Abstract
The incidence and risk factors of lung cancer in patients with idiopathic pulmonary fibrosis (IPF) have been poorly investigated.
We conducted a retrospective study of 632 patients with IPF to assess the incidence and risk factors of lung cancer development.
Seventy patients developed lung cancer over a median follow-up period of 3.8 years. The incidence density of lung cancer development was 25.2 cases per 1000 person-years. The most frequent type was squamous cell carcinoma (30%), the majority developed lung cancer in the peripheral lung (82.9%) and adjacent to usual interstitial pneumonia (75.7%). In a multivariate Cox regression hazard model, pack-years of smoking ≥35 and coexisting emphysema were associated with lung cancer development. The 1-, 3- and 5-year all-cause mortality rates after lung cancer diagnosis were 53.5%, 78.6% and 92.9%, respectively.
The incidence density of lung cancer is high in IPF patients and occurs more frequently in patients with smoking history of pack-years of smoking ≥35 and with coexisting emphysema. The majority of lung cancers develop adjacent to usual interstitial pneumonia. Knowledge of these factors may help direct efforts for early detection of lung cancer and disease management.
Short abstract
In patients with IPF, lung cancer will develop in 25.2 cases per 1000 person-years. Clinicians should pay attention to the development of lung cancer, especially in patients with ≥35 pack-years of smoking and coexisting emphysema. http://ow.ly/KLjx30hObFu
Introduction
Idiopathic pulmonary fibrosis (IPF) is a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause. It is associated with the histopathologic and/or radiologic pattern of usual interstitial pneumonia (UIP) [1, 2]. Comorbidities including lung cancer (LC), pulmonary hypertension, chronic obstructive pulmonary disease (COPD)/emphysema, pulmonary embolism and pulmonary infections can occur in IPF [1–4]. The identification and prompt treatment of comorbidities may have a clinically significant and meaningful effect on overall outcome for patients with IPF [4].
Several studies have documented that patients with IPF are at high risk for the development of LC [5–9], and in Japan, 11% of IPF patients died of LC [10]. Thus, there is a striking association between IPF and LC. The prevalence of LC in patients with IPF is reported to be 3–45.7% [9, 11–17]. However, only a few studies have evaluated the incidence of LC during IPF follow-up [7, 18–20]. Ozawa et al. [18] reported the cumulative incidence of and predictive factors for LC in IPF. The 5-year cumulative incidence rate of LC in 103 IPF patients was 15.4%, and age was an independent significant factor predicting the development of LC. In their report, they speculated that coexisting emphysema was possibly associated with the development of LC in IPF patients, and they suggested that further investigations are needed to clarify this possibility. Hyldgaard et al. [19] reported an incidence density of 36 cases per 1000 person-years of LC in 121 patients with IPF. Le Jeune et al. [7] reported an incidence density of 11.2 cases per 1000 person-years of LC in 1064 patients with IPF in a longitudinal computerised healthcare dataset in the UK. Tomassetti et al. [20] reported a cumulative incidence of 41% and 82%, respectively, at 1 and 3 years of LC in 23 patients with LC and IPF, and that patients with LC were more frequently smokers, with combined pulmonary fibrosis and emphysema (52% versus 32%) than patients with IPF only (n=158). Previous studies have reported factors such as smoking, age, gender and emphysema as risk factors for LC in IPF [11, 12, 14, 15, 20]. However, these risk factors were assessed when IPF patients had already developed LC, with only one exception in Ozawa's study [18], and may not be predictive of the risk of developing LC at the initial diagnosis of IPF. We thus thought that the incidence density of and risk factors for LC development, including emphysema, should be elucidated on a large scale.
Methods
Subjects
From January 1995 to July 2011, 910 patients with IPF were treated at our institution. Of these patients, 278 were not included: 223 had simultaneous LC at IPF diagnosis, and 10 patients were diagnosed as having microscopic polyangiitis at IPF diagnosis. The observation period of 45 patients was less than 3 months. Thus, 632 patients comprised the cohort of this study. All patients fulfilled the criteria for IPF of the American Thoracic Society (ATS) and European Respiratory Society (ERS) [2] or the official ATS/ERS/Japanese Respiratory Society/Latin American Thoracic Society statement on IPF [1]. Emphysema was considered present if low-attenuation areas were present on high-resolution computed tomography (HRCT) images. The study was approved by the institutional review board of Saitama Cardiovascular and Respiratory Center.
IPF diagnosis
Each patient was diagnosed as having IPF according to criteria used at the time of diagnosis. We checked the CT scan of all patients and diagnosed as IPF only those patients who satisfied the diagnostic criteria of 2011 and 2013 [1, 2]. All patients were evaluated using HRCT. A radiologist and a respiratory physician used HRCT to diagnose patients with UIP pattern as having IPF, and they did not include possible UIP pattern. Most patients did not undergo surgical lung biopsy.
Emphysema diagnosis
On CT scan, we defined low-attenuation areas bordered by a very thin (<1 mm) or no wall in the normal lung as emphysema.
LC diagnosis and histologic type
LC was diagnosed on the basis of pathology or cytology. When cytological findings were suggesting nonsmall cell LC, but histological type was not specified, LC was diagnosed as unclassified nonsmall cell LC.
Study design
This was a retrospective cohort study. Clinical, laboratory, radiographic, cytological and pathological data and outcome were collected from medical records. Baseline clinical parameters were obtained within 1 month of initial diagnosis. If these data were not obtained within this period, we considered them to be unknown. Then, during follow-up periods, we investigated the incidence of LC. Survival status was obtained from medical records and/or telephone interviews. We also investigated the risk factors of LC development.
Statistical analysis
Categorical baseline characteristics are summarised by frequency and per cent, and continuous characteristics are reported as mean±sd or median and range as appropriate. Group comparisons were made using Wilcoxon rank-sum test, or Fisher's exact test as appropriate. Diagnosis of LC was estimated by Kaplan–Meier analysis. Survival was evaluated using a Kaplan–Meier curve and compared between groups using log-lank tests. Cox regression analysis was used to determine whether the following factors at diagnosis increased the risk of LC: sex, age, body mass index, smoking history, pack-years of smoking, emphysema, forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1)/FVC ratio, lung diffusion capacity for carbon monoxide, partial pressure of oxygen in arterial blood, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum albumin and Krebs von den Lungen-6 (KL-6) at baseline. In all analyses, a p-value of <0.05 was considered to be statistically significant. We conducted all statistical analyses with SAS version 9.2 (SAS Institute, Cary, NC).
Results
Characteristics of patients who did not develop LC or developed LC during IPF follow-up
Of the 632 patients with IPF, the mean age was 69.5 years, 76.4% were male, 76.4% were smokers, and coexisting emphysema was present in 188 (29.7%) patients. Table 1 compares the clinical and physiological characteristics of the patients with and without LC diagnosis. At baseline, patients who later developed LC were younger, more commonly male and smoking more pack-years of cigarettes, and more frequently had emphysema, and had a better FVC % and worse FEV1/FVC.
TABLE 1.
Baseline characteristics of patients with idiopathic pulmonary fibrosis with or without lung cancer diagnosis during follow-up periods
| Characteristics | All patients | Patients with lung cancer | Patients without lung cancer | p-value |
| Patients | 632 | 70 | 562 | |
| Age years | 69.5±8.5 | 66.8±7.9 | 69.8±8.5 | 0.006 |
| Males % | 76.4 | 94.3 | 74.2 | <0.001 |
| BMI kg·m−2 | 23.2±3.1 | 23.2±2.9 | 23.2±3.1 | 0.996 |
| Smoking exposure pack-years | 35.0 (0–141) | 46.4 (0.75–120) | 31.0 (0–141) | <0.001 |
| Smokers % | 76.4 | 100 | 73.5 | <0.001 |
| Emphysema % | 29.7 | 60.0 | 26.0 | <0.001 |
| FVC % pred | 76.2±20.2 | 89.2±16.9 | 75.1±20.1 | <0.001 |
| FEV1/FVC % | 80.3±10.8 | 74.1±9.4 | 80.9±10.7 | <0.001 |
| DLCO % pred | 76.4±23.5 | 74.9±26.9 | 76.6±23.2 | 0.517 |
| PaO2 mmHg | 74.0±15.1 | 77.3±12.2 | 73.5±15.4 | 0.194 |
| ESR mm·h−1 | 34.0 (2–138) | 36.5 (5–120) | 34.0 (2–138) | 0.665 |
| CRP mg·dL−1 | 0.24 (0.00–50.68) | 0.29 (0.00–15.11) | 0.23 (0.00–50.68) | 0.819 |
| Albumin g·dL−1 | 4.0±0.5 | 4.0±0.4 | 4.0±0.5 | 0.763 |
| KL-6 U·mL−1 | 762.0 (149–9705) | 649.5 (347–2454) | 775.0 (149–9705) | 0.078 |
Data are presented as mean±sd or median (range), unless otherwise stated. BMI: body mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; PaO2: arterial oxygen tension; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; KL: Krebs von der Lungen. p-values were calculated in relation to with or without lung cancer diagnosis.
Incidence of LC
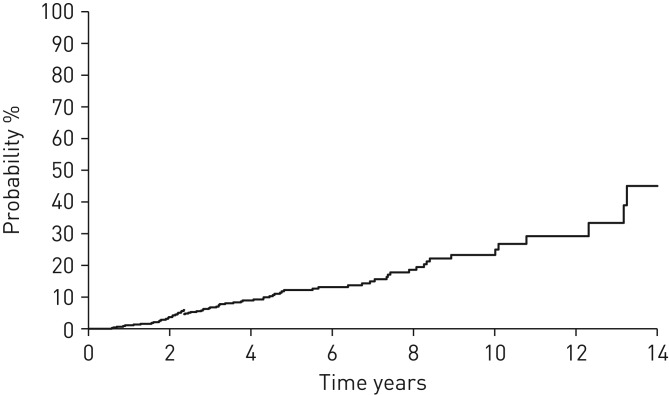
Of the 632 patients with IPF, 70 (11.1%) developed LC during median (range) follow-up periods of 3.8 (0.25–15.2) years. The 5- and 10-year cumulative LC development rates were 12.2% and 23.3%, respectively. The incidence densities of LC development in all, male and female patients were 25.2, 30.8 and 6.3 cases per 1000 person-years, respectively (figure 1).
FIGURE 1.
Kaplan–Meier curve for the time until the development of lung cancer in patients with idiopathic pulmonary fibrosis. The 5- and 10-year cumulative lung cancer development rates were 12.2% and 23.3%, respectively. The incidence density of lung cancer development was 25.2 cases per 1000 person-years.
Characteristics of LC
The mean age at LC diagnosis was 71.7 years. Of the 70 patients who developed LC, 66 (94.3%) were male, all patients were smokers, coexisting emphysema was present in 42 (60.0%) patients (table 1), and the histological types of LC in 21 (30.0%), 14 (20.0%), 14(20.0%) and 15 (21.4%) patients were squamous cell carcinoma, adenocarcinoma, small cell carcinoma and unclassified nonsmall cell LC, respectively. The locations of LC were peripheral in 58 patients (82.9%), lower lobe in 34 (48.6%) and adjacent to UIP in 53 (75.7%) patients (table 2 and figure 2). LC was diagnosed by regular follow-up radiology in 44 (62.9%) patients and by symptoms in 26 (37.1%) patients. LC stagings were stage I, II, III and IV in 22 (31.4%), 7 (10.0%), 20 (28.6%) and 21 (30.0%) patients, respectively.
TABLE 2.
Lung cancer that developed during follow-up periods in 70 patients with idiopathic pulmonary fibrosis
| Patients | 70 (100%) |
| Age years mean±sd | 71.7±7.8 |
| Histological type | |
| Squamous cell carcinoma | 21 (30.0%) |
| Adenocarcinoma | 14 (20.0%) |
| Small cell carcinoma | 14 (20.0%) |
| Unclassified nonsmall cell lung cancer | 15 (21.4%) |
| Large cell carcinoma | 2 (2.9%) |
| Others | 4 (5.7%) |
| Performance status | |
| 0 | 35 (50.0%) |
| 1 | 17 (24.3%) |
| 2 | 9 (12.9%) |
| 3 | 6 (8.6%) |
| 4 | 3 (4.3%) |
| Location | |
| Central | 12 (17.1%) |
| Peripheral | 58 (82.9%) |
| Upper lobes | 33 (47.1%) |
| Middle lobes | 3 (4.3%) |
| Lower lobes | 34 (48.6%) |
| Adjacent to usual interstitial pneumonia | 53 (75.7%) |
| Diagnosis | |
| Incidental findings | 44 (62.9%) |
| Symptoms | 26 (37.1%) |
Data are presented as n (%), unless otherwise stated.
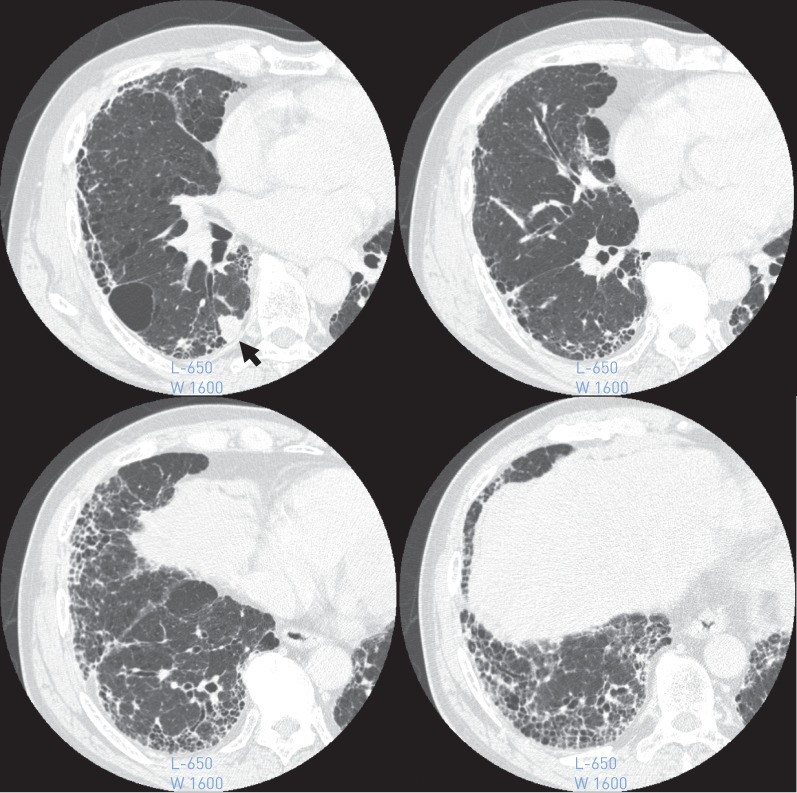
FIGURE 2.
High-resolution computed tomography image showing a peripheral right lower lobe mass (arrow) and a usual interstitial pneumonia that exists around the mass to bottom of the lung.
Risk factors for LC development
Univariate analysis indicated that being male, pack-years of smoking, emphysema and FVC % predicted ≥80% were factors associated with LC development (table 3). In a multivariate Cox proportional hazard model, pack-years of smoking ≥35 and emphysema were associated with LC development (table 4).
TABLE 3.
Univariate Cox regression analysis exploring factors associated with lung cancer diagnosis in idiopathic pulmonary fibrosis patients
| Parameter | HR (95% CI) | p-value |
| Age years | ||
| <60 | Ref. | |
| ≥60 | 1.402 (0.714–2.755) | 0.326 |
| Sex | ||
| Female | Ref. | |
| Male | 4.809 (1.752–13.199) | 0.002 |
| BMI kg·m−2 | ||
| ≥25 | Ref. | |
| ≥18.5–<25 | 1.258 (0.732–2.162) | 0.407 |
| <18.5 | 0.942 (0.218–4.083) | 0.937 |
| Unknown | 1.854 (0.246–13.954) | 0.549 |
| Smoking history | ||
| None | Ref. | |
| Smoker | >999.999 (0.000–−) | 0.985 |
| Smoking exposure pack-years | ||
| <35 | Ref. | |
| ≥35 | 3.053 (1.747–5.334) | <0.001 |
| Emphysema | ||
| None | Ref. | |
| Present | 2.944 (1.823–4.753) | <0.001 |
| FVC % pred | ||
| ≥80 | Ref. | |
| <80 | 0.449 (0.212–0.952) | 0.037 |
| Unknown | 1.639 (0.991–2.710) | 0.054 |
| FEV1/FVC % | ||
| ≥70 | Ref. | |
| <70 | 1.877 (0.963–3.656) | 0.064 |
| Unknown | 2.330 (1.382–3.926) | 0.001 |
| DLCO % pred | ||
| ≥80 | Ref. | |
| <80 | 2.179 (0.990–4.800) | 0.053 |
| Unknown | 2.574 (1.250–5.305) | 0.010 |
| PaO2 mmHg | ||
| ≥70 | Ref. | |
| <70 | 1.421 (0.628–3.216) | 0.399 |
| Unknown | 0.850 (0.504–1.433) | 0.542 |
| ESR mm·h−1 | ||
| <35 | Ref. | |
| ≥35 | 1.673 (0.946–2.959) | 0.077 |
| Unknown | 1.391 (0.774–2.501) | 0.27 |
| CRP mg·dL−1 | ||
| <0.3 | Ref. | |
| ≥0.3 | 1.217 (0.742–1.994) | 0.437 |
| Unknown | 0.983 (0.427–2.266) | 0.968 |
| Albumin g·dL−1 | ||
| ≥3.5 | Ref. | |
| <3.5 | 2.047 (0.910–4.601) | 0.083 |
| Unknown | 0.864 (0.521–1.432) | 0.571 |
| KL-6 U·mL−1 | ||
| <500 | Ref. | |
| ≥500 | 1.092 (0.493–2.420) | 0.829 |
| Unknown | 2.041 (0.985–4.227) | 0.055 |
HR: hazard ratio; BMI: body mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; PaO2: arterial oxygen tension; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; KL: Krebs von der Lungen.
TABLE 4.
Multivariate Cox regression analysis exploring factors associated with lung cancer diagnosis in idiopathic pulmonary fibrosis patients
| Parameter | HR (95% CI) | p-value |
| Smoking exposure pack-years | ||
| <35 | Ref. | |
| ≥35 | 2.354 (1.278–4.339) | 0.006 |
| Emphysema | ||
| None | Ref. | |
| Present | 2.066 (1.220–3.498) | 0.007 |
HR: hazard ratio.
Treatments of LC according to LC staging
LC treatment according to staging is reported in table 5. Of 22 patients with stage I, 14 (63.6%) patients underwent operation, and 8 (36.4) received only best supportive care. Of 7 patients with stage II, only 2 (28.6%) were operated. Of 21 patients with stage IV, 16 (76.2%) received only best supportive care. Only one patient received radiotherapy.
TABLE 5.
Lung cancer treatment according to clinical staging
| Treatment | Stage | |||
| I (n=22) | II (n=7) | III (n=20) | IV (n=21) | |
| Surgery | 14 (63.6%) | 2 (28.6%) | 4 (20.0%) | 1 (4.8%) |
| Chemotherapy | 0 (0.0%) | 2 (28.6%) | 8 (40.0%) | 4 (19.0%) |
| Radiotherapy | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) |
| Best supportive care | 8 (36.4%) | 3 (42.9%) | 7 (35.0%) | 16 (76.2%) |
Mortality in IPF patients who did not develop LC or developed LC during follow-up
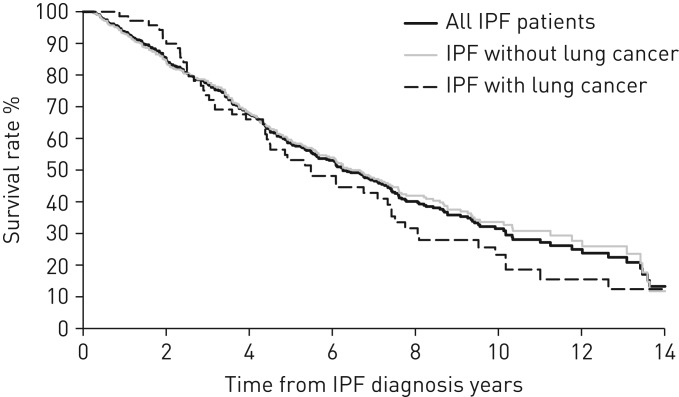
The respective 5- and 10-year all-cause mortality rates in patients who did not develop LC were 40.4% and 66.4% versus 46.9% and 76.7% in those who developed LC (p=0.262) (figure 3).
FIGURE 3.
Kaplan–Meier survival curves of all-cause mortality in patients with idiopathic pulmonary fibrosis (IPF). The 5- and 10-year mortality rates of all patients, patients who did not develop lung cancer and patients who developed lung cancer were 41.4% and 68.5%, 40.4% and 66.4%, and 46.9% and 76.7%, respectively. A log-rank test showed that the difference between survival curves of patients with or without lung cancer diagnosis was not significant (p=0.262).
Mortality after LC development
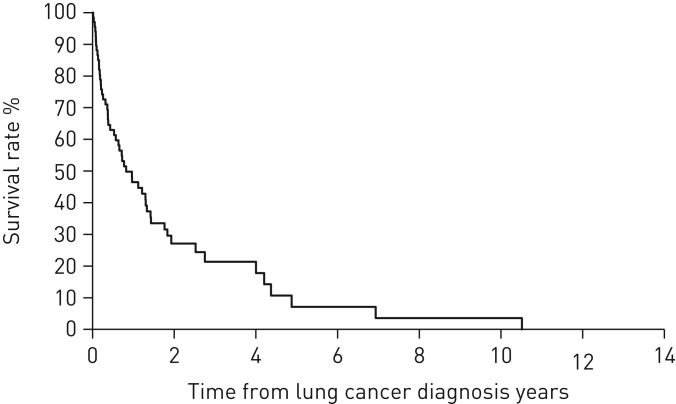
The 1-, 3- and 5-year all-cause mortality rates after LC diagnosis were 53.5%, 78.6% and 92.9%, respectively (figure 4).
FIGURE 4.
Kaplan–Meier survival curves of all-cause mortality in patients with idiopathic pulmonary fibrosis after lung cancer development. The 1-, 3- and 5-year all-cause mortality rates after lung cancer diagnosis were 53.5%, 78.6% and 92.9%, respectively.
Discussion
This long-term longitudinal study of a large cohort of patients with IPF resulted in four important findings. First, the incidence density of LC development in patients with IPF was 25.2 cases per 1000 person-years. Second, the most frequent type was squamous cell carcinoma (30%), and the majority developed LC in the peripheral lung (82.9%) and adjacent to UIP (75.7%). Third, pack-years of smoking and coexisting emphysema were associated with LC development. Fourth, the 1-year all-cause mortality rate after LC diagnosis was high (53.5%).
The incidence rate of LC in Japan in 2007 was reported to be 1.48 per 1000 person-years [21]. The incidence of LC in patients with IPF is reported to be 11.2–36 cases per 1000 person-years [7, 19]. The incidence of 25.1 cases per 1000 person-years in the present study was nearly equal to the results from previous studies. Tomassetti et al. [20] reported that 23 patients with LC and IPF were more frequently smokers, with combined pulmonary fibrosis and emphysema (52% versus 32%), than 158 patients with IPF only. However, because this study included 7 patients whose LC was diagnosed at the same time as IPF diagnosis, emphysema may not be predictive of the risk of developing LC at the initial diagnosis of IPF. In the present study, in a multivariate Cox proportional hazard model, pack-years of smoking ≥35 and emphysema were associated with LC development. Similarly, pack-years of smoking and emphysema were reported to be independently associated with LC development in COPD patients [22].
There are many possible underlying mechanisms linking LC and IPF. Epigenetic and genetic alterations, abnormal expression of microRNAs (miRNAs), cellular and molecular aberrances such as an altered response to regulatory signals, delayed apoptosis or reduced cell-to-cell communication, along with the activation of specific signalling transduction pathways, are all features that characterise the pathogenesis of both IPF and LC [23, 24]. Genetic analysis has revealed that deleterious mutations in surfactant protein A1 or A2 cause familial idiopathic interstitial pneumonia and lung cancer [25–27].
Tomassetti et al. [20] reported that survival in patients with IPF with LC was significantly worse than in patients with IPF without LC (time 0 is diagnosis of IPF for both groups). This study included 181 patients with IPF, and, of these patients, 23 developed LC. Among the 23 patients with LC and IPF, 7 (30%) were diagnosed as having LC at the same time of IPF diagnosis and the other 16 patients (70%) developed LC after diagnosis of IPF. Ozawa et al. [18] and Hyldgaard et al. [19] studied 103 and 121 IPF patients without LC at their initial diagnoses, respectively. A total of 21 and 6 patients with IPF developed LC during the observation period, respectively. There were no significant differences in survival between IPF-alone and IPF-LC patients (time 0 is diagnosis of IPF). As in the Ozawa et al. and Hyldgaard et al. studies, our study did not include IPF patients simultaneously having LC at IPF diagnosis, and the survival of patients who developed and did not develop LC was not so different.
Several studies have reported that pulmonary morbidity and mortality are substantially higher, and outcomes are much poorer, after pulmonary resection for LC among patients with IPF than among patients without IPF [28–31]. Kreuter et al. [17] reported the treatment and outcome of 42 patients with IPF and LC. Of the 42 patients, 12 (28.6%) patients underwent surgery, 11 (26.2%) radiotherapy, 6 (14.3%) radio-chemotherapy, 10 (23.8%) chemotherapy and 4 (9.5%) best supportive care. Therapy-associated toxicities were observed in 55% of the patients, the 30 day mortality among the operated patients was 25%, 4 (24%) out of 17 irradiated patients developed radiation-induced pneumonitis, and 63% of patients with chemotherapy developed chemotherapy-related toxicity. They reported that, despite the high incidence of LC therapy-related complications, a significant survival impact was not detected. Khan et al. [32] reported the treatment and outcome of 34 patients with IPF and LC. They reported that therapeutic interventions made no significant difference in the overall poor long-term survival of these patients, regardless of the stage of cancer or severity of IPF. Kenmotsu et al. [33] investigated the risk of exacerbation of interstitial lung disease for patients with LC with interstitial lung disease. Patients with UIP pattern developed cytotoxic chemotherapy-related exacerbation of interstitial lung disease more frequently than those with non-UIP pattern (30% versus 8%, p=0.005). The incidence of grade 5 pulmonary toxicities was 9% in patients with UIP pattern, compared with 3% in those with non-UIP pattern. In the present study, overall, 34 (48.6%) of the 70 patients could not receive standard-of-care treatment for LC and only received best supportive care. In our study, 2 (9.5%) of the 21 operated patients and 1 (7.1%) of the 14 patients who received chemotherapy developed acute exacerbation. Two of these patients died.
The papers reporting survival among IPF patients with LC were limited by small sample sizes. Mean survival time after LC diagnosis in 24 and 42 patients with IPF and LC was reported to be 1.6 and 1.7 years, respectively [15, 17]. The median survival time after LC diagnosis in 21 patients with IPF and LC was reported to be 1.1 years [18]. In the present study, the 1-year all-cause mortality rate after LC diagnosis was 53.5%, showing overall poor survival, like previous studies.
One limitation of this study is that it is retrospective, so some clinical and laboratory findings were not available. Second, our conclusions are limited because this study is a single-centre review. Third, the true cumulative incidence of LC may be underestimated because routine screening for LC was lacking. Fourth, there is a problem of censored cases with competing risk events. In our study, the age at IPF diagnosis of the group with LC development was younger than that of the group without LC development (medians of 67.5 years and 71.0 years, respectively) and FVC % at IPF diagnosis of the group with LC development was higher than that of the group without LC development (median of 91.3% and 74.7%, respectively). Elderly patients with depressed pulmonary function have a poor prognosis and, accordingly, it is possible that they will die before developing LC. When patients have poor prognostic factors, survival time tends to be shortened, and poor prognostic factors may reduce the risk of developing LC due to patient death before the onset of LC. The method to solve the problem of censoring by competing risk event is to constitute an event called “lung cancer development or death” using “lung cancer development” and “death”, and to analyse the occurrence and risk factors of the event. This analytical method enables the search for risk factors for which “lung cancer development or death” is high. However, this analytical method does not allow for the search of the pure risk factor of LC development. After discussing this with a statistician, we determined that it would be better to adopt Cox regression analysis of “lung cancer-free time”.
In conclusion, the incidence density of LC is high in IPF patients and occurs more frequently in patients with a smoking history of pack-years of smoking ≥35 and coexisting emphysema. The majority of LCs develop adjacent to UIP. Knowledge of these factors may help direct efforts for the early detection of LC and disease management.
Acknowledgements
We thank Tsutomu Yanagisawa and Kazuyoshi Kurashima of the Department of Respiratory Medicine, Saitama Cardiovascular and Respiratory Center, for their handling of the diagnosis and treatment of the patients with rheumatoid arthritis-associated UIP.
Footnotes
Conflict of interest: None declared
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panos RJ, Mortenson RL, Niccoli SA, et al. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med 1990; 88: 396–404. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Amatto VC, Behr J, et al. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J 2015; 46: 1113–1130. [DOI] [PubMed] [Google Scholar]
- 5.Turner-Warwick M, Lebowitz M, Burrows B, et al. Cryptogenic fibrosing alveolitis and lung cancer. Thorax 1980; 35: 496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubbard R, Venn A, Lewis S, et al. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000; 161: 5–8. [DOI] [PubMed] [Google Scholar]
- 7.Le Jeune I, Gribbin J, West J, et al. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med 2007; 101: 2534–2540. [DOI] [PubMed] [Google Scholar]
- 8.Harris JM, Johnston ID, Rudd R, et al. Cryptogenic fibrosing alveolitis and lung cancer: the BTS study. Thorax 2010; 65: 70–76. [DOI] [PubMed] [Google Scholar]
- 9.Collard HR, Ward AJ, Lanes S, et al. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ 2012; 15: 829–835. [DOI] [PubMed] [Google Scholar]
- 10.Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014; 190: 773–779. [DOI] [PubMed] [Google Scholar]
- 11.Nagai A, Chiyotani A, Nakadate T, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Tohoku J Exp Med 1992; 167: 231–237. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Im JG, Ahn JM, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: CT findings. J Comput Assist Tomogr 1996; 20: 979–982. [DOI] [PubMed] [Google Scholar]
- 13.Hironaka M, Fukayama M. Pulmonary fibrosis and lung carcinoma: a comparative study of metaplastic epithelia in honeycombed areas of usual interstitial pneumonia with or without lung carcinoma. Pathol Int 1999; 49: 1060–1066. [DOI] [PubMed] [Google Scholar]
- 14.Park J, Kim DS, Shim TS, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur Respir J 2001; 17: 1216–1219. [DOI] [PubMed] [Google Scholar]
- 15.Aubry MC, Myers JL, Douglas WW, et al. Primary pulmonary carcinoma in patients with idiopathic pulmonary fibrosis. Mayo Clin Proc 2002; 77: 763–770. [DOI] [PubMed] [Google Scholar]
- 16.Araki T, Katsura H, Sawabe M, et al. A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients. Intern Med 2003; 42: 483–489. [DOI] [PubMed] [Google Scholar]
- 17.Kreuter M, Ehlers-Tenenbaum S, Schaaf M, et al. Treatment and outcome of lung cancer in idiopathic interstitial pneumonias. Sarcoidosis Vasc Diffuse Lung Dis 2015; 31: 266–274. [PubMed] [Google Scholar]
- 18.Ozawa Y, Suda T, Naito T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009; 14: 723–728. [DOI] [PubMed] [Google Scholar]
- 19.Hyldgaard C, Hilberg O, Bendstrup E. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respir Med 2014; 108: 647–653. [DOI] [PubMed] [Google Scholar]
- 20.Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015; 147: 157–164. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda A, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2007: a study of 21 population-based cancer registries for the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2013; 43: 328–336. [DOI] [PubMed] [Google Scholar]
- 22.de-Torres JP, Wilson DO, Sanchez-Salcedo P, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med 2015; 191: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vancheri C, Failla M, Crimi N, et al. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 2010; 35: 496–504. [DOI] [PubMed] [Google Scholar]
- 24.Vancheri C. Idiopathic pulmonary fibrosis and cancer: do they really look similar? BMC Med 2015; 13: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 2009; 84: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Moorsel CH, Ten Klooster L, van Oosterhout MF, et al. SFTPA2 Mutations in familial and sporadic idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2015; 192: 1249–1252. [DOI] [PubMed] [Google Scholar]
- 27.Nathan N, Giraud V, Picard C, et al. Germline SFTPA1 mutation in familial idiopathic interstitial pneumonia and lung cancer. Hum Mol Genet 2016; 25: 1457–1467. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki H, Nagai K, Yoshida J, et al. Postoperative morbidity, mortality, and survival in lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol 2002; 81: 33–37. [DOI] [PubMed] [Google Scholar]
- 29.Chiyo M, Sekine Y, Iwata T, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg 2003; 126: 1141–1146. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe A, Higami T, Ohori S, et al. Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J Thorac Cardiovasc Surg 2008; 136: 1357–1363. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Kawai Y, Takahashi N, et al. Survival after surgery for pathologic stage IA non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann Thorac Surg 2011; 92: 1812–1817. [DOI] [PubMed] [Google Scholar]
- 32.Khan KA, Kennedy MP, Moore E, et al. Radiological characteristics, histological features and clinical outcomes of lung cancer patients with coexistent idiopathic pulmonary fibrosis. Lung 2015; 193: 71–77. [DOI] [PubMed] [Google Scholar]
- 33.Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 2011; 6: 1242–1246. [DOI] [PubMed] [Google Scholar]