Abstract
A majority of methamphetamine (Meth) abusers also abuse alcohol but the neurochemical consequences of this co-abuse are unknown. Individually, alcohol and Meth cause inflammation and long-term alterations in dopamine and serotonin signaling within the brain. Experiments were conducted to identify if serial exposure to alcohol and Meth has neurochemical consequences that are greater than after either drug alone. Male Sprague Dawley rats voluntarily drank 10% ethanol (EtOH) every other day for 4 weeks and were then exposed to a binge injection regimen of Meth (10mg/kg injected every 2 hrs, for a total of 4 injections). EtOH drinking and preference increased over the 4 weeks and caused inflammation evidenced by increases in serum and brain lipopolysaccharide (LPS) and brain cyclooxygenase-2 (COX-2) 24 hours after the last day of drinking. Meth alone depleted dopamine and serotonin in the striatum, as well as serotonin in the prefrontal cortex when measured 1 week later. In contrast, EtOH drinking alone did not affect dopamine and serotonin content in the striatum and prefrontal cortex, but prior EtOH drinking followed by injections of Meth enhanced Meth-induced depletions of dopamine, serotonin, as well as dopamine and serotonin transporter immunoreactivities in a manner that was correlated with the degree of EtOH consumption. Cyclooxygenase inhibition by ketoprofen during EtOH drinking blocked the increases in LPS and COX-2 and the enhanced decreases in dopamine and serotonin produced by Meth. Therefore, prior EtOH drinking causes an increase in inflammatory mediators that mediate a synergistic interaction with Meth to cause an enhanced neurotoxicity.
Keywords: methamphetamine, alcohol, cyclooxygenase-2, lipopolysaccharide, monoamines
Introduction
Alcohol and methamphetamine (Meth) are often abused together and present a co-morbidity such that 77% of people diagnosed with amphetamine dependence also have an alcohol use disorder (Stinson et al. 2005). Similarly, within the population of Meth users, alcohol consumption increases the probability of Meth use by four-fold (Bujarski et al. 2014). Despite this co-morbidity, little is known about the neurochemical consequences of their co-abuse even though both drugs share similar neuropharmacological and neurochemical profiles and produce oxidative stress after their combined use (Vaghef et al. 2014; Althobaiti et al. 2016).
Markers of Meth-induced neurotoxicity that are suggestive of degeneration of dopamine (DA) and serotonin (5HT) nerve terminals include long-term depletions of DA and 5HT content as well as decreases in the transporters for dopamine (DAT) and serotonin (SERT) within the brain (Seiden et al. 1976; Wagner et al. 1980; Ricaurte et al. 1980; Volkow et al. 2001). Factors known to commonly contribute to the observed nerve terminal degradation and long-term decreases in monoamine neurotransmission produced by either Meth or alcohol include reactive oxygen species (ROS), glutamate-mediated excitotoxicity, and inflammation (Mirecki et al. 2004; Nash and Yamamoto 1992; Thomas et al. 2004; Blanco et al. 2004; Vallés et al. 2004) but it remains unknown if they play a role in the possible co-morbid effects of EtOH and Meth.
Inflammation within the gastrointestinal (GI) tract is associated with excessive alcohol consumption (Fleming et al. 2001). The gut contains a GI-blood barrier comparable to the blood-brain barrier, which prevents toxins such as gram-negative bacteria containing lipopolysaccharide (LPS) from exiting the lumen and entering the bloodstream. However, the GI-blood barrier is compromised by alcohol metabolism within the intestinal epithelium resulting in degradation of tight junction proteins lining the gut mucosa (Elamin et al. 2014), an increased permeability of the intestinal wall, and the eventual entrance of LPS into the circulation (Ferrier et al. 2006). Consequently, LPS escapes the lumen and initiates a pro-inflammatory cascade (Qin and Crews 2012). In contrast, less is known about the inflammatory consequences of Meth in the periphery, but Loftis et al. (2011) observed Meth-induced increases in circulating pro-inflammatory chemokines in both rodents and humans. Moreover, case studies of human Meth users have documented evidence of ischemic colitis and other GI tract abnormalities (Johnson and Berenson 1991) that produce inflammatory mediators and can potentiate a neuroinflammatory state (Banks 2005).
The neuroinflammation that ensues from peripheral inflammation has been reported to contribute to Meth and alcohol abuse. Activation of microglia and consequently, the upregulation of cyclooxygenase-2 (COX-2) mRNA in the brain is known to play a key role in DA nerve terminal damage observed in mice after Meth exposure (Thomas et al. 2004). In contrast, COX-2 knockout mice are protected against Meth-induced toxicity (Thomas and Kuhn 2005). Alcohol consumption also increases COX-2 through activation of the toll-like receptor 4 (TLR4) by LPS to produce other pro-inflammatory mediators and apoptosis in the brain. Interestingly, TLR4-deficient mice do not exhibit increased COX-2 and apoptosis after alcohol exposure (Alfonso-Loeches et al. 2010). Thus, inflammation is a common denominator in mediating the neurotoxicity to both alcohol and Meth.
The current study employed a paradigm that approximates the serial exposure of humans to alcohol and Meth by allowing rats to voluntarily and intermittently drink ethanol (EtOH) prior to challenge injections of Meth. It was hypothesized that voluntary ethanol drinking would produce a peripheral inflammatory response that increases the vulnerability to Meth neurotoxicity evidenced by the enhancement of long-term depletions in DA and 5HT content as well as DAT and SERT. Moreover, we posited that the blockade of the inflammatory response that is restricted to the time of EtOH exposure only, would mitigate the enhanced neurotoxicity observed after subsequent exposure to Meth.
Materials and Methods
Animals
Male Sprague Dawley rats (250–300 g, Envigo, Indianapolis, IN) were allowed to acclimate to the animal colony at Indiana University for 4 days before the start of any experiments and had ad libitum access to food and water throughout the experiments. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Indiana University Institutional Animal Care and Use Committee.
Drug Treatments
Rats were housed individually and had access on alternating days to either 2 water bottles or simultaneous access to a water bottle and a bottle containing 10% EtOH as described by Li et al. (2011). To prevent position bias, the placements of the water and EtOH bottles were changed before every EtOH exposure. To calculate EtOH intake (g/kg) and preference (% total volume ingested), rats and drinking bottles were weighed at the same time every day at the end of each 24 hour access period. The amount of EtOH consumed was calculated based on the density of EtOH and normalized to the weight of the rat.
A separate, subset of rats was exposed to a 20% EtOH gavage (6g/kg) once per day for 7 days. EtOH was administered at the same time every day.
One day after the last exposure to EtOH drinking or gavage, rats were exposed to a binge Meth injection regimen. (+) Methamphetamine-hydrochloride (Sigma, St. Louis, MO, cat# M-8750) was dissolved in 0.9% saline and administered intraperitoneally at a dose of 10 mg/kg, once every 2 hr for a total of 4 injections. Control rats received 0.9% saline injections (1 mL/kg) at the same time. This paradigm of Meth exposure is comparable to that used by human Meth users (McCann et al. 1998) and produces deficits in dopamine transmission (Volkow et al. 2001; Callaghan et al. 2012). This binge injection paradigm was chosen over the self-administration procedure because it is well-suited for studying the dose-dependent pharmacological interactions with EtOH. It utilizes a well-documented and specific dose of Meth over a defined time window after the exposure to EtOH and avoids the confounds of more protracted and variable times required to train rats to self-administer a given Meth dose.
Body temperatures were monitored remotely throughout Meth injections via transponders (IPTT-300 transponder, BMDS) implanted subcutaneously and body temperatures were remotely measured every 30 min.
Ketoprofen, a nonselective cyclooxygenase inhibitor, was purchased from Sigma (St. Louis, MO, Cat. K1751), dissolved in 100% transcutol (gift from Gattefosse Corporation, Paramus, NJ, USA) and diluted 1:4 with Millipore water resulting in a final solution of 2.5 mg/mL ketoprofen in 25% transcutol. Based on a 12 hr half-life (Foster and Jamali 1988), ketoprofen (5 mg/kg) or transcutol was administered subcutaneously to rats twice per day during the 24 hr when EtOH was unavailable. This dose has been shown to attenuate Meth-induced decreases in DAT immunoreactivity (Asanuma et al. 2003).
Blood Alcohol Concentrations (BAC) and Brain Meth
BAC was measured via gas chromatography. Blood was collected in heparinized capillary tubes 15 min after the last dose of EtOH gavage or 4 hrs into the dark cycle of the drinking rats on Day 28. The time of collection was based on a pilot study showing this time point was the peak drinking period of EtOH. The blood was centrifuged for 45 seconds in a Microfuge to collect the plasma supernatant and the EtOH concentrations were analyzed via the Analox Analyzer (model GL5; Analox Instruments USA, Lunenburg, MA). EtOH concentrations are expressed as mg% (milligrams per deciliter). Brain Meth concentrations were measured within the striatum as described previously (Truong et al. 2005) with a ThemoQuest Quantum liquid chromatography tandem mass spectrometer (Thermo Electron Corporation, Walham, MA) at the University of Utah Center for Human Toxicology. The lower limit of quantitation was 0.5 ng/ml.
Lipopolysaccharide measurements
Twenty four hr after the last day of EtOH drinking, rats were euthanized. Trunk blood was collected in non-heparinized tubes and allowed to coagulate for 90 min on wet ice. The blood was then centrifuged at 3,000 × g for 10 min and supernatant was collected as serum. Brains were rapidly removed and prefrontal cortex and striatal tissue were dissected and frozen immediately on dry ice. Tissue was sonicated in RIPA buffer and protein was quantified via Bradford assay. Serum and brain endotoxin were measured using the Limulus Amebocyte Lysate QCL-1000™ (Lonza, Walkersville, MD) at 24 hrs after the last EtOH exposure as a measure of inflammation to EtOH alone that was present prior to and during the Meth injections. A subset of rats receiving ketoprofen during EtOH drinking were euthanized at the same time to assess the effects on LPS.
Western Blotting
Twenty four hr after the last day of EtOH drinking, rats were euthanized for COX-2 quantification. A subset of rats that received EtOH and Meth were decapitated at 7 days for DAT and SERT measurements. COX-2 assays were performed on prefrontal cortex and striata that were dissected upon ice and homogenized in RIPA buffer. DAT and SERT assays were conducted on crude synaptosomal fractions. Tissue was homogenized in 0.32M sucrose and centrifuged at 800 × g for 24 minutes at 4°C to pellet insoluble material. Supernatant (S1) was collected and centrifuged at 22,000 × g for 17 minutes at 4°C. The supernatant from the second spin (S2) was discarded, and the remaining pellet containing the synaptosomes was re-suspended in ice-cold Millipore-purified water (Millipore Bioscience Research Reagents, Temecula, CA). Bradford assays quantified protein content. Tissue homogenate was diluted in Novex 4X LDS sample buffer and 30μg was loaded onto a NuPAGE Novex 4–12% Bis-Tris gel for gel electrophoresis at 150V for 90 min. Proteins were then transferred onto PVDF membranes for 2 hr at 28V and incubated with primary antibody for COX-2 (1:500, anti-rabbit, Cell Signaling cat#12282S), DAT (1:1000, anti-goat, Santa Cruz cat#SC1433) or SERT (1:1000, anti-goat, Santa Cruz cat#SC1458) for 18 hr at 4°C. Membranes were washed with TBS containing 0.5% Tween (TBS-T) and incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG, 1:2500, Santa Cruz cat#SC2004 or rabbit anti-goat IgG, 1:2500, Santa Cruz cat#SC2768) for 1 hr at room temperature. Membranes were then incubated with HyGLO-enhanced chemiluminescence and imaged with a FujiFilm LAS-4000 camera. Band density was quantified using Multi-Gauge V3.1 software and normalized against β-actin (1:3000, Millipore cat#MAB1501).
Dopamine and 5HT Content
Tissue from the other hemisphere of the aforementioned rats euthanized 7 days after drug exposure was sonicated in 0.25 N perchloric acid and centrifuged at a speed of 14,000 × g for 20 min at 4°C. Supernatant was removed and injected onto a C18 column (250 × 4.6mm, 5μm particle diameter, Varian) for determination of monoamine content via high-performance liquid chromatography (HPLC) using a LC-4C amperometric detector (Bio-analytical Systems) and EZChrom ® software. The mobile phase contained citric acid anhydrous (21.0 g/L), sodium phosphate dibasic (10.65 g/L), octane sulfonic acid (470 g/L), 15% methanol, and pH 4.0. The remaining pellet after centrifugation was resuspended and dissolved in 1 N NaOH overnight at 4°C and protein content determined by the Bradford assay. DA and 5HT concentrations were normalized to total protein content. A subset of rats receiving ketoprofen during EtOH drinking were euthanized at the same time to assess the effects of ketoprofen on monoamine concentrations.
Statistical Analyses
EtOH intake and preference were analyzed using one-way repeated-measures ANOVA, whereas LPS, and COX-2 were analyzed using a t-test to compare between groups. Statistical analyses of Meth-induced monoamine and plasmalemmal transporter depletions after EtOH were conducted using either a two-way ANOVA and subsequent Tukey post hoc tests, or linear regression. A repeated-measures ANOVA was used to analyze body temperatures during Meth treatment. One-way ANOVAs were used to analyze LPS and COX-2 data with ketoprofen injections. A three-way ANOVA and subsequent Tukey post hoc analyses were performed to analyze monoamine content after the introduction of ketoprofen during EtOH drinking. All analyses were performed using SigmaPlot 13.0 software (Systat Software, SigmaPlot for Windows). All data are presented as mean±SEM. Sample sizes were chosen to result in a power of 0.80 or greater, and alpha-level in all experiments is 0.05 or less.
Results
Rats voluntarily drink 10% EtOH over 28 days
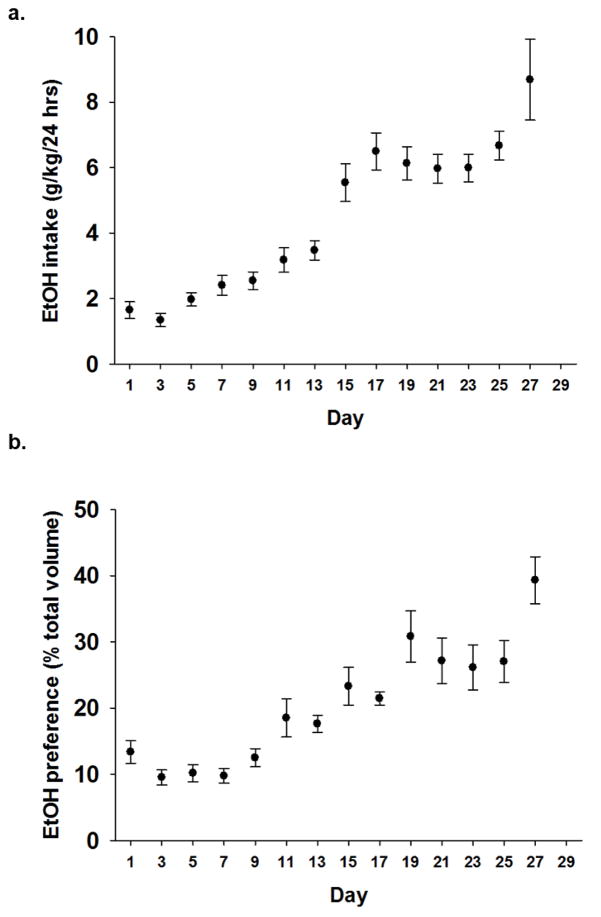
EtOH and water intake (g) were normalized to body weight (kg). A repeated-measures ANOVA revealed rats increased both intake (g/kg) and preference (% total fluid intake) for EtOH over 28 days (n=43; p<0.001; Fig. 1a–b). Importantly, total fluid intake over the 28 days did not increase. Rats reached a mean intake of 5g/kg/24hr on Day 15 and continued to escalate EtOH intake, resulting in a peak mean intake of 8.9g/kg/24hr on Day 28.
Figure 1. EtOH intake and preference are increased over 28 days.
EtOH drinking was on odd numbered days and alternated with water drinking only on even number days (not shown). a) Escalated intake of 10% EtOH (g/kg/day) over 14 24-hr exposures (p<0.001; n=43). b) Preference for 10% EtOH (% total fluid intake (EtOH+Water)) follows a similar trend over 14 24-hr exposures as compared to water intake (p<0.001; n=43).
Blood Alcohol and Brain Meth Concentrations
Blood alcohol concentrations were 146.29±2.2 mg% when measured 15 minutes after the last EtOH gavage on Day 7 and 70.88±1.9 mg% in drinking animals on Day 28. There were no significant differences between Water+Meth (2.80±0.77 ng/mg tissue) and EtOH+Meth (2.01±0.38 ng/mg tissue) in Meth concentrations within the striatum 2 hours after Meth (n=7/group).
Effects of EtOH drinking on serum LPS, brain LPS, and brain COX-2
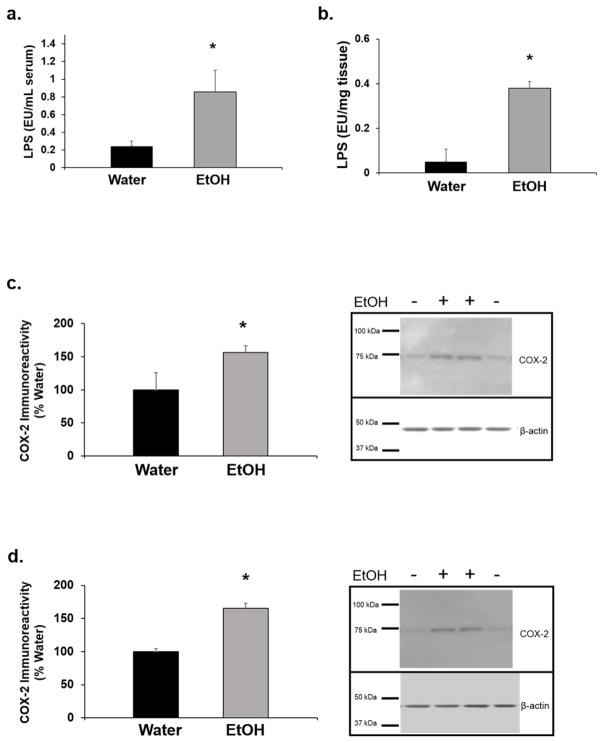
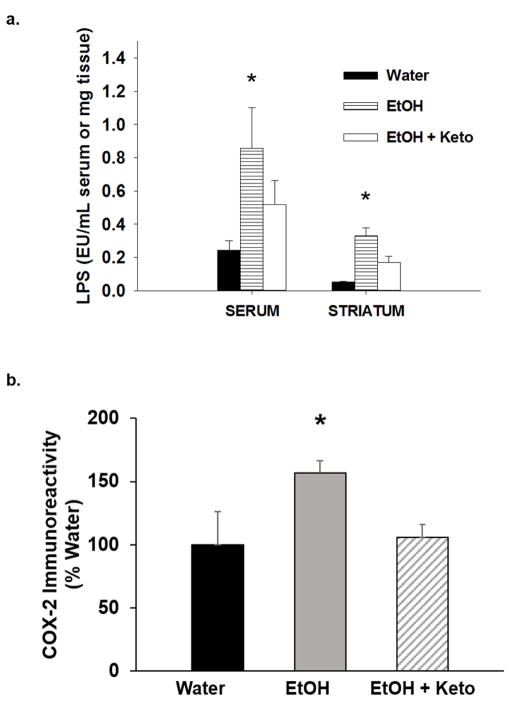
Twenty four hr after the last EtOH exposure, LPS in serum and the striatum, and COX-2 in the striatum and the prefrontal cortex were determined. EtOH drinking increased serum LPS by 4-fold (t1,18= 2.01; p<0.05; Fig. 2a) and striatal LPS by 8-fold (t1,7= 4.80; p<0.05; Fig. 2b). There was a significant increase in striatal and prefrontal cortex COX-2, which was 59.6 ±14.3% (t1,6= 3.04; p<0.05; Fig. 2c) and 65.3 ± 7.9% (t1,6= 4.87; p<0.05; Fig. 2d) greater, respectively, in EtOH-drinking rats compared to water-drinking rats. n=5–7/group.
Figure 2. EtOH drinking increases markers of inflammation.
Twenty four hr after the last day of drinking, a) serum lipopolysaccharide (LPS), b) striatal LPS, c) striatal cyclooxygenase-2 (COX-2), and d) prefrontal cortex COX-2 in EtOH-drinking rats was significantly higher compared to water-drinking rats (*p<0.05; n=5–7/group).
Effects of EtOH drinking on Meth-induced hyperthermia
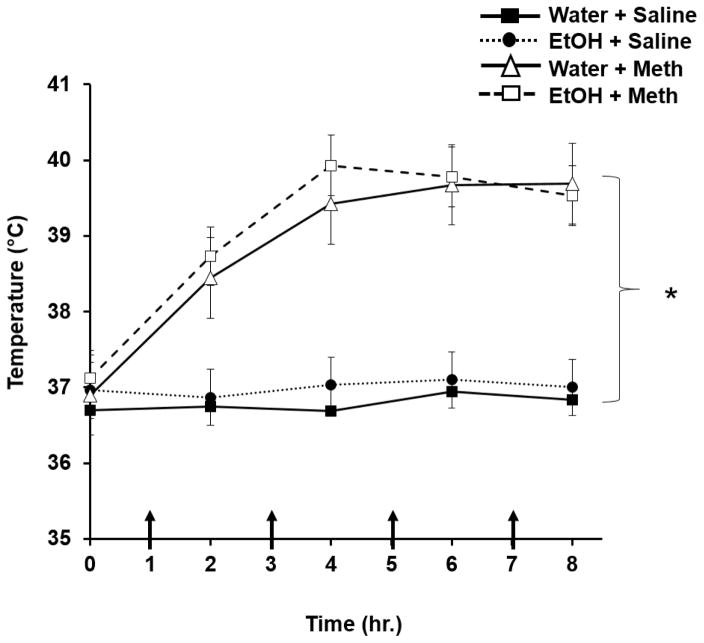
Meth-treated rats displayed elevated body temperatures compared to saline-treated rats over time (F3,176= 39.75; p<0.01; Fig. 3). However, previous exposure to EtOH did not increase body temperature compared to Water+Meth rats. Basal temperatures did not differ between Water+Meth (36.9±0.4°C) and EtOH+Meth (37.0±0.3°C) (F1,6=1.11; p>0.05; n=5/group). Meth significantly increased body temperatures for both Water+Meth (39.3±0.3°C; F1,6=78.95; p<0.05, n=6) and EtOH+Meth (39.5±0.3°C; F1,6=92.34; p<0.05; n=7) but the elevated body temperatures over the time course of Meth administration did not differ between the two groups (p>0.05). Ketoprofen administration did not affect Meth-induced hyperthermia in that the Water+Meth+ketoprofen group (39.2±0.4°C) did not differ from EtOH+Meth+ketoprofen group (39.5±0.5°C; F1,6=2.98; p>0.05; n=5–7/group).
Figure 3. Previous EtOH drinking does not increase Methamphetamine-induced hyperthermia.
Rats receiving Meth displayed higher body temperatures compared to saline-treated rats over time (*p<0.01; n=12/group) but there were no significant differences between Water+Meth and EtOH+Meth groups. Arrows denote injection times.
Effects of prior EtOH on Meth-induced neurotransmitter concentrations
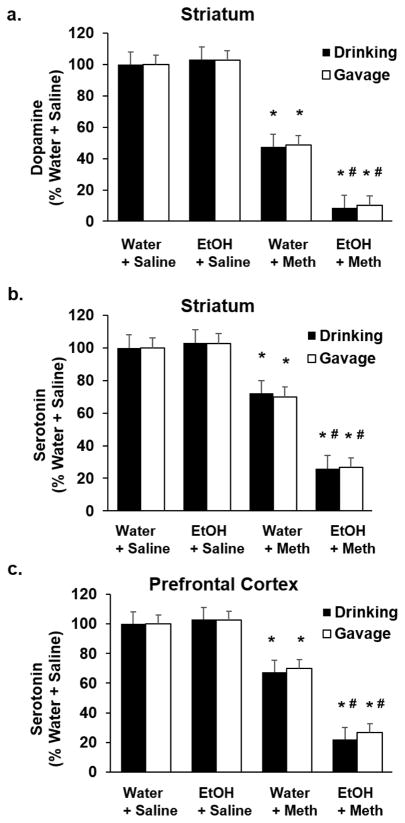
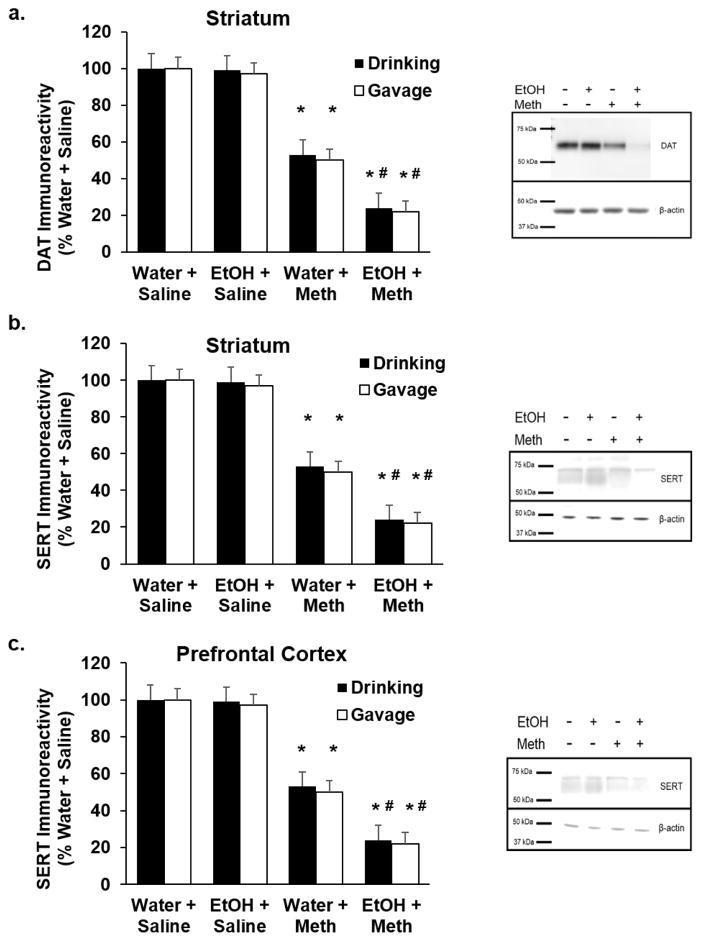
One day after the last EtOH exposure (either drinking or gavage), Meth was injected as described above to model binge Meth use, after which they were euthanized 7 days later. DA and 5HT content, as well as DAT and SERT immunoreactivities, were quantified in both the striatum and the prefrontal cortex. A two-way ANOVA revealed a significant Meth-induced decrease in striatal DA and 5HT in EtOH-drinking (DA: F1,17= 644.41; p<0.05; Fig. 4a; 5HT: F1,19= 1152.83; p<0.05; Fig. 4b) and EtOH-gavaged rats (DA: F1,19= 1066.44; p<0.05; Fig. 4a; 5HT: F1,19= 680.05; p<0.05; Fig. 4b), as well as 5HT in the prefrontal cortex (Drinking: F1,19= 969.40; p<0.05; Fig. 4c; Gavage: F1,19= 2206.78; p<0.05; Fig. 4c). DAT (Drinking: F1,19= 60.64; p<0.05; Gavage: F1,19= 65.87; p<0.05; Fig. 5a) and SERT in the striatum (Drinking: F1,9= 185.06; p<0.05; Gavage: F1,9= 203.54; p<0.05; Fig. 5b) and the prefrontal cortex (Drinking: F1,9= 183.42; p<0.05; Gavage: F1,9= 245.61; p<0.05; Fig. 5c) paralleled the depletions of DA and 5HT in EtOH-drinking rats. DA, DAT, 5HT, and SERT were depleted to a greater extent in EtOH drinking+Meth rats compared to Water+Meth rats (Tukey post hoc; DA: q=15.06; p<0.05; Fig. 4a; striatal 5HT: q=34.33; p<0.05; Fig. 4b; PFC 5HT: q=26.15; p<0.05; Fig. 4c) as well as in EtOH-gavaged rats compared to Water+Meth (DA: q=23.96; p<0.05; Fig. 4a; striatal 5HT: q=19.34; p<0.05; Fig. 4b; PFC 5HT: q=35.040; p<0.05; Fig. 4c). EtOH alone did not deplete monoamines. Representative Western blots are shown for DAT and SERT in EtOH-drinking rats (n=5–7/group).
Figure 4. Previous EtOH drinking or gavage enhances Methamphetamine-induced depletions of dopamine and serotonin in striatum and prefrontal cortex.
Seven days after Meth, there was a significant Meth-induced decrease in a) striatal dopamine, b) striatal serotonin, and c) prefrontal cortex serotonin compared to Water+Saline rats (*p<0.05; n=5–7/group, 2-way ANOVA). There were greater decreases in a) striatal dopamine and b) striatal serotonin, and c) prefrontal cortex serotonin compared to Water+Meth rats (#p<0.05; n=5–7/group, Tukey post hoc).
Figure 5. Previous EtOH drinking or gavage enhances Methamphetamine-induced depletions of dopamine transporter (DAT) and serotonin transporter (SERT) immunoreactivities.
Seven days after Meth, there was a significant Meth-induced decrease in a) striatal DAT, b) striatal SERT, and c) prefrontal cortex SERT compared to Water+Saline rats (*p<0.05; n=5–7/group, 2-way ANOVA). There were greater decreases in a) striatal DAT, b) striatal SERT and c) prefrontal cortex SERT compared to Water+Meth rats (#p<0.05; n=5–7/group, Tukey post hoc).
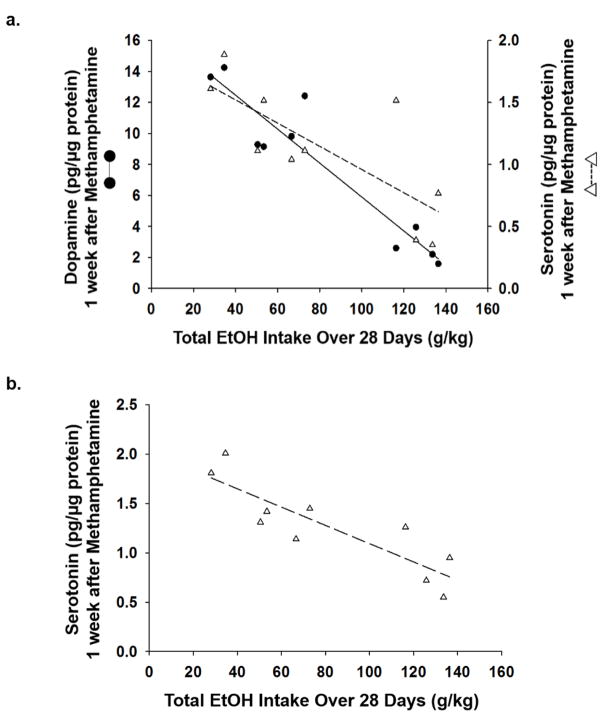
DA and 5HT content was assessed as a function of EtOH intake. Results showed a significant negative correlation between EtOH intake and changes in DA (r2=−0.89; p<0.001) and 5HT (striatum: r2=−0.58; prefrontal cortex: r2=−0.75; p<0.05) content produced by Meth (Fig. 6a–b) (n=10/group).
Figure 6. Methamphetamine-induced dopamine and serotonin depletions are dose-dependently affected by previous amount of EtOH consumed.
Linear regression showed a significant correlation between total EtOH intake over 28 days and degree of a) dopamine (r2=−0.89; p<0.001) and b) serotonin (r2=−0.58; p<0.05) depletions in the striatum, as well as c) serotonin (r2=−0.75; p<0.05) depletions in the prefrontal cortex (n=10).
Effects of COX inhibition on LPS and COX-2 after EtOH and enhanced monoamine depletions after EtOH drinking and binge Meth administration
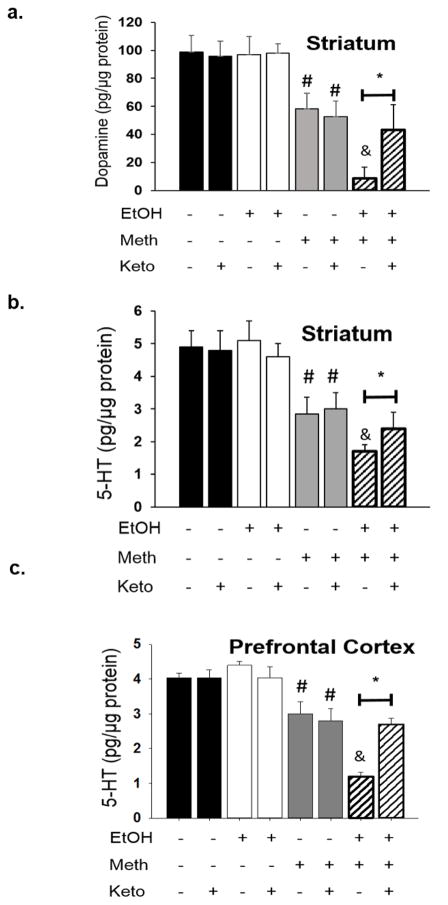
To examine the role of COX in mediating increases in inflammation after EtOH, the COX inhibitor, ketoprofen (5 mg/kg) was administered only during the days of intermittent EtOH withdrawal (28 injections total). Ketoprofen blocked EtOH-induced increases in LPS (n=4–7/group; serum: F2,10= 39.40; p<0.01; striatum: F2,11= 870.187; p<0.01; Fig. 7a) and COX-2 (n=4–7/group; F2,14= 173.82; p<0.01; Fig. 7b) at 24 hr after the last bout of EtOH. Monoamine concentrations did not differ between rats that drank EtOH and challenged with saline and those that drank just water. Rats that drank only water and were challenged with Meth showed a 50% reduction in striatal DA, a 40% reduction in 5HT within the striatum, and a 25% reduction in 5HT within the prefrontal cortex. Rats that drank EtOH and challenged with Meth showed enhanced neurotransmitter depletions evidenced by a 91% reduction in DA and 75% reduction in 5HT within the striatum and a 60% reduction in 5HT within the prefrontal cortex. Rats that received ketoprofen intermittently during the weeks of EtOH drinking did not differ compared to rats allowed to drink only water such that DA (n=5–12/group; q=24.9; p<0.01; Fig. 8a) and 5HT (n=5–12/group; striatum: q=13.62; p<0.01; Fig. 8b; prefrontal cortex: q=14.6; p<0.01; Fig. 8c) concentrations in ketoprofen-treated rats were significantly higher compared to the EtOH+Meth rats treated with vehicle.
Figure 7. Markers of inflammation are blocked by ketoprofen during EtOH drinking.
COX-2 inhibitor ketoprofen (Keto) during EtOH only blocked EtOH-induced increases in a) LPS and b) striatal COX-2 (*p<0.01; n=4–7/group; 1-way ANOVA).
Figure 8. Enhanced monoamine depletions are blocked by ketoprofen during EtOH drinking.
Meth alone resulted in significant depletions of dopamine in a) striatum, and 5-HT in b) striatum and c) prefrontal cortex (#p<0.05 vs. Water+Saline+Vehicle; 3-way ANOVA and post hoc Tukey tests; n=5–12/group). Previous exposure to EtOH significantly enhanced DA depletions in a) striatum, and 5-HT depletions in b) striatum and c) prefrontal cortex (&p<0.05 vs. Water+Meth+Vehicle). Treatment with Keto during EtOH drinking attenuated dopamine depletions in a) striatum, and 5-HT depletions in b) striatum and c) prefrontal cortex (*p<0.05 vs. EtOH+Meth+Vehicle).
Discussion
EtOH drinking by intermittent exposure over a 28 day period produced inflammation marked by elevated serum LPS and eventual increases in striatal LPS and COX-2. Furthermore, EtOH drinking synergized with the subsequent exposure to Meth and depleted DA and 5HT in the striatum, 5HT in the prefrontal cortex, and their respective transporters, DAT and SERT, in a manner that was blocked by the inhibition of COX during EtOH exposure.
Sprague Dawley rats voluntarily drank EtOH and increased their preference over 4 weeks. This escalation of drinking behavior is consistent with previous studies using a variety of rat strains that have employed a 2-bottle choice and intermittent access exposure paradigm (Simms et al. 2008; Bito-Onon et al. 2011; Li et al. 2011; Mill et al. 2013). Moreover, the amount of intake was similar to that reported in genetic alcohol preferring rats (Vengeliene et al. 2003; Bell et al. 2006). Therefore, this model of drinking has widespread applicability that can be used across multiple rat strains and is not limited to a genetic selection.
EtOH alone increased inflammation prior to Meth exposure, as indicated by increases in serum LPS at 24 hr after the last bout of EtOH drinking. This is likely due to GI barrier breakdown as LPS is associated with gram-negative bacteria and is normally restricted endogenously to the GI and respiratory tracts. Upon exposure of the GI tract to alcohol however, the lining of the GI tract is disrupted by reactive oxygen species that oxidize the microtubule skeleton of the epithelial cells and disassemble tight junctions such as occludin and claudin-3 within the small intestine (Elamin et al. 2014). The GI tract can then become permeable to LPS and enter the circulation (Ferrier et al. 2006) to activate peripheral macrophages and promote the transcription of NF-kB and secretion of inflammatory mediators such COX-2 and cytokines (Zuckerman et al. 1991; Eliopoulos et al. 2002).
The increase in inflammatory mediators is not limited to the periphery but can also readily cross the blood-brain barrier (BBB) to promote neuroinflammation (see Banks 2005 for review). The brain lymphatic system could also readily transport immune cells such as T-cells across the BBB (Louveau et al. 2015). In addition, cross-talk between peripheral and central immune cells can be initiated at the BBB where LPS binds to the TLR4 receptor on brain capillary endothelial cells that in turn, signal an immune response across the BBB and into the brain parenchyma (Zhou et al. 2009). Along these lines, the current results show that LPS and COX-2 are increased in the brain and the periphery after the serial exposure to EtOH and Meth (Fig. 2). While peripheral LPS can elicit a neuroinflammatory response indirectly through its actions at the BBB (i.e. brain capillary endothelial cells), it does not readily cross the intact BBB since only 0.025% of LPS enters the brain (Banks and Robinson 2010). Therefore, the observed increases in brain LPS could be indicative of BBB breakdown due to Meth (Sharma and Ali 2006; Northrop and Yamamoto 2012) and when combined with EtOH.
LPS can also amplify the activation of microglia upon entrance into the brain and produce neuroinflammatory responses such as increases in COX-2 (Fig. 2c–d). Previous findings have shown that COX-2-facilitated inflammation is toxic to striatal DA and/or 5HT terminals (Vallés et al. 2004; Thomas et al. 2004) and are consistent with the dramatic long-term decreases in DA and 5HT content, as well as decreases in DAT and SERT after the serial exposure to EtOH and Meth (Figs. 4–5).
Intermittent EtOH drinking by itself over the 28 day period did not affect DA and 5HT content, or DAT and SERT immunoreactivities within the brain. Meth alone produced a 45% depletion of DA and 5HT in the striatum and frontal cortex as well as a 40% depletion of DAT and SERT. Prior exposure to voluntary EtOH drinking potentiated Meth-induced depletions of the monoamines and their transporters and suggests a synergistic relationship between the two drugs that enhances neurotoxicity. Furthermore, the depletions of DA and 5HT were dose-dependent such that higher amounts of EtOH consumption produced greater decreases in DA and 5HT content in the striatum and prefrontal cortex after Meth (Fig. 6). This relationship clearly supports the interactive and causative effects of EtOH consumption on Meth-induced neurotoxicity.
The effects of alcohol on metabolism by the liver and hepatotoxicity are well-established. Similarly, we have reported that Meth also produces evidence of hepatotoxicity (Halpin and Yamamoto 2012). Thus, any interactive effects between EtOH and Meth may be due to their combined toxicity to the liver. However, voluntary EtOH drinking did not produce overt evidence of hepatotoxicity or alterations in ALT or AST (unpublished findings) and Meth concentrations in the brain were not changed by prior EtOH exposure. Thus, the synergistic depletions of monoamines observed after the serial exposure to EtOH and Meth are not due to decreased metabolism of Meth by the liver.
The finding that EtOH drinking alone increased COX-2 (Fig. 2c–d) is consistent with prior studies (Knapp and Crews 1999; Pascual et al. 2007) and suggests that prior EtOH exposure creates an inflammatory state that impacts the neurotoxic effects of Meth. The results show that ketoprofen during EtOH drinking blocked the increases in LPS and COX-2 produced by EtOH alone (Figs. 7a–b) as well as the supra-additive monoamine depletions after the challenge with Meth (Fig. 8a–c), indicating that EtOH-induced inflammation contributes to the enhanced toxicity produced by Meth. The neuroprotective effect of ketoprofen was observed despite the report that ketoprofen causes GI bleeding and ulcers (Shientag et al. 2012) through the inhibition of COX-1 (see Crofford 1997 for review); however, the gastric damage and GI barrier breakdown should have increased rather than decreased the neurotoxicity produced by EtOH and Meth. Therefore, the efficacy of ketoprofen against the neurotoxicity is probably mediated by inhibition of COX-2. Ketoprofen also attenuated increases in LPS (Fig. 7a) and may have the additional benefit of blocking LPS-induced inflammation after EtOH and Meth. Since LPS can increase COX-2 mediated inflammation, as evidenced by proinflammatory cytokines and prostaglandin production (Walton et al. 2009), and contribute to GI barrier breakdown, ketoprofen could decrease the amount of LPS translocating from the lumen to bloodstream. In addition, alterations in glutamate signaling are produced by alcohol (Crews et al. 2006) and Meth (Nash and Yamamoto 1992) and may interact with the inflammatory response to promote and enhance excitotoxicity.
Overall, the current study modeled the often observed co-exposure to alcohol and Meth. The results have identified a long-term neurochemical consequence of the co-abuse of alcohol and Meth that results in synergistic depletions of DAT, SERT, and DA and 5HT content within brain. While the current study focused logically on the brain regions and neurotransmitters typically affected by Meth, the extent of the neurotoxicity related to the combination of Meth and EtOH remains to be determined. Moreover, these studies provide the rationale for future studies using different behavioral models to examine the consequences of prior EtOH drinking on Meth self-administration and subsequent neurotoxicity. Additionally, the persistence of these effects remains to be determined but could be a long lasting/irreversible effect. The finding of a synergistic rather than an additive interaction between EtOH and Meth suggests a mechanism different than the more transient effect of Meth alone and the lack of an EtOH effect on the neurochemical parameters measured in this study. It is possible the prior exposure to Meth followed by EtOH drinking would produce an enhanced inflammatory state given that each drug alone produces inflammation. It would also be interesting to determine if a similar neurotoxicity arises if the serial exposure were reversed (i.e., Meth before EtOH drinking). In that particular scenario, the neurotoxicity might be different since inflammation is typically thought to provide a sensitizing effect. In this regard, the enhanced toxicity to Meth would require prior exposure to EtOH and its inflammatory effects rather than EtOH exposure after Meth.
Overall, the implications of the findings suggest an important antecedent role for peripheral inflammation as evidenced by increases in circulating and brain LPS after EtOH drinking that triggers the augmentation of neurotoxicity produced by Meth. Importantly, limiting ketoprofen administration to EtOH drinking effectively blocked peripheral inflammation and the subsequent enhancement of neuroinflammation and neurotoxicity observed after a Meth. Therefore, targeting peripheral inflammation may be an effective therapeutic strategy aimed at mitigating the co-morbidity associated with the abuse of alcohol and Meth.
Acknowledgments
This work was supported by NIH DA007606.
The authors would like to thank Dr. Eric Engleman and Alena Sentir for their assistance in measuring blood ethanol concentrations. Methamphetamine concentrations were determined by the University of Utah Center for Human Toxicology. This research is supported by NIH DA042737 and DA007606.
Footnotes
Compliance with Ethical Standards
The authors declare that they have no conflict of interest.
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- Althobaiti YS, Sari Y. Alcohol Interactions with Psychostimulants: An Overview of Animal and Human Studies. Journal of addiction research & therapy. 2016;7(3) doi: 10.4172/2155-6105.1000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of Neuroscience. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N. Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neuroscience letters. 2003;352:13–16. doi: 10.1016/j.neulet.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Current Pharmaceutical Design. 2005;11:973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood–brain barrier. Brain, behavior, and immunity. 2010;24:102–109. doi: 10.1016/j.bbi.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. REVIEW: The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biology. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addiction Biology. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Pascual M, Valles SL, Guerri C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-κB. Neuroreport. 2004;15:681–685. doi: 10.1097/00001756-200403220-00021. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Roche DJ, Lunny K, Moallem NR, Courtney KE, Allen V, et al. The relationship between methamphetamine and alcohol use in a community sample of methamphetamine users. Drug and Alcohol Dependence. 2014;142:127–132. doi: 10.1016/j.drugalcdep.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug and alcohol dependence. 2012;120(1):35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. Alcoholism: Clinical and Experimental Research. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. The Journal of Rheumatology Supplement. 1997;49:15–19. [PubMed] [Google Scholar]
- Elamin E, Masclee A, Troost F, Pieters HJ, Keszthelyi D, Aleksa K, et al. Ethanol impairs intestinal barrier function in humans through mitogen activated protein kinase signaling: a combined in vivo and in vitro approach. PloS One. 2014;9(9):e107421. doi: 10.1371/journal.pone.0107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. The EMBO Journal. 2002;21:4831–4840. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. The American Journal of Pathology. 2006;168:1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro-and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcoholism: Clinical and Experimental Research. 2001;25:579–589. [PubMed] [Google Scholar]
- Foster RT, Jamali F. Stereoselective pharmacokinetics of ketoprofen in the rat. Influence of route of administration. Drug Metabolism and Disposition. 1988;16:623–626. [PubMed] [Google Scholar]
- Halpin LE, Yamamoto BK. Peripheral ammonia as a mediator of methamphetamine neurotoxicity. The Journal of Neuroscience. 2012;32:13155–13163. doi: 10.1523/JNEUROSCI.2530-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TD, Berenson MM. Methamphetamine-induced ischemic colitis. Journal of Clinical Gastroenterology. 1991;13(6):687–689. doi: 10.1097/00004836-199112000-00015. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcoholism: Clinical and Experimental Research. 1999;23:633–643. [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABAA receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic–pituitary–adrenocortical axis. Addiction Biology. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotoxicity Research. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C] WIN-35,428. The Journal of Neuroscience. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill DJ, Bito-Onon JJ, Simms JA, Li R, Bartlett SE. Fischer rats consume 20% ethanol in a long-term intermittent-access two-bottle-choice paradigm. PloS One. 2013;8:e79824. doi: 10.1371/journal.pone.0079824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirecki A, Fitzmaurice P, Ang L, Kalasinsky KS, Peretti FJ, Aiken SS, et al. Brain antioxidant systems in human methamphetamine users. Journal of Neurochemistry. 2004;89:1396–1408. doi: 10.1111/j.1471-4159.2004.02434.x. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3, 4-methylenedioxymethamphetamine. Brain research. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Northrop NA, Yamamoto BK. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier. Journal of Neuroimmune Pharmacology. 2012;7:951–968. doi: 10.1007/s11481-012-9391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. European Journal of Neuroscience. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. Journal of Neuroinflammation. 2012;9(9):130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Research. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug and Alcohol Dependence. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Ali SF. Alterations in blood–brain barrier function by morphine and methamphetamine. Annals of the New York Academy of Sciences. 2006;1074:198–224. doi: 10.1196/annals.1369.020. [DOI] [PubMed] [Google Scholar]
- Shientag LJ, Wheeler SM, Garlick DS, Maranda LS. A therapeutic dose of ketoprofen causes acute gastrointestinal bleeding, erosions, and ulcers in rats. Journal of the American Association for Laboratory Animal Science. 2012;51:832–841. [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long–Evans and Wistar rats. Alcoholism: Clinical and Experimental Research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Cyclooxygenase-2 is an obligatory factor in methamphetamine-induced neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 2005;313(2):870–876. doi: 10.1124/jpet.104.080242. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. Journal of Pharmacology and Experimental Therapeutics. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, et al. Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: implications for neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 2005;314:1087–1092. doi: 10.1124/jpet.105.085951. [DOI] [PubMed] [Google Scholar]
- Vaghef L, Babri S. The effect of escalating dose, multiple binge methamphetamine regimen and alcohol combination on spatial memory and oxidative stress markers in rat brain. Journal of Alcoholism & Drug Dependence 2014 [Google Scholar]
- Vallés SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathology. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcoholism: Clinical and Experimental Research. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, … Logan J. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Research. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Walton KL, Holt L, Sartor RB. Lipopolysaccharide activates innate immune responses in murine intestinal myofibroblasts through multiple signaling pathways. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296(3):G601–G611. doi: 10.1152/ajpgi.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Andonegui G, Wong CHY, Kubes P. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. Journal of Immunology. 2009;183:5244–5250. doi: 10.4049/jimmunol.0901309. [DOI] [PubMed] [Google Scholar]
- Zuckerman SH, Evans GF, Guthrie L. Transcriptional and post-transcriptional mechanisms involved in the differential expression of LPS-induced IL-1 and TNF mRNA. Immunology. 1991;73:460. [PMC free article] [PubMed] [Google Scholar]