Abstract
The present study aimed to evaluate the immune effect of intramuscular primary immunization by the nucleic acid vaccine pcDNA/glycerophosphodiester phosphodiesterase-interleukin-2 (pcDNA/Gpd-IL-2) and enhanced immunization 2 weeks later with the combination of mucosal adjuvant CpG-oligodeoxynucleotides (ODN) and Gpd-IL-2 recombinant protein on skin infection caused by Treponema pallidum (Tp) in New Zealand rabbits. At week 8 following immunization, MTT assay was used to detect spleen cell proliferation, while enzyme-linked immunosorbent assay was performed to detect the cytokine and secretory immunoglobulin A (SIgA) levels. At week 10 after primary immunization, rabbits were inoculated with 105 Tp (Nichols strain). Alterations in the skin redness, swelling and ulceration were recorded for 0–60 days. In addition, positive rate of Tp in skin lesions and ulcer formation rate were examined using dark field and silver staining. The results indicated that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 followed by enhanced immunization via nasal feeding with mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein induced the higher levels of Tp Gpd specific antibodies, increased the secretion of IL-2 and interferon-γ, and promoted the proliferation of T cells in the first 8 weeks after immunization. Furthermore, this immunization strategy stimulated the production of mucosa specific SIgA antibody. Thus, this strategy led to the lowest Tp positive and ulcer formation rates at the Tp infection sites, as well as healing of skin lesions on the earliest time point (day 42). In conclusion, immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 followed by enhanced immunization with a combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein is an effective immune strategy to induce strong mucosal immune responses and immune protective effects.
Keywords: CpG-oligodeoxynucleotides, Gpd-interleukin-2, nucleic acid vaccine, Treponema pallidum
Introduction
Syphilis is a sexually transmitted disease with a complex and long course. The annual worldwide incidence of syphilis has increased from 8.4% in 2009 to 11.2% in 2014 (1). The infection and incidence rates of syphilis in China also demonstrated a linear upward trend, and ranked third among all infectious diseases, after viral hepatitis and pulmonary tuberculosis (2,3). Treponema pallidum (Tp), the pathogen of syphilis, has a strong invasive ability and enters the human body through intact mucosa or damaged skin at an early stage of the infection. The local mucosal effects of the body serve an important role against Tp infection at the early stages. Enhancing the effective stimulation of the mucosal immune system by vaccination and immunization strategies may more effectively induce the generation of protective immunity effect in the body; however, this process requires a suitable mucosal immune carrier.
CpG-oligodeoxynucleotides (CpG-ODN) exhibit a strong mucosal adjuvant activity, and is able to promote mucosal immunity and enhance immunogenicity. As a promising mucosal adjuvant, CpG-ODN has a synergistic effect with conventional mucosal adjuvant cholera toxin (CT) and heat labile enterotoxin (LT) and causes no toxic or side effects (4–6). Following primary immunization with nucleic acid vaccines, enhanced immunization with corresponding protein vaccines increases the immune response level and protective immunity effect (7–11).
Previous studies have demonstrated that intramuscular primary immunization by nucleic acid vaccine pcDNA/glycerophosphodiester phosphodiesterase-interleukin-2 (pcDNA/Gpd-IL-2) with mucosal adjuvant CpG-ODN via intranasal immunization does not significantly protect against Tp infection compared with multiple intramuscular immunization groups (12,13). Based on the findings of these studies the present study aimed to evaluate the immune effect of intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-interleukin-2 (pcDNA/Gpd-IL-2) and enhanced immunization via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein on skin infection caused by Tp in New Zealand rabbits.
Materials and methods
Bacterial strain and plasmids
CpG ODN (5′-TCCATGACGTTCCTGACGTT-3′) was phosphorylated and synthesized by Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Recombinant pcDNA/Gpd-IL-2 plasmid and empty plasmid pcDNA3.1(+) (Thermo Fisher Scientific, Inc.) were purified, dissolved in sterile phosphate-buffered saline (PBS) and adjusted to a concentration of 0.2 mg/ml. Recombinant pET28a/Gpd and pET28a/Gpd-IL-2 plasmids (constructed and stored successfully at the Pathogenic Biology Institute at University of South China) were transformed into BL21(DE3) strain following a previously published protocol (14). Following expression and purification of Gpd and Gpd-IL-2 recombinant proteins, their concentrations were adjusted to 0.1 mg/ml according to methods described previously (14).
Animals
A total of 108 female New Zealand rabbits (weight, 3.0–3.5 kg; age, 240 days; Department of Laboratory Animals, University of South China, Hengyang, China) were raised under a temperature of 18–20°C with 60–65% humidity, a normal 12 h light/dark cycle, and given every morning and evening (~40 grams each time) and free access to water without antibiotics. The rabbits were randomly divided into six groups of 18 animals each (Table I). At 2 days prior to immunization, the muscle of inoculation was injected with 0.25% bupivacaine hydrochloride (0.075 mg/kg; cat no. CAS18010-40-7; ApexBiotechnology Corp., Taiwan). According to previous studies (12,13), the vaccines and empty plasmids were inoculated into the left hind leg quadriceps of rabbits via muscle multi-point injection for primary immunization, and the immunization was strengthened via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein 2 weeks later (Table I). At week 8 after immunization, 3 rabbits from each group were subjected to rabbit spleen cell proliferation test, cytokine detection and secretory immunoglobulin A (SIgA) determination from nasopharyngeal and vaginal mucosa samples. The remaining 15 rabbits in each group were used in the syphilis infection model experiments and protective research. At week 10 following the primary immunization (day 0 of infection), all rabbits in the experimental groups (n=15) were inoculated with 105 Tp (Nichols strain) at 8 points on their back. Every 3 days, alterations in the skin redness, swelling and ulceration were recorded by calculating the erythematous diameter for 0–60 days. At day 21 following skin infection, a positive rate of Tp in the skin lesions and the ulcer formation rate were examined using dark field (DF) illuminations/microscopy (cat no. BM-14; Shanghai BM Optical Instruments Manufacture Company Ltd., Shanghai, China; magnification, ×1,600) and modified silver staining (Nikon ECLIPSE E100 light microscope, Nikon Corp., Tokyo, Japan; magnification, ×1,000) at 80°C for 30 sec. All animal experiments were approved by the Governing Animal Welfare Committee of the University of South China and conducted in accordance with the regulations of the institution.
Table I.
Different vaccine preparations in the different rabbit groups.
| Group | No. | Immunization strategy | pcDNA/Gpd-IL-2 vaccinea | Gpd-IL-2 recombinant proteina | CpG-ODN adjuvanta |
|---|---|---|---|---|---|
| A1 | 18 | Primary: pcDNA3.1 control (im) | 100 µg (pcDNA) | – | – |
| Secondary: pcDNA3.1 control (im) | |||||
| A2 | 18 | Primary: pcDNA/Gpd-IL-2 (im) | 100 µg | – | – |
| Secondary: pcDNA/Gpd-IL-2 (im) | |||||
| B1 | 18 | Primary: pcDNA/Gpd-IL-2 (im) | 100 µg | – | – |
| Secondary: pcDNA/Gpd-IL-2 (nasal) | |||||
| B2 | 18 | Primary: pcDNA/Gpd-IL-2 (im) | 100 µg | – | 10 µg |
| Secondary: pcDNA/Gpd-IL-2+CpGODN (nasal) | |||||
| C1 | 18 | Primary: pcDNA/Gpd-IL-2 (im) | 100 µg | 50 µg | – |
| Secondary: Gpd-IL-2 protein (nasal) | |||||
| C2 | 18 | Primary: pcDNA/Gpd-IL-2 (im) | 100 µg | 50 µg | 10 µg |
| Secondary: Gpd-IL-2 protein+CpG ODN (nasal) |
Amount per rabbit. im, intramuscular injection; nasal, nasal mucosal immunization; IL-2, interleukin-2; ODN, oligodeoxynucleotides.
Enzyme-linked immunosorbent assay (ELISA) for the detection of Tp Gpd antibodies
At weeks 0 (immunization day), 2, 4, 6 and 8 after primary immunization, 1 ml ear vein blood was collected from rabbits in each experimental group (n=18) and the serum was obtained by centrifugation at 37°C at 1,600 × g for 10 min for ELISA testing (cat no. 20140312; J&L Biological, Shanghai, China). Briefly, Tp Gpd recombinant protein was diluted with a coating buffer at a ratio of 1:50 and used to coat 96-well ELISA plates at 4°C overnight. Next, the plate was blocked with 10% bovine serum albumin (BSA) and PBS at 37°C for 1 h. Subsequent to washing with PBS for three times, gradient concentrations of rabbit serum were added to the plate, using Tp-positive serum as a positive control. Following incubation at 37°C for 1 h, the plate was washed, followed by the addition of horseradish peroxidase-labeled goat anti-rabbit IgG secondary antibody (1:2,000; cat no. 20140521, Thermo Fisher Scientific, Inc.). After incubation at 37°C for 1 h, the plate was washed with PBS again and subjected to color development with 3,3′,5,5′-tetramethylbenzidine (Shanghai Bioleaf Biotech Co., Ltd. Shanghai, China). The absorbance was read using a Multiskan reader (Thermo Fisher Scientific, Inc.) at 450 nm. Each sample was tested in triplicate.
ELISA for the detection of cytokine levels
At week 8 after immunization, 3 rabbits were randomly selected and sacrificed from each group to collect spleen specimens. The spleen was placed in precooled 5 ml D-Hank's solution (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and ground by a 200 mesh copper mesh to obtain spleen cells. Following centrifugation at 37°C and 1,600 × g for 10 min, the supernatant was discarded, followed by addition of 10 ml red blood cell lysis buffer (139.6 mM NH4Cl and 16.96 mM Tris; pH 7.2). Next, the samples were incubated at in a water bath for 10 min, followed by centrifugation at 1,600 × g and 37°C for 10 min. Subsequent to discarding the supernatant, RPMI-1640 medium containing 10% fetal bovine serum (FBS) was added to resuspend the cells for counting. Cells (6×105/well) were added into a 96-well plate and incubated at 37°C and 50 ml/l CO2 for 72 h. Purified Tp Gpd recombinant protein (20 µg/ml) was used as a stimulating antigen, while concanavalin A (ConA; 10 µg/ml; Sigma-Aldrich, Merck, Darmstadt, Germany) was used as the positive control according to a previously described protocol (14). Wells containing cells that were not stimulated by antigen were used as the negative control. The supernatant of the cell culture medium was collected for determining the concentrations of IL-2 and interferon (IFN)-γ using ELISA kits (J&L Biological, cat nos. 20140502, 20140422).
ELISA for the determination of IgA level
At week 8 following primary immunization, the trachea of the rabbits was dissected and 500 µl PBS was used to wash the nasopharynx. The washing fluid flowing through the nasopharynx was collected, and the nasopharynx was washed for three times to obtain 1,500 µl nasopharyngeal washing fluid. In order to obtain vaginal washing fluid, 500 µl PBS was injected into the vagina of fluid and the washing fluid was collected. A total of 1,500 µl vaginal washing fluid was obtained, and centrifuged at 1,600 × g and 37°C for 10 min. Purified protein was then diluted with coating buffer at a ratio of 1:50, and used to coat a 96-well ELISA plate at 4°C overnight. Next, the plate was blocked with 10% BSA and PBS at 37°C for 1 h. Subsequent to washing with PBS for 5 min three times, the nasopharyngeal and vaginal washing fluids (100 µl/well) were added to the plate in duplicate. Following incubation at 37°C for 1 h, the plate was washed with PBS, followed by addition of a horseradish peroxidase-labeled goat anti-rabbit IgA secondary antibody (1:2,000; cat no. 20140404, Thermo Fisher Scientific, Inc.). Following incubation at 37°C for 1 h, the plate was washed again and subjected to color development and reaction termination. The absorbance at 492 nm was read using a Multiskan reader (Thermo Fisher Scientific, Inc.). Each sample was tested in triplicate.
MTT assay
At week 8 following immunization, spleen cell suspension was diluted with RPMI-1640 medium containing 10% FBS to a density of 4×106/ml. The cells (100 µl/well) were added into a 96-well plate in triplicate. Purified Tp Gpd recombinant protein was used as a stimulating antigen (8 µg/ml), while ConA (5 µg/ml) was used as the positive control. Wells containing cells that were not stimulated by antigen were used as the negative control. Following incubation for 68 h, T cell proliferation was determined using MTT assay and the absorbance was obtained at 570 nm. The results were expressed in terms of the stimulation index (SI), as follows: SI=absorbance of experimental group/absorbance of control group.
Statistical analysis
The results were analyzed using SPSS statistical software (version 19.0; IBM Corp., Armonk, NY, USA). The data are expressed as the means ± standard deviation. One way analysis of variance followed by a Tukey's test was used to analyze the specific antibody titer, cytokine concentration and spleen cell SI data. The positive rate of Tp infection and ulcer formation rate were analyzed using χ2 test. P<0.05 indicated that the difference was statistical significance.
Results
Immunization strategy increases the level of specific IgG antibody in the serum of rabbits prior to the establishment of the syphilis model
Following intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization via nasal feeding with a combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein, ELISA was performed to determine the levels of specific IgG antibody in the serum of rabbits. The data revealed that the levels of specific IgG antibody against Gpd of the A2, B1, B2, C1 and C2 groups were significantly increased compared with that of the A1 group on weeks 4, 6 and 8 after immunization (P<0.001). Of note, the level of specific IgG antibody against Gpd of C2 group was significantly higher in comparison with those of A2, B1, B2 and C1 groups on weeks 4, 6 and 8 after immunization (P<0.05). In addition, the level of specific IgG antibody against Gpd of B1 group was significantly higher compared with those of A2, B2, C1 and C2 groups on weeks 4, 6 and 8 after immunization (P<0.05). However, the levels of specific IgG antibody against Gpd in A2, B2 and C1 were not significantly different from each other (P>0.05; Fig. 1). These results suggest that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein increased the level of specific IgG antibody in the serum of the rabbits.
Figure 1.
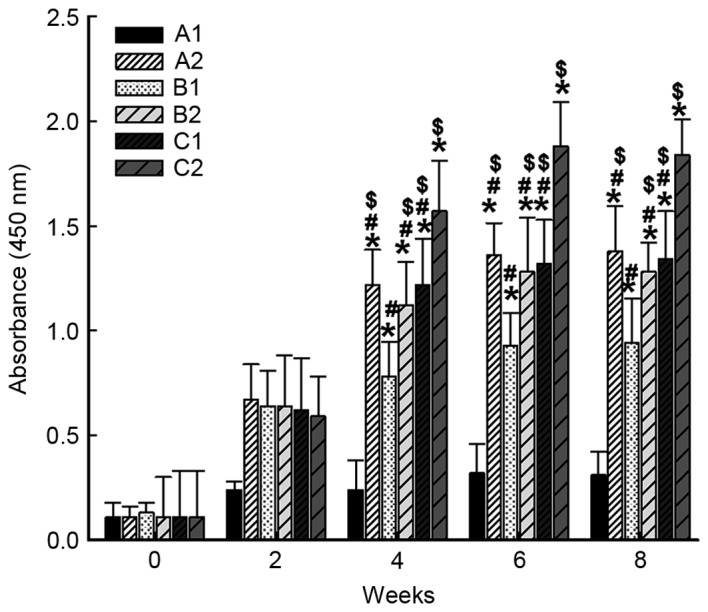
Alterations in the levels of IgG antibodies in the serum of experimental rabbits following immunization. At 0, 2, 4, 6, and 8 weeks after immunization, blood was collected from the rabbit ear veins and the anti-Tp Gpd antibody levels in the serum were determined by enzyme-linked immunosorbent assay. Absorbance was measured at 450 nm. *P<0.05 vs. A1 group at the same time point; #P<0.05 vs. C2 group at the same time point; $P<0.05 vs. B1 group at the same time point. A1, 2× intramuscular immunization with pcDNA3.1 (100 µg); A2, 2× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg); B1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg); B2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg) and CpG-ODN (10 µg); C1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg); C2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg) and CpG-ODN (10 µg); ODN, oligodeoxynucleotides; TP, Treponema pallidum; IL-2, interleukin-2; Gpd, glycerophosphodiester phosphodiesterase.
Immunization strategy elevates the concentrations of IFN-γ and IL-2 in the supernatant of spleen cells
To measure the concentrations of IFN-γ and IL-2 in the supernatant of spleen cell culture, ELISA was employed. The data demonstrated that the concentrations of IFN-γ and IL-2 in the A2, B1, B2, C1 and C2 groups were significantly higher as compared with that in the A1 group on week 8 after immunization (P<0.05). The concentration of IL-2 in B1 group was not significantly different from those in B2 (t=14.867, P=0.414) or C1 (t=31.107, P=0.101), but was significantly lower in comparison with that in the A2 (t=121.967, P<0.05) and C2 (t=151.533, P<0.05) groups (Fig. 2A). In addition, the concentration of IFN-γ in B1 group was significantly lower when compared with that in the A2 (t=85.267, P=0.002), B2 (t=68.467, P=0.009), C1 (t=58.833, P=0.021) and C2 (t=118.467, P<0.05) groups (Fig. 2B). Furthermore, the concentrations of IFN-γ and IL-2 in C2 group were significantly higher compared with those in the B2 and C1 groups (P<0.05), but were not significantly different from those in A2 group (P>0.05; Fig. 2A and B). These results indicate that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein elevated the concentrations of IFN-γ and IL-2 in the supernatant of spleen cells obtained from rabbits.
Figure 2.
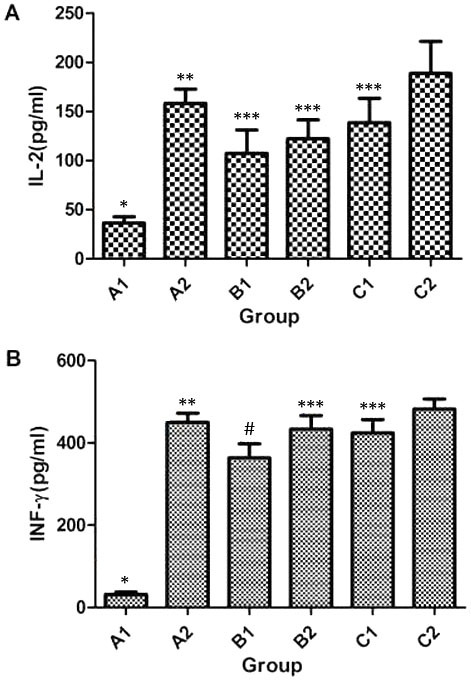
Concentrations of cytokines (A) IL-2 and (B) IFN-γ secreted from spleen cells following Tp Gpd stimulation in vitro. One-way analysis of variance was used for statistical analysis, while pairwise comparison was also performed among A1 to C2 groups. IL-2 (A1, A2, B1, B2, C1 and C2): F=17.268, P<0.05; IFN-γ (A1, A2, B1, B2, C1 and C2): F=113.727, P<0.05. A1, 2× intramuscular immunization with pcDNA3.1 (100 µg); A2, 2× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg); B1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg); B2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg) and CpG-ODN (10 µg); C1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg); C2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg) and CpG-ODN (10 µg); TP, Treponema pallidum; IL-2, interleukin-2; IFN-γ, interferon-γ; Gpd, glycerophosphodiester phosphodiesterase. *P<0.05 vs. A2/B1/B2/C1/C2; **P<0.05 vs. B1; ***P<0.05 vs. C2; #P<0.05 vs. B2/C1/C2.
Immunization strategy promotes the proliferation of spleen cells
In order to examine the proliferation of T cells following immunization, an MTT assay was conducted. The data indicated that the SI of rabbit spleen cells in the A2, B1, B2, C1 and C2 groups were significantly higher compared with that in the A1 group on week 8 after immunization (P<0.001). The SI of rabbit spleen cells in the A2 group was significantly higher than that in the B1 group (t=1.1067, P<0.05), although it was not significantly different from those in B2 (t=0.0.1867, P=0.442), C1 (t=0.4000, P=0.114) or C2 (t=0.2267, P=0.353) groups (Fig. 3). In addition, the SI of rabbit spleen cells in the B1 group was significantly lower in comparison with those of the B2, C1 and C2 groups (P<0.05). By contrast, the SI of rabbit spleen cells in the B2 group was not significantly different from those of the C1 and C2 groups (P>0.05). In the C2 group, the SI of rabbit spleen cells was significantly higher than that of C1 group (P<0.05; Fig. 3). These results suggest that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein promoted the proliferation of spleen cells.
Figure 3.

SI of spleen cells. One-way analysis of variance was used for statistical analysis, while pairwise comparison was performed among A1 to C2 groups. SI (A1, A2, B1, B2, C1 and C2): F=74.675, P<0.05. A1, 2× intramuscular immunization with pcDNA3.1 (100 µg); A2, 2× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg); B1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg); B2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg) and CpG-ODN (10 µg); C1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg); C2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg) and CpG-ODN (10 µg); SI, stimulation index. *P<0.05 vs. A2/B1/B2/C1/C2; **P<0.05 vs. B1; ***P<0.05 vs. B2/C1/C2; #P<0.05 vs. C2.
Immunization strategy stimulates the production of SIgA by the nasopharyngeal and vaginal mucosa
To investigate the levels of SIgA in the nasopharyngeal and vaginal washing fluids, ELISA was used. The data demonstrated that the levels of SIgA in the nasopharyngeal and vaginal washing fluids obtained from A2, B1, B2, C1 and C2 groups were significantly higher compared with those in the A1 group on week 8 after immunization (P<0.05; Fig. 4). In addition, the levels of SIgA in the B1, B2, C1 and C2 groups were significantly higher in comparison with those in A2 group (P<0.05). Furthermore, the levels of SIgA in the two washing fluids in B1 group were significantly lower than those obtained from B2 or C2 groups (P<0.05), but were not significantly different from the levels in the C1 group (P>0.05). In B2 group, the levels of SIgA in the fluids were significantly lower as compared with those in C2 group (P<0.05), but were not significantly different from the levels in C1 group (P>0.05). In addition, the levels of SIgA in nasopharyngeal and vaginal washing fluids from C1 group were significantly lower compared with those from C2 group (P<0.05; Fig. 4). These results indicate that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein stimulated the production of SIgA by the nasopharyngeal and vaginal mucosa.
Figure 4.

Levels of SIgA antibody in (A) nasopharyngeal and (B) vaginal fluid from immunized rabbits. One-way analysis of variance was used for statistical analysis, and pairwise comparison was performed among A1 to C2 groups. Levels of SIgA antibody in nasopharyngeal fluid (A1, A2, B1, B2, C1 and C2): F=46.703, P<0.05. Levels of SIgA antibody in vaginal fluid (A1, A2, B1, B2, C1 and C2): F=113.679, P<0.05. A1, 2× intramuscular immunization with pcDNA3.1 (100 µg); A2, 2× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg); B1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg); B2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg) and CpG-ODN (10 µg); C1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg); C2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg) and CpG-ODN (10 µg); SIgA, secretory immunoglobulin A. *P<0.05 vs. A2/B1/B2/C1/C2; **P<0.05 vs. B1/B2/C1/C2; ***P<0.05 vs. B2/C2; #P<0.05 vs. C2.
Immunization strategy decreases the Tp positive rate and ulcer formation rate at Tp infection sites
In order to compare the Tp positive rate and ulcer formation rate among groups, χ2 test was performed. The Tp DF positive rate and ulcer lesion formation rate in the A2, B1, B2, C1 and C2 groups were significantly reduced compared with those in A1 group (P<0.001). Tp DF positive rate in A2 group was significantly higher compared with those in B2 (χ2=7.467, P=0.006) and C2 (χ2=24.220, P<0.05) groups, but was not significantly different from those in B1 (χ2=0.108, P=0.743) and C1 (χ2=2.092, P=0.205) groups. However, ulcer lesion formation rate of the A2 group was only significantly higher than that of the C2 group (χ2=9.808, P=0.002), and not significantly different from those of the B1, B2 and C1 groups (P>0.05). In addition, the Tp DF positive rate and ulcer lesion formation rate in the B1 group were significantly higher in comparison with those in the B2 and C2 groups (P<0.05), but were not significantly different from those of the C1 group (P>0.05). However, the rates in B2 group were not significantly different from those of C1 group (P>0.05). Furthermore, the Tp DF positive rate of the B2 group was significantly higher compared with that of the C2 group (P<0.05), whereas the ulcer lesion formation rate did not differ significantly between these groups (P>0.05). In C1 group, the Tp DF positive rate and ulcer lesion formation rate were both significantly higher when compared with those of the C2 group (P<0.05; Table II). These results suggest that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein decreased the Tp positive rate and ulcer formation rate at Tp infection sites.
Table II.
Challenge results for immunized rabbits with Treponema pallidum (Nichols strain) spirochetes.
| Group | No. of rabbits | No. of DF+ lesions/total (%)a | P-valueb | No. of ulcerative lesions/total (%) | P-valueb |
|---|---|---|---|---|---|
| A1 | 15 | 110/120 (91.7) | P<0.001c | 106/120 (88.3) | P<0.001c |
| A2 | 15 | 22/120 (18.3) | P<0.05d | 18/120 (15.0) | P=0.002e |
| B1 | 15 | 24/120 (20.0) | P<0.05d | 22/120 (18.3) | P<0.001d |
| B2 | 15 | 8/120 (6.7) | P<0.05e | 10/120 (8.3) | |
| C1 | 15 | 14/120 (11.7) | P<0.001e | 12/120 (10.0) | P<0.05e |
| C2 | 15 | 0/120 (0.0) | 4/120 (3.3) |
Aspirates were taken from lesions and examined for the presence of Treponema pallidum under a DF microscope.
χ2 analysis comparing test values with non-immunized control values (A1, A2, B1, B2, C1 and C2). Pairwise comparison was tested in groups A1-C2. ‘A1-A2’ indicated that pairwise comparison was detected between groups A1 and A2. No. of DF+ lesions/total (%) (A1, A2, B1, B2, C1 and C2): χ2=364.463, P<0.05; no. of ulcerative lesions/total (%) (A1, A2, B1, B2, C1 and C2): χ2=406.986, P<0.05. DF, dark-field microscopy.
vs. A2/B1/B2/C1/C2.
vs. B2/C2.
vs. C2.
Immunization strategy reduces the sizes of erythematous lesions in the shortest time following immunization
To study the effect of immunization on swelling and ulcer size at different time points in each group, the erythematous diameter was calculated. The data revealed that erythema at skin lesions in each group started to appear on day 3 after immunization, with a diameter of 3–5 mm. The erythematous diameters in B2 and C2 groups reached a peak of 9.5 and 9.13 mm, respectively, on day 21, while erythema in the two groups disappeared on days 48 and 42, respectively (Fig. 5). The erythematous diameters in A2 and C1 groups reached a peak of 9.54 mm and 10.52 mm on days 15 and 18, respectively, and the erythema in the two groups disappeared on days 45 and 48, respectively. Furthermore, the erythematous diameter in B1 group reached a peak of 12.13 mm on day 18, and the erythema disappeared on day 54. Erythema in A1 group (pcDNA) appeared on day 6, with a diameter of 2.5 mm, however, it was enlarged quickly and reached a peak of 14.35 mm on day 15. Notably, the erythematous diameter of A1 group remained at 4 mm on day 60, and erythema disappeared after this time point (Fig. 5). These results indicate that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein reduced the sizes of erythematous lesions in the shortest time following immunization compared with other immunization strategies.
Figure 5.
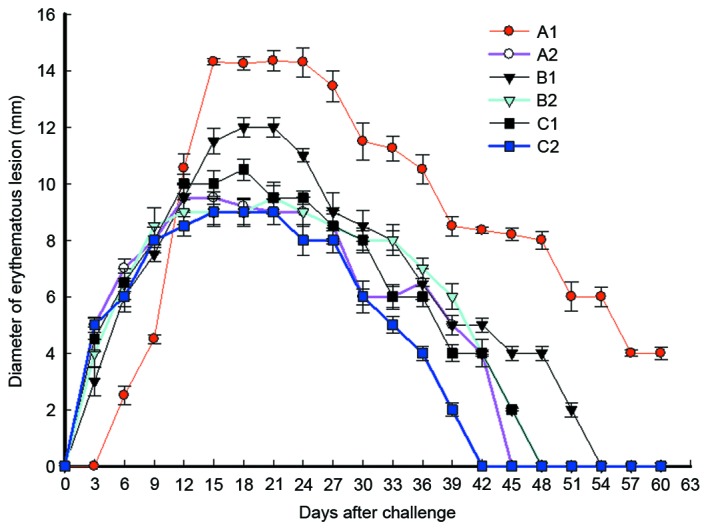
Measurements of erythematous lesion diameters in rabbits infected with Tp on week 10 after immunization at 3-day intervals (between 0–60 days). At week 10, 15 out of 18 rabbits in each group received Tp Gpd DNA vaccine immunization intradermally at eight sites on their shaved backs with 105 Tp (Nichols) spirochetes. Each bar represents the mean ± standard error of diameter of erythematous lesion. A1, 2× intramuscular immunization with pcDNA3.1 (100 µg); A2, 2× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg); B1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg); B2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with pcDNA/Gpd-IL-2 (100 µg) and CpG-ODN (10 µg); C1, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg); C2, 1× intramuscular immunization with pcDNA/Gpd-IL-2 (100 µg) + 1× nasal immunization with Gpd-IL-2 recombinant protein (100 µg) and CpG-ODN (10 µg); TP, Treponema pallidum; Gpd, glycerophosphodiester phosphodiesterase.
Discussion
Tp invades the body mainly through the urogenital tract mucosa, while systemic humoral immunity and cellular immune response are key mechanisms to prevent Tp diffusion and to finally eradicate this bacterial infection (15,16). Specific SIgA antigen in the local mucosa serves an important role in the early resistance to Tp infection (17). Therefore, Tp vaccines should stimulate both the systematic immunity and mucosal immunity. Mucosal vaccines are able to induce mucosal immunity and systematic immunity (18), and their development is easier, quicker, more reliable and cheaper in comparison with other vaccines (19). By contrast, conventional intramuscular injection of a nucleic acid vaccine is only able to induce a low level of local mucosal immunity (20).
Although enhanced immunization by nasal feeding induces the production of a certain level of SIgA in the local nasopharyngeal and vaginal mucosa, the expression of SIgA is usually transient since mucosal epithelial cells typically present a low ability for the uptake and expression of nucleic acids (21). The results of the present study demonstrated that this strategy leads to lower levels of antibody responses and cytokines, as well as to higher levels of SIgA secretion as compared with multiple intramuscular injection of pcDNA/Gpd-IL-2. However, the Tp DF positive rate and ulcer formation rate are not different between the two groups. These observations suggest that SIgA is not the only factor against early Tp infection of the skin.
CpG-ODN can be recognized by Toll-like receptor 9 on dendritic cells and B-cells to induce the production of IFN-α and chemotactic factors, as well as T-helper 1 cell immune response (22). CpG-ODN has a target site, namely pDCs, in mucosal vaccines (23), and it has been reported that CpG-ODN can increase the production of antibodies by 15 times as a vaccine adjuvant for hepatitis B vaccines (24). Consistently, the present study observed that the use of CpG-ODN as an adjuvant (B2 group) enhanced the levels of specific IgG antibody and cytokines, as well as the secretion of SIgA by the nasopharyngeal and vaginal mucosa. In addition, the Tp DF positive rate and ulcer formation rates in the group where CpG-ODN was used as an adjuvant were reduced. These results indicate that this immunization strategy induced strong specific mucosal and systematic immunities, and enhanced the immune protective effect of enhanced nucleic acid vaccine on early Tp infection in the mucosa. This further suggests that SIgA is not the only factor against mucosal Tp infection.
The procedure of immunization by vaccines is divided into three stages, including the antigen recognition stage, antigen-presenting cell activation stage, and antigen presenting and humoral and/or cellular immune response stage (25). CpG-ODN is able to serve a synergistic effect at all these three stages (26). The immunogenicity of protein complexes and peptides has been reported to be weak, and adjuvant is usually required to enhance the immune response (27). Combination of CpG-ODN with a protein antigen promotes the uptake of antigens by tissues and cells, and reduces the number of vaccinations required and immunization time (28). In the present study, it was demonstrated that the systematic immune effect in C2 group was better when compared with that in A2 and C1 groups. In addition, this strategy promoted the secretion of SIgA antibody that is important in mucosal immunity. As a result, the DF positive rate and ulcer positive rate in C2 group were lower in comparison with those in A2 group, and the lesion healing time in C2 group was shortened in comparison with the A2 group. This suggests that the protective effect of the strategy used in the C2 group was better than the strategies used in the A2 or C1 groups.
In conclusion, the present study demonstrated that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization via nasal feeding with the combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein was able to promote the immune protective effect of pcDNA/Gpd-IL-2 nucleic acid vaccines on early mucosal infection by Tp, and induce strong specific humoral immunity and cellular immunity. However, the optimization of the delivery and release system, as well as of the immunization methods, dosage, duration and times, for Tp mucosal nucleic acid vaccines require further investigation.
Acknowledgements
The present study was supported by grants from the Preclinical Medicine Hunan Provincial Key Disciplines, Hunan Provincial Key Laboratory for Special Pathogens Prevention and Control (no. 2014-5), the Hunan Provincial Cooperative Innovation Center for Molecular Target New Drug Study (no. 2014-405), the National Natural Science Foundation (nos. 81373230, 81301470 and 81273322) and the Hunan Provincial Science and Technology Department Foundation (no. 2014TT2025).
References
- 1.Shah BJ, Karia DR, Pawara CL. Syphilis: Is it making resurgence? Indian J Sex Transm Dis. 2015;36:178–181. doi: 10.4103/0253-7184.167170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Zeng L, Ren X, Geng M, Li Z, Yu H. Analysis of morbidity and mortality characteristics of the notifiable diseases reported in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:194–198. (In Chinese) [PubMed] [Google Scholar]
- 3.Zheng N, Guo Y, Padmadas S, Wang B, Wu Z. The increase of sexually transmitted infections calls for simultaneous preventive intervention for more effectively containing HIV epidemics in China. BJOG. 2014;121:35–44. doi: 10.1111/1471-0528.12999. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu K, Serada S, Kobiyama K, Nakagawa S, Morimoto A, Matsuzaki S, Ueda Y, Fujimoto M, Yoshino K, Ishii KJ, et al. CpG oligodeoxynucleotides potentiate the antitumor activity of anti-BST2 antibody. Cancer Sci. 2015;106:1474–1478. doi: 10.1111/cas.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li HT, Zhang TT, Chen ZG, Ye J, Liu H, Zou XL, Wang YH, Yang HL. Intranasal administration of CpG oligodeoxynucleotides reduces lower airway inflammation in a murine model of combined allergic rhinitis and asthma syndrome. Int Immunopharmacol. 2015;28:390–398. doi: 10.1016/j.intimp.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Iho S, Maeyama J, Suzuki F. CpG oligodeoxynucleotides as mucosal adjuvants. Hum Vaccin Immunother. 2015;11:755–760. doi: 10.1080/21645515.2014.1004033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai Y, Zhu Y, Harn DA, Wang X, Tang J, Zhao S, Lu F, Guan X. DNA Vaccination by Electroporation and Boosting with Recombinant Proteins Enhances the Efficacy of DNA Vaccines for Schistosomiasis Japonica. Clin Vaccine Immunol. 2009;16:1796–1803. doi: 10.1128/CVI.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae JY, Moon SH, Choi JA, Park JS, Hahn BS, Kim KY, Kim B, Song JY, Kwon DH, Lee SC, et al. Recombinant DNA and protein vaccines for foot-and-mouth disease induce humoral and cellular immune responses in mice. Immune Netw. 2009;9:265–273. doi: 10.4110/in.2009.9.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endmann A, Klünder K, Kapp K, Riede O, Oswald D, Talman EG, Schroff M, Kleuss C, Ruiters MH, Juhls C. Cationic lipid-formulated DNA vaccine against hepatitis B virus: Immunogenicity of MIDGE-Th1 vectors encoding small and large surface antigen in comparison to a licensed protein vaccine. PLoS One. 2014;9:e101715. doi: 10.1371/journal.pone.0101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nchinda G, Amadu D, Trumpfheller C, Mizenina O, Uberla K, Steinman RM. Dendritic cell targeted HIV gag protein vaccine provides help to a DNA vaccine including mobilization of protective CD8+ T cells; Proc Natl Acad Sci USA; 2010; pp. 4281–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Wang S, Lu S. Pilot study on the use of DNA priming immunization to Enhance Y. pestis LcrV-Specific B cell responses elicited by a recombinant LcrV protein vaccine. Vaccines (Basel) 2014;2:36–48. doi: 10.3390/vaccines2010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao F, Wang S, Zhang X, Gu W, Yu J, Liu S, Zeng T, Zhang Y, Wu Y. Protective efficacy of a Treponema pallidum Gpd DNA vaccine vectored by chitosan nanoparticles and fused with interleukin-2. Can J Microbiol. 2012;58:117–123. doi: 10.1139/w11-115. [DOI] [PubMed] [Google Scholar]
- 13.Zhao F, Liu S, Zhang X, Yu J, Zeng T, Gu W, Cao X, Chen X, Wu Y. CpG adjuvant enhances the mucosal immunogenicity and efficacy of a Treponema pallidum DNA vaccine in rabbits. Hum Vaccin Immunother. 2013;9:753–760. doi: 10.4161/hv.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Feijun, Zhang Xiaohong, Yu Jin, Liu Shuangquan, Zhang Yuejun, Wu Yimou. Expression and identification of Gpd-IL-2 recombinant protein of treponema pallidum and its immuno-competence analysis. J Med Sci Central South China. 2012;40:131–135. (In Chinese) [Google Scholar]
- 15.Syphilis. Nurs Stand. 2015;29:17. doi: 10.7748/ns.29.41.17.s20. [DOI] [PubMed] [Google Scholar]
- 16.Janier M, Hegyi V, Dupin N, Unemo M, Tiplica GS, Potočnik M, French P, Patel R. 2014 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2014;28:1581–1593. doi: 10.1111/jdv.12734. [DOI] [PubMed] [Google Scholar]
- 17.Stamm LV, Drapp RL. A synthetic lymph node containing inactivated Treponema pallidum cells elicits strong, antigen-specifichumoral and cellular immune responses in mice. Pathog Dis. 2014;70:88–94. doi: 10.1111/2049-632X.12098. [DOI] [PubMed] [Google Scholar]
- 18.Khera AK, Afkhami S, Lai R, Jeyanathan M, Zganiacz A, Mandur T, Hammill J, Damjanovic D, Xing Z. Role of B cells in mucosal vaccine-induced protective CD8+ T cell immunity against pulmonary tuberculosis. J Immunol. 2015;195:2900–2907. doi: 10.4049/jimmunol.1500981. [DOI] [PubMed] [Google Scholar]
- 19.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye T, Yue Y, Fan X, Dong C, Xu W, Xiong S. M cell-targeting strategy facilitates mucosal immune response and enhances protection against CVB3-induced viral myocarditis elicited by chitosan-DNA vaccine. Vaccine. 2014;32:4457–4465. doi: 10.1016/j.vaccine.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Umthong S, Buaklin A, Jacquet A, Sangjun N, Kerdkaew R, Patarakul K, Palaga T. Immunogenicity of a DNA and recombinant protein vaccine combining LipL32 and Loa22 for leptospirosis using chitosan as a delivery system. J Microbiol Biotechnol. 2015;25:526–536. doi: 10.4014/jmb.1408.08007. [DOI] [PubMed] [Google Scholar]
- 22.Birk R, Aderhold C, Hörmann K, Wenzel A, Kramer B, Eschenhagen T, Sommer JU. CpG-Oligodeoxynucleotides in Chronic Rhinosinusitis Cell Culture. In Vivo. 2016;30:47–52. [PubMed] [Google Scholar]
- 23.Meng Z, Zhang X, Pei R, Zhang E, Kemper T, Vollmer J, Davis HL, Glebe D, Gerlich W, Roggendorf M, et al. Combination therapy including CpG oligodeoxynucleotides and entecavir induces early viral response and enhanced inhibition of viral replication in a woodchuck model of chronic hepadnaviral infection. Antiviral Res. 2016;125:14–24. doi: 10.1016/j.antiviral.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Xiang XX, Zhou XQ, Wang JX, Xie Q, Cai X, Yu H, Zhou HJ. Effects of CpG-ODNs on phenotype and function of monocyte-derived dendritic cells in chronic hepatitis B. World J Gastroenterol. 2011;17:4825–4830. doi: 10.3748/wjg.v17.i43.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei J, Osen W, Gardyan A, Hotz-Wagenblatt A, Wei G, Gissmann L, Eichmüller S, Löchelt M. Replication-competent foamy virus vaccine vectors as novel epitope scaffolds for immunotherapy. PLoS One. 2015;10:e0138458. doi: 10.1371/journal.pone.0138458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.San R, Omán B, Gómez S, Irache JM, Espuelas S. Co-encapsulated CpG oligodeoxynucleotides and ovalbumin in PLGA microparticles; an in vitro and in vivo study. J Pharm Pharm Sci. 2014;17:541–553. doi: 10.18433/J33892. [DOI] [PubMed] [Google Scholar]
- 27.De Cesare M, Sfondrini L, Pennati M, De Marco C, Motta V, Tagliabue E, Deraco M, Balsari A, Zaffaroni N. CpG-oligodeoxynucleotides exert remarkable antitumor activity against diffuse malignant peritoneal mesothelioma orthotopic xenografts. J Transl Med. 2016;14:25. doi: 10.1186/s12967-016-0781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Zheng M, Zhu XH, Li S, Ni J, Li BL, Liu H, Gao F, Cai JM. Protective effect of CpG-oligodeoxynucleotides against low- and high-LET irradiation. Cell Physiol Biochem. 2014;34:1663–1674. doi: 10.1159/000366368. [DOI] [PubMed] [Google Scholar]


