Abstract
Eudesmin has been proven to possess anti-inflammatory effects. In the present study, the effects of eudesmin on Helicobacter pylori (H. pylori)-mediated autophagy, apoptosis, immune response and inflammation were determined in human gastric adenocarcinoma (AGS) cells in vitro and in C57BL/6 mice in vivo. Detection of the production of interleukin (IL)-8, IL-1β and immunoglobulin M (IgM) was performed using ELISA. Identification of the activation of apoptosis-associated caspase-3, −8 and −9 proteins, Bcl-2-associated X protein (Bax) and BH3 interacting domain death agonist (Bid) protein, was determined through western blot analysis. Autophagy microtubule-associated protein 1A/1B-light chain 3, isoform B (LC-3B) expression was measured using immunostaining. The results of the present study demonstrated that eudesmin inhibited the growth of H. pylori, with increased inhibition activity against antibiotic resistant strains compared with the reference strain. In addition, H. pylori-induced IL-8 secretion, LC-3B expression and apoptosis-associated protein (caspase-3, −8 and −9, Bax and Bid) activation in AGS cells was suppressed by eudesmin. Furthermore, eudesmin suppressed IL-1β and IgM production in H. pylori-infected C57BL/6 mice in vivo. In conclusion, eudesmin may be developed as a promising therapeutic agent to prevent and/or treat H. pylori-associated gastric inflammation.
Keywords: eudesmin, Helicobacter pylori, autophagy, apoptosis, inflammation
Introduction
Helicobacter pylori (H. pylori) infection is one of the most prevalent bacterial infections worldwide, and is a primary cause of gastritis, gastroduodenal ulcers and malignancies (1,2). H. pylori adhere to the gastric mucosa, inducing the production of reactive oxygen species (ROS) which damage the epithelium (3). The response of the gastric mucosal epithelium to H. pylori infection is a multistep progression reflecting the interaction of several factors, including bacterial virulence, specific receptor-linked signaling pathways and the host immune response (4,5). The first-line therapy for H. pylori infection is antibiotics, but the increasing emergence of antibiotic-resistant H. pylori strains has led to a decline in eradication rates (6,7). Therefore, developing alternative treatments for H. pylori infection is important.
Previous studies of novel H. pylori treatments have decreased H. pylori-triggered ROS production and apoptosis but enhanced autophagy (8,9). Apoptosis and autophagy are recognized to be non-inflammatory programmed cell-death (PCD) pathways (10,11). Apoptosis and autophagy serve vital roles in tissue homeostasis and in disease development in infected patients (9,10). Apoptosis (type I PCD) includes the cell-surface death receptor pathway and the mitochondrial pathway. The Fas/CD95 receptor, Fas ligand and downstream caspase-8 initiate the process of apoptosis in the death receptor-dependent pathway. The mitochondrial pathway is characterized by an increase in mitochondrial membrane permeability and the release of cytochrome c into the cytoplasm. Cytochrome c then initiates the formation of the apoptosome, which activates caspase-9. Finally, caspase-8 or −9 activate caspase-3, thus triggering apoptosis (8–10). Autophagy is a catabolic process encompassing the pathways for intracellular macromolecule degradation (9,12). Autophagy begins with the sequestration of cytoplasmic organelles in a membrane vacuole, forming an autophagosome, which then fuses with a lysosome, where the cellular materials are degraded and recycled. Increased autophagic activity is associated with cell death, and autophagy is now considered type II PCD (9,12).
Eudesmin (Fig. 1) has previously been demonstrated to exert weak toxicity in mice and no toxicity in human macrophages (13). In immunological studies, eudesmin inhibits tumor necrosis factor (TNF)-α production and T cell proliferation (14). A previous study reported that eudesmin-induced vascular relaxation of rat aorta could be facilitated by the endothelial histamine receptor-mediated release of nitric oxide and prostanoids (15). (+)-Eudesmin can induce neurite outgrowth from PC12 cell neurons by stimulating signaling upstream of the mitogen-activated protein kinase, protein kinase C and protein kinase A pathways (16). However, there are no applicable studies on eudesmin regarding the response of epithelial cells to H. pylori infection. In the present study, the effects of eudesmin, extracted from Fatsia polycarpa Hayata, on H. pylori-induced epithelial damage, as well as H. pylori colonization in vitro [human gastric adenocarcinoma (AGS) cells] and in vivo (C57BL/6 mice) was investigated.
Figure 1.
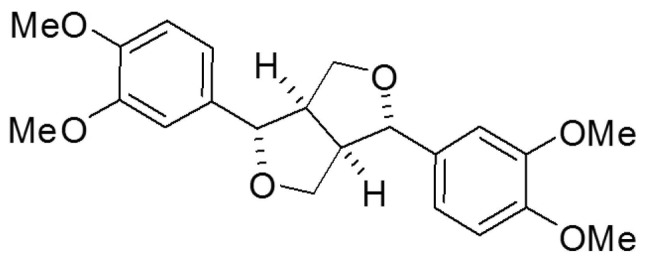
Structure of eudesmin.
Materials and methods
Isolation and identification of eudesmin
The leaves of Fatsia polycarpa Hayata were collected in November 2009 from study sites in Hehuan mountain (2105 m above sea level), Hehuanshan, Taiwan. Air-dried leaves of F. polycarpa Hayata (7 kg total) were extracted with methanol over three times, following standard extraction procedures (17). The isolated compound was identified by 1H NMR and 13C NMR spectroscopy using a Varian Inova 600 (Bruker Daltonics Inc., Billerica, MA, USA) and electrospray ionisation mass spectrometry using a Bruker Daltonics Esquire HCT (Bruker Daltonics Inc.,) as (+)-eudesmin, through comparison of spectra data in previously reported literature (18). Eudesmin was dissolved in 0.1% of dimethyl sulfoxide (DMSO) for cell culture experiments.
Bacterial strains, human cell lines and culture conditions
The H. pylori reference strain 26695 (ATCC 700392) was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). The antibiotic-resistant (metronidazole and clarithromycin) H. pylori strains V633, V1254, V1354 and V2356 were clinical isolates from previous studies (19,20). H. pylori were grown on Brucella agar (BD Biosciences, Franklin Lakes, NJ, USA) supplemented with 5% sheep blood under microaerophilic conditions at 37°C for 48–72 h. Brucella blood agar plates containing H. pylori supplement SR0147E (Thermo Fisher Scientific Oxoid Ltd., Basingstoke, UK) were used to examine the H. pylori load in infected mice under same culture condition. Salmonella enterica serovar Typhimurium (ATCC 6994), Escherichia coli (ATCC 25922) and Streptococcus aureus (ATCC 25923) were obtained from the ATCC and Pseudomonas aeruginosa (BCRC 13984) was obtained from the Bioresource Collection and Research Centre (Hsinchu, Taiwan). These bacteria were grown in Luria-Bertani medium (BD Biosciences) at 37°C for 48–72 h. Human AGS cells (CRL-1739; ATCC, Manassas, VA, USA) were purchased from ATCC and were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin.
Antimicrobial activity of eudesmin on Gram-negative and Gram-positive bacteria
The minimum inhibitory concentrations (MICs) of eudesmin were tested by a two-fold serial dilution method. Eudesmin was serially diluted with 0.1% DMSO to achieve concentrations of 500, 250, 125, 62.5, 31.25 and 15.625 µM. Equal volumes of bacterial suspension [1×106 colony forming units (CFUs)/ml] and diluted eudesmin samples were mixed and added to a 96-well plate, with an additional well containing broth only that acted as a negative control. The plate was incubated at 37°C for 24 h, following which the well containing the lowest concentration of eudesmin presenting with no visible bacterial growth was considered the MIC. The minimum bactericidal concentrations (MBCs) of eudesmin were then obtained. All samples with concentrations of eudesmin that exhibited complete inhibition of visual bacterial growth were identified and 50 µl of each culture was transferred onto a Mueller-Hinton agar plate supplemented with 5% sheep blood and incubated for 48–72 h at 37°C. The complete visual absence of bacterial colonies on the agar surface in the lowest eudesmin concentration was defined as the MBC. Each assay was repeated three times.
Scanning electron microscopy
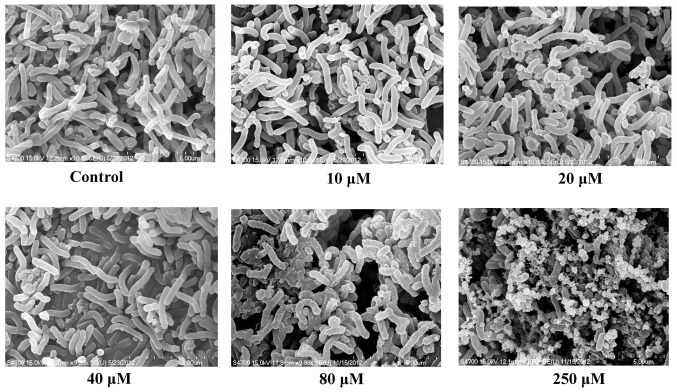
H. pylori cultures were treated with 0 (control), 10, 20, 40, 80 and 250 µM eudesmin on Brucella blood agar plates under microaerophilic conditions at 37°C for 6 h. Bacterial colonies were scraped from the plates and then washed twice in phosphate-buffered saline (PBS) and bacteria were collected by centrifugation at 15,000 × g for 10 min at room temperature. The pellets were then transferred to cover glasses and fixed using 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C for 2 h. Following rinsing with buffer, specimens were post-fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer for 1.5 h at room temperature. Specimens were subsequently dehydrated with a graded series of ethanol up to 100% ethanol. Following two exchanges of 100% acetone, specimens were critical point dried and sputter coated with gold. Specimens were then observed under a scanning electron microscope (Hitachi S-4700; Hitachi, Ltd., Tokyo, Japan) at 15 kv. Different areas (≥3) were randomly selected for image capture at magnification of ×10,000 and representative images were selected.
Cell viability assay
AGS cells were seeded into 96-well plates at a density of 1×104 cells/well and cultured in RPMI 1640 medium in an incubator containing 5% CO2 at 37°C for 18 h. Eudesmin in 0.1% DMSO at concentrations of (5, 10, 20, 40, 80, 160, 320 and 640 µM) was then added to cells and then cultured at 37°C in CO2 for 24 h. A control group, which was treated only with 0.1% DMSO, underwent the same procedures. To determine cell viability, the trypan blue exclusion test was used, where results represent the percentage of cells surviving treatment. Equal volumes of 10 µl cell suspension in PBS (pH 7.4) and trypan blue (Thermo Fisher Scientific, Inc.) were mixed. Subsequently, stained (dead) and unstained (surviving) cells were counted using a hemocytometer.
Association activity assay
AGS cells and PBS-suspended H. pylori 26695 at a multiplicity of infection (MOI) ratio of 100 were co-cultured in antibiotic-free RPMI-1640 medium supplemented with 10% FBS. The 6 concentrations of eudesmin (0, 10, 20, 40, 80 and 250 µM) were added to the culture. A control group, which was treated only with 0.1% DMSO, underwent the same procedures. Cell-associated bacteria were quantified 6 h later, following infection of the host (AGS) cells by osmotic lysis. Cell culture supernatants were removed by centrifugation at 1,500 × g for 5 min at room temperature, cells were washed with PBS twice and osmotic lysis was performed to calculate the total quantity of bacteria remaining. For this purpose, sterile water was added to the infected cells following washing, the cell lysates were re-suspended in PBS, and then plated using serial dilutions on the Brucella blood agar plates. These plates were cultured for 100 µl from each dilution at 37°C for 48 h. Bacterial cell numbers were then determined by manual colony counting. The association activity of H. pylori was determined as the mean of triplicate readings at each concentration of eudesmin. The bacteria associated with host cells included adherent and invading bacteria. The results are expressed as a percentage of the association activity of H. pylori in comparison with the control group.
Confocal fluorescence microscopy
AGS cells were grown on glass coverslips (~5×106 cells/dish) for 18 h at 37°C. H. pylori 26,695 cells were then added to cultures at an MOI ratio of 100 and grown at 37°C for 12 h. Eudesmin (10, 20, 40, 80 and 250 µM) was added to the cells, while only 0.1% DMSO was added to the control group. Cells were washed twice with PBS and fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. Cells were then washed twice in PBS and quenched with PBS (pH 7.4) containing 0.02% Triton X-100 for 30 min, prior to blocking for 30 min at room temperature in PBS with 5% non-fat dry milk. Coverslips were incubated with anti-microtubule-associated protein 1A/1B-light chain 3, isoform B (LC-3B) antibody (cat. no. NB600-1384, dilution 1:200; Novus Biologicals, LLC, Littleton, CO, USA) at room temperature for 1 h. Cells were then washed in PBS for 5 min three times and incubated with secondary anti-rabbit fluorescein isothiocyanate-labeled antibody (cat. no. NB730-F, dilution 1:10,000; Novus Biologicals, LLC) at room temperature in PBS for 1 h. Then, coverslips were washed twice in PBS and incubated with LysoTracker Red DND-99 (cat. no. L7528; Thermo Fisher Scientific, Inc.) at a dilution of 1:1,000 in PBS for 1 h, followed by incubation with 300 nM 4′,6-diamidino-2-phenylindole (cat. no. D1306; Thermo Fisher Scientific, Inc.) for 5 min at room temperature. Fluorescent signatures were then visualized using a confocal spectral microscope (Leica SP2; Leica Microsystems GmbH, Wetzlar, Germany).
Preparation of cell extracts and western blot analysis
AGS cells were seeded onto 6-well plates at a density of 5×105 cells/well for 18 h. The cells co-cultured with PBS-resuspended H. pylori at MOI of 100 were treated with varying concentrations of eudesmin (10, 20, 40, 80 and 250 µM) or 0.1% DMSO alone (control group) for 6 h in antibiotic-free RPMI 1640 supplemented with 10% FBS. Infected cells were then lysed with ice-cold lysis buffer (0.5 M Tris-HCl, pH 7.4, 10% SDS and 0.5 M dithiothreitol). Protein concentration was determined using the Bradford method (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A total of 20 µg protein samples were loaded on each lane and separated on 12% SDS-PAGE using the Hoefer miniVE system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Proteins were transferred to a Hybond-P PVDF membrane (GE Healthcare Bio-sciences) according to the manufacturer's instructions. Following the transfer, the membrane was washed with PBS and blocked for 1 h at 37°C with 5% non-fat dry milk and 0.1% Tween-20 in PBS (PBST). The primary antibodies [mouse anti-β-actin (cat. no. MAB1501; EMD Millipore, Billerica, MA, USA), rabbit anti-caspase-8 (cat. no. 25901; Abcam, Cambridge, UK), rabbit anti-BH3 interacting domain death agonist (Bid; cat. no. 2002; Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit anti-Bcl-2-associated X protein (Bax; cat. no. 2772; Cell Signaling Technology, Inc.), rabbit anti-cytochrome c (cat. no. SC-7159), and rabbit anti-caspase-9 (cat. no. SC-7885) (both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or rabbit anti-caspase-3 (cat. no. AB1899; EMD Millipore)] were added at a dilution of 1:1,000 in PBST. Blots were washed for 5 min three times in PBST and then incubated with the peroxidase-conjugated secondary antibodies [horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (cat. no. SC-2005; Santa Cruz Biotechnology, Inc.) or goat anti rabbit IgG (cat. no. 7074; Cell Signaling Technology, Inc.)] at a dilution of 1:10,000 in PBS. Following removal of the secondary antibody, blots were washed with PBST (three times, 5 min each) and then developed using a Pierce ECL-Western blotting substrate (Pierce; Thermo Fisher Scientific, Inc.). Densities of the obtained immunoblots were quantified by Kodak digital science 1D (version 2.03; Kodak, Rochester, NY, USA).
Mouse model of H. pylori infection
A total of 60 male C57BL/6 mice, obtained from the National Laboratory Animal Center (Taipei, Taiwan), weighing 20–22 g, were maintained in a pathogen-free environment and used when they reached 4 weeks of age. Mice were randomly divided into 6 groups (n=10). Mice were housed in an air-conditioned room (25±2°C) with a relative humidity of 40–70% and were subjected to a 12-h light/dark cycle. Mice had ad libitum access to tap water and a standard laboratory rodent diet. All animal-based experimental protocols were approved by the Institutional Animal Care and Use Committee of China Medical University (approval no. 101-96-N; Taichung, Taiwan) and performed according to the ethical rules and laws of China Medical University. Mice were fed a basal diet (Prolab RMH 2500, 5P14; LabDiet, St. Louis, MO, USA) for 1 week prior to use in the study. To establish H. pylori infection, all mice, apart from those in the control group, were infected with 1×109 CFU H. pylori 26695 every other day for a total of 3 doses using stomach tubes. All test samples (5, 10, 20 and 40 µM eudesmin) were dissolved in water and administered orally every day at a volume of 0.2 ml per mouse for 3 days using a stomach tube. The control group received the basal diet, without infection and administered water instead of treatment. The infection group was infected and received the basal diet but without eudesmin treatment. Stomach and blood samples were collected from all groups the day after the last treatment was administered and subsequently, the mice were humanely sacrificed by CO2 asphyxiation. Blood was taken directly from the heart via microsyringe to determine the expression of IL-1b and IgM. The stomach samples were homogenized in 1.0 ml of sterile saline, with the aid of a tissue homogenizer, at 4°C. The homogenates were then subjected to mRNA isolation using the total RNA Miniprep Purification kit (GMbiolab Co., Ltd., Taichung, Taiwan) described later on. After standing for 5 min at room temperature, supernatant of the homogenate were processed for H. pylori load.
ELISA evaluation of immune responses of H. pylori infected tissue and cells
Detection of interleukin (IL)-8 in the supernatant of H. pylori 26695 infected human AGS cells was conducted using a human IL-8 ELISA Ready-SET-Go!® kit (eBioscience, Inc., San Diego, CA, USA). IL-1β and immunoglobulin M (IgM) in H. pylori infected mice blood were measured using a mouse IL-1 β ELISA Ready-SET-Go! kit (eBioscience, Inc.) and goat anti-mouse IgM horseradish peroxidase-conjugated antibody (cat. no. A90-101P; Bethyl Laboratories, Inc., Montgomery, TX, USA), at a dilution of 1:10,000 in PBS, respectively. All kits were performed following the manual instructions. Each sample was analyzed individually. Results were calculated as the mean of triplicate readings and expressed as fold-change compared with the control group.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
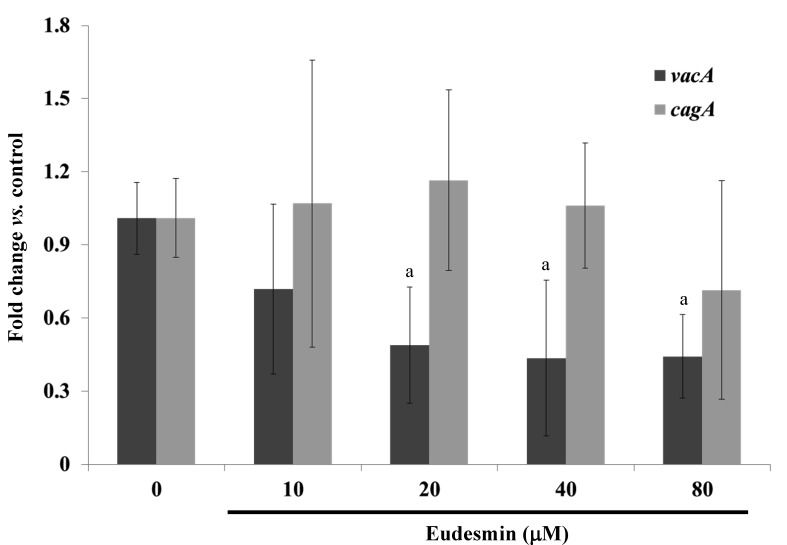
During cell culture, AGS cells and PBS-suspended H. pylori 26695 at an MOI ratio of 100 were co-cultured in antibiotic-free RPMI-1640 medium supplemented with 10% FBS. The 6 concentrations of eudesmin were added to the culture and 0.1% DMSO alone was added to the control group. Following 3 h infection, total mRNA was isolated from the AGS cells following the method previously described for detecting cytotoxin associated gene A (cagA) gene (21) and expression of the vacuolating cytotoxin A (vacA) gene (designed in this study) was also detected. For mice models of H. pylori infection, the total mRNA of stomach tissue sample homogenates was isolated using the total RNA Miniprep Purification kit (GMbiolab Co., Ltd.) and reverse transcription (RT) was performed using the Fast-Run HotStart RT-qPCR (AMV) kit (Protech Technology Enterprise Co., Ltd., Taipei, Taiwan). The kits were used following the manufacturer's instructions. The oligonucleotide primers used for RT corresponded with the murine gene sequences. All oligonucleotide primers used were synthesized by Mission Biotech Co., Ltd. (Taipei, Taiwan). RT-qPCR was performed at the following conditions: 10 min at 95°C; 40 cycles of 15 sec at 95°C; and 1 min at 60°C using 2X Power SYBR Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and 200 nM forward (F) and reverse (R) primers (vacA F, 5′-CTGGAGCCGGGAGGAAAG-3′ and R, 5′-GGCGCCATCATAAAGAGAAATTT-3′; cagA F, 5′-ATAATGCTAAATTAGACAACTTGAGCGA-3′ and R, 5′-TTAGAATAATCAACAAACATCACGCCAT-3′; 16S RNA of H. pylori F, 5′-GTGTGGGAGAGGTAGGTGGA-3′ and R, 5′-TGCGTTAGCTGCATTACTGG-3′). Each assay was run on an Applied Biosystems 7300 Real-Time PCR system (Thermo Fisher Scientific, Inc.) and the fold-changes in expression were derived using the comparative ΔΔCq method (22). 16S RNA of H. pylori served as an internal control for sample loading and mRNA integrity, as previously described (21).
Statistical analysis
The differences between the mean values of groups were evaluated by one-way analysis of variance followed by Duncan's test using SAS version 9.1 software (SAS Institute Inc., Cary, NC, USA). The results were then presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of eudesmin on H. pylori in vitro
The anti-H. pylori properties of eudesmin were tested against the reference strain 26695 and clinical isolates from H. pylori-positive patients who failed following typical antibiotic treatment in previous studies (19,20). All clinical isolate strains exhibited resistance against amoxicillin, clarithromycin and metronidazole (Table I). The MBC of eudesmin against the tested H. pylori strains are summarized in Tables I and II. Eudesmin exhibited the best bactericidal activity against antibiotic resistant strain v1254 (MBC, 2.5 µM) and strain 26695 (MBC, 10 µM). The bactericidal activity of eudesmin against Gram-negative (Pseudomonas aeruginosa, Salmonella enterica serovar Typhimurium and Escherichia coli) and Gram-positive bacteria (Streptococcus aureus) were also tested (Table II). The MBCs of eudesmin against all bacteria tested, excluding H. pylori, were >320 µM. Eudesmin exhibited a strong bacterial activity against the morphology of H. pylori in a dose-dependent manner (Fig. 2). Eudesmin at concentration of 250 µM markedly damaged the architecture of H. pylori. Furthermore, eudesmin significantly decreased the expression of vacA but not cagA, in H. pylori at concentrations ≥20 µM (P<0.05; Fig. 3). These genes are well-characterized virulence factors of H. pylori.
Table I.
Minimal bactericidal concentrations of eudesmin, amoxicillin, clarithromycin, and metronidazole against various H. pylori strains.
| Minimal bactericidal concentration of H. pylori, µM | |||||
|---|---|---|---|---|---|
| Type of antibiotic | Strain | 26695 | v633 | v1254 | v1354 |
| Eudesmin | 10 | 5 | 2.5 | 10 | |
| Amoxicillin | 0.5 | 16 | 0.25 | 2 | |
| Clarithromycin | 0.061 | 125 | 62.5 | 125 | |
| Metronidazole | 15.625 | 500 | 62.5 | 250 | |
H. pylori, Helicobacter pylori.
Table II.
Minimal bactericidal concentrations of eudesmin against different gram-negative and gram-positive bacteria.
| Minimal bactericidal concentration, µM | |||||
|---|---|---|---|---|---|
| Strain of bacteria | Helicobacter pylori | Pseudomonas aeruginosa | Salmonella enterica serovar Typhimurium | Escherichia coli | Streptococcus aureus |
| Eudesmin | 10 | >320 | >320 | >320 | >320 |
Figure 2.
Effect of eudesmin on the morphology of H. pylori. For morphological examination, H. pylori 26695 cultures were treated with 0, 10, 20, 40, 80 and 250 µM of eudesmin for 6 h. The morphological examination was determined using a scanning electron microscopy. H. pylori, Helicobacter pylori.
Figure 3.
Effect of eudesmin on H. pylori vacA and cagA mRNA expression. H. pylori 26695 cultures were treated with 0, 10, 20, 40 and 80 µM of eudesmin for 6 h. The mRNA levels of vacA and cagA were determined by reverse transcription-quantitative polymerase chain reaction. Data comparing the eudesmin-treated cells and untreated cells are presented as the mean ± SD in triplicate. aP<0.05 for vacA gene expression vs. non-treatment group. H. pylori, Helicobacter pylori.
Effect of eudesmin on H
pylori-infected human AGS cells in vitro
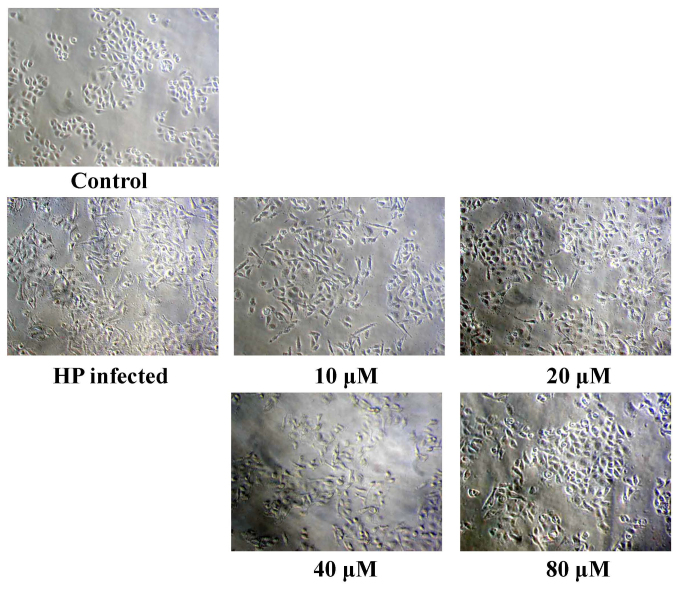
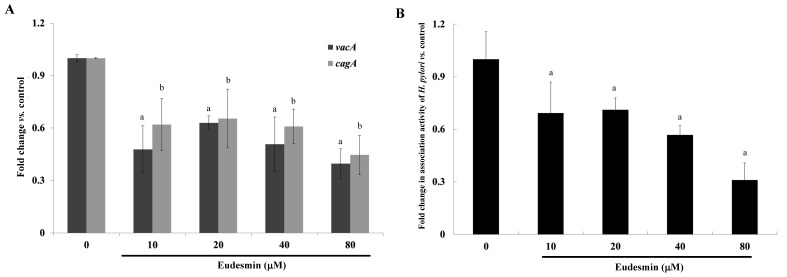
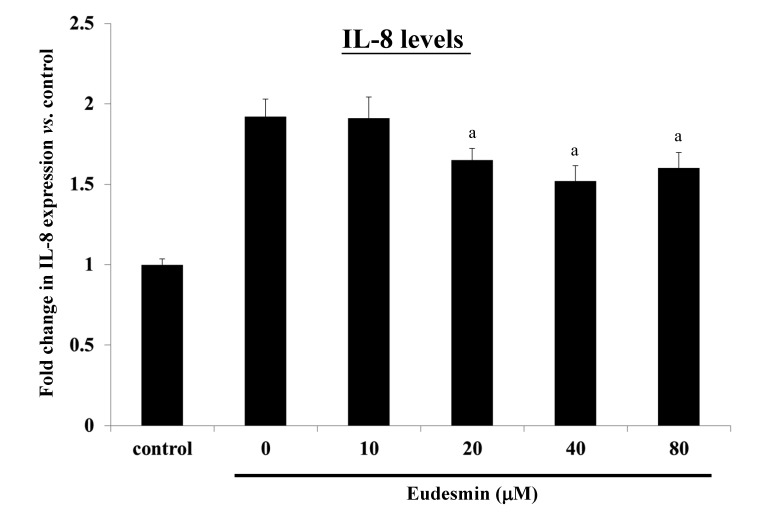
A cell viability assay was performed in order to study the cytotoxicity of eudesmin. The IC50 of eudesmin was 395 µM in AGS cells. These data suggest that eudesmin exerts weak cytotoxic activity. The results of the present study demonstrated that eudesmin reversed H. pylori-induced AGS cell morphological changes, particularly at 80 µM (Fig. 4). Eudesmin significantly decreased vacA and cagA gene expression of H. pylori-infected AGS cells compared with those of infected mice that did not receive treatment (P<0.05; Fig. 5A). In addition, at concentrations ≥10 µM, eudesmin significantly decreased the ability of H. pylori to associate with AGS cells (P<0.05; Fig. 5B). Infection with H. pylori can lead to an inflammatory response, causing increased IL-8 expression. It was observed that eudesmin significantly reduced IL-8 expression, and the inflammatory reaction, at concentrations ≥20 µM (P<0.05; Fig. 6).
Figure 4.
Effect of eudesmin on the morphology in H. pylori-infected AGS cells. For morphological examination, H. pylori 26695-infected AGS cells were treated with 0, 10, 20, 40, 80 and 250 µM of eudesmin for 6 h. HP, Helicobacter pylori.
Figure 5.
Effect of eudesmin on H. pylori vacA and cagA expression in and bacterial association to AGS cells. (A) Effect of eudesmin on vacA and cagA mRNA expression in H. pylori 26695-infected AGS cells. H. pylori-infected AGS cells were treated with 0, 10, 20, 40 and 80 µM eudesmin for 6 h. Total mRNA levels of vacA and cagA were determined by reverse transcription-quantitative polymerase chain reaction. aP<0.05 for vacA gene expression vs. non-treatment group. bP<0.05 for cagA gene expression vs. non-treatment group. (B) Effect of eudesmin on H. pylori 26695 association to AGS cells. H. pylori-infected AGS cells were treated with 0, 10, 20, 40 and 80 µM of eudesmin for 6 h. The association ability of H. pylori was determined by bacterial cell counting. Data comparing the eudesmin-treated samples and untreated samples are presented as the mean ± standard deviation in triplicate. aP<0.05 vs. untreated group. H. pylori, Helicobacter pylori.
Figure 6.
Effect of eudesmin on IL-8 production of H. pylori-infected AGS cells. H. pylori 26695-infected AGS cells were treated with 0, 10, 20, 40 and 80 µM of eudesmin for 6 h. IL-8 production by the AGS cells was determined by ELISA. Data comparing the eudesmin-treated cells and untreated cells are presented as the mean ± standard deviation in triplicate. aP<0.05 vs. untreated group. IL, interleukin; H. pylori, Helicobacter pylori.
Effect of eudesmin on autophagy and apoptosis in H. pylori-infected AGS cells in vitro
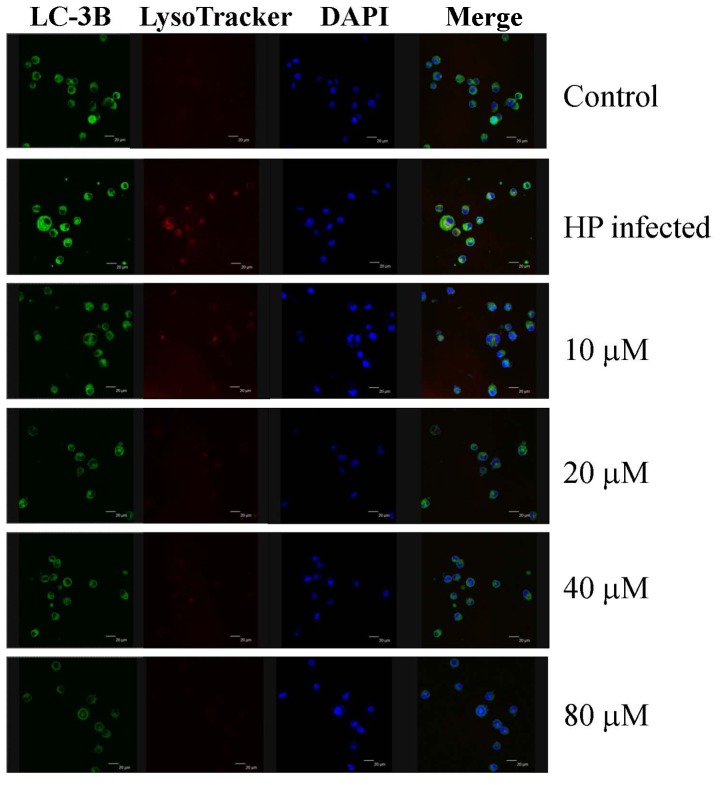
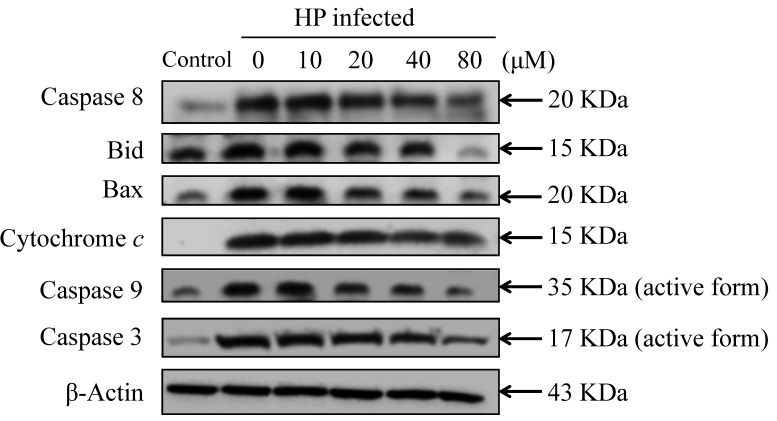
The present study investigated the effect of eudesmin on programmed cell death, through autophagy or apoptosis, in H. pylori-infected AGS cells. Eudesmin treatment between 20 and 80 µM notably decreased autophagy-associated LC-3B protein levels (Fig. 7). Treatment with 20–80 µM eudesmin inhibited the expression of apoptosis-associated caspase-8, Bid, Bax, cytochrome c, caspase-9 and −3 protein (Fig. 8). These results suggest that eudesmin inhibits autophagy and apoptosis in H. pylori-infected AGS cells.
Figure 7.
Effect of eudesmin on LC-3B expression in H. pylori-infected AGS cells. H. pylori 26695-infected AGS cells were treated with 0, 10, 20, 40 and 80 µM of eudesmin for 12 h. Cells were fixed, then stained with LC3-B antibody, and LysoTracker and DAPI dyes. LC-3B protein expression was detected by immunostaining and visualized under a confocal microscope. LC-3B, microtubule-associated protein 1A/1B-light chain 3, isoform B; DAPI, 4′,6-diamidino-2-phenylindole; HP, Helicobacter pylori.
Figure 8.
Effect of eudesmin on apoptosis-associated protein levels in H. pylori-infected AGS cells. H. pylori 26695 infected AGS cells were treated with 0, 10, 20, 40 and 80 µM of eudesmin for 6 h, then subjected to western blotting. Western blotting of caspase-8, Bid, Bax, cytochrome c, caspase-9, and −3 protein levels was performed. The β-actin was used as an internal control for equivalent protein loading. HP, Helicobacter pylori.
Effect of eudesmin on a mice model of H. pylori infection
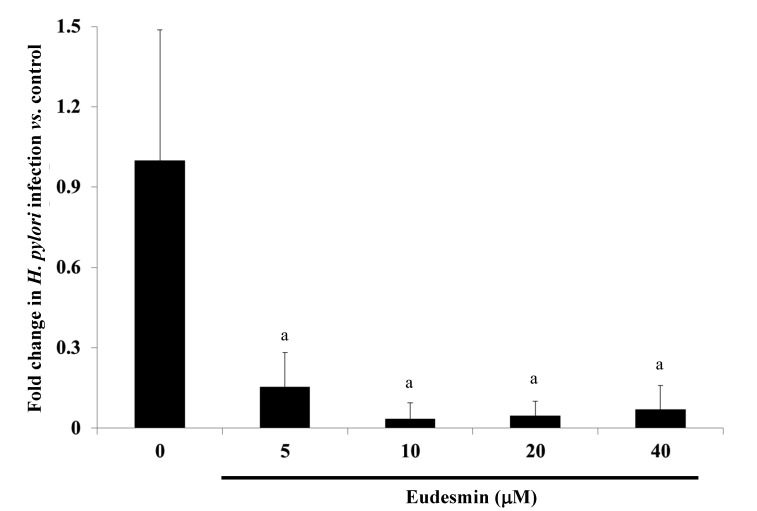
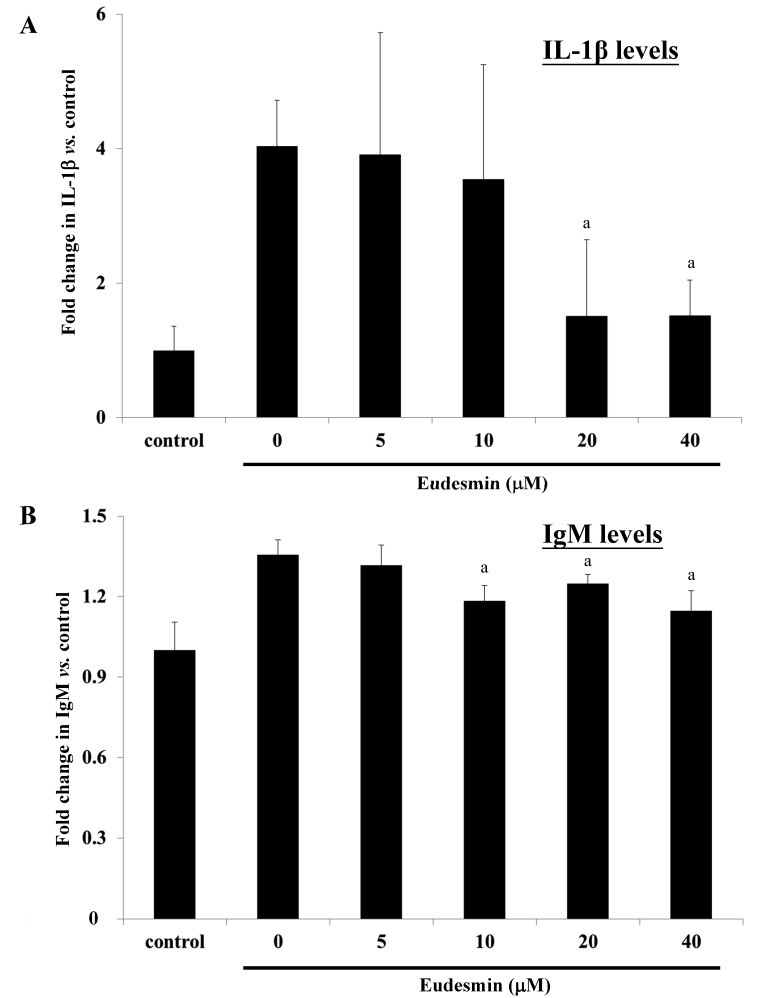
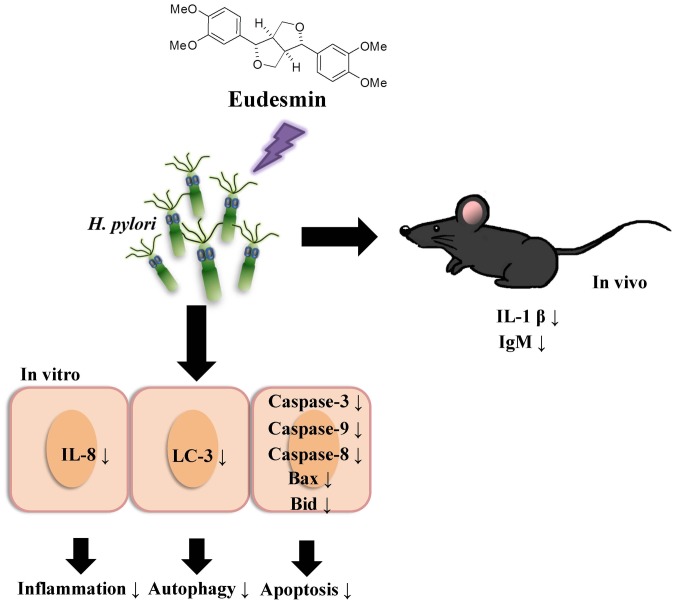
To study the effects of eudesmin in vivo, eudesmin (5, 10, 20 and 40 µM) was used to treat H. pylori-infected mice for 3 days. Results from the present study identified that the lowest dose (5 µM) of eudesmin was sufficient to significantly decrease H. pylori-load in the stomach tissue of infected mice (P<0.05; Fig. 9). In addition, serum levels of IL-1β (Fig. 10A) and IgM (Fig. 10B) in H. pylori-infected mice were significantly suppressed at concentrations of 20 and 40 µM eudesmin, respectively (P<0.05). These data suggest that eudesmin reduces inflammatory and immune responses to H. pylori infection. To summarize the aforementioned results from the in vivo and in vitro studies (Fig. 11), eudesmin may reduce the virulence of H. pylori and also suppress H. pylori induced inflammation, autophagy and apoptosis.
Figure 9.
Effect of eudesmin on H. pylori load from infected gastric tissues. H. pylori 26695-infected C57BL/6 mice were treated with 0, 5, 10, 20 and 40 µM eudesmin for 3 days. The mRNA level of H. pylori 16r RNA was determined by reverse transcription-quantitative polymerase chain reaction. Data comparing the eudesmin-treated and untreated control mice are presented as the mean ± standard deviation in triplicate. aP<0.05 vs. untreated group. H. pylori, Helicobacter pylori.
Figure 10.
Effect of eudesmin on (A) IL-1β expression and (B) IgM expression in H. pylori-infected C57BL/6 mice in vivo. H. pylori 26695-infected C57BL/6 mice were treated with 0, 5, 10, 20 and 40 µM of eudesmin for 3 days. The level of IL-1β and IgM expression was determined by ELISA. Data comparing the eudesmin-treated and non-treated mice are presented as the mean ± standard deviation in triplicate. aP<0.05 vs. non-treatment group. IL, interleukin; IgM, immunoglobulin M.
Figure 11.
Proposed mechanism by which eudesmin may suppress H. pylori-triggered inflammation, autophagy and apoptosis. H. pylori, Helicobacter pylori.
Discussion
It has previously been demonstrated that eudesmin can be isolated from a number of different plants, including Apiaceae, Rutaceae, Ochnaceae and Magnoliaceae (17,18). The present study, to the best of our knowledge, is the first to describe the isolation of eudesmin from Fatsia polycarpa Hayata. Numerous previous studies have identified the biological functions of eudesmin, including that its cytotoxic, anti-bacterial, anti-fungal and inhibitory effects on TNF-α production (13–16). However, the effects of eudesmin on H. pylori infection have not been tested previously. The present study, to the best of our knowledge, is the first to investigate the effects of eudesmin on H. pylori-infected AGS cells in vitro and to study the possible mechanisms involved in eudesmin's anti-bacterial activity.
In the present study, the lowest MBC of eudesmin was 2.5 µM against the antibiotic resistant strain v1254 of H. pylori. However, the IC50 of eudesmin was 395 mM in AGS cells and the MBC of eudesmin against other common Gram-negative and Gram-positive bacteria was >320 µM. These results suggest that eudesmin is specifically bactericidal against H. pylori but has low cytotoxicity. At a concentration of 250 µM, eudesmin could abolish the morphological structure of H. pylori under visualized under a scanning electron microscope. The mechanisms underlying the destruction of the morphology and bactericidal activity against H. pylori by eudesmin should be further investigated. Following the treatment of H. pylori 26695 with eudesmin, expression of vacA, but not cagA, was suppressed. The protease VacA is a well-known virulence factor of H. pylori, which causes infected cells to undergo apoptosis (23,24). In addition, VacA serves a role in autophagy in infected cells (24). Interestingly, in H. pylori-infected human AGS cells, eudesmin interfered with vacA and cagA gene expression. CagA protein is delivered into the host cell by a type IV secretion system of H. pylori, where it induces the expression of proinflammatory cytokines (25). Therefore, data from the present study indicates that eudesmin can cause damage to H. pylori bacteria and interfere with its expression of certain virulence factors while infection occurs.
It has previously been reported that H. pylori can induce autophagy in gastric epithelial cells and professional phagocytes (10,11). Deen et al (26) reported that H. pylori infection can induce canonical autophagy in macrophages. In addition, H. pylori has been reported to induce gastric epithelial cell apoptosis through the activation of the cell-surface death receptor pathway and the mitochondrial pathway (9,10). These pathways activate caspase-3 to initiate apoptosis. Furthermore, it has been observed that the VacA protein of H. pylori is involved in inducing apoptosis and autophagy in gastric epithelial cells during infection (12). Since eudesmin interferes with the expression of VacA, the positive outcome of eudesmin treatment in H. pylori infection models may be due to the fact that the virulence of H. pylori is attenuated. In addition, the present study identified that eudesmin decreased the expression of proteins involved in apoptosis and autophagy of H. pylori-infected AGS cells, such as LC-3B, caspase-3, caspase-9, caspase-8, Bax, and Bid, suggesting that it suppresses H. pylori-induced apoptosis and autophagy. Increased apoptosis is associated with the development of gastric carcinoma. Thus, eudesmin may prevent the development gastric carcinoma in H. pylori-infected individuals.
H. pylori infection may result in gastritis (1,2). The pathogenesis of gastritis involves the host cell's inflammatory response. Inflammation is the primary host response against microbial infections (4,5). Multiple pathways are involved that mediate the activation of caspases, which subsequently induce the secretion of pro-inflammatory cytokines, such as IL-1β and IL-8 (8). IL-1β is an important pro-inflammatory cytokine that is a powerful inhibitor of gastric acid secretion (27). It has been demonstrated that the expression of IL-1β in gastric mucosa is upregulated following H. pylori infection (8) and IL-1β may serve a central role in the initiation of the inflammatory response to infection. Previous studies have reported that H. pylori induces the expression of caspase-1 and IL-1β in macrophages and dendritic cells (4,8). The present study determined the effects of eudesmin on H. pylori-mediated inflammation in the AGS human gastric adenocarcinoma epithelial cell line. Eudesmin treatment efficiently reduced IL-8 expression by AGS cells in response to H. pylori infection. It was also observed that eudesmin decreased IL-1β expression in a mouse model of H. pylori infection.
The results of the present study indicate a proposed mechanism by which eudesmin suppresses H. pylori-triggered inflammation, autophagy and apoptosis. The results of the present study suggest that H. pylori infection induces inflammation in AGS cells, which results in an upregulation of IL-8 and IL-1β in vitro and in vivo. In conclusion, the present study demonstrates that the administration of eudesmin efficiently eradicates H. pylori and attenuates H. pylori-induced epithelial cell death through autophagy and apoptosis. The high efficacy of the eudesmin treatment observed makes eudesmin a promising novel non-antibiotic therapy of H. pylori infection.
Acknowledgements
The present study was supported by the Department of Chinese Medicine and Pharmacy, Ministry of Health and Welfare (CCMP99-RD-208), Ministry of Science and Technology, Taiwan NSC-101-2621-B-039-003, China Medical University (CMU103-S-05) and Yen Tjing Ling Medical Foundation (CI-103-21).
References
- 1.de Bernard M, Josenhans C. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2014;19(Suppl 1):S11–S18. doi: 10.1111/hel.12160. [DOI] [PubMed] [Google Scholar]
- 2.Kountouras J, Zavos C, Gavalas E, Tzilves D. Challenge in the pathogenesis of autoimmune pancreatitis: Potential role of Helicobacter pylori infection via molecular mimicry. Gastroenterology. 2007;133:368–369. doi: 10.1053/j.gastro.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 3.Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, Patel J, Dirden-Kramer B, Boldogh I, Ernst PB, Crowe SE. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun. 2007;75:4030–4039. doi: 10.1128/IAI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clevers HC, Bevins CL. Paneth cells: Maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 5.Peek RM, Jr, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Federico A, Gravina AG, Miranda A, Loguercio C, Romano M. Eradication of Helicobacter pylori infection: Which regimen first? World J Gastroenterol. 2014;20:665–672. doi: 10.3748/wjg.v20.i3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam SY, Park BJ, Ryu KH, Nam JH. Effect of Helicobacter pylori infection and its eradication on the fate of gastric polyps. Eur J Gastroenterol Hepatol. 2016;28:449–454. doi: 10.1097/MEG.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Yang HC, Shun CT, Wang TH, Chien CT, Kao JY. Catechins and sialic acid attenuate Helicobacter pylori-triggered epithelial caspase-1 activity and eradicate Helicobacter pylori infection. Evid Based Complement Alternat Med. 2013;2013:248585. doi: 10.1155/2013/248585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castano-Rodriguez N, Kaakoush NO, Goh KL, Fock KM, Mitchell HM. Autophagy in Helicobacter pylori infection and related gastric cancer. Helicobacter. 2015;20:353–369. doi: 10.1111/hel.12211. [DOI] [PubMed] [Google Scholar]
- 10.Labbé K, Saleh M. Cell death in the host response to infection. Cell Death Differ. 2008;15:1339–1349. doi: 10.1038/cdd.2008.91. [DOI] [PubMed] [Google Scholar]
- 11.Liu WH, Liu TC, Mong MC. Antibacterial effects and action modes of asiatic acid. Biomedicine (Taipei) 2015;5:16. doi: 10.7603/s40681-015-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raju D, Hussey S, Ang M, Terebiznik MR, Sibony M, Galindo-Mata E, Gupta V, Blanke SR, Delgado A, Romero-Gallo J, et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160–1171. doi: 10.1053/j.gastro.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Song Z, Liao DG, Zhang TY, Liu F, Zhuang K, Luo K, Yang L, He J, Lei JP. Anticonvulsant and sedative effects of eudesmin isolated from Acorus tatarinowii on mice and rats. Phytother Res. 2015;29:996–1003. doi: 10.1002/ptr.5337. [DOI] [PubMed] [Google Scholar]
- 14.Cho JY, Yoo ES, Baik KU, Park MH. Eudesmin inhibits tumor necrosis factor-alpha production and T cell proliferation. Arch Pharm Res. 1999;22:348–353. doi: 10.1007/BF02979056. [DOI] [PubMed] [Google Scholar]
- 15.Raimundo JM, Trindade AP, Velozo LS, Kaplan MA, Sudo RT, Zapata-Sudo G. The lignan eudesmin extracted from Piper truncatum induced vascular relaxation via activation of endothelial histamine H1 receptors. Eur J Pharmacol. 2009;606:150–154. doi: 10.1016/j.ejphar.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Yang YJ, Park JI, Lee HJ, Seo SM, Lee OK, Choi DH, Paik KH, Lee MK. Effects of (+)-eudesmin from the stem bark of magnolia kobus DC. var. borealis Sarg. on neurite outgrowth in PC12 cells. Arch Pharm Res. 2006;29:1114–1118. doi: 10.1007/BF02969301. [DOI] [PubMed] [Google Scholar]
- 17.Wang CM, Chen HT, Li TC, Weng JH, Jhan YL, Lin SX, Chou CH. The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. J Chem Ecol. 2014;40:90–98. doi: 10.1007/s10886-013-0376-y. [DOI] [PubMed] [Google Scholar]
- 18.Batista AND, Batista JM, Lopez SN, Furlan M, Cavalheiro AJ, Silva DHS, Bolzani VDS, Nunomura M, Yoshida M. Aromatic compounds from three Brazilian Lauraceae species. Quim Nova. 2010;33:321–323. doi: 10.1590/S0100-40422010000200017. [DOI] [Google Scholar]
- 19.Poon SK, Chang CS, Su J, Lai CH, Yang CC, Chen GH, Wang WC. Primary resistance to antibiotics and its clinical impact on the efficacy of Helicobacter pylori lansoprazole-based triple therapies. Aliment Pharmacol Ther. 2002;16:291–296. doi: 10.1046/j.1365-2036.2002.01184.x. [DOI] [PubMed] [Google Scholar]
- 20.Lai CH, Kuo CH, Chen PY, Poon SK, Chang CS, Wang WC. Association of antibiotic resistance and higher internalization activity in resistant Helicobacter pylori isolates. J Antimicrob Chemother. 2006;57:466–471. doi: 10.1093/jac/dki479. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Kelly LK, Ayub K, Graham DY, Go MF. Genotypes of Helicobacter pylori obtained from gastric ulcer patients taking or not taking NSAIDs. Am J Gastroenterol. 1999;94:1502–1507. doi: 10.1111/j.1572-0241.1999.01133.x. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.da Costa DM, Pereira Edos S, Rabenhorst SH. What exists beyond cagA and vacA? Helicobacter pylori genes in gastric diseases. World J Gastroenterol. 2015;21:10563–10572. doi: 10.3748/wjg.v21.i37.10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres J, Backert S. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2008;13(Suppl 1):S13–S17. doi: 10.1111/j.1523-5378.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 25.Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20:12767–12780. doi: 10.3748/wjg.v20.i36.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deen NS, Huang SJ, Gong L, Kwok T, Devenish RJ. The impact of autophagic processes on the intracellular fate of Helicobacter pylori: More tricks from an enigmatic pathogen? Autophagy. 2013;9:639–652. doi: 10.4161/auto.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]