Abstract
Background
Given that adolescence is a critical epoch in the onset of schizophrenia, studying aberrant brain changes in adolescent-onset schizophrenia, particularly in patients with drug-naive first-episode schizophrenia, is important to understand the biological mechanism of this disorder. Previous resting-state functional magnetic resonance imaging studies have shown abnormal functional connectivity in separate hemispheres in patients with adult-onset schizophrenia. Our aim to study adolescent-onset schizophrenia can provide clues for the early aetiology of schizophrenia.
Method
A total of 48 drug-naïve, first-episode, adolescent-onset schizophrenia outpatients and 31 healthy controls underwent resting-state functional magnetic resonance imaging scans. Data were subjected to voxel-mirrored homotopic connectivity and support vector machine analyses.
Results
Compared with the healthy controls, the adolescent-onset schizophrenia group showed significantly lower voxel-mirrored homotopic connectivity values in different brain regions, including the fusiform gyrus, superior temporal gyrus/insula, precentral gyrus, and precuneus. Decreased voxel-mirrored homotopic connectivity values in the superior temporal gyrus/insula were significantly correlated with Trail-Making Test: Part A performance (r = −0.437, P = .002). A combination of the voxel-mirrored homotopic connectivity values in the precentral gyrus and precuneus may be used to discriminate patients with adolescent-onset schizophrenia from controls with satisfactory classification results, which showed sensitivity of 100%, specificity of 87.09%, and accuracy of 94.93%.
Conclusion
Our findings highlight resting-state interhemispheric FC abnormalities within the sensorimotor network of patients with adolescent-onset schizophrenia and confirm the relationship between adolescent-onset schizophrenia and adult-onset schizophrenia. These findings suggest that reduced interhemispheric connectivity within the sensorimotor network has a pivotal role in the pathogenesis of schizophrenia.
Keywords: adolescent-onset schizophrenia, voxel-mirrored homotopic connectivity, functional connectivity, resting-state functional magnetic resonance imaging, support vector machine
Significance Statement
This study aimed to examine functional connectivity between homotopic brain regions in drug-naïve, first-episode patients with AOS. The present study found that patients with AOS exhibited decreased interhemispheric interactions within the sensorimotor network compared with healthy controls. This result supports the disconnection theory of schizophrenia. Patients with AOS showed a parallel deficit pattern with adult-onset schizophrenia. This pattern corresponds to the neurodevelopmental hypothesis of the disorder. Furthermore, we found that decreased interhemispheric coordination within the STG/insula was significantly correlated with processing speed deficit, indicating that STG/insula disturbances may contribute to cognitive deficits in schizophrenia. These findings suggest that reduced interhemispheric connectivity within the sensorimotor network may play a pivotal role in the pathogenesis of schizophrenia.
Introduction
Initial studies have posited that schizophrenia may be associated with failure of hemispheric dominance for language (Gur, 1977; Crow, 1997), because many of its symptoms are related to language lateralization, for example: neologism, circumlocution, and perseveration (Andreasen and Grove, 1986). Aberrant asymmetries of brain structure may disrupt the evolution of language and delay language lateralization; these 2 results may collectively contribute to the emergence of schizophrenic symptoms (Crow, 1997). However, succeeding studies have reported that individuals with schizophrenia exhibit normal auditory and language lateralization during language and cognitive processing (Magaro and Chamrad, 1983; Mohr et al., 2001). Other studies have also found that failure of language lateralization is associated with deficits in the inhibition of the right hemisphere (Sommer et al., 2001). Hence, schizophrenia may be a disorder of aberrant interhemispheric cooperation, not of abnormalities of the left hemisphere (Crow et al., 1996).
Evidence suggests that the integrity of interhemispheric cooperation is impaired in patients with schizophrenia. The concept of interhemispheric cooperation deficits in schizophrenia is consistent with the recent hypothesis of schizophrenia as a disconnectivity disorder (Friston, 1998; Stephan et al., 2006). This hypothesis conceptualizes schizophrenia as a disorder of dysfunctional connectivity that is associated with the disrupted integrity of white-matter fiber tracts, which connect homotopic brain regions and primarily mediate interhemispheric cooperation. Postmortem studies, as well as structural and diffusion imaging studies, have found reduced cellular density and volume and fractional anisotropy in white-matter fiber tracts of patients with schizophrenia (Woodruff et al., 1997; Kubicki et al., 2002, 2008; Simper et al., 2011). The results of numerous of behavioral and psychophysiological studies have revealed the role of interhemispheric cooperation in schizophrenia. Electroencephalography measurements revealed that patients with schizophrenia show abnormal bilateral interhemispheric alpha-band coherence patterns during cognitive/activation tasks (Morrison-Stewart et al., 1996). Meanwhile, event-related brain potential and behavioral measurements revealed decreased bilateral redundancy gain in patients with schizophrenia (Mohr et al., 1994; Endrass et al., 2002), the decrease of bilateral gain in patients with schizophrenia indicates the decrease of interhemispheric cooperation (Barnett et al., 2007). These results provide evidence that deficits in interhemispheric cooperation may contribute to the manifestation and cognitive deficit of schizophrenia.
Early-onset schizophrenia is defined as the onset of schizophrenia before 18 years old. It concludes childhood-onset schizophrenia, which develops before the patient is 13 years of age (Rapoport et al., 1999), and AOS, which develops in patients between 13 and 18 years of age (Fraguas et al., 2016). Cross-sectional and longitudinal studies of first-episode patients with schizophrenia have shown clinical and biological continuity between early-onset and adult-onset schizophrenia (Nicolson and Rapoport, 1999). Early-onset schizophrenia shares the same diagnostic criteria and clinical characteristics with adult-onset schizophrenia (Hollis, 2000) and exhibits higher genetic vulnerability, more distinct responses to environmental influences, and refractory treatment response to antipsychotics (Nicolson and Rapoport, 1999; Hollis, 2000) than adult-onset schizophrenia. Thus, the study of early-onset schizophrenia may reduce the heterogeneity inherent in schizophrenia, add evidence to the neurodevelopmental hypothesis of the disorder, and provide important clues to the aetiology of this disease.
AOS is similar to adult-onset schizophrenia in that the first episode of AOS that primarily occurs during late adolescence or early adulthood (Lieberman, 2006) is usually recognizable, whereas the first episode of childhood-onset schizophrenia is somewhat obscure (Arango et al., 2008). As schizophrenics may experience an entirely different neurodevelopment from exceedingly early age (Davis and Bracha, 1996), the adolescence of patients with childhood-onset schizophrenia may be distinctly different from that of patients with AOS. Moreover, the onset of schizophrenia in adolescents or young adults may also contribute to the second wave of influences of genetic and environmental factors and their interactions with earlier developmental abnormalities (Mangiarini et al., 1996; Sowell et al., 1999). Longitudinal studies have shown that the progressive loss of grey matter volume is more exaggerated in patients with childhood-onset schizophrenia than in patients with AOS (Arango et al., 2008). Some baseline studies have found that progressive changes in childhood-onset schizophrenia are inconsistent with known deficits in adult-onset schizophrenia (James et al., 2002; Moreno et al., 2005). Thus, given that childhood-onset schizophrenia and AOS may be secondary to a different primary pathology, these 2 stages should be studied separately. The majority of resting-state fMRI studies have explored functional connectivity (Whitfield-Gabrieli and Ford, 2012; Tang et al., 2013) in adult-onset patients to identify the possible brain mechanism that underlies the psychopathology of schizophrenia. Considering that AOS is similar to adult-onset schizophrenia and studies focusing on interhemispheric functional connectivity conducted on this population of patients with schizophrenic can provide early aetiology of schizophrenia, we specifically investigated AOS.
Voxel-mirrored homotopic connectivity (Zuo et al., 2010) (VMHC) is an approach that can measure functional connectivity between homotopic brain regions. This method has been well conducted in patients with schizophrenia (Hoptman et al., 2012; Guo et al., 2014b, 2017a, 2017b) and unaffected siblings (Guo et al., 2014a), depression (Guo et al., 2013a, 2013b), and somatization disorder (Su et al., 2016). Moreover, support vector machine (SVM) is a supervised classification method (Pavlidis et al., 2004) that has been successfully applied in diagnostic and prognostic problems, text categorization, handwriting digital recognition (Noble, 2006), and bioinformatics domains (Zien et al., 2000; Ding and Dubchak, 2001).
In this study, we applied VMHC to obtain homotopic functional connectivity maps for 48 patients with AOS and for 31 healthy controls. We then used the maps to quantify interhemispheric cooperation in the subjects. We used the SVM classification method to identify the optimal brain region or network that may be used to distinguish patients with AOS from healthy controls. Furthermore, we aimed to investigate the correspondence between abnormal brain alterations during adolescence with previous resting-state VMHC findings for patients with early-onset and adult-onset schizophrenia. Our results may integrate and verify the interhemispheric cooperation findings of previous resting-state fMRI studies on schizophrenia and provide clues for the early aetiology of schizophrenia.
Methods
Participants
A total of 48 right-handed outpatients with AOS were recruited from the Second Affiliated Hospital of Xinxiang Medical University, and 31 right-handed healthy adolescents were recruited from the local community through advertisements and were screened using SCID. All participants were drug-naive adolescent outpatients with first-episode schizophrenia. The study participants met the following inclusion criteria: (1) 13 to 18 years old, (2) formal education >6 years, IQ >70, (4) no experience of alcohol or substance abuse, (5) without incompatible implants, (6) no history of head injury resulting in unconsciousness, (7) all patients with AOS met the DSM-IV-TR criteria for first-episode schizophrenia with an established diagnosis for no more than 2 years, (8) all AOS subjects had no comorbid Axis I disease or illness, (9) all control subjects had no past or current diagnosis of neurological disorders, claustrophobia, or family history of hereditary neurological disorders, and (10) all participants had not received any antipsychotic or psychotropic treatment. All recruited subjects provided informed consent from themselves and their parents or legal guardians. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Xinxiang Medical University.
The symptom severity of patients with AOS was assessed using PANSS. The cognitive tests used to assess cognition performance included the Trail-Making Test: Part A (TMT-A); Brief Assessment of Cognition in Schizophrenia: Symbol Coding; Category Fluency: Animal Naming (Fluency); Hopkins Verbal Learning Test-Revised; Neuropsychological Assessment Battery: Mazes (NAB); Brief Visuospatial Memory Test-Revised; and Stroop word, color, and color-word tests (Eack et al., 2010; Brannick et al., 2011).
Data Acquisition and Preprocessing
MRI images were acquired at the Second Affiliated Hospital of Xinxiang Medical University on the same day as clinical assessment. MRI data were acquired by using a Siemens 3T Trio scanner (Siemens Medical Systems) with an 8-channel phased-array head coil. All participants were instructed to remain relaxed, stationary, motionless, supine, with eyes closed, and awake during the whole examination. Straps and foam pads were used to fix each participant’s head snugly and to limit head movement. First, localizer scans and conventional structural imaging were completed. Second, functional images were acquired through an echo-planar imaging sequence with the following parameters: TR/TE=2000/30 milliseconds, 33 slices, 64×64 matrix, 90° flip angle, field of view =220×220 mm2, interslice gap=0.6 mm, and voxel size=3.44 mm×3.44 mm×4 mm. The fMRI scan lasted 480 seconds and 240 volumes were obtained per subject.
Data were preprocessed with MATLAB (MathWorks) by using the statistical parametric mapping software package (SPM8, http://www.fil.ion.ucl.ac.uk/spm). Images were corrected for slice timing and head motion. The maximum displacement in the x, y, or z dimension of each subject should be no more than 2 mm and with an angular motion of no more than 2°. Then, functional images were normalized and resampled to 3×3×3 mm3. To decrease the effect of low-frequency drifts and physiological high-frequency noise, the images were smoothed spatially with an 8-mm FWHM Gaussian kernel, temporally bandpass filtered (0.01–0.08 Hz), and linearly detrended. Finally, linear regression was applied to remove spurious covariates and their temporal derivatives, such as 24 head motion parameters obtained by rigid body correction, the signal from a ventricular region of interest, and that from a region centered in the white matter (Fox et al., 2005). In addition, the global signal was not regressed out of the present data, because the removal of the global signal during the preprocessing of resting-state FC data remains controversial (Fox et al., 2005; Murphy et al., 2009; Saad et al., 2012).
VMHC Analysis
VMHC analysis was conducted with REST software (version 1.8). The time series for each voxel in one hemisphere was correlated with that for its homotopic voxel in the contralateral hemisphere. For each participant, homotopic FC was calculated as the Pearson correlation coefficient between the residual time series of each voxel and that of its mirrored interhemispheric voxel. The coefficients were then subjected to Fisher z-transformation. Finally, the VMHC maps were generated for all participants.
We compared the group VMHC maps with a voxel-wise 2-sample t test analysis. The level of significance was set at the corrected P<.005 level using the Gaussian random field correction method at the cluster level (voxel significance: P<.001, cluster significance: P<.005). We used age and years of education as covariates to minimize the potential confounding effects. The framewise displacement values were computed for each subject, and the mean framewise displacement was used as a covariate in the group comparison.
Statistical Analysis
Demographic and clinical data, cognitive performance, VMHC, and correlations between VMHC values and PANSS scores/cognitive performance were statistically analyzed using SPSS 19.0 software. Differences in age, years of education, and cognitive parameters between patients with AOS and matched healthy controls were determined with 2-sample t tests. Gender between the 2 groups was compared through chi-square test. Partial correlation analyses with controlled age and years of education as covariates were performed between the 2 groups with Pearson coefficient. Posthoc analysis (Bonferroni) was used to correct the level of correlation.
SVM Classification Analysis
SVM was conducted using the LIBSVM software package (http://www.csie.ntu.edu.tw/~cjlin/libsvm/). The LIBSVM classifier is trained to learn differences between groups by providing examples of the form (xi, ci), i=1, …, l, where xi∈Rn, xi represents the VMHC values of abnormal clusters and c is the class label (c=+1 for patients with AOS and c=−1 for healthy controls). The grid search method and Gaussian radial basis function kernels were used for parameter optimization (Liao and Noble, 2010). The “leave-pair-out” cross-validation approach was applied by using LIBSVM software to obtain the highest sensitivity and specificity.
Results
Demographic and Clinical Characteristics
Demographic information on age, years of education, and gender, as well as clinical information and cognitive performance, is presented in Table 1. Gender, age, and years of education were not significantly different between patients with AOS and healthy controls. However, patients with AOS showed significant deficits in global cognitive tests compared with healthy controls. The duration of illness ranged from 0.5 to 24 months. The PANSS performances of patients with AOS are also presented in Table 1.
Table 1.
Demographic and Clinical Characteristics of the Subjects
| Characteristics | AOS | HC | Statistics | p values |
|---|---|---|---|---|
| mean ± SD | mean ± SD | |||
| Sample size | 48 | 31 | ||
| Age(years) | 15.79 ± 1.64 | 15.42 ± 1.52 | 1.014 | 0.314 |
| Gender(M/F) | 21/27 | 14/17 | 0.015 | 0.903 |
| Education years(years) | 8.88 ± 1.95 | 8.44 ± 1.56 | 1.056 | 0.294 |
| TMT-A | 59.52 ± 38.63 | 40.94 ± 14.48 | 3.021 | 0.012 |
| BACS-SC | 39.44 ± 12.17 | 55.81 ± 9.84 | -6.276 | <0.001 |
| HVLT-R | 19.79 ± 6.05 | 26.13 ± 4.79 | -4.919 | <0.001 |
| BVMT-R | 18.65 ± 8.37 | 28.94 ± 5.25 | -6.716 | <0.001 |
| NAB-M | 10.31 ± 6.53 | 15.29 ± 6.40 | -3.333 | 0.001 |
| Fluency | 15.54 ± 4.61 | 18.32 ± 4.81 | -2.576 | 0.012 |
| Stroop word | 70.54 ± 20.96 | 90.58 ± 14.34 | -4.660 | <0.001 |
| Stroop color | 43.10 ± 18.10 | 62.77 ± 14.00 | -5.136 | <0.001 |
| Stroop color-word | 24.08 ± 12.05 | 34.42 ± 7.11 | -4.790 | <0.001 |
| PANSS | ||||
| Total | 75.10 ± 9.88 | |||
| Positive | 21.50 ± 5.01 | |||
| Negative | 17.92 ± 6.95 | |||
| General | 34.25 ± 5.89 | |||
| Duration of illness(months) | 5.35 ± 6.12 |
Abbreviations: AOS, adolescent-onset schizophrenia; BACS-SC, Brief Assessment of Cognition in Schizophrenia-Symbol Coding; BVMT-R, Brief Visuospatial Memory Test-Revised; CF, category fluency; HC, healthy control; HVLT-R, Hopkins Verbal Learning Test-Revised; NAB-M, Neuropsychological Assessment Battery-Mazes; PANSS, Positive and Negative Syndrome Scale; TMT-A, Trail Making Test Part A.
Group Differences in VMHC
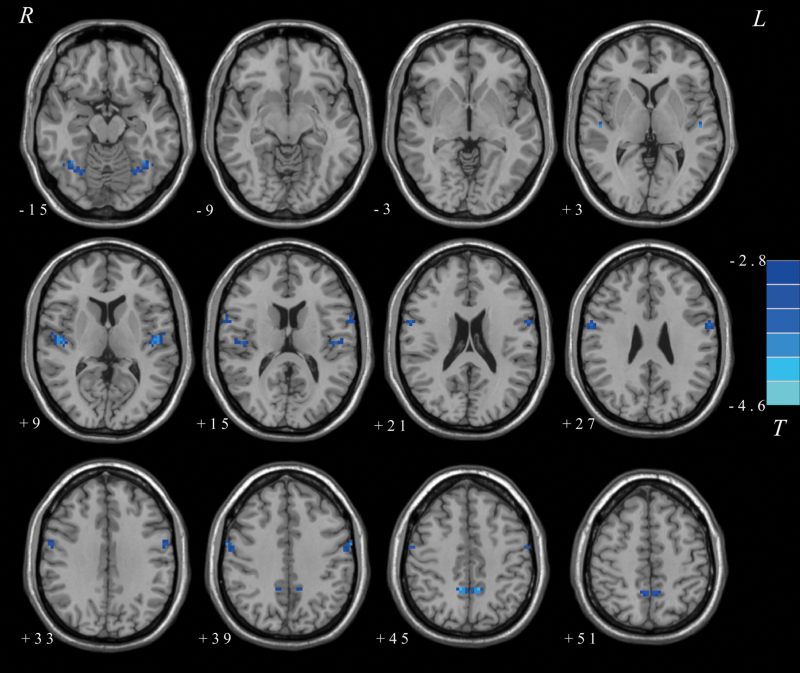
ANCOVA revealed that compared with healthy controls, patients with AOS had significantly lower VMHC values in the fusiform gyrus, superior temporal gyrus (STG)/insula, precentral gyrus, and precuneus (see detail in Table 2, Figure 1). The results implied that abnormal interhemispheric interaction patterns might primarily occur in the sensorimotor network.
Table 2.
Brain Regions with Significant VMHC Differences between Patients with and Healthy Controls
| Brain Regions | VMHC values (mean ± SD) | Number of voxels | t | p values | Peak MNI coordinate | |||
|---|---|---|---|---|---|---|---|---|
| AOS | HC | X | Y | Z | ||||
| Decreased (Patients < Controls) | ||||||||
| Fusiform Gyrus | 0.352 ± 0.201 | 0.557 ± 0.217 | 68 | -3.8871 | <0.000 | ±36 | -54 | -15 |
| Superior Temporal Gyrus/Insula | 0.358 ± 0.158 | 0.577 ± 0.278 | 86 | -4.6346 | <0.000 | ±45 | -18 | 12 |
| Precentral Gyrus | 0.392 ± 0.193 | 0.596 ± 0.218 | 116 | -3.9172 | <0.000 | ±60 | 0 | 24 |
| Precuneus | 0.614 ± 0.195 | 0.816 ± 0.214 | 62 | -4.2486 | <0.000 | ±9 | -48 | 45 |
Abbreviations: AOS, adolescent-onset schizophrenia; HC, healthy control; VMHC, voxel-mirrored homotopic connectivity.
The level of significance was set at the corrected P<.005 level using the Gaussian random field correction method at the cluster level (voxel significance: P<.001, cluster significance: P<.005).
Figure 1.
Differences in voxel-based homotopic connectivity (VMHC) between patients with adolescent-onset schizophrenia (AOS) and healthy controls. The color bar represents the t values of the VMHC group analysis.
Correlations, ROC, and SVM Results
In patients with AOS, decreased VMHC values and PANSS performances were not significantly correlated, whereas decreased VMHC values within 4 brain regions were correlated with cognitive deficits on the TMT-A and BASC-SC tests. The details of the correlations are as follows: the fusiform gyrus vs TMT-A (r=−0.287, P=.048), precentral gyrus vs TMT-A (r=−0.334, P=.020), STG/insula vs TMT-A (r=−0.437, P=.002), and STG/insula vs BASC-SC (r=0.294, P=.043). After Bonferroni correction, only decreased VMHC values in the STG/insula and TMT-A deficits were significantly correlated (r=−0.437, P=.002).
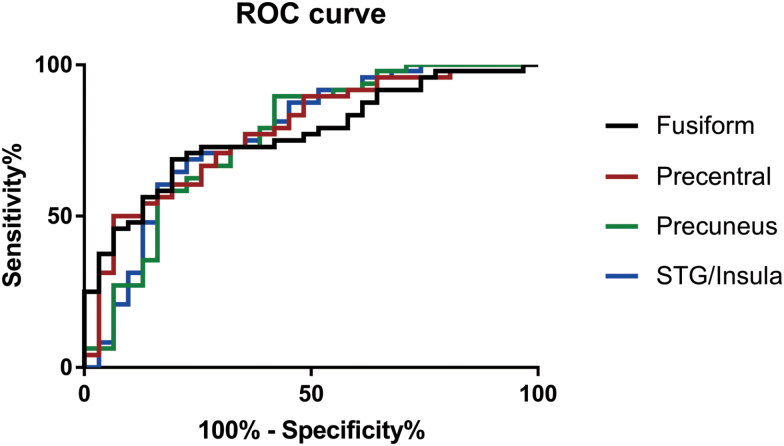
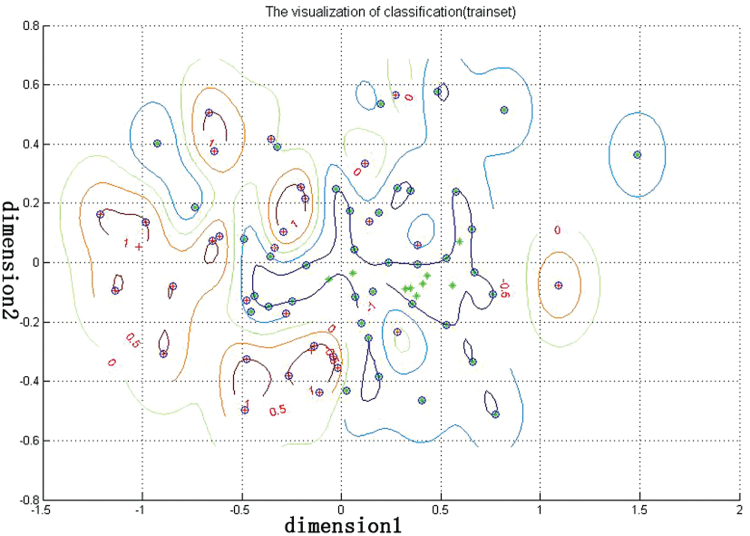
ROC analysis was used to determine the usefulness of VMHC differences in brain regions in discriminating patients with AOS from healthy controls. The AUC, cut-off value, sensitivity, and specificity of each brain region are summarized in Table 3 and Fig. 2. However, none of the VMHC abnormalities in the 4 regions could independently discriminate patients with AOS from healthy controls with satisfactory sensitivity and specificity. Thus, SVM analysis was conducted to examine whether a combination of VMHC values could be used to discriminate patients from healthy controls with optimal sensitivity and specificity (Figure 3). The sensitivity, specificity, and accuracy of each combination of 2 brain regions are summarized in Table 4. The best results were achieved when VMHC values in the precentral gyrus and the precuneus were combined. This combination showed sensitivity of 100%, specificity of 87.09%, and accuracy of 94.93%.
Table 3.
ROC Analyses of Patients with AOS and Healthy Controls
| Brain region | AUC | 95% CI | Cut-off value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Fusiform Gyrus | 0.771 | 0.668, 0.873 | 0.3932 | 0.806 | 0.688 |
| Superior Temporal Gyrus/Insula | 0.770 | 0.675, 0.883 | 0.4169 | 0.774 | 0.688 |
| Precentral Gyrus | 0.779 | 0.651, 0,877 | 0.3411 | 0.935 | 0.500 |
| Precuneus | 0.764 | 0.658, 0.883 | 0.8472 | 0.581 | 0.896 |
Abbreviations: AOS, adolescent-onset schizophrenia; AUC, area under the curve; ROC,receiver operating characteristic curve.
Figure 2.
Receiver operating characteristic curve (ROC) of the discrimination between patients with adolescent-onset schizophrenia (AOS) and healthy controls using the voxel-based homotopic connectivity (VMHC) values of different brain regions.
Figure 3.
Performance of voxel-based homotopic connectivity (VMHC)-based classification. Dimension 1 and dimension 2 represent the VMHC values in the precentral gyrus and precuneus, respectively. Red crosses represent the healthy controls, green crosses represent the adolescent-onset schizophrenia (AOS), and blue circles represent the support vectors.
Table 4.
Number of Correctly Classified Subjects and the Sensitivity, Specificity, and Accuracy of the Combination of VMHC Values in Different Brain Regions
| Brain regions combination | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| AB | 0.875 | 0.742 | 0.823 |
| AC | 0.875 | 0.516 | 0.734 |
| AD | 0.854 | 0.581 | 0.747 |
| BC | 1.000 | 0.871 | 0.949 |
| BD | 0.896 | 0.548 | 0.759 |
| CD | 0.938 | 0.677 | 0.835 |
(A) Fusiform gyrus. (B) Precentral gyrus. (C) Precuneus. (D) Superior temporal gyrus/insula. VMHC, voxel-based homotopic connectivity.
Discussion
This study aimed to examine functional connectivity between homotopic brain regions in drug-naive first-episode patients with AOS. We observed significantly decreased homotopic functional connectivity in patients with AOS compared with healthy controls. These reduced VMHC distributed over large areas, primarily including the fusiform gyrus, STG, insula, and precentral gyrus, as well as precuneus. We also observed that decreased VMHC in the STG/insula was significantly correlated with TMT-A cognitive deficits. The SVM classification result suggested that the combination of decreased VMHC of the precentral gyrus and the precuneus could be used to distinguish patients with AOS from healthy controls with the best sensitivity, specificity, and accuracy. These data added to numerous reports implicating aberrant interhemispheric cooperation in the psychopathology of schizophrenia.
The results of the VMHC approach indicated that regions with aberrant interhemispheric cooperation are mainly located in the sensorimotor network. Previous studies have reported sensory and motor abnormalities in schizophrenia. In schizophrenics, the motor cortex, like the prefrontal cortex, has decreased cortical volume and abnormal changes in associated executive functioning (Owens et al., 2012). Imaging studies have found abnormalities in cortical and subcortical motor areas (Berman et al., 2016), and eye movement studies have further revealed the motor cortex abnormalities are associated with function deficits in patients with schizophrenia (Wolff and O’Driscoll, 1999). Furthermore, increased activation in the bilateral neural network, which is involved in frontal-temporal areas, is significantly associated with auditory verbal hallucinations experience in patients with schizophrenia (Renaud et al., 2011). Diffusion measures have reinforced the potential of abnormalities in visual white-matter integrity as the mechanism that underlies visual-processing deficits (Butler et al., 2006). Additionally, the absent asymmetry of fractional anisotropy within the uncinate fasciculus in patients with schizophrenia indicates dysfunctional visual and auditory processing (Kubicki et al., 2002). The evidence added to the sensorimotor deficits in schizophrenics. Our finding about significantly decreased homotopic functional connectivity within the sensorimotor network supports the theory that schizophrenia results from the abnormal cooperation of the 2 functional hemispheres and highlights the possibility that bilateral cooperation deficits within the sensorimotor network are salient pathological clues for schizophrenia. This finding corresponds with that of previous resting-state VMHC studies on patients with adult-onset schizophrenia (Guo et al., 2017a). Thus, our study provides evidence for the continuity of interhemispheric cooperation abnormalities between AOS and adult-onset schizophrenia. However, our results are not in accordance with previous reports that VMHC patterns decreased in drug-naïve, first-episode patients with early-onset schizophrenia (Li et al., 2015). Specifically, patients with early-onset schizophrenia in the previous study, aged from 9.0 to 17.9 years, exhibited significantly lower VMHC values in the superior temporal cortex/postcentral gyrus than healthy controls (Li et al., 2015). Early-onset schizophrenia is a progressive neurodevelopmental disorder with early- and late-developmental abnormalities (Waddington et al., 1997). Early-onset schizophrenia may also associate with more salient environmental influence or greater genetic insults than late-onset schizophrenia (Nicolson and Rapoport, 1999). The significant differences in neurodevelopmental abnormalities during childhood and adolescence might contribute to the discrepancies in the results of the 2 studies. Moreover, a more appropriate sample size may yield more powerful and generalizable results.
The combination of VMHC values in the precentral gyrus and the precuneus might be an ideal index for the discrimination of patients from healthy controls in present study. This combination showed discrimination with sensitivity of 100%, specificity of 87.09%, and accuracy of 94.93%. The precentral gyrus, which is functionally identified as Brodmann’s area 4 and commonly known as the primary motor cortex, participates in somatosensory information processing and motor execution. The precuneus, which is located in the dorsal region of the posteromedial cortex between the somatosensory and visual cortex superior to the posterior cingulate and retrosplenial cortex, is implicated in multiple cognitive functions. Recent studies (Margulies et al., 2009) have suggested that the precuneus can be functionally subdivided into 4 discrete functional roles. The anterior precuneus exhibits functional connectivity with multiple motor cortex regions, whereas the remaining regions of the precuneus are associated with cognitive and visual functions (Margulies et al., 2009). The SVM results of our present study confirm that the core pathology of schizophrenia is attributed to the disturbed functional and structural integrity of the whole cerebrum and not to that of an individual brain region. The specific combination of decreased VMHC in the precentral gyrus and the precuneus is a potential biomarker that can be used to discriminate individuals with schizophrenia from healthy controls.
Compared with healthy controls, patients with AOS showed significant deficits in multiple cognitive functions, including processing speed (TMT, BASC-SC, and fluency), visual/spatial memory (BVMT), executive function/problem solving (NAB), and language (Stroop). Our findings on cognitive deficits in drug-naïve, first-episode patients with AOS paralleled those of previous works. Patients with schizophrenia exhibit severe dysfunction in global cognitive function, including executive function, attention, processing speed, verbal memory, visual memory, spatial memory, working memory, language, and motor scales (Hoff et al., 1992; Heaton et al., 1994; Mohamed et al., 1999; Robert M. Bilder et al., 2000; Pukrop et al., 2006). Cognitive deficits in the processing speed domain are correlated with decreased VMHC in the STG/insula. A growing body of interhemispheric cooperation studies suggest that cognitive processing by cooperating bilateral hemispheres is more efficient than that by an individual hemisphere (Banich and Karol, 1992; Nuechterlein et al., 2009). Thus, deficits in interhemispheric cooperation may contribute to deficits in cognitive processing. The insula is involved in information processing across various processing pathways. Its abnormal function in patients with schizophrenia indicates that functional connectivity is reduced during information processing (Sheffield et al., 2017). Furthermore, STG has been identified as a possible location for processing deficits in schizophrenia (van Tol et al., 2014). Hence, changes in the STG and insula might contribute to aberrant processing patterns in schizophrenia. Consequently, we hypothesised that abnormal functional interhemispheric cooperation within brain regions might result in specific cognitive deficits.
This study has several limitations. The relatively small sample size of this study is its first limitation. A larger size would improve the generalizability of the findings. Second, the present study assessed functional connectivity alone; however, findings would be more robust if aberrant alterations in the gray and white matter of the cerebrum are also explored. Third, this study observed aberrant cerebral changes at the age points that are correlated with the onset of illness or established schizophrenia diagnosis. Aberrant developmental alterations during puberty to adulthood in patients with AOS still need to be studied. A future longitudinal study should focus on this topic.
In conclusion, we found that patients with AOS showed dysfunctional interhemispheric cooperation within the sensorimotor network. This result supports the disconnection theory of the pathogenesis of schizophrenia. Notably, the deficit pattern of patients with AOS parallels that of patients with adult-onset schizophrenia and corresponds to the neurodevelopmental hypothesis of the disorder. A comprehensive mechanism underlies deficits in interhemispheric functional connectivity. Widespread deficits in white matter integrity most likely account for VMHC abnormalities (Woodruff et al., 1997; Kubicki et al., 2002; Catani et al., 2005). Nevertheless, future structural and functional studies should explore the other mechanisms that underlie abnormal VMHC patterns. Furthermore, decreased interhemispheric cooperation within the STG/insula is significantly correlated with processing speed deficits, indicating that disturbances in interhemispheric cooperation may contribute to cognitive deficits in schizophrenia. These findings suggest that mirror interhemispheric functional connectivity within the sensorimotor network has a pivotal role in the pathogenesis of schizophrenia.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant nos. 81571310, 81630033, 81771447, and 81471363), the National Key National Key RandD Program of China (2016YFC1307100 and 2016YFC1306900), and the Natural Science Foundation of Guangxi Province for Distinguished Young Scientists (grant no. 2014GXNSFGA118010).
Statement of Interest
None of the authors holds any actual or potential conflict of interest for this study.
Acknowledgments
We are grateful to the patients, their families, and the subjects who offered their time to participate in this study.
References
- Andreasen NC, Grove WM(1986)Thought, language, and communication in schizophrenia: diagnosis and prognosis. Schizophr Bull 12:348–359. [DOI] [PubMed] [Google Scholar]
- Arango C, Moreno C, Martinez S, Parellada M, Desco M, Moreno D, Fraguas D, Gogtay N, James A, Rapoport J(2008)Longitudinal brain changes in early-onset psychosis. Schizophr Bull 34:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Karol DL(1992)The sum of the parts does not equal the whole: evidence from bihemispheric processing. J Exp Psychol Hum Percept Perform 18:763–784. [DOI] [PubMed] [Google Scholar]
- Barnett KJ, Kirk IJ, Corballis MC(2007)Bilateral disadvantage: lack of interhemispheric cooperation in schizophrenia. Conscious Cogn 16:436–444. [DOI] [PubMed] [Google Scholar]
- Berman RA, Gotts SJ, McAdams HM, Greenstein D, Lalonde F, Clasen L, Watsky RE, Shora L, Ordonez AE, Raznahan A, Martin A, Gogtay N, Rapoport J(2016)Disrupted sensorimotor and social–cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain 139:276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JMJ, Woerner MG, Geisler S, Kane JM, Lieberman JA(2000)Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 157:549–559. [DOI] [PubMed] [Google Scholar]
- Brannick MT, Wahi MM, Goldin SB(2011)Psychometrics of Mayer-Salovey-Caruso emotional intelligence test (MSCEIT) scores. Psychol Rep 109:327–337. [DOI] [PubMed] [Google Scholar]
- Butler PD, Hoptman MJ, Nierenberg J, Foxe JJ, Javitt DC, Lim KO(2006)Visual white matter integrity in schizophrenia. Am J Psychiatry 163:2011–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH(2005)Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Crow TJ.(1997)Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci 20:339–343. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Done DJ, Sacker A(1996)Cerebral lateralization is delayed in children who later develop schizophrenia. Schizophr Res 22:181–185. [DOI] [PubMed] [Google Scholar]
- Davis JO, Bracha HS(1996)Prenatal growth markers in schizophrenia: a monozygotic co-twin control study. Am J Psychiatry 153:1166–1172 [DOI] [PubMed] [Google Scholar]
- Ding CH, Dubchak I(2001)Multi-class protein fold recognition using support vector machines and neural networks. Bioinformatics 17:349–358. [DOI] [PubMed] [Google Scholar]
- Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, Keshavan MS(2010)Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso emotional intelligence test. Schizophr Bull 36:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass T, Mohr B, Rockstroh B(2002)Reduced interhemispheric transmission in schizophrenia patients: evidence from event-related potentials. Neurosci Lett 320:57–60. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME(2005)The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas D, Diaz-Caneja CM, Pina-Camacho L, Janssen J, Arango C(2016)Progressive brain changes in children and adolescents with early-onset psychosis: a meta-analysis of longitudinal MRI studies. Schizophr Res 173:132–139. [DOI] [PubMed] [Google Scholar]
- Friston KJ.(1998)The disconnection hypothesis. Schizophr Res 30:115–125. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Xue Z, Gao K, Liu Z, Xiao C, Chen H, Zhao J (2013a) Decreased interhemispheric coordination in treatment-resistant depression: a resting-state fMRI study. PloS One 8:e71368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Dai Y, Jiang M, Zhang J, Yu L, Long L, Chen H, Gao Q, Xiao C (2013b) Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 41:24–29. [DOI] [PubMed] [Google Scholar]
- Guo W, Jiang J, Xiao C, Zhang Z, Zhang J, Yu L, Liu J, Liu G (2014a) Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr Res 152:170–175. [DOI] [PubMed] [Google Scholar]
- Guo W, Xiao C, Liu G, Wooderson SC, Zhang Z, Zhang J, Yu L, Liu J (2014b) Decreased resting-state interhemispheric coordination in first-episode, drug-naive paranoid schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48:14–19. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Chen J, Wu R, Li L, Zhang Z, Zhao J (2017a) Family-based case-control study of homotopic connectivity in first-episode, drug-naive schizophrenia at rest. Sci Rep 7:43312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Chen J, Wu R, Li L, Zhang Z, Chen H, Zhao J (2017b) Treatment effects of olanzapine on homotopic connectivity in drug-free schizophrenia at rest. World J Biol Psychiatry 7:1–9. [DOI] [PubMed] [Google Scholar]
- Gur RE.(1977)Motoric laterality imbalance in schizophrenia. A possible concomitant of left hemisphere dysfunction. Arch Gen Psychiatry 34:33–37. [DOI] [PubMed] [Google Scholar]
- Heaton R, Paulsen JS, McAdams L et al. (1994)Neuropsychological deficits in schizophrenics: relationship to age, chronicity, and dementia. Arch Gen Psychiatry 51:469–476. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE(1992)Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 149:898–903. [DOI] [PubMed] [Google Scholar]
- Hollis C.(2000)Adult outcomes of child- and adolescent-onset schizophrenia: diagnostic stability and predictive validity. Am J Psychiatry 157:1652–1659. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, D’Angelo D, Mauro CJ, Butler PD, Milham MP, Javitt DC(2012)Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr Res 141:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AC, Javaloyes A, James S, Smith DM(2002)Evidence for non-progressive changes in adolescent-onset schizophrenia: follow-up magnetic resonance imaging study. Br J Psychiatry 180:339–344. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME(2002)Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry 159:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Styner M, Bouix S, Gerig G, Markant D, Smith K, Kikinis R, McCarley RW, Shenton ME(2008)Reduced interhemispheric connectivity in schizophrenia- tractography based segmentation of the corpus callosum. Schizophr Res 106:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Xu Y, Zhang KR, Hoptman MJ, Zuo XN(2015)Homotopic connectivity in drug-naïve, first-episode, early-onset schizophrenia. J Child Psychol Psychiatry 56:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Noble WS(2010)Combining pairwise sequence similarity and support vector machines for remote protein homology detection. J Comput Biol 10:225–232. [DOI] [PubMed] [Google Scholar]
- Lieberman JA.(2006)Neurobiology and the natural history of schizophrenia. J Clin Psychiatry 67:e14. [PubMed] [Google Scholar]
- Magaro PA, Chamrad DL(1983)Information processing and lateralization in schizophrenia. Bio Psychiatry 18:29–44. [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW(1996)Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M(2009)Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A 106:20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N(1999)Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry 56:749–754. [DOI] [PubMed] [Google Scholar]
- Mohr B, Pulvermuller F, Zaidel E(1994)Lexical decision after left, right and bilateral presentation of function words, content words and non-words: evidence for interhemispheric interaction. Neuropsychologia 32:105–124. [DOI] [PubMed] [Google Scholar]
- Mohr B, Heim S, Pulvermuller F, Rockstroh B(2001)Functional asymmetry in schizophrenic patients during auditory speech processing. Schizophr Res 52:69–78. [DOI] [PubMed] [Google Scholar]
- Moreno D, Burdalo M, Reig S, Parellada M, Zabala A, Desco M, Bacabaldomero E, Arango C(2005)Structural neuroimaging in adolescents with a first psychotic episode. J Am Acad Child Adolesc Psychiatry 44:1151–1157. [DOI] [PubMed] [Google Scholar]
- Morrison-Stewart SL, Velikonja D, Corning WC, Williamson P(1996)Aberrant interhemispheric alpha coherence on electroencephalography in schizophrenic patients during activation tasks. Psychol Med 26:605–612. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA(2009)The impact of global signal regression on resting state correlations: are anti-correlated networks introduced?NeuroImage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R, Rapoport JL(1999)Childhood-onset schizophrenia: rare but worth studying. Bio Psychiatry 46:1418–1428. [DOI] [PubMed] [Google Scholar]
- Noble WS.(2006)What is a support vector machine?Nat biotechnol 24:1565–1567. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M(2009)CNTRICS final task selection: control of attention. Schizophr Bull 35:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens SF, Picchioni MM, Ettinger U, McDonald C, Walshe M, Schmechtig A, Murray RM, Rijsdijk F, Toulopoulou T(2012)Prefrontal deviations in function but not volume are putative endophenotypes for schizophrenia. Brain 135:2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Wapinski I, Noble WS(2004)Support vector machine classification on the web. Bioinformatics 20:586–587. [DOI] [PubMed] [Google Scholar]
- Pukrop R, SchultzeLutter F, Ruhrmann S, Brockhaus-Dumke A, Tendolkar I, Bechdolf A, Matuschek E, Klosterkötter J(2006)Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J Clin Exp Neuropsychol 28:1388–1407. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, Nicolson R, Bedwell J, Lenane M, Zijdenbos A, Paus T, Evans A(1999)Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 56:649–654. [DOI] [PubMed] [Google Scholar]
- Renaud J, Alexandre P, Delphine P, Pierre T(2011)Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry 168:73–81. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW(2012)Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, Clementz BA, Lerman-Sinkoff DB, Hill SK, Barch DM(2017)Transdiagnostic associations between functional brain network integrity and cognition. JAMA Psychiatry 74:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simper R, Walker MA, Black G, Di Rosa E, Crow TJ, Chance SA(2011)The relationship between callosal axons and cortical neurons in the planum temporale: alterations in schizophrenia. Neurosci Res 71:405–410. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Kahn RS(2001)Language lateralization in schizophrenia, an fMRI study. Schizophr Res 52:57–67. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW(1999)In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci 2:859–861. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ(2006)Synaptic plasticity and dysconnection in schizophrenia. Bio Psychiatry 59:929–939. [DOI] [PubMed] [Google Scholar]
- Su Q, Yao D, Jiang M, Liu F, Long L, Dai Y, Yu M, Zhang Z, Zhang J, Liu J, Xiao C, Zhao J, Guo W(2016)Decreased interhemispheric functional connectivity in insula and angular gyrus/supramarginal gyrus: significant findings in first-episode, drug-naive somatization disorder. Psychiatry Res 248:48–54. [DOI] [PubMed] [Google Scholar]
- Tang J, Liao Y, Song M, Gao JH, Zhou B, Tan C, Liu T, Tang Y, Chen J, Chen X(2013)Aberrant default mode functional connectivity in early onset schizophrenia. PloS One 8:e71061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tol MJ, Van der Meer L, Bruggeman R, Modinos G, Knegtering H, Aleman A(2014)Voxel-based gray and white matter morphometry correlates of hallucinations in schizophrenia: the superior temporal gyrus does not stand alone. Neuroimage Clin 4:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington JL, Scully PJ, Youssef HA(1997)Developmental trajectory and disease progression in schizophrenia: the conundrum, and insights from a 12-year prospective study in the Monaghan 101. Schizophr Res 23:107–118. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM(2012)Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Wolff AL, O’Driscoll GA(1999)Motor deficits and schizophrenia: the evidence from neuroleptic-naive patients and populations at risk. J Psychiatry Neurosci 24:304–314. [PMC free article] [PubMed] [Google Scholar]
- Woodruff PW, Phillips ML, Rushe T, Wright IC, Murray RM, David AS(1997)Corpus callosum size and inter-hemispheric function in schizophrenia. Schizophr Res 23:189–196. [DOI] [PubMed] [Google Scholar]
- Zien A, Ratsch G, Mika S, Scholkopf B, Lengauer T, Muller KR(2000)Engineering support vector machine kernels that recognize translation initiation sites. Bioinformatics 16:799–807. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP(2010)Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 30:15034–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]