Summary
The neurotransmitter dopamine (DA) has prominent effects in the immune system and between the immune cells, CD4+ regulatory T (Treg) lymphocytes, a specialized T‐cell subset crucial for the control of immune homeostasis, are especially sensitive to DA. Dopaminergic receptors (DR) are grouped into two families according to their pharmacological profile and main second messenger coupling: the D1‐like (D1 and D5), which activate adenylate cyclase, and the D2‐like (D2, D3 and D4), which inhibit adenylate cyclase and exist in several variants that have been associated to clinical conditions such as schizophrenia, bipolar disorder, substance abuse and addiction. We aimed to examine, in venous blood samples from healthy volunteers, the relationship between the arbitrary DR score and DR functional responses in human lymphocytes. All the samples were genotyped for selected DR gene variants (DRD1: rs4532 and rs686; DRD2: rs1800497 and rs6277; DRD3: rs6280; DRD4: rs747302 and seven 48‐base pair variable number tandem repeat (VNTR)) and a DR score was attributed to each participant. We have also tested whether DR gene polymorphisms might affect Treg cell ability to suppress effector T‐cell function. To our knowledge, this is the first study showing a correlation between DR gene variants and human T lymphocyte function. The main results are that both D1‐like and D2‐like DR are functionally active in human lymphocytes, although the D1‐like DR stimulation results in stronger effects in comparison to the D2‐like DR stimulation. In addition, it seems that the DR genetic profile may affect the ability of lymphocytes to respond to dopaminergic agents. More investigations are needed about the possible clinical relevance of such findings.
Keywords: CD4 cell, immune homeostasis, inflammation, regulatory T cell
The neurotransmitter dopamine (DA) has prominent effects in the immune system, affecting T and B lymphocytes, dendritic cells, monocytes/macrophages, neutrophils and natural killer cells.1, 2 Among lymphocytes, CD4+ regulatory T (Treg) lymphocytes, a specialized T‐cell subset crucial for the control of immune homeostasis, are especially sensitive to DA, which down‐regulates their regulatory functions acting on D1‐like dopaminergic receptors (DR).3
Dopaminergic receptors belong to the seven‐transmembrane, G‐protein coupled receptors family, and are grouped into two families according to their pharmacological profile and main second messenger coupling: the D1‐like (D1 and D5), which activate adenylate cyclase, and the D2‐like (D2, D3 and D4), which inhibit adenylate cyclase.4 DR genes exist in several variants, which have been associated with clinical conditions such as schizophrenia, bipolar disorder, substance abuse and addiction.5, 6
We recently showed that in the peripheral blood of healthy individuals CD4+ T lymphocyte count is associated with DR gene polymorphisms, and in particular with an arbitrary score based on the activity of D1‐like versus D2‐like DR (higher values corresponding to increased D1‐like DR density/responsiveness), suggesting that increased D1‐like DR activity might correlate with a higher number of circulating CD4+ T cells.6
The aim of the present investigation was first to examine the relationship between the DR score used in the previous study and DR functional responses in human lymphocytes. To this end, we collected venous blood samples from healthy volunteers, as part of a project aimed at assessing the expression and role of DR on circulating CD4+ T cells in Parkinson's disease. The study protocol was approved by the Ethics Committee of the Ospedale di Circolo and Fondazione Macchi, Varese (I).7 Lymphocytes were isolated by Ficoll‐Paque Plus density gradient centrifugation, resuspended in RPMI‐1640 medium supplemented with 10% heat‐inactivated fetal calf serum and 2 mm glutamine, at a final concentration of 1 × 106 cells/ml for subsequent culture at 37° in a moist atmosphere of 5% CO2. Cells were then stimulated for 45 min with phytohaemagglutinin (PHA) 10 μg/ml, alone or in the presence of the D1‐like DR agonist SKF‐38393, 1 μm, or the D2‐like DR agonist pramipexole, 1 μm. Cells were finally harvested and intracellular cAMP was measured using a cAMP ELISA kit (R&D Systems, Mckinley Place, NE, Minneapolis, MN). All the samples were genotyped for selected DR gene variants (DRD1: rs4532 and rs686; DRD2: rs1800497 and rs6277; DRD3: rs6280; DRD4: rs747302 and seven 48‐base pair variable number tandem repeat (VNTR)) and a DR score was attributed to each participant, as previously described.6 Briefly, +1 was assigned to each allele associated with increased D1‐like DR activity or decreased D2‐like DR activity, and −1 to each allele associated with decreased D1‐like DR activity or increased D2‐like DR activity (Table 1).5, 6 In our previous study,6 this score correlated with total lymphocytes, CD3+ and CD4+ T cells in the peripheral blood of healthy individuals.
Table 1.
Dopaminergic receptor (DR) gene variants analysed in the study and assigned scores (for detailed references see ref. 6)
| Receptor | Gene | Variant | Nucleotide change | Effects | Score |
|---|---|---|---|---|---|
| D1‐like | |||||
| D1 | DRD1 | rs4532 | −48A>G | Association with nicotine and alcohol dependence, and with tobacco smoking in schizophrenia. | +1 |
| rs686 | 62C>T | Higher gene expression and association with nicotine and alcohol dependence, and with tobacco smoking in schizophrenia. | +1 | ||
| D2‐like | |||||
| D2 | DRD2 | rs1800497 | 2137G>A | Lower striatal DR D2 density in healthy individuals. | +1 |
| rs6277 | 957C>T | Decreased mRNA stability and translation, reduced dopamine‐induced up‐regulation of DR D2 expression in vitro, and lower DR D2 expression in cortex and thalamus of healthy individuals. | +1 | ||
| D3 | DRD3 | rs6280 | 25G>A | Higher dopamine binding affinity in vitro, association with alcohol and heroin dependence. | −1 |
| D4 | DRD4 | 7 48‐base pair VNTR | Reduced mRNA levels in human post‐mortem brain tissue samples, lower response to stimulants and requirements of higher doses of methylphenidate. | +1 | |
As expected, stimulation with PHA increased intracellular cAMP by (mean ± SEM) 65·3 ± 1·6 × 10−12 mol/106 cells (n = 12, P < 0·001 versus resting cells). In comparison to PHA alone, the increase was higher in the presence of the D1‐like DR agonist SKF‐38393 (99·1 ± 2·3 × 10−12 mol/106 cells, n = 12, P < 0·001 versus PHA alone), and was lower with the D2‐like DR agonist pramipexole (56·4 ± 1·9 × 10−12 mol/106 cells, n = 12, P < 0·001 versus PHA alone) (Fig. 1a). In individual preparations, the SKF‐38393‐induced increase of cAMP correlated with the DR score: in particular, the SKF‐38393‐induced cAMP increase was higher with increasing DR score, corresponding to increased D1‐like DR density and/or activity, whereas the pramipexole‐induced decrease showed no statistically significant correlation with the DR score (Fig. 1b).
Figure 1.
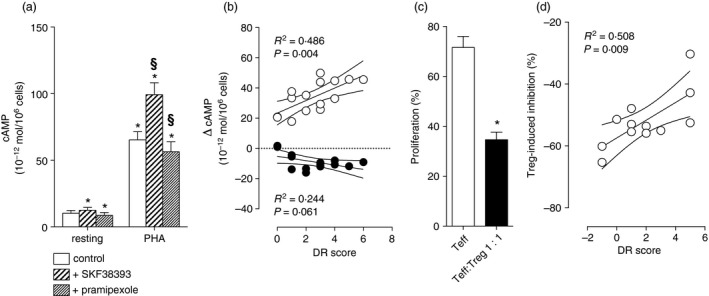
Dopaminergic receptor (DR) gene polymorphisms affect cAMP responses in peripheral blood mononuclear cells (PBMC) and regulatory T (Treg) cell‐induced inhibition of effector T (Teff) cell proliferation. (a) Stimulation with phytohaemagglutinin (PHA) increases cAMP levels in PBMC and the effect is enhanced by the D1‐like DR agonist SKF‐38393 1 μm and reduced by the D2‐like DR agonist pramipexole 1 μm. Data are means ± SEM of 15 different participants. *P < 0·05 versus resting/control and §P < 0·05 versus PHA alone. (b) Correlation between SKF‐38393‐induced increase (empty circles) or pramipexole‐induced decrease of cAMP levels in PBMC and DR score. Linear regression lines are represented together with their 95% confidence intervals. R 2 and P values are indicated in the graph. The effect of SKF‐38393 increases with increasing DR score, corresponding to increased D1‐like DR activity, whereas the effect of pramipexole shows no significant correlation with the score. (c) Proliferation of Teff cells stimulated with PHA/interleukin‐2 alone and in the presence of Treg cells 1 : 1. Data are means ± SEM of 12 different participants. *P < 0·0001 versus Teff alone. (d) Correlation between Treg‐induced inhibition of Teff cell proliferation and DR score. Linear regression lines are represented together with their 95% confidence intervals. R 2 and P values are indicated in the graph. Treg cell inhibition of Teff cell proliferation decreased with increased DR score and therefore with increased D1‐like DR activity.
In a second set of experiments, we tested whether DR gene polymorphisms might affect the ability of Treg cellsto suppress T effector (Teff) cell function. We used venous blood samples collected as described above, and Treg cells were separated from CD4+ Teff cells by means of a human CD4+ CD25+ Regulatory T cell Isolation Kit (Miltenyi Biotec, Auburn, CA, USA) as previously reported.3, 8 Treg and Teff cells were then placed in wells containing RPMI‐1640 medium supplemented with 10% heat‐inactivated fetal calf serum, 2 mm glutamine and 100 U/ml penicillin/streptomycin, at 37° in a moist atmosphere of 5% CO2. Cells were kept alone or in co‐culture (Teff : Treg ratio = 1 : 1), and treated with PHA 5 μg/ml, and interleukin‐2 40 ng/ml. Proliferation of Teff cells was measured after 5 days of culture by using flow cytometry and Cell Proliferation Dye eFluor. Acquisition was performed on a BD FACSCanto II flow cytometer (Becton Dickinson Italy, Milan, Italy) with BD FACSdiva software (version 6.1.3). Even in this set of experiments, all the samples were genotyped for the previously listed DR gene variants and a DR score was attributed to each participant.
When cultured alone, Teff proliferated up to 71·6% ± 4·4% (mean ± SEM, n = 12). Proliferation in the presence of Treg decreased to 34·7% ± 3·0% (n = 12, P < 0·0001) (Fig. 1c). In individual preparations, Treg‐induced reduction of Teff proliferation was inversely proportional to the DR score (Fig. 1d), implying that Treg cell inhibition of Teff cell proliferation decreased with increased D1‐like DR activity. Analysis of individual DR gene variants showed that the DRD1 rs4532 was strongly associated with Treg cell inhibition of Teff cell proliferation (Table 2). Co‐incubation with DA 1 μm reduced Treg‐induced inhibition of Teff cell proliferation by 63·0% ± 10·1% (P = 0·001 versus Treg alone); however, in the two individuals carrying the DRD1 rs4532 A/A genotype, dopaminergic reduction of the effects of Treg cells was only 45·7% ± 18·1%, whereas in the eight participants with the A/G phenotype it was 69·0% ± 16·8% and in the two participants with the A/A phenotype it was 65·6% ± 12·6%.
Table 2.
Dopaminergic receptor (DR) gene variants and regulatory T (Treg) inhibition of effector T (Teff) cell proliferation
| Gene | Variant | Genotype | No. of participants | Treg‐induced inhibition (%) | anova | P for trend |
|---|---|---|---|---|---|---|
| DRD1 | rs4532 | A/A | 2 | −57·7 ± 3·3 | 0·005 | 0·004 |
| A/G | 8 | −54·3 ± 5·0 | ||||
| G/G | 2 | −36·5 ± 8·8 | ||||
| rs686 | C/C | 4 | −58·4 ± 5·4 | 0·066a | na | |
| C/T | 7 | −49·5 ± 8·9 | ||||
| T/T | 1 | −42·8 | ||||
| DRD2 | rs1800497 | G/G | 8 | −52·4 ± 10·4 | 0·824a | na |
| G/A | 4 | −51·1 ± 5·6 | ||||
| A/A | 0 | = | ||||
| rs6277 | C/C | 3 | −58·9 ± 7·02 | 0·115a | na | |
| C/T | 8 | −49·2 ± 8·9 | ||||
| T/T | 1 | −52·5 | ||||
| DRD3 | rs6280 | G/G | 2 | −41·4 ± 15 | 0·152 | 0·059 |
| G/A | 5 | −52·4 ± 5·5 | ||||
| A/A | 5 | −55·7 ± 6·1 | ||||
| DRD4 | 7 48‐base pair VNTR | 7R/7R | 9 | −53·1 ± 6·1 | 0·458a | na |
| 7R/4R | 3 | −48·5 ± 15·9 | ||||
| 4R/4R | 0 | = |
anova, analysis of variance; na, not applicable.
By two‐tailed Student's t‐test.
This is the first study showing a correlation between DR gene variants and human T lymphocyte function. In our previous investigation,6 we reported an association between DR gene variants and circulating CD4+ T‐cell count, suggesting that the prevalence of D1‐like DR activity might lead to increased CD4+ T cells in peripheral blood. Interestingly, we recently reported that D1‐like DR are more expressed than D2‐like DR in human CD4+ T cells.7 DR gene variants have been studied so far mainly in relationship to substance addiction and to behavioural responses to stimulants,5, 6 and no information is currently available regarding their role in other diseases and/or in the regulation of immune cell function, despite extensive knowledge about dopaminergic regulation of adaptive and innate immunity in health and disease.2, 9, 10 In the present study, we tested the effects of DR gene variants on the short burst of cAMP production induced by PHA stimulation, which is critical for T‐cell activation,11 and on Treg‐induced inhibition of Teff cell proliferation. Indeed, we previously reported that Treg cells, in comparison to Teff cells, respond to DA with a rapid and sustained increase of cAMP.3 The present results on the effects of DR agonists on cAMP levels may suggest that both D1‐like and D2‐like DR are functionally active in human lymphocytes; however, that D1‐like DR stimulation may result in stronger effects than D2‐like DR stimulation at the second messenger level. Moreover, the correlation between D1‐like DR‐induced cAMP increase and DR score suggests that the DR genetic profile may affect the ability of lymphocytes to respond to dopaminergic agents.
The clear correlation between the DR score and Treg‐induced reduction of Teff cell proliferation strengthens previous observations about the inhibitory role of D1‐like DR on human Treg cells.3 A higher DR score indicates increased D1‐like DR activity (as confirmed by cAMP increase in total lymphocytes), and probably results in reduced regulatory functions by Treg cells. Indeed, Treg cells from individuals with higher DR score exert less inhibition on Teff cell proliferation (Fig. 1d). The DR gene variant that shows the strongest association with Treg cell function is DRD1 rs4532. In our previous study,6 we described a correlation between DRD1 rs4532 and DR D1 expression on T lymphocytes. In particular, individuals carrying the DRD1 rs4532 G/G genotype had 0·21 ± 0·05 × 109 DR D1 + CD4+ T cells/ml, individuals with the G/A genotype had 0·11 ± 0·04 × 109 DR D1 + CD4+ T cells/ml, and the only one individual with the A/A genotype had 0·03 × 109 DR D1 + CD4+ T cells/ml. In the present study, the participants with the G/G genotype had the lowest Treg cell function whereas the A/A genotype individuals showed the highest Treg cell function (Table 2). We previously showed that dopaminergic inhibition of Treg cell function occurs through the activation of D1‐like DR,3 and in agreement with those findings we now observed that the lowest Treg cell function is associated with the highest expression of D1‐like DR D1, whereas the highest Treg cell function occurs when almost no D1‐like DR D1 are expressed on CD4+ T cells. Preliminary experiments with DA confirmed the ability of this catecholamine to reduce Treg‐induced inhibition of Teff cell proliferation,3, 8 and also possibly suggested that DA‐dependent reduction of Treg cell function is dependent on the availability of D1‐like DR D1, because it was higher in the A/G and G/G genotypes and lower in the A/A genotype.
Results raise the issue of possible relationships between DRD1 genetic variants and Treg cell dysfunction‐related disease. Interestingly, DRD1 genetic variants have been associated with cancer, but it is unclear whether Treg cells contribute.12 More investigations are needed in cancer as well as in autoimmunity, organ transplantation and infectious disease, because increasing the knowledge about the role of DR genetic variants in the modulation of T lymphocyte function and in particular of Treg cells might result in better understanding of individual variability of immune responses in health and disease, providing the basis for the development of tailored therapeutic approaches.
Author contribution
MC and FM contributed to the study conception and design; MF, NK and ER contributed to the acquisition of data; and MC, NK, MF and FM performed the analysis and interpretation of data. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and declare that they have confidence in the integrity of the contributions of their co‐authors.
Disclosure
The authors declare that they have no relevant conflicts of interest.
Acknowledgements
The authors are indebted to the personal involvement of the individuals who participated in this study. This study was supported by a grant from Fondazione CARIPLO to Marco Cosentino (Project 2011‐0504: Dopaminergic modulation of CD4+ T lymphocytes: relevance for neurodegeneration and neuroprotection in Parkinson's disease – The dopaminergic neuro‐immune connection). Natasa Kustrimovic had a postdoctoral fellow appointment supported by the grant. The authors wish to express their gratefulness to Dr Iva Aleksic and to Dr Alessandra Luini (Centre for Research in Medical Pharmacology, University of Insubria) for their skilful technical collaboration, and to Ms Paola Gervasini (Centre for Research in Medical Pharmacology, University of Insubria) for her valuable collaboration in the administrative management and reporting of the grant.
References
- 1. Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun 2010; 24:525–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cosentino M, Marino F. Adrenergic and dopaminergic modulation of immunity in multiple sclerosis: teaching old drugs new tricks? J Neuroimmune Pharmacol 2013; 8:163–79. [DOI] [PubMed] [Google Scholar]
- 3. Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E et al Human CD4+ CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 2007; 109:632–42. [DOI] [PubMed] [Google Scholar]
- 4. Beaulieu J‐M, Gainetdinov RR. The physiology, signalling, and pharmacology of dopamine receptors. Pharmacol Rev 2011; 63:182–217. [DOI] [PubMed] [Google Scholar]
- 5. Wong AH, Buckle CE, Van Tol HH. Polymorphisms in dopamine receptors: what do they tell us? Eur J Pharmacol 2000; 410:183–203. [DOI] [PubMed] [Google Scholar]
- 6. Cosentino M, Ferrari M, Kustrimovic N, Rasini E, Marino F. Influence of dopamine receptor gene polymorphisms on circulating T lymphocytes: a pilot study in healthy subjects. Hum Immunol 2015; 76:747–52. [DOI] [PubMed] [Google Scholar]
- 7. Kustrimovic N, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F et al Dopaminergic receptors on CD4+ T naive and memory lymphocytes correlate with motor impairment in patients with Parkinson's disease. Sci Rep 2016; 6:33738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosentino M, Zaffaroni M, Trojano M, Giorelli M, Pica C, Rasini E et al Dopaminergic modulation of CD4+ CD25high regulatory T lymphocytes in multiple sclerosis patients during interferon‐β therapy. NeuroImmunoModulation 2012; 19:283–92. [DOI] [PubMed] [Google Scholar]
- 9. Levite M, Marino F, Cosentino M. Dopamine, T cells and multiple sclerosis (MS). J Neural Transm 2017; 124:525–42. [DOI] [PubMed] [Google Scholar]
- 10. Pinoli M, Marino F, Cosentino M. Dopaminergic regulation of innate immunity: a review. J Neuroimmune Pharmacol 2017; doi: 10.1007/s11481‐017‐9749‐2. [DOI] [PubMed] [Google Scholar]
- 11. Arumugham VB, Baldari CT. cAMP: a multifaceted modulator of immune synapse assembly and T cell activation. J Leukoc Biol 2017; 101:1301–16. [DOI] [PubMed] [Google Scholar]
- 12. Robles AI, Yang P, Jen J, McClary AC, Calhoun K, Bowman ED et al A DRD1 polymorphism predisposes to lung cancer among those exposed to secondhand smoke during childhood. Cancer Prev Res (Phila) 2014; 7:1210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


