ABSTRACT
Bordetella bronchiseptica, a Gram-negative bacterium, causes chronic respiratory tract infections in a wide variety of mammalian hosts, including humans (albeit rarely). We recently designed Bordetella pertussis and Bordetella parapertussis experimental vaccines based on outer membrane vesicles (OMVs) derived from each pathogen, and we obtained protection against the respective infections in mice. Here, we demonstrated that OMVs derived from virulent-phase B. bronchiseptica (OMVBbvir+) protected mice against sublethal infections with different B. bronchiseptica strains, two isolated from farm animals and one isolated from a human patient. In all infections, we observed that the B. bronchiseptica loads were significantly reduced in the lungs of vaccinated animals; the lung-recovered CFU were decreased by ≥4 log units, compared with those detected in the lungs of nonimmunized animals (P < 0.001). In the OMVBbvir+-immunized mice, we detected IgG antibody titers against B. bronchiseptica whole-cell lysates, along with an immune serum having bacterial killing activity that both recognized B. bronchiseptica lipopolysaccharides and polypeptides such as GroEL and outer membrane protein C (OMPc) and demonstrated an essential protective capacity against B. bronchiseptica infection, as detected by passive in vivo transfer experiments. Stimulation of cultured splenocytes from immunized mice with OMVBbvir+ resulted in interleukin 5 (IL-5), gamma interferon (IFN-γ), and IL-17 production, indicating that the vesicles induced mixed Th2, Th1, and Th17 T-cell immune responses. We detected, by adoptive transfer assays, that spleen cells from OMVBbvir+-immunized mice also contributed to the observed protection against B. bronchiseptica infection. OMVs from avirulent-phase B. bronchiseptica and the resulting induced immune sera were also able to protect mice against B. bronchiseptica infection.
IMPORTANCE Bordetella bronchiseptica, a Gram-negative bacterium, causes chronic respiratory tract infections in a wide variety of mammalian hosts, including humans (albeit rarely). Several vaccines aimed at preventing B. bronchiseptica infection have been developed and used, but a safe effective vaccine is still needed. The significance and relevance of our research lie in the characterization of the OMVs derived from B. bronchiseptica as the source of a new experimental vaccine. We demonstrated here that our formulation based on OMVs derived from virulent-phase B. bronchiseptica (OMVBbvir+) was effective against infections caused by B. bronchiseptica isolates obtained from different hosts (farm animals and a human patient). In vitro and in vivo characterization of humoral and cellular immune responses induced by the OMVBbvir+ vaccine enabled a better understanding of the mechanism of protection necessary to control B. bronchiseptica infection. Here we also demonstrated that OMVs derived from B. bronchiseptica in the avirulent phase and the corresponding induced humoral immune response were able to protect mice from B. bronchiseptica infection. This realization provides the basis for the development of novel vaccines not only against the acute stages of the disease but also against stages of the disease or the infectious cycle in which avirulence factors could play a role.
KEYWORDS: Bordetella bronchiseptica, outer membrane vesicles, vaccine, phenotypic phases
INTRODUCTION
Bordetella bronchiseptica is a Gram-negative bacterium that causes respiratory diseases in a variety of mammalian hosts (1). Although this pathogen rarely infects humans, certain reports have indicated that B. bronchiseptica can infect immunocompromised patients or those with underlying respiratory diseases (2–4). The respiratory infections caused by this zoonotic pathogen could also become chronic, although with few or no symptoms (5, 6). The persistence of B. bronchiseptica in hosts seems to be facilitated through modification of the expression of bacterial constituents mainly controlled by a two-component regulatory system encoded by the bvgAS locus (7, 8). This system senses signals from the external environment, regulates the expression of hundreds of genes, and controls different phenotypic phases (9).
The prophylaxis of diseases caused by B. bronchiseptica is achieved through vaccination, but no satisfactory vaccine to confer protection in animals against acute or chronic infections caused by B. bronchiseptica has been developed to date. Some of the current vaccines are composed of either killed wild-type bacterial strains (administered parentally) or live attenuated strains (administered intranasally) (10, 11). Most of the vaccines containing the killed bacteria induce high serum antibody titers but do not always provide effective protection against infection (10). Data on the safety and efficacy of live attenuated vaccines are scarce. Moreover, this kind of vaccine is not well accepted because the strains included in the vaccines may revert to full or partial virulence, since the basis of the original attenuation is still unknown. Regarding acellular B. bronchiseptica vaccines, one is composed of the immunogenic Bordetella colonization factor A protein, while others contain pertactin (PRN), an outer membrane protein that is a highly immunogenic virulence factor (12–15). Although these vaccines appear to resolve mainly issues related to adverse side reactions, no conclusive evidence has been garnered to support their immunogenicity (13, 16). Therefore, the identification of appropriate bacterial components for the development of a new vaccine is still needed. In this search, the characteristic constituents of the avirulent phase could be included in evaluations, since this phase seems to be involved in the infectious process (6, 8, 17).
In the work reported here, we investigated whether a vaccine based on outer membrane vesicles (OMVs) derived from B. bronchiseptica in either the virulent or avirulent phenotypic phase would be able to generate protective immunity against infections caused by B. bronchiseptica. OMV-based vaccines against Bordetella pertussis or Bordetella parapertussis infections were recently developed by our group (18–22). The administration of OMV-based vaccines confers complete protection against B. pertussis or B. parapertussis in mice. The protection against B. pertussis is long lasting and is mediated by both antibodies and CD4+ T cells (20). We made the interesting observation that the protective capacity of OMVs obtained from a B. pertussis strain that expressed the avirulent phenotype was lower than that of OMVs from virulent-phase B. pertussis; nevertheless, the two were protective in the mouse model used (23).
These results, in combination, permit the hypothesis that OMVs derived from B. bronchiseptica from either the virulent phase or the avirulent phase could constitute a suitable candidate for a vaccine against bordetellosis. The findings described here support this hypothesis, since the protection experiments performed in the murine intranasal challenge model demonstrated that the OMV vaccine derived from virulent B. bronchiseptica was able to effect a significant decrease in the lung colonization of different B. bronchiseptica strains obtained from different hosts, i.e., farm animals or a human. Furthermore, by performing in vitro and in vivo experiments, we determined that both a humoral response possessing killing capacity and immune splenic cells contributed to the protection induced by the OMVBbvir+ vaccines. Moreover, we also observed that protective capacity could be induced with a vaccine formulated from OMVs obtained from B. bronchiseptica in the avirulent phase.
RESULTS
Isolation and characterization of OMVs obtained from B. bronchiseptica 9.73 grown in the virulent phase.
The OMV samples obtained from B. bronchiseptica 9.73 grown in the virulent phase (OMVBbvir+) were negatively stained and examined by electron microscopy (Fig. 1A). The procedure was repeated at least eight times, and in all the samples the size range (i.e., 50 to 150 nm) was both consistent from batch to batch and similar to that described previously for OMV preparations derived from B. pertussis (22). To further characterize these OMVBbvir+ samples, one-dimensional electrophoresis (Fig. 1B) and immunoblotting (Fig. 1C) were performed. With this assay, we could determine that the OMVBbvir+ isolates contained adenylate cyclase-hemolysin (AC-Hly), PRN, and fimbrial serotype 2 (FIM2) (Fig. 1C).
FIG 1.
(A) Transmission electron microscopic image of the negatively stained preparations obtained from B. bronchiseptica 9.73 in the virulent phase (OMVBbvir+) (scale bar, 200 nm). (B) Analysis of OMVBbvir+ by 12.5% (wt/vol) SDS-PAGE. The bands were visualized by staining with Coomassie brilliant blue R-250. Molecular masses are indicated on the left. (C) Immunoblots of OMVBbvir+ and purified proteins, indicating the bands binding to anti-AC-Hly, anti-PRN, and anti-FIM2 polyclonal mouse antibodies. The sources of the samples are indicated above the panels.
IL-6 levels after immunization.
Usually after systemic immunization of an animal, increases in the levels of proinflammatory cytokines can be detected. The levels are related to the proinflammatory capability of the formulation employed. Interleukin 6 (IL-6) is among the proinflammatory cytokines usually chosen as indicators of this activity (24). In our experiments, the OMVBbvir+-containing formulations induced levels of IL-6 (425.54 ± 34.00 pg/ml) that were significantly lower than those detected with the B. bronchiseptica whole-cell vaccine (53,673.08 ± 5,987.44 pg/ml).
Protection against intranasal B. bronchiseptica challenge after vaccination with OMVBbvir+.
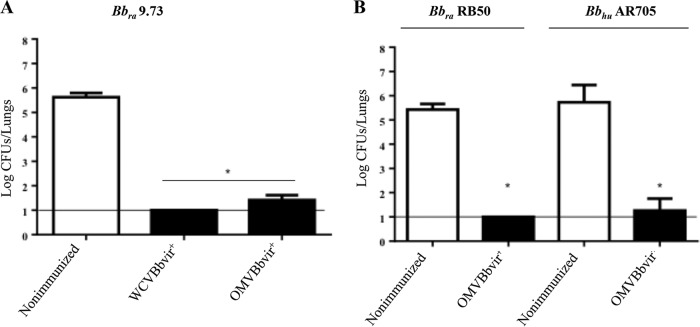
To evaluate the protective capability of the OMVBbvir+ vaccine against a B. bronchiseptica challenge, we used a murine model of intranasal infection. The CFU recovered from the lungs of OMVBbvir+-immunized mice were compared with those recovered from mice immunized with the B. bronchiseptica 9.73 whole-cell vaccine prepared in our laboratory from wild-type bacteria in the virulent phase (WCVBbvir+) (Fig. 2A). Significant differences (P < 0.001) in lung bacterial counts between the OMVBbvir+-immunized animals and nonimmunized mice were obtained (Fig. 2A). The numbers of colonies recovered from the lungs of the OMVBbvir+-immunized mice on day 7 after challenge were at least 4 log units lower than those recovered from the nonimmunized animals and similar to those recovered from the WCVBbvir+-immunized mice (Fig. 2A).
FIG 2.
Effect of i.p. immunization with active OMVBbvir+ in the mouse intranasal challenge model. WCVBbvir+ was used as a positive control. (A) B. bronchiseptica 9.73 (Bbra9.73) was used as the challenge bacteria (5 × 105 CFU, in 40 μl). (B) B. bronchiseptica RB50 (BbraRB50) and the human clinical isolate B. bronchiseptica AR705 (BbhuAR705) were also used as challenge bacteria (5 × 105 CFU, in 40 μl). In all instances, three biological replicates were performed, with the results from a representative one being presented. Results depicted are the means of four mice per group at 7 days after challenge. The horizontal lines indicate the lower limit of detection. In both panels, the numbers of bacteria recovered from the mouse lungs, expressed as the mean ± standard error of the mean (SEM) (error bars) of log10 CFU in the lungs, are plotted on the ordinates for the samples from the lungs of the experimental groups indicated on the abscissas. The Bronchiseptica strains used for the challenges are indicated above the panels. *, significant differences (P < 0.001).
We next sought to investigate whether the protective capability induced by the OMVBbvir+ vaccine extended to strains other than B. bronchiseptica 9.73. To that end, we performed in vivo protection assays using two other strains of B. bronchiseptica for challenge, one isolated from a farm animal (rabbit B. bronchiseptica RB50) and the other recovered from a pediatric patient with cystic fibrosis (human B. bronchiseptica AR705). Mice were immunized twice with the OMVBbvir+ formulation and then challenged, 2 weeks after the second immunization, with a sublethal dose of either rabbit B. bronchiseptica RB50 or human B. bronchiseptica AR705. As a negative control, we used nonimmunized mice. For both strains used in the bacterial challenge, significant differences (P < 0.001) in lung B. bronchiseptica CFU counts of more than 4 log units between the immunized animals and the negative control group were obtained (Fig. 2B).
Characterization of the humoral immune response induced by OMVBbvir+.
To characterize the immune response induced by the OMVBbvir+ vaccine derived from B. bronchiseptica 9.73, antibody titers were quantitatively measured (Table 1). In comparison with the antibody levels detected in negative-control animals, higher serum levels of specific IgG were found 14 days after the OMV priming (Table 1). Further tests were then conducted to determine the antibody subtypes. Mice immunized with OMVBbvir+ produced high titers of specific IgG1 and IgG2a antibodies. The specific IgG1 titer was higher than the specific IgG2a titer (IgG1/IgG2a ratio of 2.2) (Table 1). Examination of these IgG subclasses indicated that the mice had responded to the OMVBbvir+ vaccination with a mixed Th1/Th2 profile but mainly with a skewed Th2-type immune response.
TABLE 1.
Serum IgG and isotype-specific antibody titers
| Antibody profile | OMVBbvir+ immunized | Nonimmunized |
|---|---|---|
| Antibody titer (mean ± SEM) | ||
| Total IgG | 259.6 ± 32.4 | 9.3 |
| IgG2a | 250.2 ± 71.9 | NDa |
| IgG1 | 558.7 ± 124.3 | 11.3 |
| IgG1/IgG2a ratio | 2.2 |
ND, not detected.
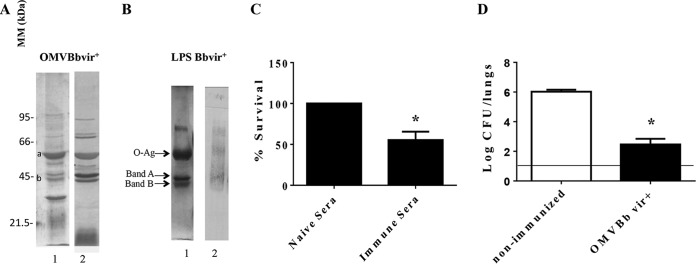
To identify the main immunogenic proteins present in the OMVs, we analyzed the antibody profile by immune proteomics. This analysis was achieved by examining the reactivity of sera induced by the OMVBbvir+ vaccine against proteins from the OMVs derived from B. bronchiseptica. Figure 3A shows protein bands detected in OMVBbvir+ that cross-reacted with sera induced by vaccination with OMVs. The identity of some of those antigens was determined after the selected immunoreactive bands were excised from the one-dimensional electrophoretic gels and then analyzed by mass spectrometry. Bands a and b in Fig. 3A, for example, were identified as the GroEL-like protein and outer membrane protein C (OMPc), respectively. The mass spectrometric identification of the protein indicated as GroEL was subsequently confirmed by immunoreactivity with a GroEL-specific antibody (data not shown).
FIG 3.
(A) Analysis of OMVBbvir+ antigenic reactivity by 12.5% (wt/vol) SDS-PAGE, visualized by staining with Coomassie brilliant blue (lane 1). Molecular masses are indicated on the left. Immunoblots with the OMVBbvir+ protein sample were probed with polyclonal antisera obtained from mice immunized with OMVBbvir+ (lane 2). Bands a and b were identified as GroEL and OMPc, respectively. (B) Analysis of purified B. bronchiseptica LPS by 15% (wt/vol) SDS-PAGE (lane 1) and by immunoblotting with the polyclonal antisera obtained from mice immunized with OMVBbvir+ (lane 2). The arrows indicate the locations of the O antigen and bands A and B that were recognized by the antisera used for immunoblotting in lane 2. (C) In vitro killing assay of B. bronchiseptica with immune sera derived from OMVBbvir+-vaccinated animals and from nonimmunized mice. Three biological replicates were performed, with the results from a representative one being presented. The percent survival of the bacteria is plotted on the ordinate for each of the sera indicated on the abscissa. *, significant difference (P < 0.05). (D) Effect of passive immunization with sera collected from OMVBbvir+-immunized mice. B. bronchiseptica 9.73 was used as the challenge bacteria (5 × 105 CFU, in 40 μl). Three biological replicates were performed, with the results from a representative one being presented. The data are the mean values from four mice per group at 7 days after challenge. The horizontal line indicates the lower limit of detection. The number of bacteria recovered from the mouse lungs, expressed as the mean ± SEM (error bars) of log10 CFU in the lungs, is plotted on the ordinate for each of the experimental groups indicated on the abscissa. *, significant difference (P < 0.001).
To investigate the presence of specific antibodies against the lipopolysaccharide (LPS) in the OMVBbvir+-induced immune serum, we performed a Western blot analysis to determine the mobility on SDS-PAGE of the purified wild-type B. bronchiseptica 9.73 LPS. The three expected bands were observed, that is, a diffuse band (the lipid A–3-deoxy-d-manno-octulosonic acid [KDO] core O antigen) containing the O antigen (a single-sugar polymer consisting of 2,3-dideoxy-di-N-acetylgalactosaminuronic acid) and two faster migrating bands, i.e., band A (the lipid A-KDO core) and band B (lipid A-KDO) (25). As anticipated, these LPS-associated bands were recognized by the immune serum induced by OMVBbvir+ vaccination (Fig. 3B).
Since the activated components of complement had been reported previously to mediate, at least in part, the killing of B. bronchiseptica bacteria by direct bacterial lysis, we next performed a killing assay with immune serum (26). For this determination, a suspension of 500 B. bronchiseptica 9.73 bacteria was incubated in 50 μl of 90% (vol/vol) serum in phosphate-buffered saline (PBS), to ensure that the serum components were not limiting. This assay revealed that the B. bronchiseptica 9.73 bacteria were sensitive to the immune sera (to a degree of ∼50% survival) but resistant to naive serum (i.e., 100% survival) (Fig. 3C). In control experiments using heat-inactivated sera, we found 100% bacterial survival for both immune and naive sera.
To examine the specific roles of antibodies in the control and clearance of B. bronchiseptica, serum from naive or immunized animals was adoptively transferred into naive animals 24 h before challenge with B. bronchiseptica (5 × 105 CFU B. bronchiseptica 9.73). This OMVBbvir+-induced serum, collected from the mice 14 days after the second dose, cleared B. bronchiseptica from the mouse lungs by day 7 after the inoculation (at a reduction of 3.5 log units), whereas naive serum had no significant effect (Fig. 3D). All of the results presented here indicated that the OMVBbvir+ vaccine induced robust humoral immunity that yielded protection against B. bronchiseptica, at least partially, as a consequence of the killing activity of the serum.
Induction of mixed Th1, Th2, and Th17 immune responses by OMVBbvir+ vaccination.
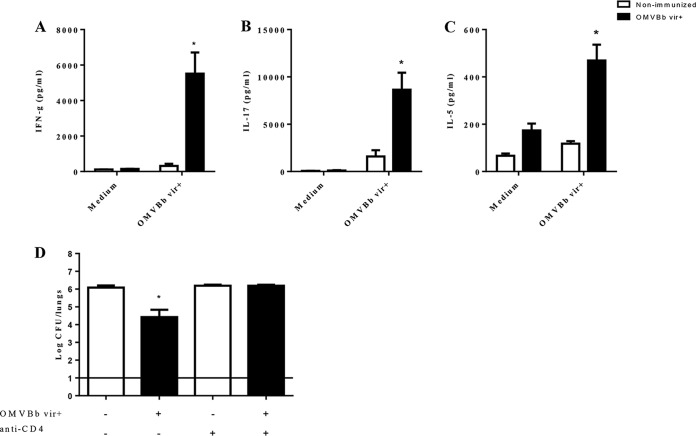
To characterize the T-cell profile induced by the OMVBbvir+ vaccine, we determined the levels of gamma interferon (IFN-γ), IL-17, and IL-5 (markers of the Th1, Th17, and Th2 immune responses, respectively) produced by stimulated spleen cell cultures (Fig. 4). Lymphocyte proliferation assays revealed that OMVBbvir+ vaccination effectively stimulated lymphocyte proliferation (data not shown). Two months after immunization, higher concentrations of IFN-γ (5,514.48 ± 1,198.51 pg/ml) (Fig. 4A) and IL-17 (8,645.97 ± 1,796.29 pg/ml) (Fig. 4B) were produced by spleen cells from OMVBbvir+-immunized mice, compared with those present in nonimmunized mice (Fig. 4). IL-5 was also detected (469.14 ± 67.03 pg/ml) in the supernatants of stimulated splenocytes from OMVBbvir+-immunized mice (Fig. 4C). All of these findings strongly indicate that OMVBbvir+ vaccination induces a mixed Th1-Th17-Th2 spleen cell profile.
FIG 4.
(A to C) Cytokine production by splenocytes from immunized mice. BALB/c mice were immunized with two doses of OMVBbvir+ or were left nonimmunized. Two months after the final immunization, the mice were sacrificed, and their cultured spleen cells were stimulated with OMVBbvir+ or simply incubated in the culture medium (negative control). After 72 h, the concentrations of IFN-γ (A), IL-17 (B), and IL-5 (C) in the culture supernatants were determined by ELISA. The results are expressed as the means ± standard errors of three experiments, with 4 mice per group. Significant differences between nonimmunized and immunized mice were analyzed for each cytokine (P ≤ 0.01 [A] or P < 0.001 [B and C]). In each of the three panels, the concentration of the cytokine that was assayed in the culture supernatant, as indicated on the ordinate, is plotted for the experimental condition (exposure to medium or vesicles) indicated on the abscissa. (D) Effects of passive immunization with spleen cells collected from OMVBbvir+-immunized mice with or without depletion of CD4+ T cells. Donor animals were injected i.p. with anti-CD4 antibody or the corresponding isotype control 24 h before the spleens were collected. After the i.p. injection of spleen cells from OMVBbvir+-immunized or nonimmunized mice, as indicated on the abscissa, the recipient mice were challenged with B. bronchiseptica 9.73 (5 × 105 CFU, in 40 μl). Three biological replicates were performed, with the results from a representative one being presented. The data represent the means for 4 mice per group, at 7 days after challenge. The horizontal line indicates the lower limit of detection. The numbers of bacteria recovered from the mouse lungs, expressed as the mean ± SEM (error bars) of log10 CFU in the lungs, are plotted on the ordinate for the samples from the lungs of mice that received spleen cells from the sources indicated on the abscissa. In all four panels, the white bars indicate spleen cells from nonimmunized mice and the black bars those from immunized mice. *, significant differences (P < 0.001).
To investigate the role of OMVBbvir+-induced immune spleen cells in protection, we injected BALB/c mice intraperitoneally (i.p.) with 5 × 106 intact spleen cells from nonimmunized animals or from mice that had been immunized with OMVBbvir+ vaccine 2 weeks earlier. The mice were infected 24 h later with 5 × 105 CFU of B. bronchiseptica 9.73 and then were sacrificed 7 days later for determination of the numbers of CFU in the lungs (Fig. 4D). The transfer of spleen cells from immunized animals, but not from nonimmunized mice, resulted in a reduction of bacterial colonization of approximately 2 log units. To look specifically at the function of CD4+ T cells in the protection against B. bronchiseptica colonization, spleen cells from mice immunized with OMVBbvir+ and treated with anti-CD4 antibody were administered to naive mice 24 h before challenge with a sublethal dose of B. bronchiseptica. Depletion of CD4+ T cells in the spleens of the donor animals increased the counts of bacteria recovered from the lungs of the recipient animals. The bacterial counts detected in those animals were similar to those found in the lungs of mice that had received the spleen cells of naive animals (Fig. 3D). All of these results indicate that immunity mediated by spleen cells, particularly CD4+ T cells, plays a role in the protection against B. bronchiseptica induced by the OMVBbvir+ vaccine.
Protection against intranasal B. bronchiseptica challenge after vaccination with OMVs from B. bronchiseptica blocked in the avirulent phase.
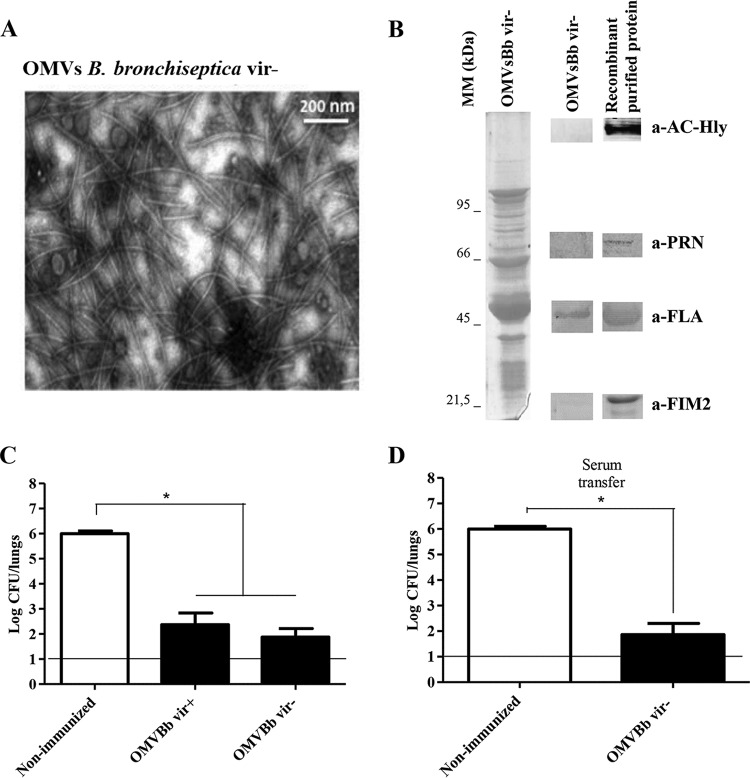
Since the avirulent phase of B. bronchiseptica could be involved in some stage of the infectious cycle (i.e., during chronic infection), we sought to evaluate whether an experimental vaccine containing the OMVs derived from B. bronchiseptica 9.73 blocked in the avirulent phase (OMVBbvir−) was able to confer protection against B. bronchiseptica infection. To this end, OMVs were obtained from a mutant, constructed previously in our laboratory, that was defective in the two-component signal transduction regulatory system BvgA of B. bronchiseptica (27). The characterization of OMVBbvir− revealed no differences from OMVBbvir+ in the size distribution, as evaluated by electron microscopy (Fig. 5A), but certain changes in the electrophoretic profile were detected, even though the major components were the same as those of OMVBbvir+ (Fig. 5B, left). The lack of expression of the main virulence factors and the expression of flagellin as an avirulent marker were evident upon immunoblotting (Fig. 5B, right). After this initial characterization of the OMVs, intranasal B. bronchiseptica challenges were performed. In those experiments, we analyzed the effects of two previous administrations of the OMVBbvir− preparation on subsequent colonization of the lungs of the experimental mice by B. bronchiseptica strain 9.73 (∼106 CFU in 40 μl) (Fig. 5C and D). The results were compared with those obtained for mice that had been preimmunized with the OMVBbvir+ vaccine (Fig. 5C). PBS-injected mice served as negative controls. Significant differences (P < 0.001) in lung bacterial counts between the immunized animals and the negative-control group were observed (Fig. 5). Of major significance was the observation that protection against B. bronchiseptica challenge was also achieved, at the same level, with OMVBbvir− immunization. The number of CFU recovered from the lungs at day 7 after challenge similarly decreased by at least 4 orders of magnitude, compared to values for nonimmunized mice (Fig. 5C).
FIG 5.
(A) Transmission electron microscopic image of negatively stained OMVs obtained from B. bronchiseptica 9.73 in the avirulent phase (OMVBbvir−) (scale bar, 200 nm). (B) Analysis of OMVBbvir− by 12.5% (wt/vol) SDS-PAGE, with visualization with Coomassie brilliant blue. Molecular masses are indicated on the left. On the right, immunoblots of OMVBbvir− and the purified recombinant proteins AC-Hly, PRN, flagellin (FLA), and FIM2, as detected with the respective specific polyclonal mouse antibodies, are shown. (C) Effect of active i.p. immunization with OMVBbvir− in the mouse intranasal challenge model. (D) Effect of passive immunization with sera collected from OMVBbvir−-immunized mice. For panels C and D, B. bronchiseptica 9.73 was used as the challenge bacteria (1 × 106 CFU, in 40 μl). Three biological replicates were performed, with the results from a representative one being presented. The data represent the means for 4 mice per group, at 7 days after challenge. The horizontal lines indicate the lower limit of detection. The numbers of bacteria recovered from the mouse lungs, expressed as the mean ± SEM (error bars) of log10 CFU in the lungs, are plotted on the ordinates for the lung samples from the mice receiving the immunogen, in the form of vesicles (C) or serum (D), indicated on the abscissas. *, significant differences (P < 0.001).
For this experimental vaccine, we also evaluated the specific roles of induced antibodies in the control and clearance of B. bronchiseptica. For that purpose, serum from naive or immunized animals was adoptively transferred into naive animals 24 h before a challenge with B. bronchiseptica (5 × 105 CFU of B. bronchiseptica 9.73). As seen with the OMVBbvir+ vaccine, the OMVBbvir−-induced serum collected from mice 14 days after the second dose cleared B. bronchiseptica (reduction of 3.5 log units) from the lungs of the challenged mice by day 7 postinoculation, whereas the naive serum had no significant effect (Fig. 5D). The results presented in this section clearly indicate that the OMVBbvir− vaccine and the induced humoral immunity possessed the capability of protecting mice against infections caused by B. bronchiseptica.
DISCUSSION
In this report, we describe the development and evaluation of OMVs obtained from B. bronchiseptica as vaccines against lung colonization. As we observed previously for OMVs derived from B. pertussis, the sizes of the OMVs from B. bronchiseptica 9.73 (with dimensions of 50 to 150 nm) were consistent from batch to batch. Furthermore, well-known principal B. bronchiseptica surface immunogens, such as PRN, AC-Hly, and FIM2, were detected in the OMVs through immunoblotting. The OMVBbvir+ thus characterized was then used in a murine model to examine its safety and protective capability. In the first experiments described, we performed comparisons with a whole-cell vaccine prepared in our laboratory, since a previous report indicated that the same type of formulation in dogs reduced the disease burden and the lesions in the vaccinated animals, relative to those of infected naive controls (10). We observed that two doses of our experimental OMVBbvir+ vaccine administered 2 weeks before B. bronchiseptica challenge fully protected the BALB/c mice against the colonization of different B. bronchiseptica strains obtained from diverse hosts, with two representative strains (one from a farm animal and one from a human patient) being shown. Regarding the safety of these preparations, in comparison with the whole-cell vaccine, we observed only a minimal increase in the levels of the proinflammatory cytokine IL-6 in serum just after OMV vaccination. These results would position the OMV-based vaccines above the classic cellular preparations, as OMVBbvir+ induces equal levels of protection but with adequate levels of safety. Another significant and relevant result detected with our experimental vaccine was the ability to induce protection against different isolates of B. bronchiseptica obtained from diverse hosts. This result becomes especially pertinent upon consideration of the diversity of genotypes already reported for B. bronchiseptica (28, 29), since this finding indicates that the formulation of a specific OMV-based vaccine for each specific isolate would be unnecessary.
In the present work, we also noted that the antibody titers of OMVBbvir+-vaccinated mice were higher on day 14 after the second dose and before B. bronchiseptica challenge than were those of the nonimmunized control group. Moreover, this OMVBbvir+ vaccine–induced immune serum recognized a group of antigens that included OMPc and the GroEL-like protein along with LPS. The fact that OMPc and the GroEL-like protein had been detected previously in mice immunized with OMVs derived from B. pertussis (23) was of interest to us. As discussed in a previous publication, both proteins in Bordetella and other microorganisms were described as promising vaccine targets (30, 31). With immunoblotting assays, we also determined that OMVBbvir+ was able to induce antibodies against LPS, an immunogenic bacterial component with proven protective capability (32). Based on the detection of these protection-inducing immunogens, we next sought to investigate whether the OMVBbvir+ vaccine-induced antibodies alone were sufficient to confer protection against B. bronchiseptica. The passive transfer assays we performed demonstrated that the vaccine-induced serum reduced the number of viable bacteria in the lungs by ∼4 log units. This protective role of the antibodies was in accordance with previous results reported by other authors (33). In fact, protection against B. bronchiseptica infection was demonstrated with antibodies induced by either infection or vaccination (33), through mechanisms that appeared to be different; whereas the immunity induced by previous infection offered significant protection even in the absence of complement or the induction of the monocyte IgG receptors FcγRs, vaccination-induced protection required both complement and FcγRs (33).
In addition, we found that CD4+ T cells contributed to the protection exhibited by the OMVBbvir+-based vaccine. The protection against B. bronchiseptica infection induced by the OMV-based vaccine seems to involve a dual mechanism involving both humoral and cellular immune responses, as indicated by adoptive transfer experiments.
Based on our previous results with OMVs derived from B. pertussis in the avirulent phase (23) and taking into account the potential role of that phase in the B. bronchiseptica infectious process (i.e., chronic infection), we decided to evaluate whether OMVs derived from B. bronchiseptica blocked in the avirulent phase (OMVBbvir−) were also able to induce protection. The OMVBbvir− formulation indeed proved to be protective, as judged by the significant (P < 0.001) decrease of 4 log units in the lung bacterial counts observed for avirulent OMVBbvir−-immunized mice, compared with the nonimmunized control group (Fig. 5C and D). The detected flagellum of B. bronchiseptica in the OMVBbvir− preparation probably contributes to the protective capacity of the OMVBbvir− formulation, since the flagellum has been described as a potent proinflammatory factor that induces expression of chemokines, cytokines, and the host defense gene (34). In addition, it was demonstrated that B. bronchiseptica flagellin is able to signal effectively through both human and mouse Toll-like receptor 5 (34). The protective effect is elicited by nonreplicative avirulent components that have thus far not been reported as being capable of conferring protection against B. bronchiseptica. The only study regarding a vaccine with avirulent B. bronchiseptica that we identified was performed in dogs with a live intact avirulent strain (35). Moreover, the authors demonstrated that the vaccinated dogs were protected against colonization as soon as 48 h after immunization (35).
Considered together, the results presented here provide clear evidence that the OMVs derived from B. bronchiseptica exhibit a high level of protection against B. bronchiseptica that is not dependent on the expression of bacterial virulence factors. Moreover, we demonstrate that the protection induced by these OMV-based vaccines is mediated mainly by antibodies but also by CD4+ T cells.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bordetella bronchiseptica strain 9.73 (isolated from a rabbit) (36) and a mutant derivative strain defective in expression of the BvgA protein (blocked in the avirulent phase) (27) were used throughout this study. Bordetella bronchiseptica strains were grown on Bordet-Gengou agar (Difco, Houston, TX, USA) supplemented with 10% (vol/vol) defibrinated sheep blood (from Laboratorio Argentino S.A.). For challenges in the animal experiments, the strains B. bronchiseptica 9.73 and B. bronchiseptica RB50 from rabbits (kindly provided by Peggy Cotter of the University of North Carolina) and B. bronchiseptica AR705 (an Argentinian clinical isolate obtained from a pediatric patient with cystic fibrosis) were also used.
Isolation of OMVs.
To obtain OMVs from the bacterial cells, we used the method previously described by us (18, 22, 37). In brief, culture samples from the decelerating growth phase of the bacteria were centrifuged at 10,000 × g for 20 min at 4°C, and the pellet obtained was resuspended in 20 mM Tris-HCl, 2 mM EDTA (pH 8.5) (TE buffer). Of the resulting pellet, approximately 1 g (wet weight) was resuspended in 5 ml of TE buffer. OMV release was promoted by sonication in ice water; the cells were then removed by centrifugation at 10,000 × g for 30 min, and the OMV supernatant was concentrated by ultracentrifugation at 40,000 × g for 3 h. The OMVs thus obtained were stored at 4°C. Thereafter, the OMVs were examined by electron microscopy after negative staining (22).
Protein assay.
Protein contents were estimated with the Bradford method, with bovine serum albumin (BSA) as the standard (38).
One-dimensional electrophoresis and immunoblotting.
OMV proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Immobilon P; Millipore), and probed with either a polyclonal anti-AC-Hly antibody (1:300), an anti-PRN antibody (1:500), an anti-FIM2 antibody (1:500), an anti-flagellin (1:2,000) antibody, or anti-GroEL, an antibody against a protein analogous to the Escherichia coli chaperonin GroEL (1:5,000), followed by incubation with anti-mouse IgG conjugated with alkaline phosphatase, at a 1:1,000 dilution. Nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate were used as the phosphatase substrates, according to the manufacturer's protocol (Biodynamics SRL, Buenos Aires, Argentina). Some of the proteins present in the vesicles were identified by mass spectrometry after the initial separation by one-dimensional electrophoresis, as described previously (39, 40). Bordetella bronchiseptica LPS electrophoresis was performed at room temperature and constant voltage. The LPSs were visualized by the Bio-Rad silver staining technique.
Formulation of vaccines.
To use the OMVs as acellular vaccines, the vesicle preparations were detoxified by mixing with aqueous formaldehyde (0.37% [vol/vol]) and incubation at 37°C overnight, and aluminum hydroxide (0.2 mg/ml) was then added as an adjuvant. To prepare the whole-cell vaccine (WCVBbvir+), a suspension containing 2 × 1010 CFU/ml heat-killed (56°C for 20 min) B. bronchiseptica 9.73 was detoxified in the same manner and then mixed with aluminum hydroxide (0.2 mg/ml) as an adjuvant.
Expression of inflammatory markers upon systemic delivery of OMVs.
In order to assess the proinflammatory capacity of the OMV-based vaccine formulation, mouse blood samples were collected 4 h after each immunization, by submandibular bleeding. Serum IL-6 levels were measured by an enzyme-linked immunosorbent assay (ELISA) with the BD OptiEIA kit (BD Biosciences, San Jose, CA, USA), according to the manufacturer's instructions.
Active immunization and intranasal challenge.
Four-week-old female BALB/c mice obtained from Instituto Biológico Argentino SAIC (Argentina) were used for all assays. As described previously (40), the immunization protocols included a two-dose schedule, with the formulations described above, over a period of 2 weeks. Two weeks after the second immunization, mice were subjected to a nasal challenge with a sublethal dose (105 to 106 CFU, in 40 μl) of a B. bronchiseptica strain. The lungs of the challenged mice were excised and collected for bacterial counting 7 days after the challenge. The numbers of CFU were determined as described previously (40). At least three biological replicates were performed.
ELISA.
Plates were coated with sonicated B. bronchiseptica (whole-cell lysates) in 0.5 M carbonate buffer (pH 9.5) in an overnight incubation at 4°C and then were blocked with 3% (vol/vol) skim milk in blocking buffer for 2 h at 37°C before incubation with serially diluted mouse serum samples for 1 h at 37°C. The bound IgG was detected after a 2-h incubation with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG at a titer of 1:20,000 (Thermo Fisher Scientific, Buenos Aires, Argentina). For measurement of IgG isotypes, bound antibody was detected with HRP-labeled subclass-specific anti-mouse IgG1 (1:8,000) or IgG2a (1:1,000) (Sigma-Aldrich). As the substrate, 1.0 mg/ml o-phenylendiamine (OPD) (Bio Basic Canada Inc.) in 0.1 M citrate phosphate buffer (pH 5.0) containing 0.1% hydrogen peroxide was used. Optical densities (ODs) were measured at 492 nm with a Titertek Multiskan model 340 microplate reader (ICN), and OD values were plotted as a function of log (serum dilution)−1. The inflection point of the curve was determined with GraphPad Prism software. Titers were defined as the reciprocal of the serum dilution giving an OD corresponding to the inflection point of the curve.
Bactericidal assay.
The bactericidal activity of sera collected from mice 2 weeks after immunization with the OMVBbvir+ vaccine (i.e., immune sera) and sera collected from nonimmunized mice (i.e., naive sera) was tested in vitro. Immune and naive sera inactivated by heat and PBS were used as controls. Virulent B. bronchiseptica 9.73 was grown on Bordet-Gengou agar and diluted to 1 × 105 CFU/ml in PBS containing 0.05 M MgCl2 and 0.15 mM CaCl2. Forty-five microliters of serum or PBS was mixed with 5 μl of a suspension containing 500 CFU of bacteria. After 1 h of incubation at 37°C, serial dilutions of the samples were spread on Bordet-Gengou agar plates and incubated for 48 to 72 h, to determine the CFU. At least three biological replicates were performed.
Analysis of cellular responses elicited by vaccination.
The cellular responses were analyzed as described previously (20). In brief, spleen cells from mice immunized with the OMV-based vaccine were harvested 8 weeks after the last immunization and seeded in 48-well culture plates at 106 cells per well, in a volume of 500 μl of RPMI 1640 cell culture medium supplemented with 10% (vol/vol) fetal bovine serum (Invitrogen, Buenos Aires, Argentina) and containing 100 IU/ml penicillin and 100 μg/ml streptomycin. All spleen cells were either stimulated with OMVs derived from B. bronchiseptica (5 μg/ml) or exposed to medium alone. Supernatants were removed after 72 h of incubation at 37°C in an atmosphere of 5% CO2, and the production of IFN-γ, IL-17, and IL-5 was determined by ELISA (BD Biosciences), according to the protocol specified by the manufacturer.
Adoptive transfer.
Pooled sera (100 μl) or spleen cells (5 × 106 cells) from nonimmunized mice or mice immunized with the OMV-based vaccine 2 weeks previously were transferred i.p. to female BALB/c mice. Twenty-four hours thereafter, the mice were infected with a sublethal dose (105 to 106 CFU, in 40 μl) of B. bronchiseptica 9.73, and subsequent protection was assessed by determining the numbers of CFU in the mouse lungs 7 days after the challenge.
In order to evaluate the contribution of CD4+ T cells to the protection, adoptive transfer assays were also performed using spleen cells obtained from animals immunized with OMVBbvir+ and depleted of CD4+ T cells. Depletion of CD4+ T cells in vaccinated mice was performed by i.p. injection of the monoclonal antibody (61.2 mg/ml) from the GK1.5 hybridoma specific for CD4. The dosing schedule consisted of administration of 200 μl of the antibody to the donor animals the day before spleens were collected for the passive immunization protocol. After spleen cell transfer, receptor animals received two doses of anti-CD4 antibody to ensure depletion. Depletion of CD4+ T cells was confirmed by ≥95% reductions of the lymphocyte CD4+ populations in blood and spleen, as determined by flow cytometry. Donor mice treated with a control isotype (IgG2bk) were included in the assays for comparison.
Statistical analysis.
The data were evaluated statistically by one-way analysis of variance (ANOVA), followed by the Tukey post hoc test, using GraphPad Prism software. Differences were considered significant at P values of <0.05.
ACKNOWLEDGMENTS
This work was supported by grants from ANPCyT, CONICET, and CICBA (Argentina) to D.H. D.B., M.E.G., and D.H. are members of the Scientific Career staff of CONICET. M.E.Z. and E.B. are fellows and C.V. is a professional of CONICET.
Donald F. Haggerty, a retired academic investigator and native English speaker, edited the final version of the manuscript.
REFERENCES
- 1.Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yacoub AT, Katayama M, Tran J, Zadikany R, Kandula M, Greene J. 2014. Bordetella bronchiseptica in the immunosuppressed population: a case series and review. Mediterr J Hematol Infect Dis 6:e2014031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng VL, Boggs JM, York MK, Golden JA, Hollander H, Hadley WK. 1992. Recovery of Bordetella bronchiseptica from patients with AIDS. Clin Infect Dis 15:376–377. doi: 10.1093/clinids/15.2.376. [DOI] [PubMed] [Google Scholar]
- 4.Decker GR, Lavelle JP, Kumar PN, Pierce PF. 1991. Pneumonia due to Bordetella bronchiseptica in a patient with AIDS. Rev Infect Dis 13:1250–1251. doi: 10.1093/clinids/13.6.1250. [DOI] [PubMed] [Google Scholar]
- 5.Goodnow RA. 1980. Biology of Bordetella bronchiseptica. Microbiol Rev 44:722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wernli D, Emonet S, Schrenzel J, Harbarth S. 2011. Evaluation of eight cases of confirmed Bordetella bronchiseptica infection and colonization over a 15-year period. Clin Microbiol Infect 17:201–203. doi: 10.1111/j.1469-0691.2010.03258.x. [DOI] [PubMed] [Google Scholar]
- 7.Gueirard P, Ave P, Balazuc AM, Thiberge S, Huerre M, Milon G, Guiso N. 2003. Bordetella bronchiseptica persists in the nasal cavities of mice and triggers early delivery of dendritic cells in the lymph nodes draining the lower and upper respiratory tract. Infect Immun 71:4137–4143. doi: 10.1128/IAI.71.7.4137-4143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gueirard P, Weber C, Le Coustumier A, Guiso N. 1995. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J Clin Microbiol 33:2002–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotter PA, Jones AM. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol 11:367–373. doi: 10.1016/S0966-842X(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 10.Ellis JA. 2015. How well do vaccines for Bordetella bronchiseptica work in dogs? A critical review of the literature 1977–2014. Vet J 204:5–16. doi: 10.1016/j.tvjl.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson A, Roberts M. 2002. Use of a rationally attenuated Bordetella bronchiseptica as a live mucosal vaccine and vector for heterologous antigens. Vaccine 20:2325–2335. doi: 10.1016/S0264-410X(02)00118-4. [DOI] [PubMed] [Google Scholar]
- 12.Sukumar N, Love CF, Conover MS, Kock ND, Dubey P, Deora R. 2009. Active and passive immunizations with Bordetella colonization factor A protect mice against respiratory challenge with Bordetella bronchiseptica. Infect Immun 77:885–895. doi: 10.1128/IAI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis J, Rhodes C, Lacoste S, Krakowka S. 2014. Antibody responses to Bordetella bronchiseptica in vaccinated and infected dogs. Can Vet J 55:857–864. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Z, Wang C, Xue Y, Ding K, Zhang C, Cheng X, Li Y, Liu Y, Wu T. 2010. Immunogenicity and protective efficacy of pertactin recombinants against Bordetella bronchiseptica challenge. Wei Sheng Wu Xue Bao 50:1239–1245. (In Chinese.) [PubMed] [Google Scholar]
- 15.Zhao Z, Xue Y, Tang X, Wu B, Cheng X, He Q, Zhang C, Guo A, Jin M, Chen H. 2009. Immunogenicity of recombinant protective antigen and efficacy against intranasal challenge with Bordetella bronchiseptica. Vaccine 27:2523–2528. doi: 10.1016/j.vaccine.2008.09.091. [DOI] [PubMed] [Google Scholar]
- 16.Davis R, Jayappa H, Abdelmagid OY, Armstrong R, Sweeney D, Lehr C. 2007. Comparison of the mucosal immune response in dogs vaccinated with either an intranasal avirulent live culture or a subcutaneous antigen extract vaccine of Bordetella bronchiseptica. Vet Ther 8:32–40. [PubMed] [Google Scholar]
- 17.King AA, Shrestha S, Harvill ET, Bjornstad ON. 2009. Evolution of acute infections and the invasion-persistence trade-off. Am Nat 173:446–455. doi: 10.1086/597217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asensio CJ, Gaillard ME, Moreno G, Bottero D, Zurita E, Rumbo M, van der Ley P, van der Ark A, Hozbor D. 2011. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 29:1649–1656. doi: 10.1016/j.vaccine.2010.12.068. [DOI] [PubMed] [Google Scholar]
- 19.Bottero D, Gaillard ME, Errea A, Moreno G, Zurita E, Pianciola L, Rumbo M, Hozbor D. 2013. Outer membrane vesicles derived from Bordetella parapertussis as an acellular vaccine against Bordetella parapertussis and Bordetella pertussis infection. Vaccine 31:5262–5268. doi: 10.1016/j.vaccine.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 20.Bottero D, Gaillard ME, Zurita E, Moreno G, Martinez DS, Bartel E, Carriquiriborde F, Errea A, Castuma C, Rumbo M, Hozbor D. 2016. Characterization of the immune response induced by pertussis OMVs-based vaccine. Vaccine 34:3303–3309. doi: 10.1016/j.vaccine.2016.04.079. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard ME, Bottero D, Errea A, Ormazabal M, Zurita ME, Moreno G, Castuma C, Bartel E, Flores D, van der Ley P, van der Ark A, Hozbor D. 2014. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine 32:931–937. doi: 10.1016/j.vaccine.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 22.Roberts R, Moreno G, Bottero D, Gaillard ME, Fingermann M, Graieb A, Rumbo M, Hozbor D. 2008. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 26:4639–4646. doi: 10.1016/j.vaccine.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Ormazabal M, Bartel E, Gaillard ME, Bottero D, Errea A, Zurita ME, Moreno G, Rumbo M, Castuma C, Flores D, Martín MJ, Hozbor D. 2014. Characterization of the key antigenic components of pertussis vaccine based on outer membrane vesicles. Vaccine 32:6084–6090. doi: 10.1016/j.vaccine.2014.08.084. [DOI] [PubMed] [Google Scholar]
- 24.Geurtsen J, Banus HA, Gremmer ER, Ferguson H, de la Fonteyne-Blankestijn LJ, Vermeulen JP, Dormans JA, Tommassen J, van der Ley P, Mooi FR. 2007. Lipopolysaccharide analogs improve efficacy of acellular pertussis vaccine and reduce type I hypersensitivity in mice. Clin Vaccine Immunol 14:821–829. doi: 10.1128/CVI.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston A, Petersen BO, Duus JO, Kubler-Kielb J, Ben-Menachem G, Li J, Vinogradov E. 2006. Complete structures of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharides. J Biol Chem 281:18135–18144. doi: 10.1074/jbc.M513904200. [DOI] [PubMed] [Google Scholar]
- 26.Gopinathan L, Kirimanjeswara GS, Wolfe DN, Kelley ML, Harvill ET. 2007. Different mechanisms of vaccine-induced and infection-induced immunity to Bordetella bronchiseptica. Microbes Infect 9:442–448. doi: 10.1016/j.micinf.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez J, Sisti F, Bottero D, Gaillard ME, Hozbor D. 2005. Constitutive expression of bvgR-repressed factors is not detrimental to the Bordetella bronchiseptica-host interaction. Res Microbiol 156:843–850. doi: 10.1016/j.resmic.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Khattak MN, Matthews RC. 1993. Genetic relatedness of Bordetella species as determined by macrorestriction digests resolved by pulsed-field gel electrophoresis. Int J Syst Bacteriol 43:659–664. doi: 10.1099/00207713-43-4-659. [DOI] [PubMed] [Google Scholar]
- 29.Register KB, Boisvert A, Ackermann MR. 1997. Use of ribotyping to distinguish Bordetella bronchiseptica isolates. Int J Syst Bacteriol 47:678–683. doi: 10.1099/00207713-47-3-678. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M, Dixit A. 2016. Immune response characterization and vaccine potential of a recombinant chimera comprising B-cell epitope of Aeromonas hydrophila outer membrane protein C and LTB. Vaccine 34:6259–6266. doi: 10.1016/j.vaccine.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Chen Z, Tan C, Liu W, Xu Z, Zhou R, Chen H. 2012. Immunogenic characterization of outer membrane porins OmpC and OmpF of porcine extraintestinal pathogenic Escherichia coli. FEMS Microbiol Lett 337:104–111. doi: 10.1111/1574-6968.12013. [DOI] [PubMed] [Google Scholar]
- 32.Sisti F, Fernandez J, Cordero A, Casabuono A, Couto A, Hozbor D. 2017. Modifications of Bordetella bronchiseptica core lipopolysaccharide influence immune response without affecting protective activity. Bioorg Med Chem Lett 27:432–436. doi: 10.1016/j.bmcl.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 33.Kirimanjeswara GS, Mann PB, Harvill ET. 2003. Role of antibodies in immunity to Bordetella infections. Infect Immun 71:1719–1724. doi: 10.1128/IAI.71.4.1719-1724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Boado YS, Cobb LM, Deora R. 2005. Bordetella bronchiseptica flagellin is a proinflammatory determinant for airway epithelial cells. Infect Immun 73:7525–7534. doi: 10.1128/IAI.73.11.7525-7534.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bey RF, Shade FJ, Goodnow RA, Johnson RC. 1981. Intranasal vaccination of dogs with liver avirulent Bordetella bronchiseptica: correlation of serum agglutination titer and the formation of secretory IgA with protection against experimentally induced infectious tracheobronchitis. Am J Vet Res 42:1130–1132. [PubMed] [Google Scholar]
- 36.Gueirard P, Guiso N. 1993. Virulence of Bordetella bronchiseptica: role of adenylate cyclase-hemolysin. Infect Immun 61:4072–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hozbor D, Rodriguez ME, Fernandez J, Lagares A, Guiso N, Yantorno O. 1999. Release of outer membrane vesicles from Bordetella pertussis. Curr Microbiol 38:273–278. doi: 10.1007/PL00006801. [DOI] [PubMed] [Google Scholar]
- 38.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Bottero D, Gaillard ME, Basile LA, Fritz M, Hozbor DF. 2012. Genotypic and phenotypic characterization of Bordetella pertussis strains used in different vaccine formulations in Latin America. J Appl Microbiol 112:1266–1276. doi: 10.1111/j.1365-2672.2012.05299.x. [DOI] [PubMed] [Google Scholar]
- 40.Bottero D, Gaillard ME, Fingermann M, Weltman G, Fernandez J, Sisti F, Graieb A, Roberts R, Rico O, Ríos G, Regueira M, Binsztein N, Hozbor D. 2007. Pulsed-field gel electrophoresis, pertactin, pertussis toxin S1 subunit polymorphisms, and surfaceome analysis of vaccine and clinical Bordetella pertussis strains. Clin Vaccine Immunol 14:1490–1498. doi: 10.1128/CVI.00177-07. [DOI] [PMC free article] [PubMed] [Google Scholar]