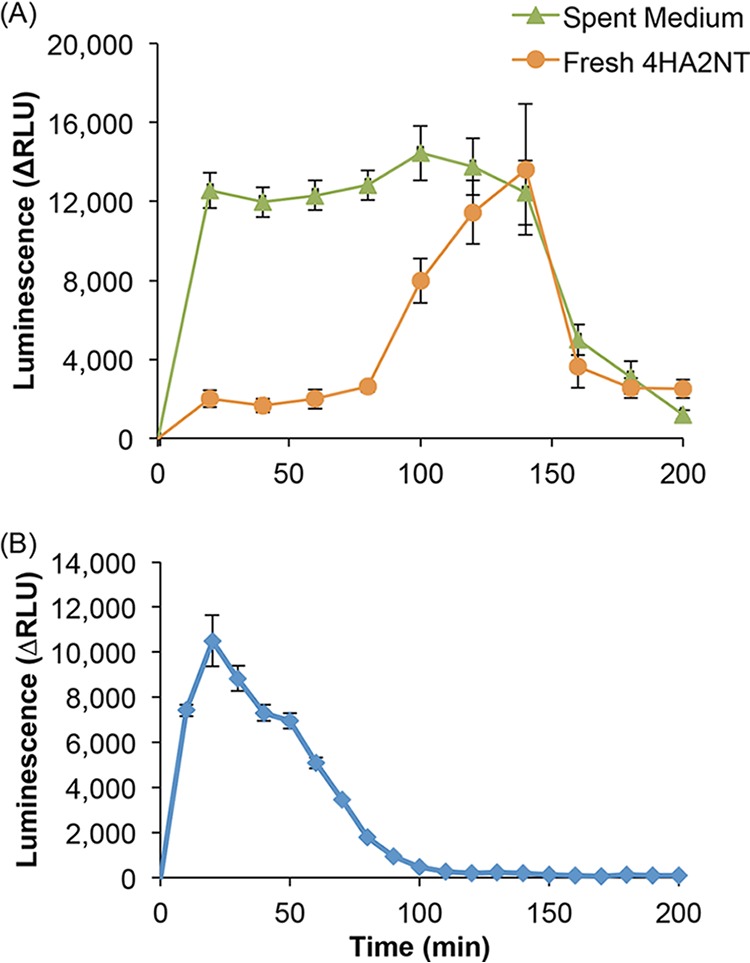
Induction of yqjF by 4HA2NT and by THT. (A) Luminescent response of yqjF::luxCDABE to 50 mg/liter 4HA2NT and to a spent medium (collected after 120-min incubation of WT E. coli with 4HA2NT) containing its transformation products. (B) Luminescent response of yqjF::luxCDABE to 12.5 mg/liter THT.
DISCUSSION
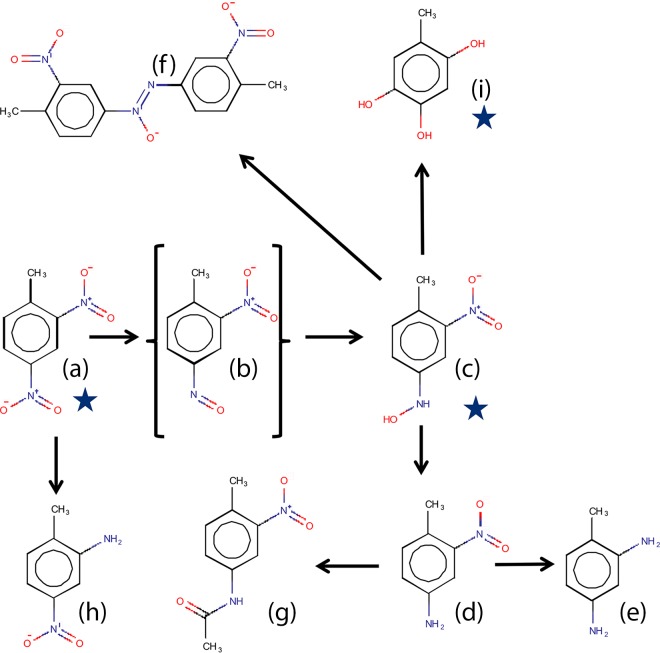
We show in this communication that DNT is metabolized by E. coli and that its foremost transformation pathway is reductive. We have identified the main metabolites in the initial stages of this pathway and have described their accumulation in the growth medium. We also propose the existence of an additional, presently hypothetical, nonreductive pathway, a product of which promotes the transcription of yqjF. Our data support the biotransformation scheme presented in Fig. 6, which includes the reduction of DNT to 4HA2NT, hypothetically through a 4-nitroso-2-nitrotoluene (4NO2NT) intermediate, followed by further reduction to 4A2NT, which is then either reduced to DAT or acetylated to 4Acm2NT. In addition, at least some of the 4HA2NT is condensed to produce 4,4′-DM-3,3′-DNAB and probably an additional isomer of this compound. This pathway is somewhat similar to that described by McCormick et al. (8) in the fungus Mucrosporium. We further suggest that some of the 4HA2NT is transformed to THT by reduction of the remaining nitro group, followed by mono- and dioxygenation of the carbon atoms bearing the resulting amine and hydroxylamino groups. A similar mechanism for the oxygenation of aminoaromatic compounds to catechols has been previously described (21–24). A BLAST analysis (25) performed to identify E. coli homologs to methylaniline dioxygenase and flavin monooxygenase genes from other species revealed several E. coli proteins that exhibited high sequence resemblance (see Table S1 in the supplemental material), indicating that such processes may take place also in this bacterium. Further research is necessary to substantiate this hypothetical pathway. Several studies (7, 26) demonstrated that the E. coli nitroreductases NfsA and NfsB exhibit a significantly greater reduction rate of the nitro derivatives on the para position than on the ortho position of TNT. Assuming a similar reduction mechanism for DNT, this could explain the accumulation of 4A2NT rather than 2A4NT.
FIG 6.
Suggested pathway for DNT metabolism in E. coli. DNT (a) is reduced by nitroreductases to 4NO2NT (b), 4HA2NT (c), 4A2NT (d), and finally to DAT (e). Reduction of DNT to 2A4NT (h) is less substantial. 4HA2NT (c) is either condensed to yield 4,4′-DM-3,3′-DNAB (f), or transformed to THT (i) by hypothesized mono- and deoxygenation steps. 4A2NT is acetylated to 4Acm2NT (g). The product labeled “b” is hypothetical. Starred compounds induced the yqjF gene promoter.
The metabolites' accumulation pattern of the nitroreductase-deficient mutants suggests that nfsA and nfsB are both involved in the reduction of DNT. While single knockout mutations of either gene resulted in only a minimal inhibition of the DNT biotransformation rate, a mutant in which both genes were missing exhibited very restricted DNT reduction. However, whereas a single knockout of nfsB resulted in a significantly inhibited 4HA2NT accumulation, a mutant lacking nfsA was still able to produce this compound almost at the same rate as the wild-type strain. The effects of these mutations on yqjF activation by DNT and 4HA2NT suggest that while NfsB is a necessary element in DNT and 4HA2NT metabolism to THT, NfsA is not essential in this process. Supporting evidence is provided by the similarity between the mutation effects obtained for yqjF induction by DNT and 4HA2NT in the absence of both nitroreductases and in the absence of NfsB, suggesting a minor role of NfsA in this route. It is highly probable that in the absence of NfsB, NfsA acts as the main nitroreductase in DNT biotransformation, allowing significant removal of DNT but shifting it into a pathway that does not produce THT. When tested directly with THT, induction of yqjF in nitroreductase-deficient strains was not significantly affected, which supports the assumption that THT is indeed the direct inducer of yqjF. It should be noted that NfsA may also have a role in the reduction of quinone intermediates (27), such as 2H5MQ, but further research is clearly needed for its characterization.
The actual role of yqjF in DNT metabolism remains uncertain. Daley et al. (28), based on bioinformatics data, postulated that this gene encodes a transmembrane quinol oxidase subunit, which could explain the strong response of the yqjF-based reporter toward quinol and its derivatives, including THT. This is supported by recent findings regarding the regulatory protein YhaJ, which regulates the expression of yqjF and several other genes, all related to the biotransformation of quinol-like compounds (29). While this may suggest a possible role of YqjF in the downstream transformation of THT, a deletion of yqjF did not result in any significant effect on the yqjF::lux reporter's response toward DNT (our unpublished data). It is possible, however, that this lack of effect may be due to other quinol oxidases such as subunits of the cytochrome bo oxidase (CyoABCD), cytochrome bd-I oxidase (CydABX), or cytochrome bd-II oxidase (AppCD) picking up the burden of THT degradation in yqjF mutants. Future studies will reveal the subsequent fate of THT and possibly shed additional light on the degree to which DNT is metabolized in E. coli. Only with such information will it be possible to close the apparently incomplete “mass balance” emerging from currently available data (as seen in Fig. 3 and elsewhere).
In summary, we have shown that E. coli reduces DNT in a reductive pathway, initiated by the NfsA and NfsB nitroreductases; we further suggest that an oxidative pathway diverges from this route to produce THT, which in turn activates the yqjF-based DNT bioreporter. In addition to highlighting previously unknown metabolic activities of E. coli, our enhanced understanding of this process may serve to improve the performance of yqjF-based bioreporters of buried landmines and other explosive devices.
MATERIALS AND METHODS
Chemicals and media.
2,4-Dinitrotoluene (DNT), 2,4-diaminotoluene (DAT), 4-acetamido-2-nitrotoluene (4Acm2NT), 4-amino-2-nitrotoluene (4A2NT), and 2-amino-4-nitrotoluene (2A4NT) were of the highest analytical grade and were all purchased from Sigma-Aldrich. 4-Hydroxylamino-2-nitrotoluene (4HA2NT) was synthesized by Yoel Sasson from the Hebrew University's Institute of Chemistry. 4,4′-Dimethyl-3,3′-dinitroazoxybenzene (4,4′-DM-3,3′-DNAB) and 4-methyl-5-nitrocatechol (4M5NC) were purchased from Toronto Research Chemicals Inc. (Toronto, Ontario, Canada). 2,4,5-Trihydroxytoluene (THT) was purchased from Akos GmbH (Steinen, Germany). Hydrophobic chemicals were dissolved in ethanol or acetonitrile, and hydrophilic chemicals were dissolved in double-distilled H2O. Chemicals were kept in the dark and used up to 7 days after stock preparation. Methanol used during microanalysis of metabolic products was liquid chromatography/mass spectrometry (LC/MS) grade.
M9 minimal medium was used for transformation assays, conducted in batch mode in Erlenmeyer flasks. The medium contained (per liter) 12.8 g Na2HPO4·7H2O, 3 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl, 2 ml 1 M MgSO4·7H2O, 0.1 ml 1 M CaCl2, and 10 ml 20% (wt/vol) glucose. Lysogeny broth (LB), used for routine growth and culture maintenance, contained (per liter) 10 g Bacto tryptone, 5 g Bacto yeast extract, and 5 g NaCl. Phosphate-buffered saline (PBS; pH = 7.0), used for CFU quantification, contained (per liter) 8 g NaCl, 0.2 g KCl, 1.42 g Na2HPO4, and 0.24 g KH2PO4.
Bacterial strains and plasmids.
Bioluminescence generated by E. coli strains harboring plasmid pBR2TTS-yqjF::luxCDABE (4) served as an indication of the degree of yqjF activation. A library containing E. coli clones with single (the “Keio collection” [30]) or with double (4) gene deletions was employed to assess the roles of various genes in the DNT biotransformation process and the potential of the transformation products to induce yqjF. E. coli strain BW25113 (wild-type strain of the Keio collection [30]) transformed with the same plasmid served as a control in all experiments. E. coli strains and plasmids used in the course of this study are listed in Table 4.
TABLE 4.
E. coli strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| BW25113a | Keio collection wild-type E. coli K-12 strain | 30 |
| BW25113ΔnfsB | BW25113 with nfsB deletion | 30 |
| BW25113ΔnfsA | BW25113 with nfsA deletion | 30 |
| BW25113ΔnfsB ΔnfsA | BW25113 with nfsB and nfsA deletions | 4 |
| Plasmidb | ||
| pBR2TTS-yqjF::luxCDABE | pBR322 derivative carrying yqjF::luxCDABE | 4 |
Medium contained kanamycin (30 μg/ml).
Medium contained ampicillin (100 μg/ml) for maintenance of bioluminescent plasmids.
Growth of E. coli on DNT as the sole C or N source.
E. coli strain BW25113 was incubated overnight (ON) at 37°C with shaking in M9 medium, diluted 1/100 in fresh M9, and then grown under the same conditions to an optical density at 600 nm (OD600) of ca. 0.2 (early exponential growth phase). Five milliliters of the culture was then centrifuged (10 min, 4,000 rpm, 4°C), the supernatant was discarded, and the pellet was resuspended either in M9 medium or in a modified M9 medium lacking either glucose as a carbon source, NH4Cl as a nitrogen source, or both. DNT (27,000 mg/liter) dissolved in ethanol was added to a concentration of 50 mg/liter. Control samples, to which DNT was not added, were supplemented with an identical volume of ethanol. The cultures were incubated for 21 h at 37°C with shaking (200 rpm), at which time culture aliquots were serially diluted in PBS and spread on LB agar plates. CFU were counted after ON incubation at 37°C. Growth kinetics of a control sample containing NH4Cl, glucose, and DNT (see Fig. S5b in the supplemental material) indicated that the culture was at the late stationary phase at the time of sample extraction.
Monitoring yqjF induction by DNT and by related compounds.
The tested E. coli strains harboring plasmid pBR2TTS-yqjF::luxCDABE were incubated ON with shaking at 37°C in LB medium supplemented with 100 μg/ml ampicillin. The culture was diluted 1/50 in M9 medium and regrown at 37°C with shaking to the early exponential growth phase (OD600, ca. 0.2). Culture aliquots (50 μl) were combined in an opaque white 96-well microtiter plate (Greiner Bio-One) with 50 μl of the tested chemical at twice the desired final concentration. Light emission was measured using a VICTOR2 plate reader (Wallac, Turku, Finland), and results are presented in the instrument's arbitrary relative luminescence units (RLU). Each measurement was preceded by vigorous shaking of the plate to maintain aerobic conditions and a homogenous suspension in each well. The magnitude of induction was calculated as the ΔRLU, representing the difference between luminescence intensity in the tested reaction mixture and that of the untreated control at the same time point. An effect of a mutation (ME) was calculated according to the following equation: ME (%) = {[Max0–900 min(ΔRLUmutant)/Max0–900 min(ΔRLUWT)] × 100} − 100, where Max0–900 min represents the maximal ΔRLU measured in the course of a 900-min incubation. Mutation effects that were less than −50% or more than +50% were considered to be significant negative and positive effects, respectively. Bacterial growth in the microtiter plate in the course of the experiment was minimal (Fig. S5a).
Monitoring yqjF induction by a spent medium.
A wild-type E. coli culture was incubated ON with shaking at 37°C, diluted 1/50 in fresh M9 medium, and regrown at 37°C with shaking to the early exponential growth phase (OD600, ca. 0.15). The culture was then supplemented with DNT (100 mg/liter). Medium samples were drawn at various time intervals and filtered with a 0.22-μm syringe filter. The filtered medium was used to spike the yqjF bioreporter in a manner similar to that of DNT (described in the previous section). The early and late responses of yqjF, the former due to the DNT metabolites present in the medium and the latter due to DNT that was not metabolized during the first stage of the experiment, were measured.
Identification and quantification of DNT biotransformation products.
For characterization of DNT metabolites, bacteria were incubated overnight in LB medium supplemented with the appropriate antibiotic at 37°C with shaking. The cells were diluted 1/100 in 20 ml of M9 medium in a 100-ml Erlenmeyer flask and then regrown at 37°C with shaking (200 rpm) to early exponential growth phase (OD600, ca. 0.2). The culture was spiked with either DNT or a DNT metabolite, the transformation of which was studied, and then further incubated under the same conditions. Growth under these conditions was exponential for the first 250 min (Fig. S5b). Samples were withdrawn at various time intervals and filtered (Millex 0.22-μm polyvinylidene difluoride [PVDF] syringe filter; Merck Millipore); the filtrate was kept at 4°C in the dark until analyzed.
DNT and its metabolites were identified by liquid chromatography/mass spectrometry (LC/MS) using an Agilent 1200 system composed of an Agilent 6520 Accurate-Mass Q-TOF LC/MS apparatus equipped with an electrospray ionization source (ESI) and a UV detector (Agilent Technologies, United States). High-performance liquid chromatography (HPLC) separation was performed with an Agilent Zorbax Eclipse XDB-C18 column (2.1 mm by 50 mm). The analytical method was modified from the U.S. Environmental Protection Agency Method 8330B (31) for the detection of explosives by HPLC. The injection volume was 10 μl, the flow rate 0.2 ml/min, the oven temperature was maintained at 40°C, and the capillary voltage was 4,000 V. The desolvation gas temperature was 350°C, and the gas flow rate was 10 liters/min. Negative ionization was applied, with the solvent gradient described in Table S2 in the supplemental material. UV absorbance at 254 nm was measured for all samples.
To further validate the identification of THT in the growth medium, we applied an MS/MS analysis using the same instrumentation, in which the suspected mass was targeted and fragmented. A sample (80 μl) was injected, and collision energy of 10 mV was applied.
Supplementary Material
ACKNOWLEDGMENTS
Research was partially supported by the Minerva Center for Bio-Hybrid Complex Systems and by the by NATO Science for Peace and Security Programme project 985042.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01729-17.
REFERENCES
- 1.McLean IG. 2003. Mine detection dogs: training, operations and odour detection. Geneva International Centre for Humanitarian Demining, Geneva, Switzerland. [Google Scholar]
- 2.MacDonald J, Lockwood J, McFee J, Altshuler T, Broach T. 2003. Alternatives for landmine detection. Rand Corporation, Arlington, VA. [Google Scholar]
- 3.Jenkins TF, Leggett DC, Miyares PH, Walsh ME, Ranney TA, Cragin JH, George V. 2001. Chemical signatures of TNT-filled land mines. Talanta 54:501–513. doi: 10.1016/S0039-9140(00)00547-6. [DOI] [PubMed] [Google Scholar]
- 4.Yagur-Kroll S, Lalush C, Rosen R, Bachar N, Moskovitz Y, Belkin S. 2014. Escherichia coli bioreporters for the detection of 2,4-dinitrotoluene and 2,4,6-trinitrotoluene. Appl Microbiol Biotechnol 98:885–895. doi: 10.1007/s00253-013-4888-8. [DOI] [PubMed] [Google Scholar]
- 5.Yagur-Kroll S, Amiel E, Rosen R, Belkin S. 2015. Detection of 2,4-dinitrotoluene and 2,4,6-trinitrotoluene by an Escherichia coli bioreporter: performance enhancement by directed evolution. Appl Microbiol Biotechnol 99:7177–88. doi: 10.1007/s00253-015-6607-0. [DOI] [PubMed] [Google Scholar]
- 6.Belkin S, Yagur-Kroll S, Kabessa Y, Korouma V, Septon T, Anati Y, Zohar-Perez C, Rabinovitz Z, Nussinovitch A, Agranat AJ. 2017. Remote detection of buried landmines using a bacterial sensor. Nat Biotechnol 35:308–310. doi: 10.1038/nbt.3791. [DOI] [PubMed] [Google Scholar]
- 7.Yin H, Wood TK, Smets BF. 2005. Reductive transformation of TNT by Escherichia coli: pathway description. Appl Microbiol Biotechnol 67:397–404. doi: 10.1007/s00253-004-1736-x. [DOI] [PubMed] [Google Scholar]
- 8.McCormick NG, Cornell JH, Kaplan AM. 1978. Identification of biotransformation products from 2,4-dinitrotoluene. Appl Environ Microbiol 35:945–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes JB, Wang CY, Zhang C. 1999. Anaerobic biotransformation of 2,4-dinitrotoluene and 2,6-dinitrotoluene by Clostridium acetobutylicum: a pathway through dihydroxylamino intermediates. Environ Sci Technol 33:1065–1070. doi: 10.1021/es9809915. [DOI] [Google Scholar]
- 10.Noguera DR, Freedman DL. 1996. Reduction and acetylation of 2,4-dinitrotoluene by a Pseudomonas aeruginosa strain. Appl Environ Microbiol 62:2257–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudcova T, Halecky M, Kozliak E, Stiborova M, Paca J. 2011. Aerobic degradation of 2,4-dinitrotoluene by individual bacterial strains and defined mixed population in submerged cultures. J Hazard Mater 192:605–613. doi: 10.1016/j.jhazmat.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 12.Aburto-Medina A, Taha M, Shahsavari E, Ball AS. 2017. Degradation of the dinitrotoluene isomers 2,4- and 2,6-DNT: appraising the role of microorganisms, vol 1, p 5–20. In Anjum NA, Gill SS, Tuteja N (ed), Enhancing cleanup of environmental pollutants. Springer, Berlin, Germany. [Google Scholar]
- 13.Liu D, Thomson K, Anderson AC. 1984. Identification of nitroso compounds from biotransformation of 2,4-dinitrotoluene. Appl Environ Microbiol 47:1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Race PR, Lovering AL, Green RM, Ossor A, White SA, Searle PF, Wrighton CJ, Hyde EI. 2005. Structural and mechanistic studies of Escherichia coli nitroreductase with the antibiotic nitrofurazone—reversed binding orientations in different redox states of the enzyme. J Biol Chem 280:13256–13264. doi: 10.1074/jbc.M409652200. [DOI] [PubMed] [Google Scholar]
- 15.Suen W, Spain J. 1993. Cloning and characterization of Pseudomonas sp. strain DNT genes for 2,4-dinitrotoluene degradation. J Bacteriol 175:1831–1837. doi: 10.1128/jb.175.6.1831-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roldán MD, Pérez-Reinado E, Castillo F, Moreno-Vivián C. 2008. Reduction of polynitroaromatic compounds: the bacterial nitroreductases. FEMS Microbiol Rev 32:474–500. doi: 10.1111/j.1574-6976.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- 17.Spanggord RJ, Spain J, Nishino S, Mortelmans K. 1991. Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol 57:3200–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leungsakul T, Keenan BG, Yin H, Smets BF, Wood TK. 2005. Saturation mutagenesis of 2,4-DNT dioxygenase of Burkholderia sp. strain DNT for enhanced dinitrotoluene degradation. Biotechnol Bioeng 92:416–426. doi: 10.1002/bit.20602. [DOI] [PubMed] [Google Scholar]
- 19.Snellinx Z, Taghavi S, Vangronsveld J, van der Lelie D. 2003. Microbial consortia that degrade 2,4-DNT by interspecies metabolism: isolation and characterisation. Biodegradation 14:19–29. doi: 10.1023/A:1023539104747. [DOI] [PubMed] [Google Scholar]
- 20.Boopathy R, Kulpa C. 1993. Nitroaromatic compounds serve as nitrogen source for Desulfovibrio sp. (B strain). Can J Microbiol 39:430–433. doi: 10.1139/m93-062. [DOI] [PubMed] [Google Scholar]
- 21.Aoki K, Ohtsuka K, Shinke R, Nishira H. 1984. Rapid biodegradation of aniline by Frateuria species ANA-18 and its aniline metabolism. Agric Biol Chem 48:865–872. [Google Scholar]
- 22.Konopka A, Knight D, Turco RF. 1989. Characterization of a Pseudomonas sp. capable of aniline degradation in the presence of secondary carbon sources. Appl Environ Microbiol 55:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeyer J, Wasserfallen A, Timmis KN. 1985. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol 50:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs K, Schreiner A, Lingens F. 1991. Degradation of 2-methylaniline and chlorinated isomers of 2-methylaniline by Rhodococcus rhodochrous strain CTM. Microbiology 137:2033–2039. [DOI] [PubMed] [Google Scholar]
- 25.Gish W. 1993. Identification of protein coding regions by database similarity search. Nat Genet 3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 26.Bai J, Yang J, Liu P, Yang Q. 2015. Transformation pathway of 2,4,6-trinitrotoluene by Escherichia coli nitroreductases and improvement of activity using structure-based mutagenesis. Process Biochem 50:705–711. doi: 10.1016/j.procbio.2015.01.029. [DOI] [Google Scholar]
- 27.Valiauga B, Williams EM, Ackerley DF, Čėnas N. 2017. Reduction of quinones and nitroaromatic compounds by Escherichia coli nitroreductase A (NfsA): characterization of kinetics and substrate specificity. Arch Biochem Biophys 614:14–22. doi: 10.1016/j.abb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Daley DO, Rapp M, Granseth E, Melén K, Drew D, Von Heijne G. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 308:1321–1323. doi: 10.1126/science.1109730. [DOI] [PubMed] [Google Scholar]
- 29.Palevsky N, Shemer B, Connolly JP, Belkin S. 2016. The highly conserved Escherichia coli transcription factor YhaJ regulates aromatic compound degradation. Front Microbiol 7:1490. doi: 10.3389/fmicb.2016.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Environmental Protection Agency. 2006. Method 8330B (SW-846): nitroaromatics, nitramines, and nitrate esters by high performance liquid chromatography (HPLC), revision 2. US Environmental Protection Agency, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.