Abstract
The aim of the present study was to investigate the value of the albumin-bilirubin (ALBI) score in the assessment of the disease conditions of hepatitis B virus (HBV)-related acute-on-chronic liver failure (HBV-ACLF), HBV-related liver cirrhosis (HBV-LC) and HBV-related hepatocellular carcinoma (HBV-HCC). A total of 395 patients with HBV-ACLF, HBV-LC, or HBV-HCC were retrospectively studied. The ALBI, Child-Turcotte-Pugh (CTP), and Model for End-Stage Liver Disease (MELD) scores of the patients were calculated, and the relationships between the ALBI score and the CTP and MELD scores were investigated. Furthermore, the ALBI grading was tested for the evaluation of the severity and stages of HBV-ACLF, HBV-LC, and HBV-HCC, especially when classifying the clinical stages of HBV-ACLF. The mean ALBI scores of the HBV-ACLF, HBV-LC, and HBV-HCC patients were −1.17±0.55, −1.76±0.66 and −2.59±0.62, respectively; the mean CTP scores were 10.70±1.81, 8.19±1.25 and 5.81±1.22, respectively; and the mean MELD scores were 19.93±7.44, 11.10±4.39 and 7.01±3.22, respectively. The ALBI scores were positively correlated with the CTP and MELD scores. The mean ALBI score and the frequency of grade 3 disease were higher in HBV-ACLF patients than in patients with HBV-LC or HBV-HCC. A later HBV-ACLF stage resulted in a higher frequency of ALBI grades of 3. In conclusion, ALBI scores exhibited parallel tendencies to the CTP and MELD scores in HBV-ACLF, HBV-LC, and HBV-HCC patients; thus, ALBI grading may be a simple but applicable method for the evaluation of the functional status of patients with HBV-related end-stage liver diseases.
Keywords: albumin-bilirubin score, hepatitis B virus, acute-on-chronic liver failure, liver cirrhosis, hepatocellular carcinoma
Introduction
Hepatitis B virus (HBV) infection is a worldwide epidemic. According to the World Health Organization (WHO), ~2 billion people worldwide have been infected with HBV, of whom 240 million have chronic HBV infection (1). In total, ~650,000 people succumb to mortality as a result of liver failure (LF), liver cirrhosis (LC) or hepatocellular carcinoma (HCC) due to HBV infection each year (2). China has a high prevalence of HBV infection, with ~93 million patients with hepatitis B, and 60% of LC cases and 80% of HCC cases caused by HBV infection (3). HBV-related acute-on-chronic LF (HBV-ACLF), HBV-related LC (HBV-LC), and HBV-related HCC (HBV-HCC) represent severe end-stage liver diseases with rapid progression and extremely high mortality (4). Early identification and precise evaluation of the severity and stages of these diseases may facilitate timely and proper clinical treatment, thereby reducing mortality.
Multiple scoring systems are available for assessing liver function and the severity of liver injury, including the the Child-Turcotte-Pugh (CTP) score; the Model for End-Stage Liver Disease (MELD) score; and the MELD-sodium (MELD-Na) and integrated MELD (iMELD) scores, which were established based on the original MELD score (5–7). The CTP and MELD scoring systems are most commonly used; however, these scoring systems require multiple variants and have certain limitations. The CTP scoring system, with a total of 15 points, has a relatively narrow grading range, and its assessment indicators of ascites and hepatic encephalopathy are strongly subjective, lacking objective quantification (8). The indicators of the MELD scoring system are all objective, and the inclusion of creatinine (Cr) reflects the effect of complicating hepatorenal syndrome on the prognosis of LC patients to a certain extent (9). However, the MELD score is typically significantly affected by large variations in the international normalized ratio (INR) due to measurement by different laboratories with different methods (10). Moreover, the MELD scoring system does not take the effects of basic disease history, age, bleeding, ascites, bacterial infection, or hepatopulmonary syndrome, among other factors, into account when assessing disease outcomes, and the effects of different treatment regimens on outcomes is not considered either (11).
The albumin (Alb)-bilirubin (ALBI) score is a newly developed, simple, and objective scoring system for assessing the severity of liver function damage via only two indicators: bilirubin and Alb. The ALBI score may be used to evaluate the liver function damage and prognosis of patients with liver cancer (12,13). This score has been reported to have predictive value for in-hospital mortality in patients with primary biliary cirrhosis or LC combined with upper gastrointestinal bleeding (14,15). Few studies on the value of the ALBI score in assessing the conditions of liver function damage in various HBV-related liver diseases have been performed (16,17). The present study retrospectively analyzed data from patients with HBV-ACLF, HBV-LC or HBV-HCC treated in the Department of Infectious Diseases of Taihe Hospital (Shiyan, China) and evaluated the value of the ALBI score in assessing liver function damage occurring in these HBV-related end-stage liver diseases.
Materials and methods
Patients
Patients with HBV-related end-stage liver diseases admitted to the Department of Infectious Diseases of Taihe Hospital between November 2013 and October 2016 were enrolled in the present retrospective study. In total, there were 138 HBV-ACLF patients (age, 45.80±11.01 years; male:female, 111:27), 130 HBV-LC patients (age, 49.00±11.13 years; male:female, 86:44), and 127 HBV-HCC patients (age, 53.61±10.45 years; male:female, 118:9). The present study conformed to ethical requirements and was approved by the Ethics Committee of Taihe Hospital.
Inclusion and exclusion criteria
The inclusion criteria were as follows: The diagnostic criteria for hepatitis B were based on ‘The Guideline of Prevention and Treatment for Chronic Hepatitis B: A 2015 Update’ (18). The ACLF diagnostic criteria were based on the ‘Diagnostic and Treatment Guidelines for Liver Failure (2012 version)’ (19). Due to the chronic liver disease, acute or subacute clinical liver function, decompensation syndrome occurred in the short term and manifested as: i) Extreme weakness with obvious gastrointestinal symptoms; ii) rapid jaundice, serum total bilirubin (TBil) levels >10 times the upper limit of normal [defined as 0–17.1 µmol/l (18)], or a daily increase ≥17.1 µmol/l; iii) bleeding tendency, prothrombin activity (PTA) ≤40% (or INR ≥1.5) with exclusion of other causes; iv) decompensated ascites; and v) with or without hepatic encephalopathy. Patients with HBV-ACLF were divided into early, middle, and late stages upon admission based on the previously mentioned guidelines. Early stage was diagnosed as follows: i) Extreme fatigue with serious gastrointestinal symptoms such as obvious anorexia, vomiting and abdominal distension; ii) progressively increasing jaundice (serum TBil ≥171 µmol/l or a daily increase ≥17.1 µmol/l); iii) bleeding tendency, 30% <PTA ≤40% (or 1.5 <INR ≤1.9); and iv) no hepatic encephalopathy or other complications. Middle stage was diagnosed as liver failure aggravated beyond that of the early stage with one of the following two conditions: i) Hepatic encephalopathy below degree II and/or obvious ascites and infection; and ii) bleeding tendency (bleeding or blood stasis), 20% <PTA ≤30% (or 1.9 <INR ≤2.6). Late stage was diagnosed when the disease was further aggravated beyond that of the middle stage with a severe bleeding tendency, PTA ≤20% (or INR >2.6), and at least one of the following four symptoms was observed: Hepatorenal syndrome, upper gastrointestinal bleeding, severe infection or degree II hepatic encephalopathy.
The exclusion criteria were as follows: Patients with viral hepatitis other than hepatitis B, alcoholic liver disease, autoimmune liver disease, or drug-induced liver injury; patients with other concomitant severe primary diseases of the heart, lung, or other organs; patients with metastatic HCC; and patients with primary carcinomas in other organs.
Additionally, patients with concurrent liver tumors or tumors in other organs were excluded from the HBV-ACLF group, patients with concomitant liver tumors or liver failure were excluded from the HBV-LC group and patients with liver failure were excluded from the HBV-HCC group.
Data collection
The parameters of TBil, Alb, Cr, prothrombin time (PT) and INR required for ALBI, CTP, and MELD scoring were all measured in the Department of Laboratory Tests at Taihe Hospital within 24 h following admission. The ascites conditions were also evaluated by imaging examinations within 24 h following admission. Data were retrieved from the electronic medical record system of Taihe Hospital and then validated via the clinical test data system and imaging system of the hospital.
ALBI, CTP, and MELD scoring criteria
The ALBI score was calculated as: ALBI = [Log10TBil (µmol/l) × 0.66] + [Alb (g/l) x-0.085], wherein the ALBI grades included grades 1 (ALBI score ≤-2.6), 2 (−2.59 <ALBI score <-1.39), and 3 (ALBI score ≥-1.39) (20).
The CTP score was the sum of the scores of five items (ascites, hepatic encephalopathy, TBil, Alb, and PT extension time). Each item consisted of 1–3 points, for a total maximum score of 15 points (21,22).
The MELD score was calculated as follows: MELD=3.8 × loge [TBil (mg/dl)] + 11.2 × loge (INR) + 9.6 × loge [Cr(mg/dl)] + 6.4 × etiology. Cholestasis and alcoholic liver disease had a value of 0 and other etiologies had a value of 1 (23).
Statistical analysis
The statistical analysis was performed with GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as the mean ± standard deviation. Multiple sample means of the measurement data were compared using one-way analysis of variance, and Scheffe's post hoc tests when appropriate. Pairwise comparisons were conducted using a Student's t-test and count data were compared using the chi-squared test. Linear regression analysis was used to compare the correlation between ALBI score and CTP or MELD scores. P<0.05 was considered to indicate a statistically significant difference.
Results
HBV-ACLF patients exhibit higher ALBI, CTP, and MELD scores than patients with HBV-LC or HBV-HCC
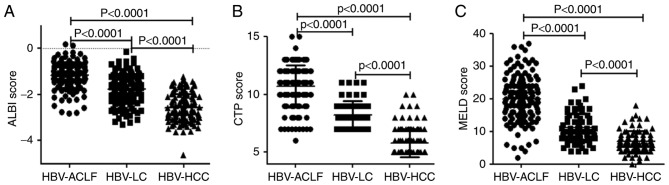
The mean ALBI scores of the HBV-ACLF, HBV-LC, and HBV-HCC patients were −1.17±0.55, −1.76±0.66 and −2.59±0.62, respectively. Additionally, the respective mean CTP scores were 10.70±1.81, 8.19±1.25 and 5.81±1.22, and the respective mean MELD scores were 19.93±7.44, 11.10±4.39 and 7.01±3.22. The HBV-ACLF patients had the highest scores for all three scoring systems and predominantly exhibited an ALBI grade of 3, whereas the lowest scores for all three scoring systems were demonstrated by HBV-HCC patients, who mainly exhibited an ALBI grade of 1. Intergroup comparisons of the ALBI, CTP, and MELD scores indicated statistically significant differences between all groups (P<0.0001; Fig. 1; Table I).
Figure 1.
Comparison of the scoring systems among HBV-ACLF, HBV-LC, and HBV-HCC patients. Comparison of the (A) ALBI score, (B) CTP score and (C) MELD score. Data are presented as the mean ± standard deviation. HBV, hepatitis B virus; HBV-ACLF, HBV-related acute-on-chronic liver failure; HBV-LC, HBV-related liver cirrhosis; HBV-HCC, HBV-related hepatocellular carcinoma; ALBI, albumin-bilirubin; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease.
Table I.
The ALBI grades of patients with HBV-ACLF, HBV-LC, and HBV-HCC.
| Grade 1 | Grade 2 | Grade 3 | |||||
|---|---|---|---|---|---|---|---|
| Group | Patients (n) | Patients (n) | Constituent frequency (%) | Patients (n) | Constituent frequency (%) | Patients (n) | Constituent frequency (%) |
| HBV-ACLF | 138 | 3 | 2.17 | 35 | 25.36 | 100 | 72.46 |
| HBV-LC | 130 | 20 | 15.38 | 71 | 54.62 | 39 | 30.00 |
| HBV-HCC | 127 | 72 | 56.69 | 54 | 42.52 | 1 | 0.79 |
ALBI, albumin-bilirubin; HBV, hepatitis B virus; HBV-ACLF, HBV-related acute-on-chronic liver failure; HBV-LC, HBV-related liver cirrhosis; HBV-HCC, HBV-related hepatocellular carcinoma.
ALBI scores are positively correlated with MELD scores
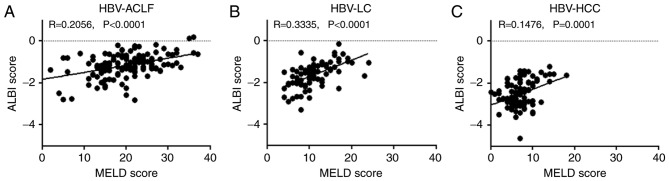
A positive correlation was observed between the ALBI score and the MELD score for HBV-ACLF, HBV-LC, and HBV-HCC patients, with correlation coefficients of 0.2056, 0.3335 and 0.1476, respectively (P<0.01; Fig. 2).
Figure 2.
Association between the ALBI score and the MELD score. Associations between ALBI scores and MELD scores in patients with (A) HBV-ACLF, (B) HBV-LC and (C) HBV-HCC. HBV, hepatitis B virus; HBV-ACLF, HBV-related acute-on-chronic liver failure; HBV-LC, HBV-related liver cirrhosis; HBV-HCC, HBV-related hepatocellular carcinoma; ALBI, albumin-bilirubin; MELD, model for end-stage liver disease.
ALBI scores are positively correlated with CTP scores
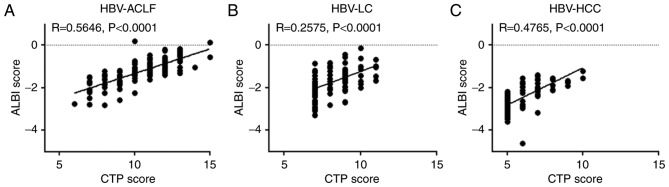
A positive correlation was also observed between the ALBI and CTP scores of HBV-ACLF, HBV-LC, and HBV-HCC patients, with correlation coefficients of 0.5646, 0.2575 and 0.4765, respectively (P<0.0001; Fig. 3). Furthermore, the ALBI grading of HBV-ACLF patients was more complex than CTP grading. The only patient with a CTP grade of A exhibited an ALBI grade of 1, and the majority of patients with a CTP grade of C had an ALBI grade of 3, whereas patients with a CTP grade of B had varying ALBI grades (Table II).
Figure 3.
Association between the ALBI and CTP scores. Associations between ALBI scores and CTP scores in patients with (A) HBV-ACLF, (B) HBV-LC and (C) HBV-HCC. HBV, hepatitis B virus; HBV-ACLF, HBV-related acute-on-chronic liver failure; HBV-LC, HBV-related liver cirrhosis; HBV-HCC, HBV-related hepatocellular carcinoma; ALBI, albumin-bilirubin; CTP, Child-Turcotte-Pugh.
Table II.
The association between the CTP and ALBI grades in patients with HBV-ACLF.
| ALBI grade 1 | ALBI grade 2 | ALBI grade 3 | |||||
|---|---|---|---|---|---|---|---|
| Group | Patients (n) | Patients (n) | Constituent frequency (%) | Patients (n) | Constituent frequency (%) | Patients (n) | Constituent frequency (%) |
| CTP grade A | 1 | 1 | 100 | 0 | 0 | 0 | 0 |
| CTP grade B | 31 | 2 | 6.45 | 26 | 83.87 | 3 | 9.68 |
| CTP grade C | 106 | 0 | 0 | 9 | 8.49 | 97 | 91.51 |
CTP, Child-Turcotte-Pugh; ALBI, albumin-bilirubin; HBV-ACLF, hepatitis B virus-related acute-on-chronic liver failure.
ALBI score is associated with the clinical stages of HBV-ACLF patients
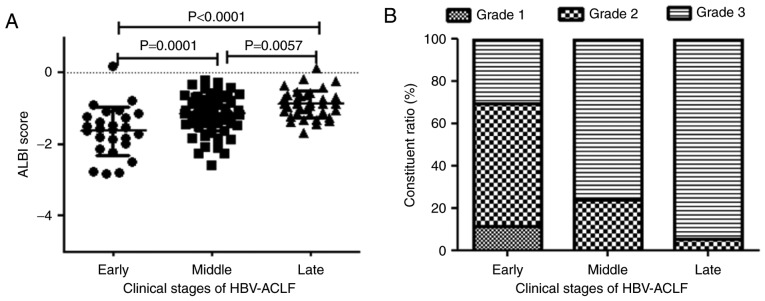
The mean ALBI scores of patients with early-, middle- and late-stage HBV-ACLF were −1.63±0.68, −1.14±0.47 and −0.88 ± 0.39, respectively. A later stage of HBV-ACLF resulted in a higher ALBI score and a greater frequency of an ALBI grade of 3 (P<0.01; Table III; Fig. 4).
Table III.
The ALBI score and grades of HBV-ACLF patients in different clinical stages.
| Grade 1 | Grade 2 | Grade 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Clinical stage | Patients (n) | ALBI score (mean ± SD) | Patients (n) | Constituent frequency (%) | Patients (n) | Constituent frequency (%) | Patients (n) | Constituent frequency (%) |
| Early stage | 26 | −1.63±0.68 | 3 | 11.54 | 15 | 57.69 | 8 | 30.77 |
| Middle stage | 76 | −1.14±0.47 | 0 | 0 | 18 | 23.68 | 58 | 76.32 |
| Late stage | 36 | −0.88±0.39 | 0 | 0 | 2 | 5.56 | 34 | 94.44 |
ALBI, albumin-bilirubin; HBV-ACLF, hepatitis B virus-related acute-on-chronic liver failure; SD, standard deviation.
Figure 4.
ALBI score and grade of HBV-ACLF patients in different clinical stages. (A) The ALBI score in the early, middle, and late stages of HBV-ACLF. (B) The ALBI grade in the early, middle, and late stages of HBV-ACLF. Data are presented as the mean ± standard deviation. ALBI, albumin-bilirubin; HBV-ACLF, hepatitis B virus-related acute-on-chronic liver failure.
Discussion
HBV-ACLF, HBV-LC, and HBV-HCC are the main clinical types of end-stage liver diseases. Given the severity of the diseases and their poor prognoses, a precise assessment of the disease conditions is pivotal for proper treatment planning and accurate prognosis prediction. Scoring systems, such as the CTP and MELD scores, have been used to assess the severity and prognosis of end-stage liver diseases, although their multiple requirements restrain their usage. In contrast, the ALBI score requires only two factors, which are also objective and easily available. The ALBI score has recently been validated to assess the severity of liver function damage in patients with HCC (12). The ALBI score has also been shown to be more accurate than the CTP score in predicting in-hospital mortality following hepatic carcinectomy in HCC patients (20). For the assessment of liver function in patients with liver cancer and treated with sorafenib, the resolution of the ALBI score is similar to that of the CTP score for patients with a CTP grade of A, whereas the ALBI grading is superior to the CTP grading for all HCC patients (21). The ALBI score has a predictive efficacy similar to that of the CTP and MELD scores in terms of predicting in-hospital mortality in LC patients with acute upper gastrointestinal bleeding and in ACLF patients with concurrent LC (22,24). In the present study, the ALBI score was compared with the CTP and MELD scores to assess their value in evaluating the liver function of patients with HBV-ACLF, HBV-LC, or HBV-HCC.
ACLF is an acute severe liver injury that occurs due to chronic liver disease. ACLF results in massive liver cell necrosis and dysfunctions in liver synthesis, detoxification, excretion, and biotransformation, with a mortality rate >50% (25). The present study demonstrated that, among the patients with end-stage liver diseases, HBV-ACLF patients had the highest ALBI scores, which was consistent with the CTP and MELD scores. The differences in the ALBI, CTP, and MELD scores among the three groups suggested that the degrees of liver function damage vary among HBV-ACLF, HBV-LC, and HBV-HCC patients. The highest ALBI, CTP, and MELD scores, observed in HBV-ACLF patients, represent the most severe liver injury, and the lowest scores, observed in HBV-HCC patients, represent the least severe liver injury. Intergroup differences measured using the ALBI scoring system were consistent with the differences measured using the CTP and MELD scoring systems, which suggested that the ALBI score may also reflect liver function damage that occurs in HBV-HCC, HBV-LC and HBV-ACLF patients. Similar to the CTP scoring system, the ALBI scoring system divided the patients into three grades according to disease severity, with higher ALBI grades indicating a more serious disease status. In the present study, analysis of the ALBI grades of HBV-ACLF, HBV-LC, and HBV-HCC patients revealed that the HBV-ACLF group predominantly exhibited grades of 3 and that the HBV-HCC group mainly exhibited grades of 1. Therefore, the ALBI classification accurately reflects liver injury severity in HBV-ACLF, HBV-LC, and HBV-HCC patients.
Additionally, the present study demonstrated that the ALBI scores of HBV-ACLF, HBV-LC, or HBV-HCC patients were positively correlated with the CTP and MELD scores, further validating that the ALBI score and the CTP and MELD scores had similar efficacy in assessing liver injury in patients with HBV-related end-stage liver diseases. Comparison of the CTP and ALBI grading in patients with HBV-ACLF demonstrated that all CTP grade A patients had an ALBI grade of 1, whereas the patients with CTP grades of B and C had varying ALBI grades, suggesting that the ALBI score may be more accurate than the CTP score.
For HBV-ACLF patients, later stages indicate more severe disease conditions. To further investigate the association between the ALBI score and disease severity, the ALBI score and the ALBI grade of HBV-ACLF patients in different disease stages were compared. The results showed significant differences in the ALBI scores and the constitutive grades among the early, middle, and late stages of liver failure, with the later stages associated with higher ALBI scores. ALBI grade 1 was only observed among early-stage HBV-ACLF patients, whereas all middle-stage patients exhibited ALBI grade 2 or grade 3 and the late-stage patients predominantly exhibited grade 3. This finding suggests that ALBI scoring and grading reflect the severity of liver function damage in HBV-ACLF patients and are positively correlated with the severity of liver disease, with more severe liver injury associated with higher ALBI scores and grades.
In conclusion, similar to the CTP and MELD scoring systems, the ALBI score is a good indicator of the severity of liver function damage in patients with HBV-ACLF, HBV-LC, or HBV-HCC. Different diseases and even different stages of the same disease exhibit different ALBI scores, and ALBI grading may accurately classify disease severity, with higher ALBI grades indicating more severe disease conditions. Given that the ALBI score requires only two simple factors and displays versatility in evaluating the severity of HBV-related end-stage liver diseases, it is expected to be extensively clinically applied as a simple scoring system. However, the present study was limited, as the values of the ALBI score for the evaluation of HBV-related end-stage liver diseases were only investigated at a single center at a single time point (admission). Multi-center studies with large sample sizes are required to further validate the efficacy and prognostic role of the ALBI score in other types of liver diseases.
Acknowledgements
The present study was partly supported by the National Natural Science Foundation of China (grant no. 81541140), the Natural Science Foundation of Hubei Province of China (grant no. 2014CFB645), the Research and Development Project of Science and Technology Plan of Hubei Provinc e (grant no. 2011BCB030), the Foundation for Innovative Research Team of Hubei University of Medicine (grant no. 2014CXG05) and the Key Program for Precision Medicine of Taihe Hospital (grant no. 2016JZ05).
References
- 1.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: The major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Cheng XP, Zhao J, Chen Y, Meng FK, Xu B, Yu HW, Meng QH, Liu YM, Zhang SB, Meng S, et al. Comparison of the ability of the PDD-ICG clearance test, CTP, MELD, and MELD-Na to predict short-term and medium-term mortality in patients with decompensated hepatitis B cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:444–448. doi: 10.1097/MEG.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling Q, Dai H, Zhuang R, Shen T, Wang W, Xu X, Zheng S. Predicting short-term survival after liver transplantation on eight score systems: A national report from China liver transplant registry. Sci Rep. 2017;7:42253. doi: 10.1038/srep42253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Ghannam MT, Hassanien MH, El-Talkawy MD, Saleem A, Sabry AI, Abu TH. Performance of disease-specific scoring models in intensive care patients with severe liver diseases. J Clin Diagn Res. 2017;11:OC12–OC16. doi: 10.7860/JCDR/2017/24543.9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110–122. doi: 10.1055/s-2008-1040325. [DOI] [PubMed] [Google Scholar]
- 9.Hong G, Lee KW, Suh S, Yoo T, Kim H, Park MS, Choi Y, Yi NJ, Suh KS. The model for end-stage liver disease score-based system predicts short term mortality better than the current Child-Turcotte-Pugh score-based allocation system during waiting for deceased liver transplantation. J Korean Med Sci. 2013;28:1207–1212. doi: 10.3346/jkms.2013.28.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porte RJ, Lisman T, Tripodi A, Caldwell SH, Trotter JF, Coagulation in Liver Disease Study Group The international normalized ratio (INR) in the MELD score: Problems and solutions. Am J Transplant. 2010;10:1349–1353. doi: 10.1111/j.1600-6143.2010.03064.x. [DOI] [PubMed] [Google Scholar]
- 11.Vitale A, Bertacco A, Gambato M, D'Amico F, Morales RR, Frigo AC, Zanus G, Burra P, Angeli P, Cillo U. Model for end-stage liver disease-sodium and survival benefit in liver transplantation. Transpl Int. 2013;26:138–144. doi: 10.1111/tri.12008. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyoda H, Lai PB, O'Beirne J, Chong CC, Berhane S, Reeves H, Manas D, Fox RP, Yeo W, Mo F, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: Application of the ALBI grade. Br J Cancer. 2016;114:744–750. doi: 10.1038/bjc.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan AW, Chan RC, Wong GL, Wong VW, Choi PC, Chan HL, To KF. New simple prognostic score for primary biliary cirrhosis: Albumin-bilirubin score. J Gastroenterol Hepatol. 2015;30:1391–1396. doi: 10.1111/jgh.12938. [DOI] [PubMed] [Google Scholar]
- 15.Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J, Peng Y, Li J, Deng H, Guo X. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk J Gastroenterol. 2016;27:180–186. doi: 10.5152/tjg.2016.15502. [DOI] [PubMed] [Google Scholar]
- 16.Chen RC, Cai YJ, Wu JM, Wang XD, Song M, Wang YQ, Zheng MH, Chen YP, Lin Z, Shi KQ. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis B-related cirrhosis. J Viral Hepat. 2017;24:238–245. doi: 10.1111/jvh.12638. [DOI] [PubMed] [Google Scholar]
- 17.Chen B, Lin S. Albumin-bilirubin (ALBI) score at admission predicts possible outcomes in patients with acute-on-chronic liver failure. Medicine (Baltimore) 2017;96:e7142. doi: 10.1097/MD.0000000000007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou JL, Lai W. The guideline of prevention and treatment for chronic hepatitis B: A 2015 update. Zhonghua Gan Zang Bing Za Zhi. 2015;23:888–905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Liver Failure And Artificial Liver Group CSOI and Severe Liver Diseases And Artificial Liver Group CSOH, corp-author. Diagnostic and treatment guidelines for liver failure (2012 version) Zhonghua Gan Zang Bing Za Zhi. 2013;21:177–183. [PubMed] [Google Scholar]
- 20.Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, Ma L, Qi LN, Ou BN, Li LQ. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103:725–734. doi: 10.1002/bjs.10095. [DOI] [PubMed] [Google Scholar]
- 21.Edeline J, Blanc JF, Johnson P, Campillo-Gimenez B, Ross P, Ma YT, King J, Hubner RA, Sumpter K, Darby S, et al. A multicentre comparison between Child Pugh and Albumin-Bilirubin scores in patients treated with sorafenib for Hepatocellular Carcinoma. Liver Int. 2016;36:1821–1828. doi: 10.1111/liv.13170. [DOI] [PubMed] [Google Scholar]
- 22.Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J, Peng Y, Li J, Deng H, Guo X. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk J Gastroenterol. 2016;27:180–186. doi: 10.5152/tjg.2016.15502. [DOI] [PubMed] [Google Scholar]
- 23.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 24.Peng Y, Qi X, Tang S, Deng H, Li J, Ning Z, Dai J, Hou F, Zhao J, Wang R, et al. Child-Pugh, MELD, and ALBI scores for predicting the in-hospital mortality in cirrhotic patients with acute-on-chronic liver failure. Expert Rev Gastroenterol Hepatol. 2016;10:971–980. doi: 10.1080/17474124.2016.1177788. [DOI] [PubMed] [Google Scholar]
- 25.Garg H, Kumar A, Garg V, Sharma P, Sharma BC, Sarin SK. Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis. 2012;44:166–171. doi: 10.1016/j.dld.2011.08.029. [DOI] [PubMed] [Google Scholar]