Abstract
The aim of the present study was to investigate the effect of a Traditional Chinese Herbal Medicine (TCHM), named Jinwei Tang on histone deacetylase 2 (HDAC2) and its role in the regulation of corticosteroid resistance in a rat model of chronic obstructive pulmonary disease (COPD). Male Wistar rats were divided into five groups (each n=10): COPD group, established by the intratracheal instillation of lipopolysaccharide and passive smoke exposure, and control, budesonide, theophylline + budesonide and Jinwei Tang + budesonide groups. Lung function was measured, lung tissue histopathology was examined and HDAC2 expression in the lung was assessed by immunohistochemistry. In addition, protein levels of interleukin-8 (IL-8), tumor necrosis factor (TNF)-α and HDAC2 in lung homogenate were quantified by ELISA. The rat COPD model exhibited alterations of the ratio of forced expiratory volume in 0.2 sec (FEV0.2) to the forced vital capacity, FEV0.2, dynamic compliance and airway resistance. HDAC2 expression was markedly reduced in the lung tissue of the COPD group compared with the control group, and treatment with Jinwei Tang + budesonide or theophylline + budesonide resulted in significant attenuation of the reduction of HDAC2 expression in the lungs (P<0.05). However, treatment with budesonide alone did not significantly alter HDAC2 expression. In the Jinwei Tang + budesonide and theophylline + budesonide groups, IL-8 and TNF-α expression was significantly decreased (P<0.05) and the HDAC2 level increased (P<0.05) compared with that in the COPD group. In conclusion, Jinwei Tang modulates airway inflammation and may enhance the anti-inflammatory effect of glucocorticoid through the upregulation of HADC2 expression in a rat model of COPD.
Keywords: traditional Chinese herbal medicine, chronic obstructive pulmonary disease, corticosteroid resistance, histone deacetylase
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic inflammation affecting the airway, lung parenchyma and pulmonary vascular system (1). Infiltration of inflammatory cells such as alveolar macrophages, T lymphocytes, particularly CD8+ T cells, and neutrophils leads to the release of inflammatory mediators, including leukotriene B4 (LTB4), interleukin-8 (IL-8) and tumor necrosis factor (TNF)-α (2,3). These inflammatory mediators are considered to contribute to the pathogenesis of COPD (4). In addition, inhalation of toxic substances such as cigarette smoke may further exacerbate airway inflammation-induced injury and tissue remodeling (5,6).
Since COPD is characterized by chronic airway inflammation and consequent tissue remodeling, anti-inflammatory therapy is considered a priority for COPD treatment. In this regard, glucocorticoids are among the most rapid and effective anti-inflammatory drugs used for the treatment of chronic inflammatory airway disease (6). However, unlike asthma, COPD is often unresponsive to glucocorticoid therapy (7,8). The mechanisms of corticosteroid resistance may involve the imbalance of histone acetylation/deacetylation, oxidative stress and genetic or epigenetic alterations (9,10). In this study, histone deacetylase 2 (HDAC2) dysfunction is thought to play an important role in the development of corticosteroid resistance in COPD (8,9,11,12). Thus, increasing HDAC2 activity may be a promising strategy to overcome corticosteroid resistance in COPD. Existing treatments such as theophylline, nortriptyline, macrolides and selective phosphatidylinositol-3-kinase-δ inhibitors have been reported to increase HDAC2 activity effectively (13). In support of this concept, Hsieh et al accessed two public traditional Chinese medicine (TCM) databases and retrieved the chemical constituents and TCM characteristics of 3,294 TCM medicinal agents (14). They identified that 1,170/3,294 (36%) of the TCM medicinal agents interact with human histone-modifying enzymes and that 56% of the histone-modifying materials promote chromatin condensation. Furthermore, analysis of TCM formulas revealed that 99% of 200 government-approved TCM formulas are histone-modifying and the synergy of the TCM medicinal agents in a formula involved mostly concurrent DNA methyltransferase (DNMT) and HDAC inhibition, co-inhibition of histone acetylation and H3S10 phosphorylation, or co-inhibition of H3K4 demethylation and H3K36 demethylation (14).
In addition to Western medicine, traditional Chinese herbal medicine (TCHM) has long been used for the clinical treatment of COPD (15,16) and the mechanisms underlying the effects of TCHM on COPD therapy have been widely studied in pre-clinical animal models of COPD (17,18). In this study, TCHM such as Bufei Jianpi, Bufei Yishen and Yiqi Zishen granules, and Liuweibuqi capsule have been demonstrated to effectively improve lung function, increase skeletal muscle strength, bone mass density and bone mineral content, and reduce airway inflammation through different mechanisms, including the inhibition of inflammatory cytokines, regulation of matrix metalloproteinases (MMPs) and their biological inhibitors (tissue inhibitors of MMPs), and modulation of the transforming growth factor-β1/Smad signal pathway (17–22). Moreover, it has been shown that glucocorticoids used in combination with certain TCHMs have a marked effect in the prevention and treatment of COPD (23–27) or asthma (28). In this regard, the combination of a Xiang Sha Liu Jun Zi Tang, salmeterol xinafoate and fluticasone propionate inhalation powder has been demonstrated to improve the clinical symptoms and lung function in patients with stable COPD (25). Moreover, the TCHM Liujunzi decoction used in combination with salmeterol xinafoate and fluticasone propionate inhalation powder significantly improved the symptoms and lung function of patients with COPD by upregulating airway HDAC (in the sputum) and downregulating IL-8 and TNF-α (23). Recently, it has been reported that a TCHM, You-Gui-Wan, reduced inflammation and eosinophil infiltration into the lung tissues through increasing HDAC and decreasing histone acetyltransferase activities in memory T lymphocytes (28).
Therefore, in the present study, the effects of on pulmonary function Jinwei Tang, that in TCM is considered to ‘support qi, nourish yin, activate blood circulation and dispel phlegm’ were investigated. In addition, HDAC2 expression in lung tissue, and protein levels of IL-8, TNF-α and HDAC2 in lung tissue homogenates of rat models of COPD were analyzed.
Materials and methods
Animals
Fifty male Wistar rats (3 months old; body weight, 220±10 g) of specific pathogen-free grade were purchased from Si Bei Fu Experimental Animal Technology (Beijing, China). All experiments followed a protocol approved by the Animal Care Ethics Committee of Beijing University of Traditional Chinese Medicine (Beijing, China).
Materials and equipment
Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Aminophylline was purchased from Zizhu Pharmaceutical Co., Ltd. (Beijing, China). Budesonide suspension for inhalation was purchased from AstraZeneca (Shanghai, China). The TCHM Jinwei Tang was made by a combination of Jinshui Liujun Jian and Weijing Tang, and consisted of Danggui (Angelica sinensis root) 15 g, Shudi (Rehmannia root) 15 g, Chenpi (dried orange peel) 12 g, Qingbanxia (tuber of pinellia) 12 g, Fulin (sclerotium of poria coco) 30 g, Lugen (reed stems) 15 g, Taoren (peach seed) 12 g, Chaoyiyiren (coix seed) 15 g, Beishashen (littoralis root) 15 g, Maidong (Ophiopogon japonicas root) 15 g, Shenghuangqi (Astragalus root) 30 g, Danshen (Salvia root) 30 g, Zhimahuang (Chinese ephedra) 6 g and Zhigancao (licorice root) 6 g. All herbs were purchased from the Pharmacy of Traditional Chinese Medicine at the Third Hospital of Beijing University of Traditional Chinese Medicine (Beijing, China). The Rat IL-8 ELISA kit (cat. no. E02I0056) was purchased from Shanghai BlueGene Biotech Co., Ltd. (Shanghai, China). The Rat TNF-α ELISA kit (cat. no. 70-EK382) and rat HDAC2 ELISA kit (cat. no. SEC210Ra) were boh purchased from Beijing Bioway Biotech Group Co., Ltd. (Beijing, China). PARI BOY SX (085G3005) nebulizer was purchased from PARI Medical Holding GmbH (Starnberg, Germany). Daqianmen cigarettes were purchased from Shanghai Tobacco Group Ltd. (Shanghai, China). Image Pro Plus 6.0 data analysis software was purchased from Media Cybernetics (Rockville, MD, USA).
Grouping and treatment
Rat models of COPD were prepared by intratracheal instillation of LPS and passive smoke exposure as described previously (24,25). The rats were divided randomly into five groups (each n=10): COPD group, which received intratracheal instillation of LPS (200 µl; 1 mg/ml) on days 1 and 14, plus passive smoke exposure with 12 Daqianmen cigarettes in a 72-l closed box for 30 min (smoke concentration, 5%) in the morning on days 2–13 and 15–28; control group, which did not receive any treatment; budesonide group, which underwent the same modeling procedure as the COPD model group, and also inhaled budesonide (2 mg) every afternoon in a 28-l closed box connected to a PARI BOY SX (085G3005) nebulizer from day 8 of the COPD model; theophylline + budesonide group, which underwent the same modeling procedure as the COPD model group, and also received aminophylline (25 mg/kg; 1 ml/100 g) treatment by gavage prior to passive smoke exposure daily from day 8 of the COPD model followed by inhalation of budesonide (2 mg) every afternoon in a 28-l closed box; TCHM + budesonide group, which underwent the same modeling procedure as the COPD model group, and also underwent treatment with granules of the TCHM (3.6 g/kg) by gavage daily prior to passive smoke exposure from day 8 of the COPD model followed by inhalation of budesonide (2 mg) every afternoon in a 28-l closed box.
Rat lung function test
Eight rats were randomly selected from each group after 28 days of cigarette smoke exposure. A tracheotomy was conducted following intraperitoneal anesthesia with 10% pentobarbital sodium. Lung function was tested as described previously (29). Briefly, following the tracheotomy, the animal was ventilated and placed into a body plethysmography box (Beijing Bestlab High-Tech Co., Ltd., Beijing, China). Normal breathing was recorded first, and the forced vital capacity (FVC) limit was set to five-fold higher than the tidal volume (1 ml, 5 ml/kg). The animals were allowed to breath normally, the FVC of air was then passively delivered to the lung by a ventilator, and expiration was performed using negative pressure (−25 cmH2O), which was considered as a one-time lung function test. Following 20 sec of normal breathing, the FVC was delivered again to measure the lung function. The lung functions tested included the ratio of forced expiratory volume in 0.2 sec (FEV0.2) to FVC (FEV0.2/FVC), FEV0.2, expiratory resistance (Re) and lung dynamic compliance (Cydn). In total, the lung function test was performed five times for each animal. Data were analyzed using AniRes 2005 software version 1 (Beijing Beilangbo Technology Co. Ltd., Beijing, China (30).
Tissue sample collection and homogenate preparation
Following the lung function test, the rats were sacrificed by abdominal aorta venesection. The right lower lung tissue was taken for pathology and immunohistochemistry of HDAC2 as described below. The left lung was homogenized with protein extraction buffer containing a cocktail of proteinase inhibitors (cat. no. 78430; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at a final concentration of 10% homogenate (w/v) and used for quantification of IL-8, TNF-α and HDAC2 by ELISA following the manufacturer's protocol.
Histopathology and immunohistochemistry
The right lung of each animal was fixed with 10% formalin for 24 h at 4°C and embedded in paraffin. Lung tissue slices (5 µm) were either stained with hematoxylin (5 min at room temperature) and eosin (2 min at room temperature) for histopathological examination or immunostained for HDAC2, using anti-HDAC2 antibody (Wuhan Boster Biological Technology, Ltd., Wuhan, China). Briefly, tissues were deparaffinized and rehydrated followed by washing with PBS. Endogenous oxygenase was blocked with 3% H2O2 and goat serum (Invitrogen; Thermo Fisher Scientific, Inc.) for 20 min at room temperature and incubated with anti-HDAC2 antibodies (cat. no. Q92769; 1:100 dilution) at 4°C overnight. Following washing, biotinylated secondary antibodies (cat. no. 85-6643; 1:100 dilution; Invitrogen; Thermo Fisher Scientifc, Inc.) were allowed to react at room temperature for 15 min followed by binding to horseradish peroxidase-avidin conjugation for 15 min. HDAC2 staining was visualized by DAB development (Wuhan Boster Biological Technology, Ltd.). Images were obtained under a Bx-41 fluorescence microscope and Nikon Digital Sight Color CCD camera (both from (Olympus Corporation, Tokyo, Japan). Intensity of the positive staining was analyzed with Image Pro Plus software version 6.0 (Media Cybernetics, Inc.).
Statistical analysis
Data are expressed as mean ± standard deviation and analyzed with Statistics Product and Service Solutions 13.0 software (SPSS v.13.0 for Windows; SPSS, Inc., Chicago, IL, USA). One-way analysis of variance followed by Tukey correction was used to evaluate the statistical significance of differences among the groups. Linear regression was performed to test for correlations between HDAC2 and TNF-α or IL-8. P<0.05 was considered to indicate a statistically significant difference.
Results
Lung function tests
Rat lung function was assessed and analyzed. As shown in Table I, FEV0.2/FVC, FEV0.2 and Cydn were significantly decreased, while Re was significantly increased in the COPD group compared with the control group (P<0.001). The TCHM + budesonide and theophylline + budesonide treatments were able to significantly block the changes of FEV0.2/FVC, FEV0.2, Cydn and Re (P<0.01), while budesonide alone slightly but not significantly improved the lung function parameters.
Table I.
Comparison of rat lung functions (mean ± standard deviation, n=8).
| Group | FEV0.2/FVC (%) | FEV0.2 (ml) | Re (sec·cm H2O/ml) | Cydn (ml/cm H2O) |
|---|---|---|---|---|
| Control | 93.25±4.86a | 2.99±0.31a | 0.52±0.06a | 0.209±0.017a |
| COPD | 84.36±2.74 | 2.27±0.27 | 0.69±0.13 | 0.144±0.032 |
| Budesonide | 85.47±2.99 | 2.37±0.30 | 0.66±0.04 | 0.153±0.031 |
| TCHM + budesonide | 89.37±4.89b | 2.71±0.30c | 0.55±0.08c | 0.185±0.021c |
| Theophylline + budesonide | 89.39±4.56b | 2.68±0.29c | 0.58±0.07c | 0.182±0.026c |
P<0.001
P<0.05
P<0.01 and vs. the COPD group. COPD, chronic obstructive pulmonary disease; FEV0.2, forced expiratory volume in 0.2 sec; FVC, forced vital capacity; Re, expiratory resistance; Cdyn, lung dynamic compliance.
Lung tissue histopathology
Control group
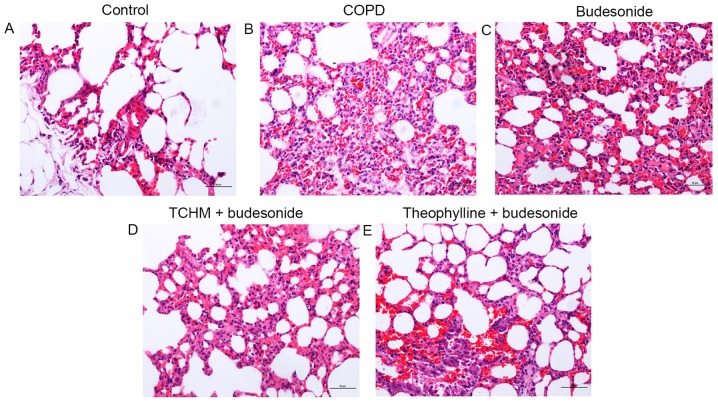
The bronchial lumen and alveolar structure appeared normal in this group (Fig. 1A). Neither inflammatory cells in the submucosa nor inflammatory exudate in the bronchial and alveolar cavity was observed (Fig. 1A).
Figure 1.
Histopathological images. Lung tissue slices from each treatment group were stained with hematoxylin and eosin and photographic images were captured under a microscope. Magnification, ×200. (A) Control group, (B) COPD group, (C) budesonide group, (D) TCHM + budesonide group and (E) theophylline + budesonide group. One representative image from each group is shown (n=8). COPD, chronic obstructive pulmonary disease; TCHM, traditional Chinese herbal medicine.
COPD group
The bronchial lumen was deformed and damaged as characterized by goblet cell hyperplasia and inflammatory cell infiltration. There were exfoliated epithelial cells and inflammatory exudate in the bronchial cavity. The alveolar septum was thickened as a result of compensatory emphysema. Infiltration of lymphocytes, plasma cells, neutrophils and eosinophils in the alveolar wall and alveolar interstitial tissue was noted in this group (Fig. 1B).
Budesonide group
The bronchial lumen was deformed and damaged. There were exfoliated epithelial cells and inflammatory exudate in the bronchial cavity with inflammatory cell infiltration. The alveolar septum was significantly expanded, with the appearance of bronchiectasis. Inflammation was severe in the alveolar interstitial tissue (Fig. 1C).
TCHM + budesonide group
The small bronchial wall was intact. Exfoliated epithelial cells and inflammatory exudate in the bronchial cavity were significantly reduced. Inflammation of the alveolar walls was also significantly reduced (Fig. 1D).
Theophylline + budesonide group
The bronchial lumen was deformed. However, there were fewer exfoliated epithelial cells, less inflammatory exudate in the bronchial cavity, and reduced infiltration of inflammatory cells than were observed in the COPD group. The alveolar wall collapsed due to emphysema, but pulmonary alveolar inflammation was mitigated (Fig. 1E).
HDAC2 immunohistochemistry
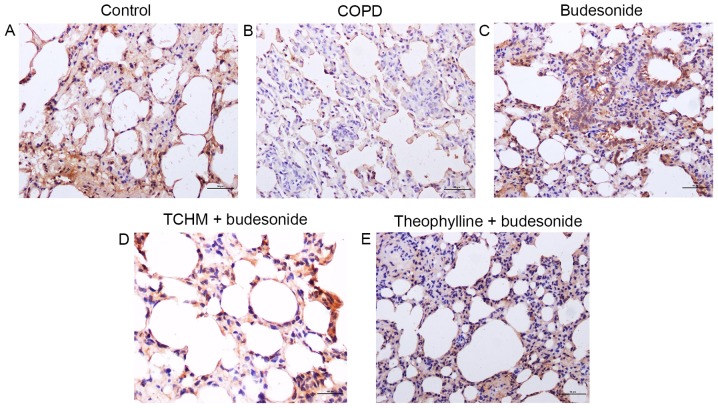
The expression of HDAC2 in the lungs of each group was determined by immunohistochemistry and is presented in Fig. 2. HDAC2 was strongly expressed in the control rat lung (mean density, 0.077; Fig. 2A), while it was markedly suppressed in the lungs of the COPD group (mean density, 0.012; Fig. 2B). While treatment with budesonide alone partially blocked the suppression of HDAC2 expression in response to LPS and cigarette smoke exposure (mean density, 0.032; Fig. 2C), TCHM + budesonide not only reduced changes of the alveolar structure but also further blocked the reduction of HDAC2 expression in response to LPS and cigarette smoke exposure (mean density, 0.039; Fig. 2D). Similarly, the regulatory effect of theophylline + budesonide on HDAC2 expression (mean density, 0.037; Fig. 2E) in response to LPS and cigarette smoke exposure was also evident.
Figure 2.
HDAC2 immunohistochemistry. Lung tissue slices from each treatment group were immunostained for HDAC2. Magnification, ×200. (A) Control, (B) COPD, (C) budesonide, (D) TCHM + budesonide and (E) theophylline + budesonide groups. One representative image from each group is shown (n=8). HDAC2, histone deacetylase 2; COPD, chronic obstructive pulmonary disease; TCHM, traditional Chinese herbal medicine.
Quantification of HDAC2, IL-8 and TNF-α in rat lung tissue homogenate
As shown in Table II, in the lung tissue homogenates of the COPD group compared with the control group, HDAC2 expression was significantly decreased while IL-8 and TNF-α levels were significantly increased (P<0.001). Treatment with TCHM + budesonide or theophylline + budesonide significantly blocked the suppression of HDAC2 expression by LPS plus cigarette smoke exposure (P<0.01), while budesonide alone had no effect on HDAC2. Similarly, in the TCHM + budesonide and theophylline + budesonide groups, the LPS plus cigarette smoke-stimulated TNF-α expression was significantly reduced (P<0.05 vs. the COPD group), while budesonide alone did not significantly affect TNF-α levels. TCHM + budesonide significantly inhibited IL-8 protein release in response to LPS and cigarette smoke exposure (P<0.05), while neither budesonide alone nor theophylline + budesonide had a significant effect on IL-8.
Table II.
Levels of HDAC2, IL-8 and TNF-α in rat lung tissue (mean ± standard deviation, n=8).
| Group | HDAC2 (ng/ml) | IL-8 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|
| Control | 0.507±0.063a | 241.30±17.18a | 64.72±11.13a |
| COPD | 0.348±0.031 | 306.94±36.99 | 96.49±10.61 |
| Budesonide | 0.369±0.027 | 298.64±42.09 | 94.64±5.56 |
| TCHM + budesonide | 0.444±0.066b | 274.87±21.96c | 77.57±8.54b |
| Theophylline + budesonide | 0.442±0.068b | 278.74±20.79 | 78.86±9.02c |
P<0.001
P<0.01
P<0.05 vs. the COPD group. COPD, chronic obstructive pulmonary disease; HDAC2, histone deacetylase 2; IL, interleukin; TNF, tumor necrosis factor.
Correlation between HDAC2 and IL-8 or TNF-α
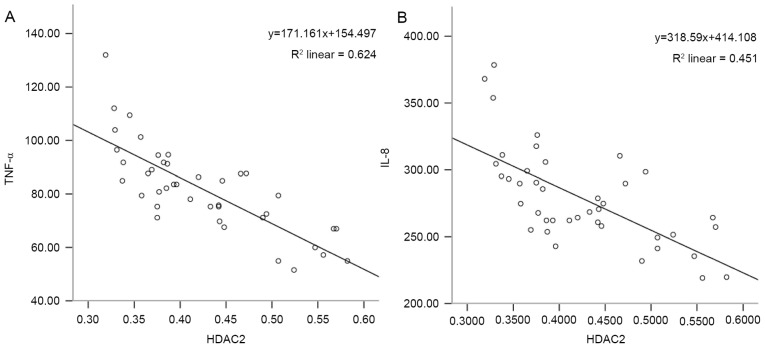
The correlation of HDAC2 with IL-8 or TNF-α levels in the rat lungs was analyzed. As shown in Fig. 3, the level of HDAC2 was negatively correlated with the level of IL-8 (r=−0.672, P<0.001; Fig. 3A) and the level of TNF-α (r=−0.790, P<0.001; Fig. 3B).
Figure 3.
Correlation of HDAC2 with IL-8 or TNF-α. Correlation between HADC2 and (A) TNF-α and (B) IL-8. HDAC2, histone deacetylase 2; IL, interleukin; TNF, tumor necrosis factor.
Discussion
In the present study, in order to investigate the role of Jinwei Tang in regulating the effect of budesonide on airway inflammation, a COPD model characterized by airway inflammation was prepared in rats by a combination of intratracheal LPS instillation and cigarette smoke exposure. As demonstrated by lung function tests as well as by histopathological examination, the rat COPD model was successfully created in the current study. In addition, the expression of inflammatory cytokines (IL-8 and TNF-α) and HDAC2 was significantly altered in the lungs of COPD models compared with those in control lungs. Furthermore, the expression of IL-8 and TNF-α in the lung tissue was negatively correlated with HDAC2 expression, suggesting that HDAC2 is involved in the modulation of inflammatory cytokine release in the context of airway inflammation.
Since HDAC dysfunction may be associated with corticosteroid resistance in COPD (8,9,11,12), in the present study, the synergetic effect of the TCHM and glucocorticoid on the expression of HDAC2 as well as inflammatory mediators (IL-8 and TNF-α) in the lungs of the COPD rat model was further explored. The TCHM plus budesonide significantly blocked the suppression of HDAC2 expression in the lungs in response to the intratracheal instillation of LPS plus cigarette smoke exposure, while budesonide treatment alone did not. Theophylline plus budesonide had a similar effect as the TCHM plus budesonide. In addition, the TCHM plus budesonide significantly inhibited the synthesis of the inflammatory mediators IL-8 and TNF-α in the lungs of rats exposed to LPS and cigarette smoke. These findings suggest that the TCHM augmented the anti-inflammatory effect of the glucocorticoid through modulating HDAC2 expression in lungs affected by COPD.
The dynamic balance between histone acetylation by histone acetyltransferase and histone deacetylation by HDAC is crucial in maintaining homeostasis, particularly in chronic inflammation. It is known that the anti-inflammatory effect of glucocorticoid largely depends on the HDAC2 level and its activity and that the reduced expression and activity of HDAC2 contribute to the development of glucocorticoid resistance in COPD (9). In this study, the expression and activity of HDAC2 protein as well as HDAC mRNA have been found to be reduced in bronchial biopsies, bronchoalveolar lavage macrophages and peripheral lung tissue obtained from patients with COPD; moreover, the reduction in HDAC2 correlates with disease severity (31). Consistent with this, the current study demonstrated that HDAC2 was significantly suppressed in response to intratracheal LPS instillation and cigarette smoke exposure.
It has been reported that when HDAC2 activity is decreased, corticosteroid function is also reduced; and when the activity of HDAC2 is restored or protected, the sensitivity to corticosteroid is recovered (28). Selective activation of HDAC2 can be achieved with low therapeutic concentrations of theophylline, which restores HDAC2 activity in macrophages from patients with COPD to normal and reverses corticosteroid resistance (8,10,13). Consistent with these studies, the ability of theophylline plus budesonide to restore HDAC2 expression in the lung tissue of rat COPD models was also observed in the current study. The TCHM plus budesonide was also able to rescue HDAC2 expression in the lung tissue of rats in response to LPS and cigarette smoke exposure, suggesting that the TCHM targets HDAC2 as well.
TCHMs have been demonstrated to serve an important role in the prevention and treatment of COPD (21,32). Previous experiments with animal models of COPD have shown that TCHM, including Bufei Yishen granules and Liujunzi Tang, are able to improve pulmonary function in the COPD rat model as well as reduce pathological changes resulting from airway remodeling (32,33). The current study demonstrated that budesonide plus the TCHM, Jinwei Tang significantly decreased the secretion of IL-8 and TNF-α and inhibited the aggregation of neutrophils, suggesting that the TCHM relieves inflammation in the COPD model. Another TCHM, Jiawei Yuepi plus Banxia decoction, has been shown to reduce lung inflammation in a rat COPD model through inhibiting IL-8 and TNF-α in a concentration-dependent manner (34). In addition, the TCHM Baibunongjian decoction inhibited the release of TNF-α, IL-8 and LTB4 and regulated airway remodeling in a rat COPD model (35). Moreover, the rectal delivery of another TCHM (Pingchuan decoction) significantly inhibited the inflammatory cytokines TNF-α and IL-8 but stimulated vascular endothelial growth factor in a rat model of COPD exacerbation (36). Furthermore, the TCHMs Ailuokechuanning and Shenhayifei capsule have been reported to inhibit airway inflammation through regulating phospho-p38 mitogen-activated protein kinase signaling and myeloperoxidase activity as well as reducing the release of IL-17 and TNF-α in rat models of COPD (37,38). The present study further demonstrated that the TCHM, Jinwei Tang modulates HDAC2 expression in response to LPS and cigarette smoke exposure by attenuating glucocorticoid resistance in the treatment of chronic airway inflammation.
In conclusion, the current study demonstrated that the TCHM, Jinwei Tang was able to rescue HDAC2 expression from the inhibitory effect of LPS plus cigarette smoke exposure, and augment the inhibitory effect of glucocorticoid on airway inflammation as evidenced by significant suppression of IL-8 and TNF-α in the lungs of a rat model of COPD. The findings of the current study indicate that this TCHM can effectively modulate the anti-inflammatory effect of glucocorticoid in the context of chronic airway inflammation, including COPD.
Acknowledgements
The present study was supported by Beijing University of Traditional Chinese Medicine Foundation (grant no. 2014-JYBZZ-JS-066).
References
- 1.Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, Cooper CB, Comellas A, Couper DJ, Curtis JL, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: An analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61:448–454. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease; Proc Am Thorac Soc; 2006; pp. 245–251. [DOI] [PubMed] [Google Scholar]
- 4.MacNee W. Pathogenesis of chronic obstructive pulmonary disease; Proc Am Thorac Soc; 2005; pp. 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crotty Alexander LE, Shin S, Hwang JH. Inflammatory diseases of the lung induced by conventional cigarette smoke: A review. Chest. 2015;148:1307–1322. doi: 10.1378/chest.15-0409. [DOI] [PubMed] [Google Scholar]
- 6.Newton R. Anti-inflammatory glucocorticoids: Changing concepts. Eur J Pharmacol. 2014;724:231–236. doi: 10.1016/j.ejphar.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Ammit AJ. Glucocorticoid insensitivity as a source of drug targets for respiratory disease. Curr Opin Pharmacol. 2013;13:370–376. doi: 10.1016/j.coph.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra D, Thimmulappa RK, Mercado N, Ito K, Kombairaju P, Kumar S, Ma J, Feller-Kopman D, Wise R, Barnes P, Biswal S. Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients. J Clin Invest. 2011;121:4289–4302. doi: 10.1172/JCI45144. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131:636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 10.Hakim A, Adcock IM, Usmani OS. Corticosteroid resistance and novel anti-inflammatory therapies in chronic obstructive pulmonary disease: Current evidence and future direction. Drugs. 2012;72:1299–1312. doi: 10.2165/11634350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Koenderman L, Chilvers ER. Future treatment in patients with chronic obstructive pulmonary disease: To reverse or not to reverse steroid resistance - that is the question. J Allergy Clin Immunol. 2014;134:323–324. doi: 10.1016/j.jaci.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Li Q, Gong Y, Ren L, Wan H, Deng W. Low-dose theophylline restores corticosteroid responsiveness in rats with smoke-induced airway inflammation. Can J Physiol Pharmacol. 2012;90:895–902. doi: 10.1139/y2012-079. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ. Development of new drugs for COPD. Curr Med Chem. 2013;20:1531–1540. doi: 10.2174/0929867311320120005. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh HY, Chiu PH, Wang SC. Histone modifications and traditional Chinese medicinals. BMC Complement Altern Med. 2013;13:115. doi: 10.1186/1472-6882-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haifeng W, Hailong Z, Jiansheng L, Xueqing Y, Suyun L, Bin L, Yang X, Yunping B. Effectiveness and safety of traditional Chinese medicine on stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. Complement Ther Med. 2015;23:603–611. doi: 10.1016/j.ctim.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Coyle M, Shergis JL, Liu S, Wu L, Zhang AL, Guo X, Lu C, Xue CC. Safety of chinese herbal medicine for chronic obstructive pulmonary disease. Evid Based Complement Alternat Med. 2015;2015:380678. doi: 10.1155/2015/380678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y, Li Y, Sun Y, Mao J, Yao F, Tian Y, Wang L, Li L, Li S, Li J. Bufei Jianpi granules improve skeletal muscle and mitochondrial dysfunction in rats with chronic obstructive pulmonary disease. BMC Complement Altern Med. 2015;15:51. doi: 10.1186/s12906-015-0559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian Y, Li Y, Li J, Xie Y, Wang M, Dong Y, Li L, Mao J, Wang L, Luo S. Bufei Yishen granule combined with acupoint sticking improves pulmonary function and morphormetry in chronic obstructive pulmonary disease rats. BMC Complement Altern Med. 2015;15:266. doi: 10.1186/s12906-015-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Yang L, Yao Q, Li Y, Tian Y, Li S, Jiang S, Wang Y, Li X, Guo Z. Effects and mechanism of bufei yishen formula in a rat chronic obstructive pulmonary disease model. Evid Based Complement Alternat Med. 2014;2014:381976. doi: 10.1155/2014/381976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Li JS, Li WW, Li SY, Tian YG, Lu XF, Jiang SL, Wang Y. Long-term effects of three Tiao-Bu Fei-Shen therapies on NF-κB/TGF-β1/smad2 signaling in rats with chronic obstructive pulmonary disease. BMC Complement Altern Med. 2014;14:140. doi: 10.1186/1472-6882-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Li Z, Liu X, Peng Q, Li F, Li D, Wang C. Effect of Liuweibuqi capsule, a Chinese patent medicine, on the JAK1/STAT3 pathway and MMP9/TIMP1 in a chronic obstructive pulmonary disease rat model. J Tradit Chin Med. 2015;35:54–62. doi: 10.1016/S0254-6272(15)30009-1. [DOI] [PubMed] [Google Scholar]
- 22.Yange T, Ya L, Jiansheng L, Suyun L, Suli J, Ying W, Xiaofan L, Weiwei L. Effects of therapies for regulating and reinforcing lung and kidney on osteoporosis in rats with chronic obstructive pulmonary disease. J Tradit Chin Med. 2015;35:175–183. doi: 10.1016/S0254-6272(15)30025-X. [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Dai Y, Zhou LH. Clincal Observation on TCM Combined with western medicine in treatment of COPD with qi deficiency and blood stasis syndrome. J Emerg Traditi Chin Med. 2013;22:301–302. [Google Scholar]
- 24.Chen L, Zhang GL, Chen M, Ji JX, Wang SG. The curative effect of Liujunzi decoction in the treatment of chronic obstructive pulmonary disease patients in peroid with lung sleep deficiency type. Zhong Yi Yao Dao Bao. 2015;21:79–81. (In Chinese) [Google Scholar]
- 25.Pei XJ, Lu YX, Zhang LD, Tan MC, Shi W. Clinical observation of modified Liujunzi decoction for respiratory muscle fatigue of stable phase patients with chronic obstructive pulmonary disease. Xin Zhong Yi. 2014;46:59–61. (In Chinese) [Google Scholar]
- 26.Shan L. The intervention effect of supplemeting qi, activating blood circulation, resolving phlegm and its different combination regimens on COPD Rats. Guangzhou Univ Chin Med. 2012;70:1. [Google Scholar]
- 27.Wang LD, Pan W. Treatment for 36 cases of chronic obstructive pulmonary disease at mild and remission stages with salmeterol fluticasone and mixture of replenising Qi and nourishing yin. West J Tradit Chin Med. 2012;25:76–77. [Google Scholar]
- 28.Zhang HP, Fu JJ, Fan T, Zhang WB, Wang ZL, Wang L, Wang G. Histone deacetylation of memory T lymphocytes by You-Gui-Wan alleviates allergen-induced eosinophilic airway inflammation in asthma. Chin Med. 2015;10:9. doi: 10.1186/s13020-015-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tazaki G, Kondo T, Tajiri S, Tsuji C, Shioya S, Tanigaki T. Functional residual capacity and airway resistance in rats of COPD model induced by systemic hyaluronidase. Tokai J Exp Clin Med. 2006;31:125–127. [PubMed] [Google Scholar]
- 30.An ZP. BS: Lung function test in wistar rats. Chin J Lab Anim Sci. 2013;12:102–104. [Google Scholar]
- 31.Mercado N, To Y, Ito K, Barnes PJ. Nortriptyline reverses corticosteroid insensitivity by inhibition of phosphoinositide-3-kinase-δ. J Pharmacol Exp Ther. 2011;337:465–470. doi: 10.1124/jpet.110.175950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou R, Luo F, Lei H, Zhang K, Liu J, He H, Gao J, Chang X, He L, Ji H, et al. Liujunzi Tang, a famous traditional Chinese medicine, ameliorates cigarette smoke-induced mouse model of COPD. J Ethnopharmacol. 2016;193:643–651. doi: 10.1016/j.jep.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Tian YG, Li JS, Dong YQ, Wang MH, Feng SX, Li LL, Mao J, Wang LL, Luo S. Bufei Yishen granules combined with acupoint sticking therapy suppress oxidative stress in chronic obstructive pulmonary disease rats: Via regulating peroxisome proliferator-activated receptor-gamma signaling. J Ethnopharmacol. 2016;193:354–361. doi: 10.1016/j.jep.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Li X, Qin Y. Exploration of regularity of treating AECOPD based on cluster analysis. J Emerg Tradit Chin Med. 2014;23:1436–1437. [Google Scholar]
- 35.Wang ZY, Gu C. Effect of BaiBuNong decoction on rat COPD model histopathology and inflammatory mediators. J Shandong Tradit Chin Med. 2014;33:1010–1013. [Google Scholar]
- 36.Wang YX, XM, Zhao QP. Effect of rectal delivery Pingchuan decoction on TNF-α, IL-8 and VEGF in rat COPD model. J Guangming Tradit Chin Med. 2014;29:705–707. [Google Scholar]
- 37.Lei ZL, GY, Zhong HW. Effect of ShenHaYiFei capsule on TNF-α expression in rat COPD model. Henan Tradit Chin Med. 2014;34:822–825. [Google Scholar]
- 38.Shang LX, WY, Zhang LZ. Effect of AiLuoKeChuanNing on inflammatory mediators and oxidative stress in rat COPD model. Chin J Exp Tradit Med Formulae. 2014;24:168–171. [Google Scholar]