abstract
Background
Rats emit 50-kHz ultrasonic vocalizations (USVs) to communicate positive emotional states, and these USVs are increasingly being investigated in preclinical studies on reward and motivation. Although it is the activation of dopamine receptors that initiates the emission of 50-kHz USVs, non-dopaminergic mechanisms may modulate calling in the 50 kHz frequency band. To further elucidate these mechanisms, the present study investigated whether the pharmacological manipulation of glucocorticoid signaling influenced calling.
Methods
Rats were administered corticosterone (1–5 mg/kg, s.c.), the glucocorticoid receptor antagonist mifepristone (40 or 100 mg/kg, s.c.), or the corticosterone synthesis inhibitor metyrapone (50 or 100 mg/kg, i.p.). The effects of these drugs on calling initiation and on calling recorded during nonaggressive social contacts or after the administration of amphetamine (0.25 or 1 mg/kg, i.p.) were then evaluated.
Results
Corticosterone failed to initiate the emission of 50-kHz USVs and did not influence pro-social and amphetamine-stimulated calling. Similarly, mifepristone and metyrapone did not initiate calling. However, metyrapone suppressed pro-social calling and calling stimulated by a moderate dose (1 mg/kg, i.p.) of amphetamine. Conversely, mifepristone attenuated calling stimulated by a low (0.25 mg/kg, i.p.), but not moderate (1 mg/kg, i.p.), dose of amphetamine and had no influence on pro-social calling.
Conclusions
The present results demonstrate that glucocorticoid signaling modulates calling in the 50 kHz frequency band only in certain conditions and suggest that mechanisms different from the inhibition of corticosterone synthesis may participate in the suppression of calling by metyrapone.
Keywords: amphetamine, corticosterone, metyrapone, mifepristone, rat communication
Significance Statement
Ultrasonic vocalizations (USVs) of 50 kHz are a behavioral marker of reward in rats and are increasingly being investigated in preclinical studies on affect and emotion. On this basis, it appears of interest to elucidate the mechanisms that participate in the emission of 50-kHz USVs. Several preclinical and clinical studies indicate that glucocorticoid receptors may play a major role in the regulation of the emotional state. Therefore, we evaluated whether the emission of 50-kHz USVs could be influenced by drugs that modulate glucocorticoid-mediated signaling. The results of this study may be relevant not only to further elucidate the neuropharmacology of 50-kHz USVs, but also may provide further insight into the interplay between glucocorticoids and positive affect.
Introduction
Rats emit the so-called 50-kHz ultrasonic vocalizations (USVs), which are acoustic signals contained within the 35- to 80-kHz frequency range, to communicate positive emotional states to conspecifics (Panksepp, 2005; Schwarting et al., 2007; Brudzynski, 2013, 2015). On this basis, preclinical studies on reward and motivation are increasingly investigating the situations that are associated with calling in the 50 kHz frequency band and the mechanisms that underlie this behavior (Barker et al., 2015; Rippberger et al., 2015; Simola, 2015).
The emission of 50-kHz USVs is initiated by the activation of dopamine (DA) receptors in the shell of the nucleus accumbens (NAc) (Burgdorf et al., 2001; Thompson et al., 2006). Nevertheless, either the activation or the antagonism of nondopaminergic receptors may modulate calling in the 50 kHz frequency band (Fu and Brudzynski, 1994; Wright et al., 2012; Costa et al., 2015; Hamed et al., 2015; Wöhr et al., 2015; Simola et al., 2010, 2014, 2016). In this regard, it is noteworthy that rewarding stimuli of a different nature may elevate the plasma levels of corticosterone in rats (Szechtman et al., 1974; Knych and Eisenberg, 1979; Moldow and Fischman, 1987; Buwalda et al., 2012; Egan and Ulrich-Lai, 2015). Endogenous corticosterone, in turn, modulates the release of DA in the NAc shell that is elicited by rewarding stimuli (Barrot et al., 2000). Moreover, additional studies indicate that the administration of exogenous corticosterone may stimulate DA release in the NAc shell and may shape the effects that rewarding stimuli elicit on the emotional state of rats (Piazza et al., 1991; Piazza and LeMoal, 1996; Pecoraro et al., 2005; Der-Avakian et al., 2006). Taken together, these findings may suggest that glucocorticoid receptors could modulate calling in rats that are exposed to rewarding stimuli.
To date, the only studies that provide some evidence of a possible relationship between glucocorticoid signaling and calling in the 50 kHz frequency band were performed in rats previously and repeatedly exposed to stress, which elevates the plasma levels of corticosterone (Smith and Gala, 1977; Giralt et al., 1987). Thus, stress-exposed rats emitted a lower number of 50-kHz USVs in situations that may be rewarding, such as heterospecific playful contacts (“tickling”) or amphetamine administration, compared with stress-naïve rats (Popik et al., 2012, 2014; Kõiv et al., 2016). Moreover, administration of the corticosterone synthesis inhibitor metyrapone during stress imposition restored calling in response to tickling (Popik et al., 2014). Nevertheless, it has to be remarked that some of the behavioral effects of stress do not depend on the elevation in plasma corticosterone (Ulrich-Lai and Herman, 2009; Mei and Li, 2013). Besides, a previous study has found high levels of calling in stress-naïve rats that were placed in an enriched environment, a situation that elevates the plasma levels of corticosterone (Perez-Sepulveda et al., 2013). However, that study did not clarify whether it was the activation of glucocorticoid receptors by corticosterone that enhanced calling. Therefore, investigating how glucocorticoid signaling modulates the emission of 50-kHz USVs in stress-naïve rats appears to be relevant. Elucidating this issue may further clarify the mechanisms that participate in calling, as well as the interplay between corticosterone and positive affect.
To this end, we administered corticosterone, the glucocorticoid receptor antagonist mifepristone, or the corticosterone synthesis inhibitor metyrapone to rats. Afterwards, we evaluated whether these drugs initiated the emission of 50-kHz USVs and/or affected calling that was recorded during nonaggressive social contacts or after the administration of amphetamine, two situations that may be rewarding for rats (Burgdorf et al., 2001, 2009). Moreover, we evaluated whether the administration of spironolactone, an antagonist of the mineralocorticoid receptors, affected pro-social and amphetamine-stimulated calling to investigate the mechanisms underlying the effects of metyrapone. In fact, metyrapone may have a greater inhibitory effect on the synthesis of the mineralocorticoid hormone aldosterone than on the synthesis of corticosterone (Rigel et al., 2010; Daniel and Newell-Price, 2015).
Methods
Animals
A total of 110 male Sprague–Dawley rats (Harlan, Italy) weighing 150 to 200 g at the beginning of the experiments were used. Rats were housed in groups of 4 to 6 in standard polycarbonate cages with sawdust bedding and maintained on a 12-hour-light/dark cycle (lights on at 8:00 am). Food and water were freely available, except during USV recordings, which were performed between 12:00 and 4:00 pm. All experiments were conducted in accordance with the guidelines for animal experimentation of the EU directives (2010/63/EU; L.276; 22/09/2010) and with the guidelines approved by the Ethical Committee of the University of Cagliari. Experiments were designed to minimize animal discomfort to the least extent possible and to reduce the number of animals used.
Doses and Administration Times of Drugs
Corticosterone, mifepristone, and spironolactone were purchased from Sequoia Research Products Ltd. D-amphetamine (sulfate) and metyrapone were purchased from Sigma-Aldrich. Corticosterone, mifepristone, and spironolactone were dissolved in 10:90% dimethyl sulfoxide (DMSO)/polyethylene glycol (PEG). Corticosterone was administered at doses of 1, 2.5, or 5 mg/kg (Deroche et al., 1992, 1997; Dietz et al., 2007; Mantsch et al., 1998; Mei and Li, 2013). Mifepristone was administered at doses of 40 or 100 mg/kg (Heikinheimo et al., 1994; De Vries et al., 1996). Spironolactone was administered at doses of 50 or 75 mg/kg (Achterberg et al., 2014). Metyrapone was dissolved in 40:60% PEG/distilled water and administered at doses of 50 or 100 mg/kg (Calvo et al., 1998; Wright et al., 2006). Amphetamine was dissolved in distilled water and administered at doses of 0.25 or 1 mg/kg, both calculated as salt (Simola and Morelli, 2015; Wright et al., 2010). Corticosterone, mifepristone, and spironolactone were administered s.c. in a volume of 1 mL/kg. Amphetamine and metyrapone were administered i.p. in a volume of 3 mL/kg.
Corticosterone (or its vehicle) was administered immediately before recordings in the experiments that evaluated calling initiation, or 10 minutes before recordings in the experiments that evaluated pro-social and amphetamine-stimulated calling (Der-Avakian et al., 2006; Scherer et al., 2011). Metyrapone (or its vehicle) was always administered 90 minutes before recordings (Calvo et al., 1998; Wright et al., 2006). Mifepristone and spironolactone (or their vehicle) were always administered 45 minutes before recordings (De Vries et al., 1996; Fiancette et al., 2010; Mei and Li, 2013; Achterberg et al., 2014; Hofford et al., 2015). Amphetamine was always administered immediately before recordings (Simola et al., 2012). The supplementary Methods describe how the doses and administration times of drugs were selected.
Experimental Plan
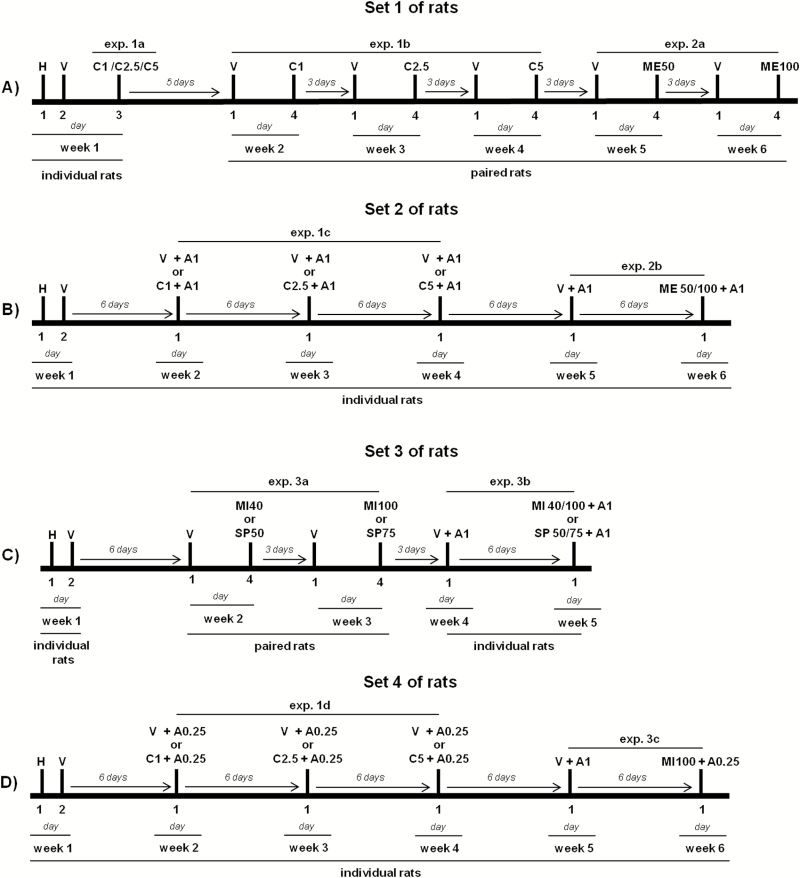
A series of experiments was performed to evaluate whether the pharmacological manipulation of glucocorticoid signaling influenced the emission of 50-kHz USVs in different conditions. Rats received the repeated administration of one or two of the following drugs: corticosterone, metyrapone, mifepristone, and spironolactone. Each drug was administered alone or in combination with amphetamine. Repeated drug administrations in the same rats were performed to reduce the number of animals used in the study. Moreover, drugs were always given at ascending doses. This was done to expose rats to the same experimental conditions across recordings in the attempt to reduce the variability of the results. Finally, 4 sets of rats were used to reduce the possible carryover effects that could arise from repeated drug administration. Rats in each set were drug-naïve before the beginning of the experiments. The individual experiments are listed below and are grouped according to the drugs administered. The timeline of experiments performed in each set of rats is described in Figure 1.
Figure 1.
Timeline of the experiments. Four sets of rats were used in this study, and the figure indicates the experiments performed in each set of rats. In the first week, rats in each set were individually housed during recordings. They were habituated (30 minutes) to the test cage on day 1, to evaluate basal calling, and received vehicle (10:90% dimethyl sulfoxide [DMSO]/polyethylene glycol [PEG]) on day 2. Afterwards, rats received pharmacological treatments and were housed either individually or in pairs during recording, as outlined in the figure. Ultrasonic vocalization (USV) recordings were performed on each testing day and lasted for 2 hours in rats individually housed and 15 minutes in rats housed in pairs. A, amphetamine; C, corticosterone; H, habituation to the test-cage; ME, metyrapone; MI, mifepristone; SP, spironolactone; V, vehicle. The numbers indicate the doses of each drug (in mg/kg). The effects of metyrapone, mifepristone, and spironolactone on calling initiation were evaluated in experiment 2b and 3b, before the administration of amphetamine.
Experiments That Evaluated the Effects of Corticosterone on Calling in the 50 kHz Band
Experiment 1a: calling initiation. Rats were individually housed during recordings and received the acute administration of corticosterone (1, 2.5, or 5 mg/kg, s.c.) (Figure 1A). n=10 for each dose of corticosterone.
Experiment 1b: pro-social calling. Rats were housed in pairs during recordings to engage in social contacts and evaluated for 3 consecutive weeks. In each week, rats received vehicle (10:90%, DMSO:PEG) on the first day and corticosterone (1, 2.5, or 5 mg/kg, s.c.) on the fourth day (Figure 1A). n=15 pairs for each evaluation.
Experiments 1c and d: amphetamine-stimulated calling. Rats were individually housed during recordings and evaluated for 3 consecutive weeks. In each week, rats received corticosterone (1, 2.5, or 5 mg/kg, s.c.) followed by amphetamine 10 minutes later. Amphetamine was administered at either a moderate dose (1 mg/kg, i.p.) (Figure 1B) or low dose (0.25 mg/kg, i.p.) (Figure 1D). n=10 for each dose of corticosterone.
Experiments That Evaluated the Effects of Metyrapone on Calling in the 50 kHz Band
Experiment 2a: pro-social calling. Rats were housed in pairs during recordings to engage in social contacts and evaluated for 2 consecutive weeks. In each week, rats received vehicle (40:60%, PEG: distilled water) on the first day and metyrapone (50 or 100 mg/kg, s.c.) on the fourth day (Figure 1A). n=15 pairs for each evaluation.
Experiment 2b: calling initiation and amphetamine-stimulated calling. Rats were individually housed during recordings and evaluated for 2 consecutive weeks. In the first week, all rats were administered vehicle (40:60% PEG: distilled water), placed in the test cage for 90 minutes, and thereafter received amphetamine (1 mg/kg, i.p.) to obtain a baseline level of calling. In the second week, rats were administered metyrapone (50 or 100 mg/kg, i.p., n=10 for each dose) and placed in the test cage for 90 minutes to evaluate calling initiation. Thereafter, metyrapone-treated rats received a moderate dose of amphetamine (1 mg/kg, i.p.) (Figure 1B).
Experiments That Evaluated the Effects of Mifepristone and Spironolactone on Calling in the 50 kHz Band
Experiment 3a: pro-social calling. Rats were housed in pairs during recordings to engage in social contacts and evaluated for 3 consecutive weeks. In each week, rats received vehicle (10:90%, DMSO:PEG) and either mifepristone (40 or 100 mg/kg, s.c.) or spironolactone (50 or 75 mg/kg, s.c.) on the fourth day (Figure 1C). n=10 pairs for each evaluation.
Experiment 3b: calling initiation and amphetamine-stimulated calling. Rats were individually housed during recordings and evaluated for 2 consecutive weeks. In the first week, all rats were administered vehicle (10:90%, DMSO:PEG), placed in the test cage for 45 minutes, and thereafter received amphetamine (1 mg/kg, i.p.) to obtain a baseline level of calling. In the second week, rats were administered either mifepristone (40 or 100 mg/kg, s.c., n=10 for each dose) or spironolactone (50 or 75 mg/kg, s.c., n=10 for each dose) and placed in the test cage for 45 minutes to evaluate calling initiation. Thereafter, mifepristone- and spironolactone-treated rats received a moderate dose of amphetamine (1 mg/kg, i.p., Figure 1C).
Experiment 3c: calling stimulated by a low dose of amphetamine. Rats were individually housed during recordings and evaluated for 2 consecutive weeks. In the first week, rats were treated as described for experiment 3b. In the second week, rats were treated with mifepristone (100 mg/kg, s.c., n=20), placed in the test cage for 45 minutes, and thereafter received a low dose of amphetamine (0.25 mg/kg, i.p., Figure 1D).
In all the experiments that evaluated pro-social calling, rats were always paired with a different rat and both rats were taken from different cages.
Recording of USVs
Rats were placed in Plexiglas cylinders (diameter, 25 cm; height, 30 cm). Thereafter, USVs were recorded by means of ultrasonic devices (CM16/CMPA microphones, UltraSoundGate 116 Hb; Avisoft), as described elsewhere (Simola et al., 2012). Recordings lasted for 2 hours in rats individually housed and 15 minutes in rats housed in pairs. All the recording times were subdivided into 5-minute intervals. The supplementary Methods report further details on the recording procedures. Supplementary Figure 1 shows some examples of 50-kHz USVs recorded in this study.
Statistical Analysis
USV recordings were converted into spectrograms by means of the software SASLab Pro 4.52 (Avisoft) with the following settings: 512 Fast Fourier Transform-length, Hamming window, and 75% overlap frame set-up. Spectrograms were visually inspected by an experienced experimenter, and SASLab Pro 4.52 was used to calculate the number of vocalizations after manual cleaning of all the signals that could not be univocally classified as 50-kHz USVs (Simola et al., 2012).
Means ± SEM of the numbers of 50-kHz USVs were calculated for all the experiments. USV data collected in each experiment were tested for normality and homoscedasticity with Levene’s test. Logarithmic transformation was applied to preserve homoscedasticity, when necessary. A constant of +1 was added to all data subjected to logarithmic transformation. Figures report raw data for clarity. The analysis of spontaneous calling recorded during habituation in each set of experiments revealed no outliers (data not shown). Moreover, neither aggressive interactions between rats nor emission of aversive 22-kHz USVs (Brudzynski, 2007) were observed in the present study. Therefore, all the rats used were included in the statistical analysis. The total number of calls emitted and the time course of calling was evaluated for each experiment with 2-way (treatment×time) ANOVA to reveal significant differences between pharmacological treatments and vehicle administration. Moreover, data collected in each experiment were subdivided into low vocalization (LV) and high vocalization (HV) groups, based on the median value of the number of 50-kHz USVs emitted. Such a subdivision was done to clarify whether ceiling and/or floor effects could affect the results observed in all rats. Separate ANOVAs were run for all rats and for the LV and the HV groups of rats. Significance was always set at P<.05 and ANOVAs were followed by Tukey’s posthoc tests, when appropriate. For the sake of conciseness, the main effects of ANOVA are reported in supplementary Table 1. Statistical results for the time course of calling recorded in each administration day are described in the legends of the supplementary figures, when appropriate. Statistical analysis was performed with Statistica (Statsoft) and QI Macros (KnowWare International) for Windows.
Results
This section describes the effects of pharmacological treatment on the total number of 50-kHz USVs emitted in each testing day. Supplementary Figures 2 to 8 demonstrate the effects of pharmacological treatment on the time course of calling in each testing day.
Effects of Corticosterone on Calling Initiation in Rats Individually Housed during Recording
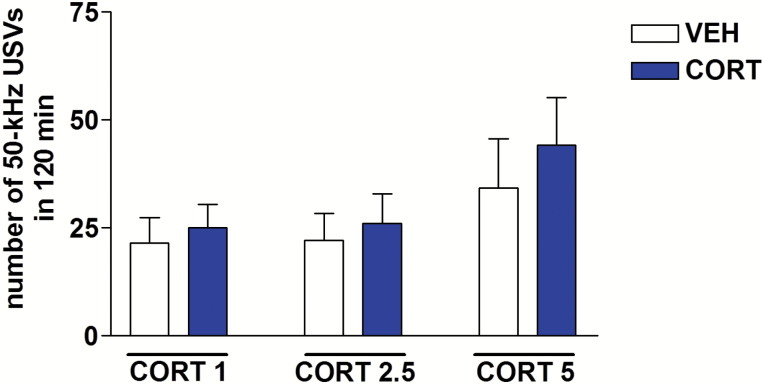
Acute administration of corticosterone (1, 2.5, or 5 mg/kg, s.c.) did not modify the number of 50-kHz USVs emitted by rats, compared with vehicle administration. Two-way ANOVA showed no significant effect of treatment (F5,53=1.41, P=.23) (Figure 2).
Figure 2.
Effects of corticosterone on calling initiation. Acute corticosterone (1–5 mg/kg, s.c.) did not initiate calling. The number of 50-kHz ultrasonic vocalizations (USVs) recorded after corticosterone administration was comparable with that recorded after vehicle administration. CORT, corticosterone; VEH, vehicle. n=10.
Effects of Corticosterone on Prosocial Calling in Rats Housed in Pairs during Recording
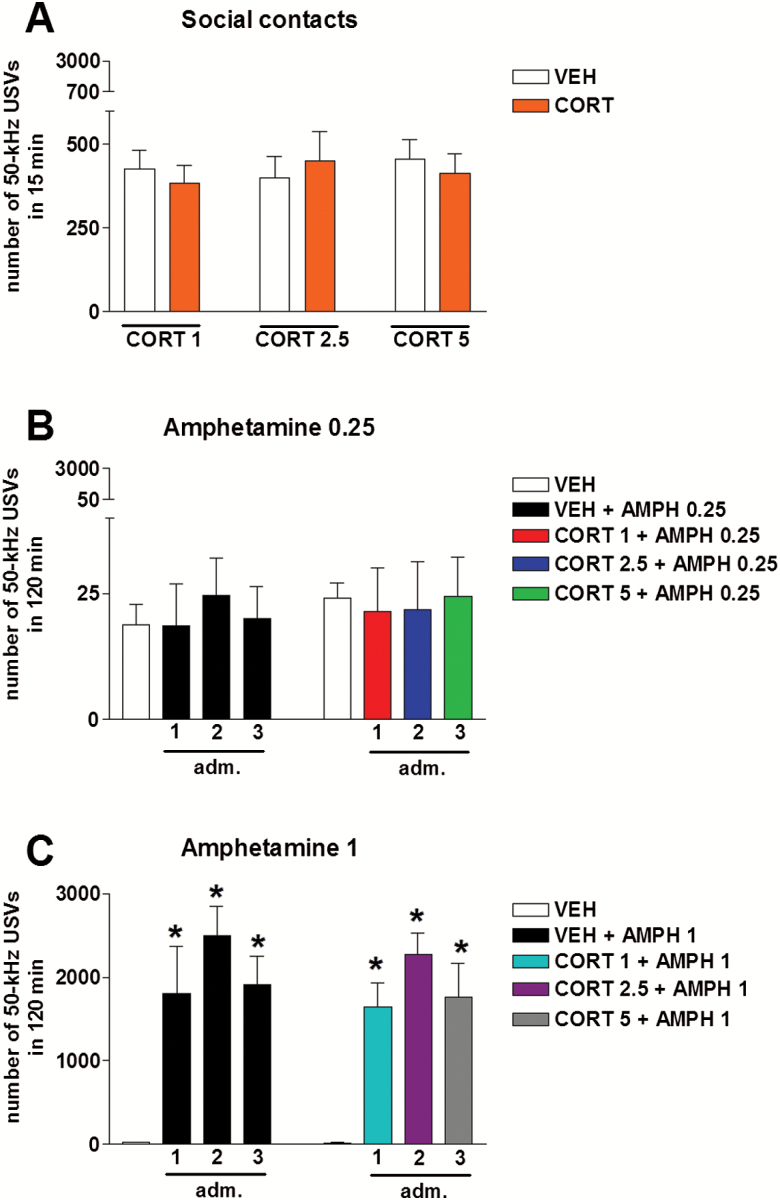
Administration of escalating doses of corticosterone (1, 2.5, or 5 mg/kg, s.c., once a week) did not modify the number of 50-kHz USVs emitted by rats that were allowed to engage in social contacts, compared with vehicle administration. Two-way ANOVA showed no significant effect of treatment (F5,84=0.31, P=.91) (Figure 3A).
Figure 3.
Effects of corticosterone on pro-social and amphetamine-stimulated calling. Escalating doses of corticosterone (1–5 mg/kg, s.c.) did not modify the number of 50-kHz USVs emitted by rats that were housed in pairs during recording, compared with vehicle administration (A). Repeated administration of a low dose of amphetamine (0.25 mg/kg, i.p., once a week for 3 weeks) did not modify the number of 50-kHz ultrasonic vocalizations (USVs) emitted by rats that were individually housed during recording, compared with vehicle administration (B). Conversely, repeated administration of a moderate dose of amphetamine (1 mg/kg, i.p., once a week for 3 weeks) significantly elevated the number of 50-kHz USVs emitted by rats that were individually housed during recording, compared with vehicle administration (C). Coadministration of escalating doses of corticosterone (1–5 mg/kg, s.c.) did not influence the effects of amphetamine on calling (B–C). adm., administration; AMPH, amphetamine; CORT, corticosterone; VEH, vehicle. * P<.05 vs VEH. n=15 for rats housed in pairs. n=10 for rats individually housed.
Effects of Corticosterone on Calling Stimulated by Amphetamine in Rats Individually Housed during Recording
Repeated administration of a low dose of amphetamine (0.25 mg/kg, i.p., once a week for 3 weeks) did not modify the number of 50-kHz USVs emitted by rats compared with vehicle administration. A similar lack of effect was observed when escalating doses of corticosterone (1–5 mg/kg, s.c.) were co-administered with amphetamine (0.25 mg/kg, i.p.). Two-way ANOVA showed no significant effect of treatment (F7,72=0.56, P=.79) (Figure 3B).
Conversely, repeated administration of a moderate dose of amphetamine (1 mg/kg, i.p., once a week for 3 weeks) significantly modified the number of 50-kHz USVs emitted by rats, compared with vehicle administration. Similar effects were observed when escalating doses of corticosterone (1–5 mg/kg, s.c.) were co-administered with amphetamine (1 mg/kg, i.p.). Two-way ANOVA showed a significant effect of treatment: (F7,72=29.82, P<.01). Tukey’s test showed that both amphetamine and corticosterone + amphetamine elevated the number of calls. However, corticosterone + amphetamine increased the number of 50-kHz USVs emitted in a fashion similar to amphetamine alone (Figure 3C).
Effects of Metyrapone on Calling Initiation in Rats Individually Housed during Recording
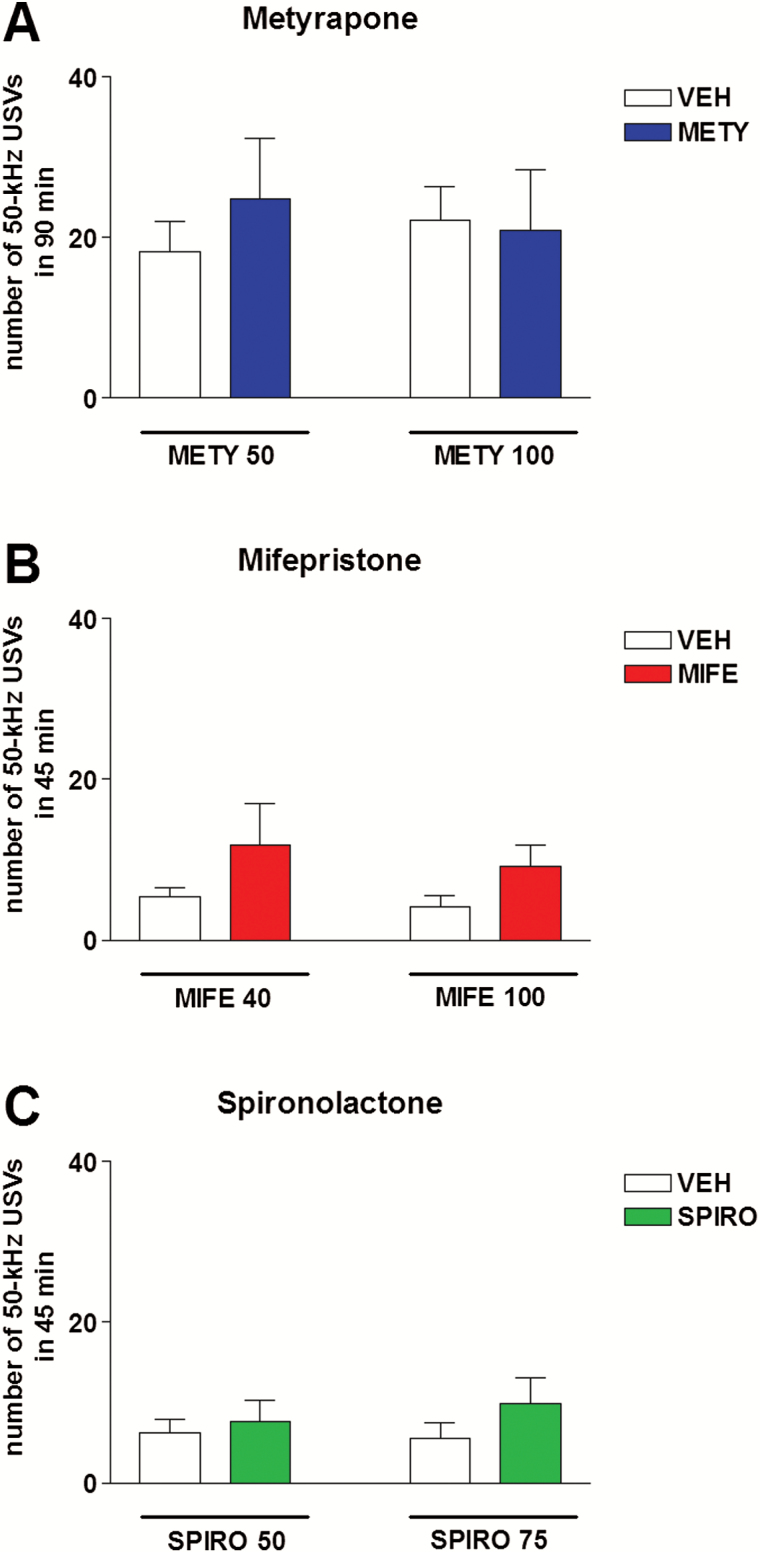
Acute administration of metyrapone (50 or 100 mg/kg, i.p.) did not modify the number of 50-kHz USVs emitted by rats, compared with vehicle administration. Two-way ANOVA showed no significant effect of treatment (F3,36=0.26, P=.86) (Figure 4A).
Figure 4.
Effects of metyrapone, mifepristone, and spironolactone on calling initiation. Acute metyrapone (50 or 100 mg/kg, i.p.) (A), mifepristone (40 or 100 mg/kg, s.c.) (B), or spironolactone (50 or 75 mg/kg, s.c.) (C) did not initiate calling. The number of 50-kHz ultrasonic vocalizations (USVs) recorded after the administration of each drug was comparable with that recorded after vehicle administration. METY, metyrapone; MIFE, mifepristone; SPIRO, spironolactone; VEH, vehicle. n=10.
Effects of Metyrapone on Prosocial Calling in Rats Housed in Pairs during Recording
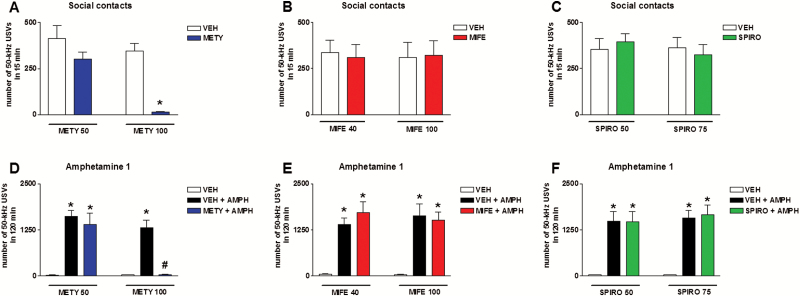
Administration of escalating doses of metyrapone (50 or 100 mg/kg, i.p., once a week) modified the number of 50-kHz USVs emitted by rats that were allowed to engage in social contacts, compared with vehicle administration. Two-way ANOVA showed a significant effect of treatment (F3,56=72.96, P<.01). Moreover, Tukey’s test showed that metyrapone (100 mg/kg, i.p.) significantly reduced the number of calls (Figure 5A).
Figure 5.
Effects of metyrapone, mifepristone, and spironolactone on pro-social and amphetamine-stimulated calling. Escalating doses of metyrapone (50 or 100 mg/kg, i.p., once a week) almost completely suppressed the number of 50-kHz ultrasonic vocalizations (USVs) emitted by rats that were housed in pairs during recording, compared with vehicle administration (A). Conversely, escalating doses of mifepristone (40 or 100 mg/kg, s.c., once a week) or spironolactone (50 or 75 mg/kg, s.c., once a week) did not affect pro-social calling (B–C). In rats that were individually housed during recordings, co-administration of metyrapone (100 mg/kg, i.p.) almost completely suppressed the number of 50-kHz USVs recorded after the administration of amphetamine (1 mg/kg, i.p.), compared with vehicle co-administration (D). Conversely, co-administration of mifepristone (40 or 100 mg/kg, s.c.) or spironolactone (50 or 75 mg/kg, s.c.) had no influence on amphetamine-stimulated calling (E–F). AMPH, amphetamine; METY, metyrapone; MIFE, mifepristone; SPIRO, spironolactone; VEH, vehicle. *P<.05 vs VEH; #P<.05 vs VEH + AMPH. n=10.
Effects of Metyrapone on Calling Stimulated by Amphetamine in Rats Individually Housed during Recording
Co-administration of metyrapone (50 or 100 mg/kg, i.p.) modified the number of 50-kHz USVs emitted by rats that were treated with amphetamine (1 mg/kg, i.p.), compared with vehicle co-administration. Two-way ANOVA showed a significant effect of treatment (F5,54=35.43, P<.01). Moreover, Tukey’s test showed that metyrapone (100 mg/kg, i.p.) significantly reduced the number of calls (Figure 5D).
Effects of Mifepristone and Spironolactone on Calling Initiation in Rats Individually Housed during Recording
Acute administration of mifepristone (40 or 100 mg/kg, s.c.) or spironolactone (50 or 75 mg/kg, s.c.) did not modify the number of 50-kHz USVs emitted by rats, compared with vehicle administration. Two-way ANOVA showed no significant effects of treatment for mifepristone (F3,36=1.77, P=.17) and spironolactone (F3,36=0.46, P=.71) (Figure 4B–C).
Effects of Mifepristone and Spironolactone on Prosocial Calling in Rats Housed in Pairs during Recording
Administration of escalating doses of mifepristone (40 or 100 mg/kg, s.c., once a week) or spironolactone (50 or 75 mg/kg, s.c., once a week) did not modify the number of 50-kHz USVs emitted by rats that were allowed to engage in social contacts, compared with vehicle administration. Two-way ANOVA showed no significant effect of treatment for mifepristone (F3,36=0.38, P=.77) and spironolactone (F3,36=0.50, P=.68) (Figure 5B–C).
Effects of Mifepristone and Spironolactone on Calling Stimulated by Amphetamine in Rats Individually Housed during Recording
Co-administration of mifepristone (40 or 100 mg/kg, s.c.) did not modify the number of 50-kHz USVs emitted by rats that were treated with amphetamine (1 mg/kg, i.p.), compared with vehicle co-administration. Thus, two-way ANOVA showed a significant effect of treatment (F5,54=33.95, P<.01). However, Tukey’s test showed that mifepristone + amphetamine increased the number of 50-kHz USVs emitted in a fashion similar to amphetamine alone (Fig 5E). Nevertheless, co-administration of mifepristone (100 mg/kg, s.c.) influenced calling recorded after the administration of a low dose of amphetamine (0.25 mg/kg, i.p.) (see below).
Co-administration of spironolactone (50 or 75 mg/kg, s.c.) did not modify the number of 50-kHz USVs emitted by rats that were treated with amphetamine (1 mg/kg, i.p.), compared with vehicle co-administration. Thus, two-way ANOVA showed a significant effect of treatment (F5,54=42.04, P<.01). However, Tukey’s test showed that spironolactone + amphetamine increased the number of 50-kHz USVs in a fashion similar to amphetamine alone (Figure 5F).
Emission of 50-kHz USVs in the LV and HV Groups of Rats
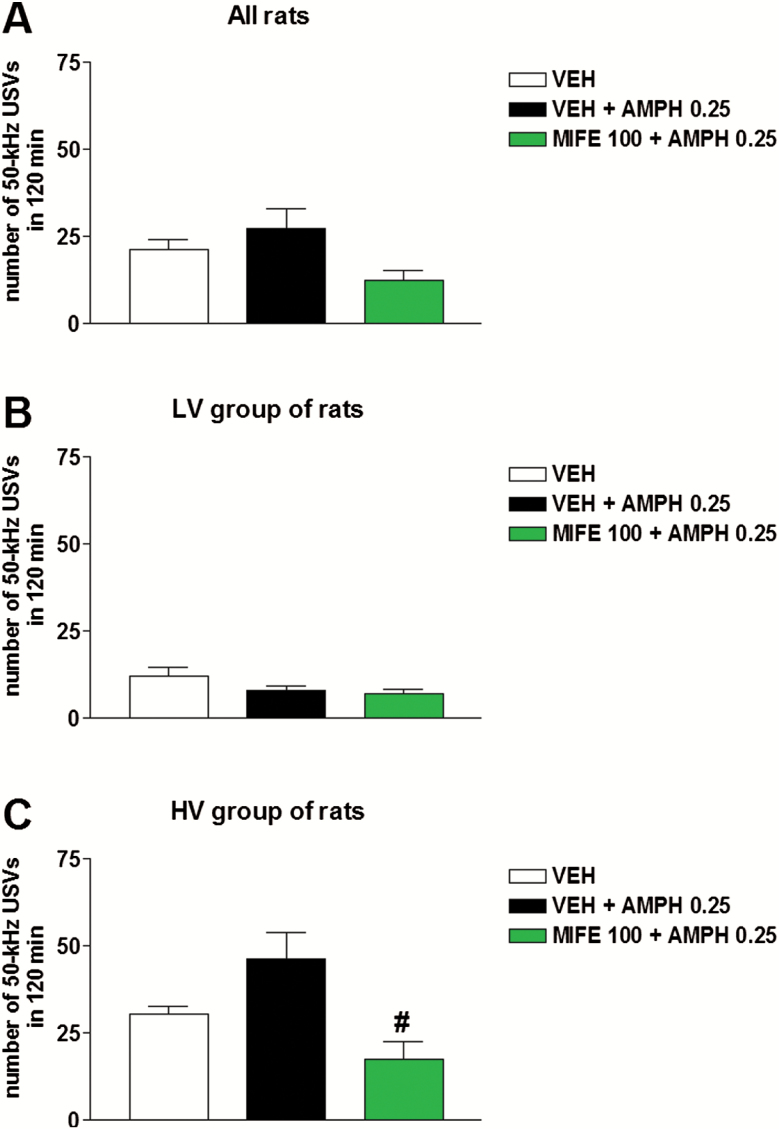
Co-administration of mifepristone (100 mg/kg, s.c.) modified the number of 50-kHz USVs emitted in the HV group of rats treated with a low dose of amphetamine (0.25 mg/kg, i.p.). Two-way ANOVA showed a significant effect of treatment (F2,27=5.03, P=.01) (Figure 6). Moreover, Tukey’s test revealed that the number of calls emitted after mifepristone + amphetamine was lower than the number of calls emitted after amphetamine alone. The other results obtained in the LV and HV groups of rats were in line with the results obtained in the totality of rats (data not shown).
Figure 6.
Effects of mifepristone on calling in rats treated with a low dose of amphetamine and individually housed during recordings. Co-administration of mifepristone (100 mg/kg, s.c.) had no influence on calling recorded after the administration of amphetamine (0.25 mg/kg, i.p.) in all rats and in the LV group (A–B). Conversely, mifepristone co-administration reduced the number of 50-kHz ultrasonic vocalizations (USVs) recorded after the administration of amphetamine in the HV group of rats (C). AMPH, amphetamine; HV, high vocalization; LV, low vocalization; MIFE, mifepristone; VEH, vehicle. #P<.05 vs VEH + AMPH. n=20 for all rats. n=10 for the LV and HV groups of rats.
Discussion
The present study evaluated whether the pharmacological manipulation of glucocorticoid signaling initiated the emission of 50-kHz USVs and/or influenced calling in situations that may be rewarding for rats. The results obtained showed that metyrapone and mifepristone affected calling, although mifepristone did so only in certain conditions. Conversely, corticosterone had no influence on calling.
Previous studies in rats have reported that corticosterone administration does not induce conditioned place preference (CPP) (Dietz et al., 2007; Mei and Li, 2013). These results would suggest that exogenous corticosterone does not elicit rewarding effects in the rat. In agreement with this view, the present study found that corticosterone administration did not initiate the emission of 50-kHz USVs, which are a behavioral marker of reward in rats (Panksepp, 2005; Schwarting et al., 2007; Brudzynski, 2013, 2015). Similarly, corticosterone administration did not affect calling during nonaggressive social contacts, a situation that may induce a positive emotional state in rats (Burgdorf et al., 2009). Besides, another previous investigation found that rats after co-administration of corticosterone and morphine, displayed a more marked CPP, compared with those that received morphine alone (Der-Avakian et al., 2006). This finding would suggest that corticosterone may amplify the rewarding effects of morphine. However, corticosterone strengthened morphine-induced CPP in rats that were previously exposed to stress, but not in stress-naïve rats (Der-Avakian et al., 2006). Interestingly, we found that corticosterone administration did not affect calling in stress-naïve rats treated with amphetamine. Therefore, and taken together, the results of the present study may suggest that in stress-naïve rats corticosterone administration neither induces a positive emotional state nor amplifies the effects that rewarding stimuli have on the emotional state.
Similar to corticosterone, mifepristone administration did not initiate calling in the 50 kHz frequency band. This finding would suggest that the antagonism of glucocorticoid receptors does not induce a positive emotional state in rats. Nevertheless, mifepristone affected calling, as it reduced the number of 50-kHz USVs emitted after amphetamine administration. In this regard, it is noteworthy that the suppression of endogenous corticosterone by adrenalectomy attenuates DA release in the NAc shell of rats treated with drugs of abuse (Barrot et al., 2000). Moreover, the activation of DA receptors in the NAc shell is the key mechanism that initiates calling (Burgdorf et al., 2001; Thompson et al., 2006). Therefore, it is conceivable that mifepristone reduced calling in amphetamine-treated rats, because it attenuated DA signaling in the NAc shell. However, mifepristone affected calling only in the HV group of rats treated with a low dose of amphetamine (0.25 mg/kg). Hence, these results indicate that the antagonism of glucocorticoid receptors modulates calling only in certain conditions.
Homospecific social contacts and amphetamine administration increase the plasma levels of corticosterone in rats (Knych and Eisenberg, 1979; File, 1980). Nevertheless, the rise in plasma corticosterone might not necessarily predict the affective valence of stimuli. Conversely, it could reflect the metabolic demands of neuronal circuits subsequent to the behavioral activation that is induced by salient stimuli (Buwalda et al., 2012; Egan and Ulrich-Lai, 2015). In agreement with the latter view, it may be speculated that either the stimulation or the antagonism of glucocorticoid receptors would not modify the affective properties of rewarding stimuli in stress-naïve rats. This hypothesis may explain why corticosterone and mifepristone did not affect calling during social contacts or after the administration of a moderate dose of amphetamine (1 mg/kg). Alternatively, it may be speculated that glucocorticoid receptor activation by endogenous corticosterone is an ancillary mechanism that sustains calling in situations featuring the emission of a low number of 50-kHz USVs (i.e., the administration of a low amphetamine dose). Besides, it cannot be ruled out that glucocorticoid receptors influence calling emitted by stress-naïve rats in situations different from those evaluated here. In fact, corticosterone may elicit dissimilar effects on the emotional state of rats depending on the situations considered (Piazza et al., 1991, 1996; Deroche et al., 1997; Mantsch et al., 1998; Pecoraro et al., 2005; Buwalda et al., 2012; Olausson et al., 2013).
The present study also found that metyrapone did not initiate the emission of 50-kHz USVs when administered at doses that inhibit corticosterone synthesis in rats. However, metyrapone almost completely suppressed calling recorded during nonaggressive social contacts or after the administration of a moderate dose of amphetamine (1 mg/kg). Since mifepristone did not affect calling in the same situations, we investigated whether mechanisms other than the inhibition of corticosterone synthesis participated in the effects of metyrapone observed here. In particular, we evaluated the effects of mineralocorticoid receptor antagonism on calling in the 50 kHz frequency band. In fact, metyrapone inhibits the synthesis of the mineralocorticoid hormone aldosterone (Rigel et al., 2010; Daniel and Newell-Price, 2015). Moreover, studies in cocaine-treated mice have demonstrated that the mineralocorticoid receptor antagonist spironolactone may affect the emotional state (Fiancette et al., 2010). However, spironolactone did not influence pro-social and amphetamine-stimulated calling, and it also failed to initiate the emission of 50-kHz USVs. In this regard, it is noteworthy that metyrapone inhibits the synthesis of both corticosterone and aldosterone (Daniel and Newell-Price, 2015). Therefore, it could be hypothesized that mifepristone and spironolactone did not suppress calling because they partially reproduced the effects of metyrapone. However, we have found in preliminary experiments that the co-administration of mifepristone and spironolactone did not attenuate amphetamine-stimulated calling (see supplementary Table 2). Moreover, previous studies have demonstrated that metyrapone may exert extra-adrenal effects in rats (Shaham et al., 1997; Guerin et al., 2014; Schmoutz et al., 2014). Therefore, we may suggest that mechanisms of actions other than the inhibition of steroidogenesis may participate in the suppression of calling by metyrapone. In this regard, it is also noteworthy that the present study employed a protocol of repeated metyrapone administration, at least in the rats that were housed in pairs during recording. Therefore, such an administration protocol could have favored the sensitization in the inhibitory effects of metyrapone on steroidogenesis and/or the emergence of additional mechanisms of action of metyrapone.
The effects of metyrapone observed here may appear to be in contrast to earlier findings in rats exposed to immobilization stress (Popik et al., 2014). Thus, rats that were previously and repeatedly immobilized emitted a lower number of 50-kHz USVs when later subjected to tickling, compared with stress-naïve rats. Metyrapone administration concomitantly with immobilization did not further suppress tickling-stimulated calling, but rather restored it. Conversely, metyrapone did not affect tickling-stimulated calling in stress-naïve rats. In this regard, it is noteworthy that the study by Popik and coworkers employed a protocol of repeated metyrapone administration throughout 7 consecutive days. Conversely, in the present study metyrapone was given in a single or double, but spaced, administration. Notably, spaced and continuous administration of metyrapone may elicit dissimilar behavioral effects in rats (Shaham et al., 1997). Moreover, the study by Popik and coworkers evaluated the effects of metyrapone in a situation (tickling) that is different from those examined in the present study. Nevertheless, the present results are of great relevance as they demonstrate, for the first time, that metyrapone affects the emission of 50-kHz USVs in stress-naïve rats. Moreover, the present results further elucidate the effects that metyrapone may have on the emotional state of rats, and can contribute to the preclinical characterization of the anti-addiction and antidepressant properties of metyrapone, which have been previously described (Murphy, 1997; Healy et al., 1999; Goeders and Guerin, 2008; Guerin et al., 2014).
In conclusion, the present study further characterizes the mechanisms that regulate the emission of 50-kHz USVs and the interplay between glucocorticoid signaling and positive affect. The results obtained may help to define the usefulness of 50-kHz USVs in studying the modifications in the emotional state of rats that can be induced by natural and pharmacological rewards.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Funding
This work was supported by intramural funds from the University of Cagliari: Contributo di Ateneo per la Ricerca (CAR) 2015; Fondo Integrativo per la Ricerca (FIR) 2016/2017; awarded to N. Simola and M. Morelli and by funds from Fondazione Banco di Sardegna (project no. 2014.0395) awarded to N. Simola.
Statement of Interest
None.
Supplementary Material
Acknowledgments
The authors are grateful to Silvia Piras for her help with the recordings of vocalizations and collection of data. The authors acknowledge that the manuscript has been edited for language by Medical Edit, Leicester, UK. The authors are entirely responsible for the scientific content of the paper. N. Simola gratefully acknowledges the financial support of the Autonomous Region of Sardinia (L.R. n 7/2007-2015)
References
- Achterberg EJ, Trezza V, Vanderschuren LJ(2014)Glucocorticoid receptor disrupts the reconsolidation of social reward-related memories in rats. Behav Pharmacol 25:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, West MO(2015)Ultrasonic vocalizations as a measure of affect in preclinical models of drug abuse: a review of current findings. Curr Neuropharmacol 13:193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rougé-Pont F, Le Moal M, Piazza PV(2000)The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci 12:973–979. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM.(2007)Ultrasonic calls of rats as indicator variables of negative or positive states: acetylcholine-dopamine interaction and acoustic coding. Behav Brain Res 182: 261–273. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM.(2013)Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol 23:310–317. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM.(2015)Pharmacology of ultrasonic vocalizations in adult rats: significance, call classification and neural substrate. Curr Neuropharmacol 13:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S(2001)Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci 115:940–944. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Brudzynski SM, Beinfeld MC, Cromwell HC, Kroes RA, Moskal JR(2009)The effects of selective breeding for differential rates of 50-kHz ultrasonic on emotional behavior in rats. Dev Psychobiol 51:34–46. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Scholte J, de Boer SF, Coppens CM, Koolhaas JM(2012)The acute glucocorticoid stress response does not differentiate between rewarding and aversive social stimuli in rats. Horm Behav 61:218–226. [DOI] [PubMed] [Google Scholar]
- Calvo N, Martijena ID, Molina VA, Volosin M(1998)Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Res 800:227–235. [DOI] [PubMed] [Google Scholar]
- Costa G, Morelli M, Simola N(2015)Involvement of glutamate NMDA receptors in the acute, long-term, and conditioned effects of amphetamine on rat 50 kHz ultrasonic vocalizations. Int J Neuropsychopharmacol 18:pyv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E, Newell-Price JD(2015)Therapy of endocrine disease: steroidogenesis enzyme inhibitors in Cushing’s syndrome. Eur J Endocrinol 172:263–280. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Bland ST, Schmid MJ, Watkins LR, Spencer RL, Maier SF(2006)The role of glucocorticoids in the uncontrollable stress-induced potentiation of nucleus accumbens shell dopamine and conditioned place preference responses to morphine. Psychoneuroendocrinology 31:653–663. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Maccari S, Le Moal M, Simon H(1992)Repeated corticosterone administration sensitizes the locomotor response to amphetamine. Brain Res 584:309–313. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV(1997)Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther 281:1401–1407. [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Tjon GH, Nestby P, Mulder AH, Vanderschuren LJ(1996)Mifepristone prevents the expression of long-term behavioural sensitization to amphetamine. Eur J Pharmacol 307:3–4. [DOI] [PubMed] [Google Scholar]
- Dietz D, Wang H, Kabbaj M(2007)Corticosterone fails to produce conditioned place preference or conditioned place aversion in rats. Behav Brain Res 181:287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AE, Ulrich-Lai YM(2015)Activation of physiological stress responses by a natural reward: Novel vs repeated sucrose intake. Physiol Behav 150:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiancette JF, Balado E, Piazza PV, Deroche-Gamonet V(2010)Mifepristone and spironolactone differently alter cocaine intravenous self-administration and cocaine-induced locomotion in C57BL/6J mice. Addict Biol 15:81–87. [DOI] [PubMed] [Google Scholar]
- File SE.(1980)The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods 2:219–238. [DOI] [PubMed] [Google Scholar]
- Fu XW, Brudzynski SM(1994)High-frequency ultrasonic vocalization induced by intracerebral glutamate in rats. Pharmacol Biochem Behav 49:835–841. [DOI] [PubMed] [Google Scholar]
- Giralt M, Garcia-Marquez C, Armario A(1987)Previous chronic ACTH administration does not protect against the effects of acute or chronic stress in male rats. Physiol Behav 40:165–170. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF(2008)Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacol Biochem Behav 91:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin GF, Schmoutz CD, Goeders NE(2014)The extra-adrenal effects of metyrapone and oxazepam on ongoing cocaine self-administration. Brain Res 1575:45–54. [DOI] [PubMed] [Google Scholar]
- Hamed A, Szyndler J, Taracha E, Turzyńska D, Sobolewska A, Lehner M, Krząścik P, Daszczuk P(2015)κ-opioid receptor as a key mediator in the regulation of appetitive 50-kHz ultrasonic vocalizations. Psychopharmacology 232:1941–1955. [DOI] [PubMed] [Google Scholar]
- Healy DG, Harkin A, Cryan JF, Kelly JP, Leonard BE(1999)Metyrapone displays antidepressant-like properties in preclinical paradigms. Psychopharmacology 145:303–308. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O, Pesonen U, Huupponen R, Koulu M, Lähteenmäki P(1994)Hepatic metabolism and distribution of mifepristone and its metabolites in rats. Hum Reprod 9Suppl 1:40–46. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Prendergast MA, Bardo MT(2015)Pharmacological manipulation of glucocorticoid receptors differentially affects cocaine self-administration in environmentally enriched and isolated rats. Behav Brain Res 283:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knych ET, Eisenberg RM(1979)Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology 29:110–118. [DOI] [PubMed] [Google Scholar]
- Kõiv K, Metelitsa M, Vares M, Tiitsaar K, Raudkivi K, Jaako K, Vulla K, Shimmo R, Harro J(2016)Chronic variable stress prevents amphetamine-elicited 50-kHz calls in rats with low positive affectivity. Eur Neuropsychopharmacol 26:631–643. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Saphier D, Goeders NE(1998)Corticosterone facilitates the acquisition of cocaine self-administration in rats: opposite effects of the type II glucocorticoid receptor agonist dexamethasone. J Pharmacol Exp Ther 287:72–80. [PubMed] [Google Scholar]
- Mei YY, Li JS(2013)Involvements of stress hormones in the restraint-induced conditioned place preference. Behav Brain Res 256:662–668. [DOI] [PubMed] [Google Scholar]
- Moldow RL, Fischman AJ(1987)Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides 8:819–822. [DOI] [PubMed] [Google Scholar]
- Murphy BE.(1997)Antiglucocorticoid therapies in major depression: a review. Psychoneuroendocrinology 22Suppl 1:125–132. [PubMed] [Google Scholar]
- Olausson P, Kiraly DD, Gourley SL, Taylor JR(2013)Persistent effects of prior chronic exposure to corticosterone on reward-related learning and motivation in rodents. Psychopharmacology 225:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J.(2005)Beyond a joke: from animal laughter to human joy?Science 308:62–63. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Gomez F, la Fleur S, Roy M, Dallman MF(2005)Single, but not multiple pairings of sucrose and corticosterone enhance memory for sucrose drinking and amplify remote reward relativity effects. Neurobiol Learn Mem 83:188–195. [DOI] [PubMed] [Google Scholar]
- Perez-Sepulveda JA, Flagel SB, Garcia-Fuster MJ, Slusky RJ, Aldridge JW, Watson S, Akil H(2013)Differential impact of a complex environment on positive affect in an animal of individual differences in emotionality. Neuroscience 248:436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML(1996)Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 36:359–378. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminière JM, Le Moal M, Mormède P, Simon H(1991)Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci USA 88:2088–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rougé-Pont F, Deroche V, Maccari S, Simon H, Le Moal M(1996)Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci USA 93:8716–8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Kos T, Pluta H, Nikiforuk A, Rojek K, Ryguła R(2014)Inhibition of the glucocorticoid synthesis reverses stress-induced decrease in rat’s 50-kHz ultrasonic vocalizations. Behav Brain Res 260:53–57. [DOI] [PubMed] [Google Scholar]
- Popik P, Potasiewicz A, Pluta H, Zieniewicz A(2012)High-frequency ultrasonic vocalizations in rats in response to tickling: the effects of restraint stress. Behav Brain Res 234:223–227. [DOI] [PubMed] [Google Scholar]
- Rigel DF, Fu F, Beil M, Hu CW, Liang G, Jeng AY(2010)Pharmacodynamic and pharmacokinetic characterization of the aldosterone synthase inhibitor FAD286 in two rodent models of hyperaldosteronism: comparison with the 11b-hydroxylase inhibitor metyrapone. J Pharmacol Exp Ther 334:232–243. [DOI] [PubMed] [Google Scholar]
- Rippberger H, van Gaalen MM, Schwarting RK, Wohr M(2015)Environmental and pharmacological modulation of amphetamine-induced 50-kHz ultrasonic vocalizations in rats. Curr Neuropharmacol 13:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer IJ, Holmes PV, Harris RB(2011)The importance of corticosterone inmediating restraint-induced weight loss in rats. Physiol Behav 102:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoutz CD, Guerin GF, Goeders NE(2014)Role of GABA-active neurosteroids in the efficacy of metyrapone against cocaine addiction. Behav Brain Res 271:269–276. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Jegan N, Wöhr M(2007)Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav Brain Res 182:208–222. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J(1997)Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17:2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola N.(2015)Rat ultrasonic vocalizations and behavioral neuropharmacology: from the screening of drugs to the study of disease. Curr Neuropharmacol 13:164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola N, Ma ST, Schallert T(2010)Influence of acute caffeine on 50-kHz ultrasonic vocalizations in male adult rats and relevance to caffeine-mediated psychopharmacological effects. Int J Neuropsychopharmacol 13:123–132. [DOI] [PubMed] [Google Scholar]
- Simola N, Fenu S, Costa G, Pinna A, Plumitallo A, Morelli M(2012)Pharmacological characterization of 50-kHz ultrasonic vocalizations in rats: comparison of the effects of different psychoactive drugs and relevance in drug-induced reward. Neuropharmacology 63:224–234. [DOI] [PubMed] [Google Scholar]
- Simola N, Frau L, Plumitallo A, Morelli M(2014)Direct and long-lasting effects elicited by repeated drug administration on 50-kHz ultrasonic vocalizations are regulated differently: implications for the study of the affective properties of drugs of abuse. Int J Neuropsychopharmacol 17:429–441. [DOI] [PubMed] [Google Scholar]
- Simola N, Morelli M(2015)Repeated amphetamine administration and long-term effects on 50-kHz ultrasonic vocalizations: possible relevance to the motivational and dopamine-stimulating properties of the drug. Eur Neuropsychopharmacol 25:343–355. [DOI] [PubMed] [Google Scholar]
- Simola N, Costa G, Morelli M(2016)Activation of adenosine A₂A receptors suppresses the emission of pro-social and drug-stimulated 50-kHz ultrasonic vocalizations in rats: possible relevance to reward and motivation. Psychopharmacology 233:507–519. [DOI] [PubMed] [Google Scholar]
- Smith SW, Gala RR(1977)Influence of restraint on plasma prolactin and corticosterone in female rats. J Endocrinol 74:303–314. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Lambrou PJ, Caggiula AR, Redgate ES(1974)Plasma corticosterone levels during sexual behavior in male rats. Horm Behav 5:191–200 [DOI] [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM(2006)Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res 168:64–73. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP(2009)Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Rippberger H, Schwarting RK, van Gaalen MM(2015)Critical involvement of 5-HT2C receptor function in amphetamine-induced 50-kHz ultrasonic vocalizations in rats. Psychopharmacology 232:1817–1829. [DOI] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD(2006)Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur J Neurosci 24:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PB(2010)Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology 211:1–13. [DOI] [PubMed] [Google Scholar]
- Wright JM, Dobosiewicz MR, Clarke PB(2012)α- and β-Adrenergic receptors differentially modulate the emission of spontaneous and amphetamine-induced 50-kHz ultrasonic vocalizations in adult rats. Neuropsychopharmacology 37:808–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.