Abstract
Background
(R)-Ketamine exhibits rapid and sustained antidepressant effects in animal models of depression. It is stereoselectively metabolized to (R)-norketamine and subsequently to (2R,6R)-hydroxynorketamine in the liver. The metabolism of ketamine to hydroxynorketamine was recently demonstrated to be essential for ketamine’s antidepressant actions. However, no study has compared the antidepressant effects of these 3 compounds in animal models of depression.
Methods
The effects of a single i.p. injection of (R)-ketamine, (R)-norketamine, and (2R,6R)-hydroxynorketamine in a rat learned helplessness model were examined.
Results
A single dose of (R)-ketamine (20 mg/kg) showed an antidepressant effect in the rat learned helplessness model. In contrast, neither (R)-norketamine (20 mg/kg) nor (2R,6R)-hydroxynorketamine (20 and 40 mg/kg) did so.
Conclusions
Unlike (R)-ketamine, its metabolite (2R,6R)-hydroxynorketamine did not show antidepressant actions in the rat learned helplessness model. Therefore, it is unlikely that the metabolism of ketamine to hydroxynorketamine is essential for ketamine’s antidepressant actions.
Keywords: metabolism; (R)-ketamine; (R)-norketamine (2R,6R)-hydroxynorketamine; learned helplessness
Significance Statement
The rapid and sustained antidepressant effects of ketamine in patients with treatment-resistant depression are the most important discovery in the field of depression research in a half-century. However, the precise mechanisms underlying the antidepressant effects of ketamine remain unknown. A recent study (Zanos et al., 2016) reported that the metabolism of ketamine to hydroxynorketamine (HNK) is essential for ketamine’s antidepressant effects. In particular, (2R,6R)-HNK, a metabolite of (R)-ketamine, plays a key role in the antidepressant actions. However, here we report that, unlike (R)-ketamine, its metabolites (R)-norketamine and (2R,6R)-HNK did not elicit antidepressant effects in a rat learned helplessness model. It is, therefore, unlikely that the metabolism of ketamine to HNK is necessary for ketamine’s antidepressant actions.
Introduction
Recently conducted meta-analyses revealed that the N-methyl-D-aspartate receptor antagonist ketamine exhibits rapid and sustained antidepressant effects in patients with treatment-resistant depression (Newport et al, 2015; Kishimoto et al, 2016). Thus, ketamine is the most attractive antidepressant for the treatment of treatment-resistant depression (Monteggia and Zarate, 2015; Duman et al., 2016; Hashimoto, 2016b), although the precise mechanisms underlying its antidepressant actions remain unknown.
(R,S)-Ketamine is a racemic mixture containing equal parts of (R)-ketamine and (S)-ketamine. (S)-Ketamine shows approximately 3- to 4-fold greater anesthetic potency and greater undesirable psychotomimetic side effects than (R)-ketamine (Domino et al., 2010). Several groups including our own have demonstrated that (R)-ketamine showed greater potency and longer-lasting antidepressant effects than (S)-ketamine in animal models of depression (Zhang et al., 2014; Yang et al., 2015, 2017, 2018; Zanos et al., 2016; Fukumoto et al., 2017). Unlike (S)-ketamine, (R)-ketamine does not induce psychotomimetic side effects or exhibit abuse potential in rodents (Yang et al., 2015). Furthermore, single or repeated intermittent administration of (S)-ketamine, but not of (R)-ketamine, resulted in the loss of parvalbumin immunoreactivity in the prefrontal cortex and hippocampus (Yang et al., 2015, 2016). Moreover, with the results using [11C]raclopride and positron emission tomography, we reported a marked reduction of dopamine D2/3 receptor binding in the conscious monkey striatum after a single infusion of (S)-ketamine, but not of (R)-ketamine (Hashimoto et al., 2017). These findings suggest that (S)-ketamine, but not (R)-ketamine, can cause a marked release of dopamine from presynaptic terminals, which is associated with acute psychotomimetic effects (Hashimoto et al., 2017). Taking these findings together, (R)-ketamine could be a potentially safer antidepressant without detrimental side effects in humans than (S)-ketamine (Hashimoto, 2016a, 2016b, 2017).
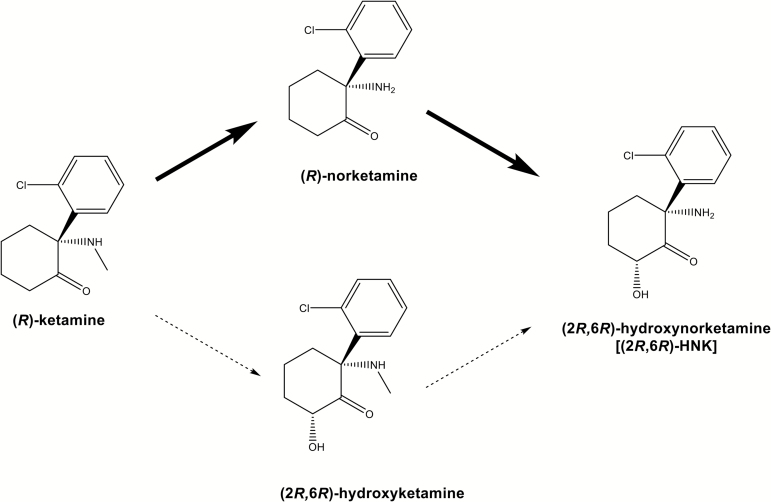
It is well known that ketamine is rapidly metabolized into norketamine and subsequently into hydroxynorketamine (HNK) by microsomal cytochrome P450 enzymes (through N-demethylation and hydroxylation) in the liver (Figure 1) (Turfus et al., 2009; Zhao et al., 2012; Zanos et al., 2016; Hashimoto, 2017). The metabolism of ketamine to HNK was also recently demonstrated to be essential for the antidepressant actions of ketamine (Zanos et al., 2016). In particular, (2R,6R)-HNK, a metabolite from (R)-ketamine, plays a key role in the antidepressant actions (Zanos et al., 2016). However, increasing attention has been drawn to the antidepressant actions of (2R,6R)-HNK (Abdallah, 2017). In the present study, we examined the effects of a single systemic injection of (R)-ketamine and its two major metabolites, (R)-norketamine and (2R,6R)-HNK, in a rat learned helplessness (LH) model.
Figure 1.
Metabolism of (R)-ketamine in the liver. In the liver, (R)-ketamine is metabolized to (R)-norketamine (major pathway) and (2R,6R)-hydroxyketamine (minor pathway), subsequently (2R,6R)-hydroxynorketamine (HNK).
Methods and Materials
Animals
Male Sprague-Dawley rats (200–230 g, 7 weeks old; Charles-River Japan) were used. The animals were housed under a 12-h-light/-dark cycle with free access to food and water. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee (permission no: 28–394 and 29–328). All efforts were made to minimize suffering.
Drugs
(R)-Ketamine hydrochloride was prepared by recrystallization of (R,S)-ketamine (Ketalar, ketamine hydrochloride, Daiichi Sankyo Pharmaceutical Ltd) and D-(-)-tartaric acid, as described previously (Zhang et al., 2014). (R)-Norketamine hydrochloride was prepared as described previously (Zanos et al., 2016). The purity of these stereoisomers was determined by a high-performance liquid chromatography (CHIRALPAK IA, column size: 250x4.6 mm, mobile phase: n-hexane/dichloromethane/diethylamine (75/25/0.1), Daicel Corporation). (2R,6R)-HNK hydrochloride was provided from Taisho Pharmaceutical Ltd as reported previously (Zanos et al., 2016). (R)-Ketamine, (R)-norketamine, and (2R,6R)-HNK were dissolved in 0.9% NaCl. Other compounds were purchased commercially. The doses of (R)-ketamine and its metabolites were selected as previously reported (Yang et al., 2015, 2017; Zanos et al., 2016).
Stress Paradigm (LH Model)
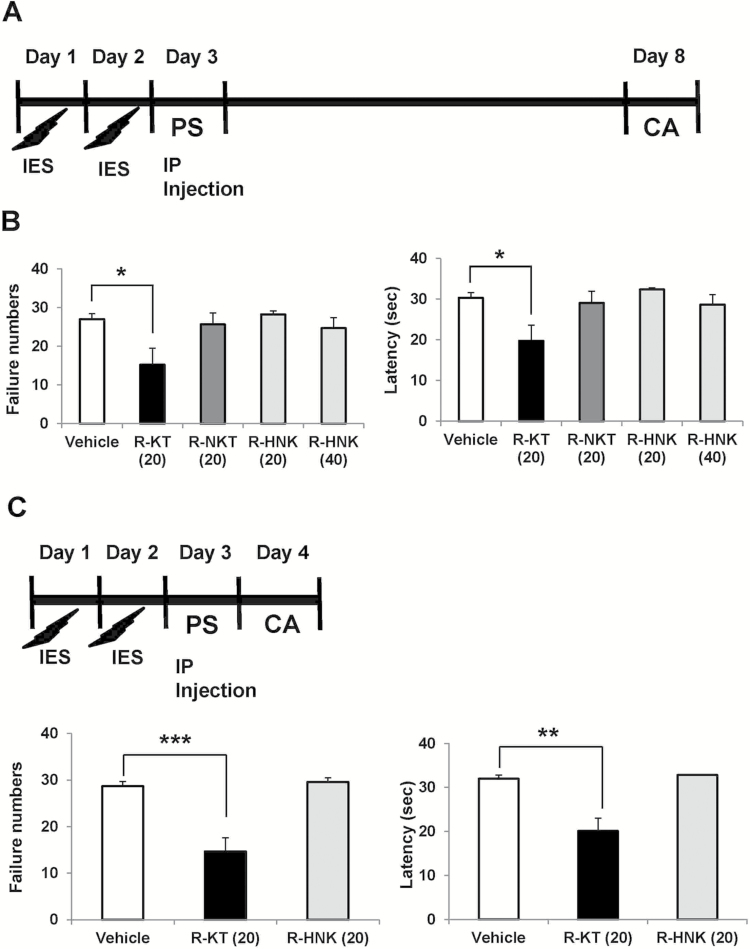
To create an LH paradigm, the animals are initially exposed to uncontrollable stress. When the animal is later placed in a situation where the shock is controllable (escapable), the animal not only fails to acquire the escape response but also often makes no efforts to escape the shock at all. The LH behavioral tests were performed using the Gemini Avoidance System (San Diego Instruments) (Shirayama et al., 2015, 2017). This apparatus is divided into 2 compartments by a retractable door. On days 1 and 2, the rats were subjected to 30 inescapable electric foot-shocks (0.65 mA, 30-second duration, administered at random intervals averaging 18–42 seconds) (Figure 2A, C). On day 3, a 2-way conditioned avoidance test was performed as a post-shock test to determine whether the rats would exhibit the predicted escape deficits (Figure 2A, C). This screening session consisted of 30 trials in which electric foot-shocks (0.65 mA, 6-second duration, administered at random intervals with a mean of 30 seconds) were preceded by a 3-second conditioned stimulus tone that remained on until the shock was terminated. Rats with more than 25 escape failures among the 30 trials were regarded as having reached the LH criterion and were used in further experiments. Approximately 65% of the rats met this criterion.
Figure 2.
Effects of a single injection of (R)-ketamine, (R)-norketamine, and (2R,6R)- HNK in a rat LH model. (A) Rats received inescapable electric shock (IES) treatments on 2 days (days 1 and 2), passed a post-shock test (PS) on day 3, and were designated as learned helplessness (LH) rats with depression-like phenotype. On day 3, vehicle (saline: 2 mL/kg), (R)-ketamine (20 mg/kg), (R)-norketamine (20 mg/kg), or (2R,6R)- HNK (20 and 40 mg/kg) was administered i.p. into LH rats. On day 8 (5 days after a single injection), conditioned avoidance (CA) test to study the antidepressant effect was performed. (B) The failure number of LH (1-way ANOVA: F4,24 = 3.755, P = .0167). The escape latency of LH (1-way ANOVA: F4,24 = 3.973, P = .013). Data are shown as mean ± SEM (n = 5–8). The number in the parenthesis is the dose (mg/kg). *P < .05 compared with vehicle-treated group. (C) Rats received IES treatments on 2 days (days 1 and 2), passed a PS on day 3, and were designated as LH rats with depression-like phenotype. On day 3, either vehicle (saline: 2 mL/kg), (R)-ketamine (20 mg/kg), or (2R,6R)- HNK (20 mg/kg) was administered i.p. into LH rats. CA test was performed on day 4 (24 hours after a single injection). (D) The failure number of LH (1-way ANOVA: F2,14 = 13.52, P < .0001). The escape latency of LH (1-way ANOVA: F2,14 = 14.73, P = .0004). Data are shown as mean ± SEM (n = 5 or 6). The number in the parenthesis is the dose (mg/kg). **P < .01, ***P < .001 compared with vehicle-treated group. R-KT: (R)-ketamine, R-NKT: (R)-norketamine, R-HNK: (2R,6R)-hydroxynorketamine.
In the experiment 1, on day 3, the rats received i.p. injection of saline (2 mL/kg), (R)-ketamine (20 mg/kg), (R)-norketamine (20 mg/kg), or (2R,6R)-HNK (20 and 40 mg/kg) (Figure 2A). On day 8 (5 days after a single injection), a 2-way conditioned avoidance test was performed (Figure 2A). In the experiment 2, on day 3, the rats received i.p. injection of saline (2 mL/kg), (R)-ketamine (20 mg/kg), or (2R,6R)-HNK (20mg/kg) (Figure 2C). On day 4 (24 hours after a single injection), a 2-way conditioned avoidance test was performed (Figure 2C). This test session consisted of 30 trials in which electric foot-shocks (0.65 mA, 30-second duration, administered at random intervals with a mean of 30 seconds) were preceded by a 3-second conditioned stimulus tone that remained on until the shock was terminated. The number of escape failures and the latency until escape for each of the 30 trials were recorded by the Gemini Avoidance System.
Statistical Analysis
The data are shown as the mean ± SEM. The analyses were performed using GraphPad Prism 5 (GraphPad Software Inc). The data were analyzed using 1-way ANOVA, followed by posthoc Tukey test. The criterion for significance was P<.05.
Results
Effects of a Single Intraperitoneal Injection of (R)-Ketamine, (R)-Norketamine, and (2R,6R)-HNK in LH Rats
Experiment 1
To examine the antidepressant effects of (R)-ketamine and its 2 metabolites in a LH model, saline (2 mL/kg), (R)-ketamine (20 mg/kg), (R)-norketamine (20 mg/kg), or (2R,6R)-HNK (20 and 40 mg/kg) was administered i.p. into the LH rats. The LH rats that received a single injection of (R)-ketamine (20 mg/kg, 5 days after a single injection) exhibited significant improvements in their conditioned avoidance test results, relative to vehicle-treated LH rats (Figure 2A,B). In contrast, a single administration of (R)-norketamine (20 mg/kg), or (2R,6R)-HNK (20 and 40 mg/kg) did not improve their conditioned avoidance test results in LH rats (Figure 2A,B).
Experiment 2
The LH rats that received a single injection of (R)-ketamine (20 mg/kg, 24 hours after a single injection) exhibited significant improvements in their conditioned avoidance test results relative to vehicle-treated LH rats (Figure 2C,D). In contrast, (2R,6R)-HNK (20 mg/kg, 24 hours after a single injection) did not improve their conditioned avoidance test results in LH rats (Figure 2C,D).
Discussion
In the present study, we established that a single systemic (R)-ketamine injection (24 hours and 5 days after a single injection) showed antidepressant effects in a rat LH model of depression, whereas a single systemic injection of (R)-norketamine or (2R,6R)-HNK did not. It should be noted that a higher dose (40 mg/kg) of (2R,6R)-HNK did not have antidepressant effects. We have also recently reported the potent and longer-lasting antidepressant effects of (R)-ketamine in a social defeat stress model of depression, although (2R,6R)-HNK did not have antidepressant effects (Yang et al., 2017). Collectively, it seems that unlike (R)-ketamine, (2R,6R)-HNK does not have an antidepressant effect in rodent models of depression, inconsistent with the findings by Zanos et al. (2016).
Zanos et al. (2016) reported more potent antidepressant effects of (2R,6R)-HNK, which is exclusively derived from (R)-ketamine. A single injection of (2R,6R)-HNK (10 or 20 mg/kg) reversed chronic corticosterone-induced anhedonia assessed with the sucrose preference and female urine sniffing behavioral tasks as well as social avoidance induced by chronic social defeat stress (Zanos et al., 2016). They reported sustained (24 hours) antidepressant effects of (2R,6R)-HNK in LH model (Zanos et al, 2016). However, we could not find sustained (24 hours) antidepressant effects of (2R,6R)-HNK (20 mg/kg) in the LH model, although (R)-ketamine (20 mg/kg) showed sustained (24 hours) antidepressant effects in the same model (Figure 2D). Thus, we were unable to detect antidepressant activity induced by (2R,6R)-HNK in any of our 3 models (inflammation, social defeat stress, and LH), although rapid and sustained antidepressant effects were detected for (R)-ketamine (Yang et al., 2017; this study). The reasons for this discrepancy (Zanos et al., 2016, vs Yang et al., 2017, and this study) remain unknown. Nonetheless, our negative findings regarding the lack of an antidepressant effect of (2R,6R)-HNK in rodents with depression-like phenotype need to be replicated by other groups in future studies.
It is well known that (R)-ketamine is stereoselectively N-demethylated by liver microsomal cytochrome P450 into (R)-norketamine (Hijazi and Boulieu, 2002; Desta et al., 2012; Zanos et al., 2016) (Figure 1). (R)-Norketamine is further metabolized to (2R,6R)-HNK arising from hydroxylation of the cyclohexanone ring (Figure 1). In addition to N-demethylation, (R)-ketamine is also metabolized by the hydroxylation of the cyclohexanone ring to produce (2R,6R)-hydroxyketamine. (2R,6R)-HNK is also prepared by the N-demethylation of (2R,6R)-hydroxyketamine (Desta et al., 2012; Zanos et al., 2016) (Figure 1). A study showed that the plasma levels of norketamine and HNK were higher after a single injection of ketamine, although plasma levels of hydroxyketamine were very low (Zanos et al., 2016), suggesting that the metabolism of (R)-ketamine to (2R,6R)-HNK via (R)-norketamine is the major pathway of (R)-ketamine in mice (Figure 1). Therefore, it is noteworthy that (R)-norketamine and (2R,6R)-HNK, the two major metabolites from (R)-ketamine, did not exert antidepressant effects, although antidepressant effects of (R)-ketamine were detected in the same model.
We have recently reported that a single bilateral infusion of (R)-ketamine into the infralimbic (IL) area of the medial prefrontal cortex (mPFC) and the DG and CA3 of the hippocampus exerted antidepressant effects in LH rats (Shirayama and Hashimoto, 2017). Furthermore, a study showed that neuronal inactivation of the IL of mPFC completely blocked the antidepressant effects of (R,S)-ketamine, and that microinfusion of (R,S)-ketamine into IL of mPFC produced an antidepressant effect in control unstressed rats (Fuchikami et al., 2015). These findings suggest a crucial role for the IL area of the mPFC, DG, and CA3 in the antidepressant action of (R)-ketamine itself (NOT metabolite) in a rat LH model, since (R)-ketamine (or (R,S)-ketamine) might not be metabolized in the brain. Given the key role of hepatic cytochrome P450 enzymes in ketamine metabolism (Turfus et al., 2009; Zhao et al., 2012; Zanos et al., 2016), it is unlikely that the metabolism of (R)-ketamine in the liver plays a role in the antidepressant actions of (R)-ketamine.
In conclusion, unlike (R)-ketamine, neither (R)-norketamine nor (2R,6R)-HNK elicited antidepressant effects in LH model, suggesting that the metabolism of (R)-ketamine might not play a key role in its robust antidepressant action.
Statement of Interest
Dr. Shirayama has received research support from Eli Lilly, Eisai, MSD, Pfizer, and Mitsubishi-Tanabe. Dr. Hashimoto has received research support from Dainippon-Sumitomo, Mochida, Otsuka, and Taisho. Dr. Hashimoto is an inventor on a filed patent application on “The use of (R)-ketamine in the treatment of psychiatric diseases” by Chiba University.
Acknowledgments
We thank Dr. Shigeyuki Chaki (Taisho Pharmaceutical Co, Ltd) for providing (2R,6R)-HNK. We also thank Yuko Fujita (Chiba University) for her technical assistance.
This study was supported by Strategic Research Program for Brain Sciences, AMED, Japan (to K.H.).
References
- Abdallah CG.(2017)What’s the buzz about hydroxynorketamine? Is it the history, the story, the debate, or the promise?Biol Psychiatry 81:61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SL, Sanghvi M, Goldberg ME, Torjman MC, Wainer IW(2012)Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica 42:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF.(2010)Taming the ketamine tiger. 1965. Anesthesiology 113:678–684 [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH(2016)Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, Aghajanian GK, Duman RS(2015)Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci USA 112:8106–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, Chaki S(2017)Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 361:9–16. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2016a) R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol Med 46:2449–2451. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2016b) Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Expert Opin Ther Targets 20:1389–1392. [DOI] [PubMed] [Google Scholar]
- Hashimoto K.(2017)Rapid antidepressant activity of ketamine beyond NMDA receptor. In: The NMDA Receptors (Hashimoto K, ed), pp 69–81. New York: Humana Press. [Google Scholar]
- Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H(2017)Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 267:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi Y, Boulieu R(2002)Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 30:853–858. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU(2016)Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46:1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Zarate C Jr(2015)Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol 30:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, APA Council of Research Task Force on Novel Biomarkers and Treatments (2015)Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K(2017)Effects of a single bilateral infusion of R-ketamine in the brain regions of a learned helplessness model of depression. Eur Arch Psychiatry Clin Neurosci 267:177–182. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Yang C, Zhang JC, Ren Q, Yao W, Hashimoto K(2015)Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur Neuropsychopharmacol 25:2449–2458. [DOI] [PubMed] [Google Scholar]
- Turfus SC, Parkin MC, Cowan DA, Halket JM, Smith NW, Braithwaite RA, Elliot SP, Steventon GB, Kicman AT(2009)Use of human microsomes and deuterated substrates: an alternative approach for the identification of novel metabolites of ketamine by mass spectrometry. Drug Metab Dispos 37:1769–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K(2015)R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Han M, Zhang JC, Ren Q, Hashimoto K(2016)Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatric Res 239:281–283. [DOI] [PubMed] [Google Scholar]
- Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K (2017) (R)-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry 82:43–44. [DOI] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K (2018) Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83:18–28. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD(2016)NMDAR-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Venkata SL, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA Jr, Mager DE, Wainer IW(2012)Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J Clin Pharmacol 74:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K(2014)R(-)-ketamine shows greater potency and longer lasting antidepressant effects than S(+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]