Abstract
Identification of novel factors critical for epithelial to mesenchymal transition (EMT) and cancer initiating cell (CIC) formation may aid in the identification of novel therapeutics for the treatment of endometrial cancer. The present study demonstrated that L1 cell adhesion molecule (CAM) is critical for EMT and formation of CICs in endometrial cancer. Overexpression of L1CAM may promote EMT with increased formation of CICs in HEC-1A endometrial cancer cells. CICs and mesenchymal status resist chemotherapeutic drugs and may regenerate the various cell types in tumors, thereby resulting in relapse of the disease. The present study demonstrated that overexpressing L1CAM promoted paclitaxel resistance and regulated paclitaxel resistance-associated microRNA expression in HEC-1A cells. Furthermore, it was demonstrated that overexpressing L1CAM promoted anoikis resistance in HEC-1A cells. This link between L1CAM and EMT/CICs may provide a novel target for advancing anticancer therapy.
Keywords: endometrial cancer, cancer initiating cells (CICs), epithelial to mesenchymal transition (EMT), L1CAM, paclitaxel resistance
Introduction
Endometrial carcinoma (EC) is the most common gynecologic malignancy and is associated with a poor prognosis when diagnosed at an advanced stage (1). Endometrial cancer is traditionally classified into type I and type II subtypes (2). Type I cancers account for 80–85% of EC cases, are of endometrioid histology, more often well differentiated and associate with favorable prognosis (2). In contrast the type II cancers are non-endometrioid carcinomas, poorly differentiated and associate with poorer survival (2). However, patients with deep myometrial invasion, poor differentiation, serous or clear cell histology or extension of disease to other organs or lymph nodes within the pelvic region are at higher risk for disease recurrence (3,4). Therefore, it is imperative to find new therapeutic targets to elaborate the molecular mechanisms underlying progression of endometrial carcinogenesis.
L1 cell adhesion molecule (L1CAM, CD171) is a 200–220-kDa transmembrane glycoprotein composed of 6 immunoglobulin-like domains, 5 fibronectin-type III domains, a transmembrane stretch, and a short cytoplasmic tail (5). L1CAM was originally identified as a neural cell adhesion molecule in the central nervous system that plays an important role in initiating cerebellar cell migration and neurite outgrowth (6). L1CAM expression is also found in other cell types such as lymphoid and myelomonocytic cells, kidney tubule epithelial cells, and intestinal crypt cells (7–10). In addition, L1CAM expression has been identified in a variety of tumor types and correlates with poor prognosis and metastasis (11). L1CAM functions mostly in proliferation, migration, invasion, and survival through L1CAM homophilic interaction or heterophilic interactions with other cell adhesion molecules, integrins, or growth factor receptor, while the cellular properties are not homogeneous among different types of cancers (12). Recently, it has been reported that L1CAM was involved in progression of endometrial cancer (13).
Cyclophilin A (CypA) is a highly abundant protein, accounting for up to ~0.6% of the total cytosolic protein content (14). CypA is involved in a growing number of biological processes, including protein folding, signal transduction, viral infection, trafficking, receptor assembly, immune response, and transcription regulation (15). Although several proteins have been identified to interact with CypA (16–19), the underlying mechanism of the CypA action and the physiological implications of the interactions remain in most cases unknown. CypA exhibits peptidyl-prolyl cis-trans isomerase (PPIase) activity by catalyzing cis-trans isomerization of peptide bonds preceding proline residues (20). CypA can in principle act as an enzyme or a binding partner (21) in mediating the biological processes.
In this study, we showed that L1CAM promotes EMT with increased characteristics of CICs and paclitaxel resistance in human endometrial cancer.
Materials and methods
HEC-1A cells line
HEC-1A cells were obtained from Peking Union Medical College (Beijing, China). Briefly, cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma, Shanghai, China) containing 10% fetal bovine serum (FBS; Shanghai ExCell Biology, China) and 100 mg/ml penicillin and streptomycin (Gibco, Shanghai, China) at 37°C in a humidified atmosphere with 5% CO2.
L1CAM expressing plasmids/empty vectors and transfection experiments
L1CAM expressing plasmids and empty vectors (pcDNA3.1) were obtained from Tiangen (Beijing, China). Transfections were performed with Lipofctamine 2000 transfection reagent (Invitrogen, Carlsbad, USA) following the manufacturers' protocols.
Western blot analysis
It was performed as described previously (22,23). Total protein was prepared using extraction buffer comprising NaCl/Pi containing 0.5% Triton X-100, 1 mM EDTA, 1 mM phenylmethyl sulfonyl fluoride, and complete protease inhibitors (Roche). The concentration of each protein lysate was determined using a BCA™ protein assay kit (Thermo Scientific, Rockford, IL, USA). Equal amounts of total protein were subjected to 12% SDS/PAGE. Then samples were transferred to nitrocellulose membranes and blocked for 60 min at room temperature in 5% skim milk powder (w/v) in NaCl/Pi. The membranes were immunoblotted using primary anti-body anti-L1CAM (1:500; Abcam, Cambridge, MA, USA), anti-E-cadherin (1:500; Abcam, Cambridge, MA, USA), anti-Vimentin (1:500; Abcam, Cambridge, MA, USA), anti-Musashi-1 (1:500; Abcam, Cambridge, MA, USA), anti-CD133 (1:500; Abcam, Cambridge, MA, USA), anti-Cyclophilin A (1:500; Abcam, Cambridge, MA, USA) and anti-β-actin (1:500; Abcam, Cambridge, MA, USA) overnight at 4°C, anti-rabbit secondary antibodies (1:10,000; Abcam, Cambridge, MA, USA) were used for 30 min at room temperature. The specific proteins were visualized by Odyssey™ Infrared Imaging System (Gene Company, Lincoln, NE, USA). β-actin expression was used as an internal control to show equal loading of the protein samples.
Immunofluorescence staining
It was performed as described previously (24,25). Cells were plated on glass coverslips in six-well plates and transfected as indicated. At 48 h after transfection, the cells were fixed in 4% paraformaldehyde for 15 min, and then blocked with goat serum blocking solution for 20 min at room temperature. Coverslips were stained with the mentioned antibody mentioned anti-L1CAM antibodies (1:500; Abcam, Cambridge, MA, USA), After washing three times with NaCl/Pi, cells were incubated with appropriate secondary antibodies (Abcam, Cambridge, MA, USA) for 30 min at 37°C. 4′6-diamidino-2-phenylindole (DAPI) staining (blue) was used to indicate nuclei. Microscopic analysis was performed with a confocal laser-scanning microscope (Leica Microsystems, Bensheim, Germany). Fluorescence intensities were calculated from a few viewing areas for 300 cells per coverslip and analyzed by ImageJ 1.37v software (http://rsb.info.nih.gov/ij/index.html).
Quantitative real-time RT-PCR (qRT-PCR)
Quantitative real-time RT-PCR were described before (21). The specific primer sets for PCR were as follows: GAPDH, forward primer: 5′-GAAGGTGAAGGTCGGAGTCA-3′, and reverse primer 5′-GAAGATGGTGATGGGATTTC-3′; E-Cadherin, forward primer 5′-TCAACGATCCTGACCAGCAGTTCG-3′ and reverse primer 5′-GGTGAACCATCATCTGTGGCGATG-3′; N-cadherin, forward primer 5′-CATCCCTCCAATCAACTTGC-3′ and reverse primer 5′-ATGTGCCCTCAAATGAAACC-3′; Vimentin, forward primer 5′-GACAATGCGTCTCTGGCACGTCTT-3′ and reverse primer 5′-TCCTCCGCCTCCTGCAGGTTCTT-3′; ZEB1, forward primer 5′-TTAGTTGCTCCCTGTGCAGTT-3′ and reverse primer 5′-TAGGAGCCAGAATGGGAAAAG-3′. GAPDH was a loading control.
MTT assay
The proliferation of cells was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay (Sigma, St Louis, MO, USA). The MTT analysis was performed as described previously (26–29). In brief, the cells were plated in 96-well plates in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum at a density of 8×103 cells per well at 37°C in a 5% CO2 incubator for 12 h. Cells were transfected with L1CAM expressing plasmid or empty vectors for 24 h and then were treated with different doses of paclitaxel (10−4-102). After 24 h, MTT (5 mg·ml−1) was added to the wells (20 µl per well). The plates were incubated in a cell incubator for 4 h, then the supernatant was removed and 150 µl of dimethyl sulfoxide was added to each well. After incubation for 10 min, the absorbance of each well was measured using a Synergy™ 4 (BioTek Instruments, Winooski, VT, USA) with a wavelength of 570 nm, with the reference wavelength set at 630 nm. Absorbance was directly proportional to the number of survival cells.
Sphere formation assay
It was performed as described previously (30). Cells (103/ml) in serum-free RPMI1640/1 mM Na-pyruvate were seeded on 0.5% agar precoated 6-well plates. After 1 week, half the medium was exchanged every third day. Single spheres were picked and counted.
Anoikis assays
It was performed as described previously (31). Anoikis resistance was evaluated by seeding 7.5×104 cells in ultralow attachment plates (Corning). After 24 h of anchorage-independent culture, cells were transfected as indicated and resuspended in 0.4% trypan blue (Sigma, St. Louis, MO, USA) and cell viability was assessed.
miRNA microarray
It was performed as described previously (32). Total RNA from cultured cells, with efficient recovery of small RNAs, was isolated using the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA). cRNA for each sample was synthesized by using 3′ IVT EXPRESS KIT (Affymetrix, Santa Clara, CA, USA) according to the manufacturer's protocols. The purified cRNA was fragmented by incubation in fragmentation buffer (provided in the 3′IVT express kit) at 95°C for 35 min and chilled on ice. The fragmented labeled cRNA was applied to MicroRNA2.0 Array (Affymetrix, Santa Clara, CA, USA) and hybridized in Genechip hybridization oven 640 (Affymetrix, Santa Clara, CA, USA) at 45°C for 18 h. After washing and staining in Genechip fluidics station 450 (Affymetrix, Santa Clara, CA, USA), the arrays were scanned by using Genechip scanner 3000 (Affymetrix, Santa Clara, CA, USA). The gene expressions levels of samples were normalized and compared by using Partek GS 6.5 (Partek, Inc, St. Louis, MO, USA). Average-linkage hierarchical clustering of the data was applied by using the Cluster [Eisen et al (33), Stanford, Stanford University, CA, USA; http://rana.lbl.gov] and the results were displayed by using TreeView [Eisen et al (33), Stanford, Stanford University, CA, USA; http://rana.lbl.gov].
Statistical analysis
Results were analyzed using SAS software (9.4). Data were presented as mean ± standard error of the mean (SEM) of separate experiments (n=3). P-values less than 0.05 were considered to be significant.
Results
L1CAM promotes EMT in endometrial cancer HEC-1A cells
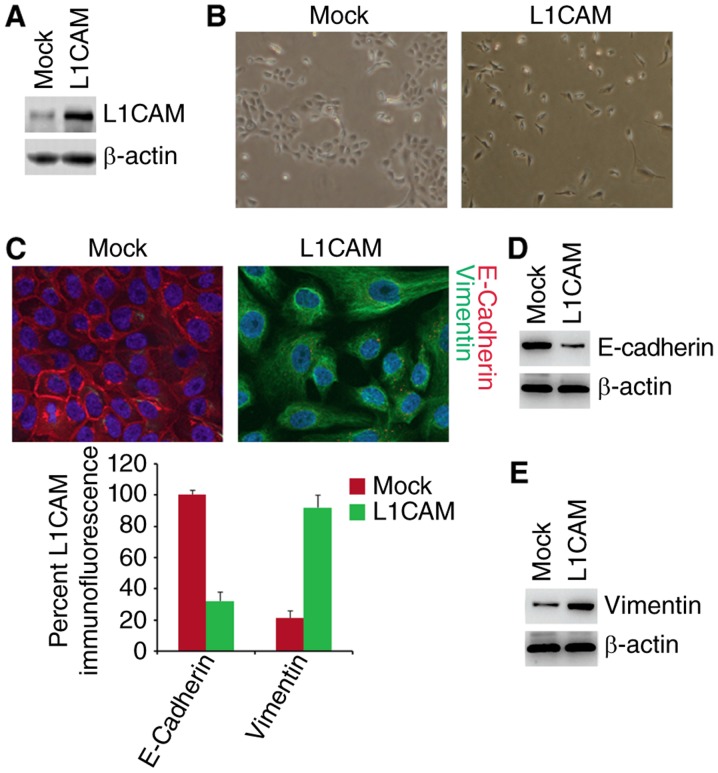
To investigate whether L1CAM can affect epithelial or mesenchymal status of HEC-1A cells, we performed western blot to test whether L1CAM expressing plasmids could express L1CAM protein in HEC-1A cells. The results of western blot showed that L1CAM expressing plasmids can significantly up-regulate L1CAM protein expression in the cells (Fig. 1A). To determine whether L1CAM can promote EMT, we transfected HEC-1A cells with L1CAM expressing plasmids and then observed that its overexpression promoted evident changes in the cells morphology (EMT, epithelial to mesenchymal transiton) (Fig. 1B). To confirm that the changes of morphology are induced by EMT, we performed immunoflurescence analysis to detect epithelial and mesenchymal markers of HEC-1A cells transfected with L1CAM expressing plasmids and empty vectors. We found that that the E-Cadherin protein (epithelial marker) was inhibited and Vimentin protein (mesenchymal marker) were induced by L1CAM in HEC-1A cells (Fig. 1C). To further analyze whether L1CAM could affect E-Cadherin and Vimentin protein, we used western blotting to detect their expression in the cells transfected with L1CAM expressing plasmids and empty vectors. The results demonstrated that E-Cadherin was downregulated and Vimentin was upregulated by L1CAM (Fig. 1D and E). We also performed real-time PCR to detect epithelial and mesenchymal markers. As anticipated, we found that epithelial marker (E-cadherin) was downregulated and mesenchymal markers (such as N-Cadherin, Vimentin, and ZEB1) was upregulated by L1CAM in HEC-1A cells (Fig. 1F).
Figure 1.
L1CAM promotes EMT in endometrial cancer HEC-1A cells. (A) Western blot of L1CAM in L1CAM expressing plasmids and empty vectors (mock) transfected HEC-1A cells. n=3. (B) HEC-1A cells transfected as indicated were photographed after 72 h of transfection. n=3. (C) Immunofluorescence assay of E-cadherin and vimentin in L1CAM expressing plasmids and empty vectors (mock) transfected HEC-1A cells. n=3. (D) Western blot of E-cadherin in L1CAM expressing plasmids and empty vectors (mock) transfected HEC-1A cells. n=3. (E) Western blot of vimentin in L1CAM expressing plasmids and empty vectors (mock) transfected HEC-1A cells. n=3.
L1CAM promotes formation of CICs in HEC-1A cells
EMT can contribute to increased formation of CICs in cancer cells (34–37). To determine whether L1CAM could affect characteristics of CICs, we performed sphere forming assay to evaluate the formation of CICs in HEC-1A cells. The results of sphere forming assay showed that formation of spheres were increased by L1CAM in HEC-1A cells (Fig. 2A). Moreover, we performed western blot to detect whether L1CAM could regulate CICs markers-Musashi-1 and CD133 expression in the cells. We found that Musashi-1 and CD133 protein were evidently upregulated by L1CAM in HEC-1A cells (Fig. 2B and C).
Figure 2.
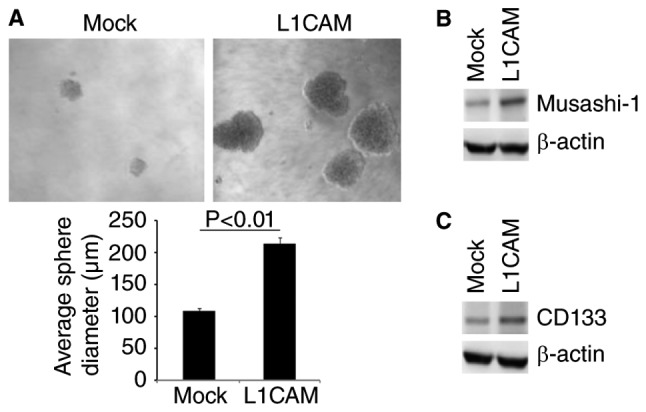
L1CAM promotes formation of CICs in endometrial cancer HEC-1A cells. (A) Sphere growth of HEC-1A cells transfected with L1CAM expressing plasmids and empty vectors (mock). (B) Western blot of Musashi-1 in L1CAM expressing plasmids and empty vectors (mock) transfected HEC-1A cells. n=3. (C) Western blot of CD133 in L1CAM expressing plasmids and empty vectors (mock) transfected HEC-1A cells. n=3.
L1CAM promotes paclitaxel resistance in human endometrial cancer HEC-1A cells
To further identify whether L1CAM can affect paclitaxel efficacy in HEC-1A cells, we performed MTT assay in HEC-1A cells treated as indicated (Fig. 3A). The results showed that overexpressing L1CAM could promote paclitaxel resistance (Fig. 3A). In addition, we performed western blot to analyze cyclophilin A protein expression in L1CAM expressing plasmids and empty vectors transfected HEC-1A cells. We found that cyclophilin A protein can be increased by L1CAM (Fig. 3B).
Figure 3.
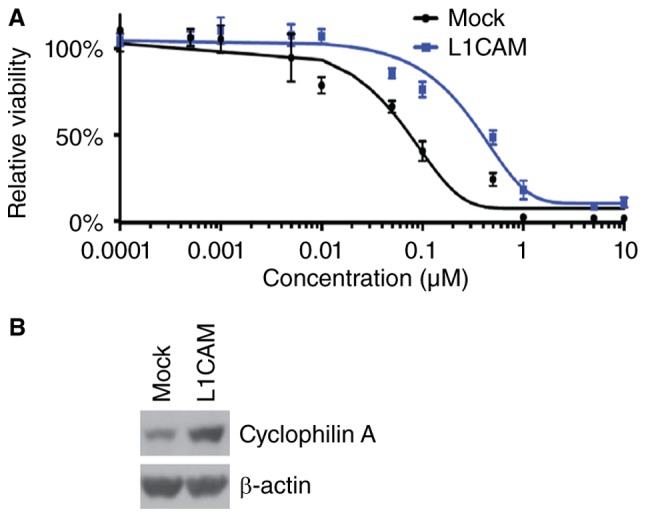
L1CAM promotes paclitaxel resistance in endometrial cancer HEC-1A cells. (A) MTT of HEC-1A cells transfected with L1CAM expressing plasmids and empty vectors (mock) were treated with different concentration of paclitaxel. n=3. (B) Western blot of cyclophilin A in L1CAM and empty vectors (mock) transfected HEC-1A cells. n=3.
L1CAM regulates paclitaxel resistance-associated microRNAs expression in HEC-1A cells
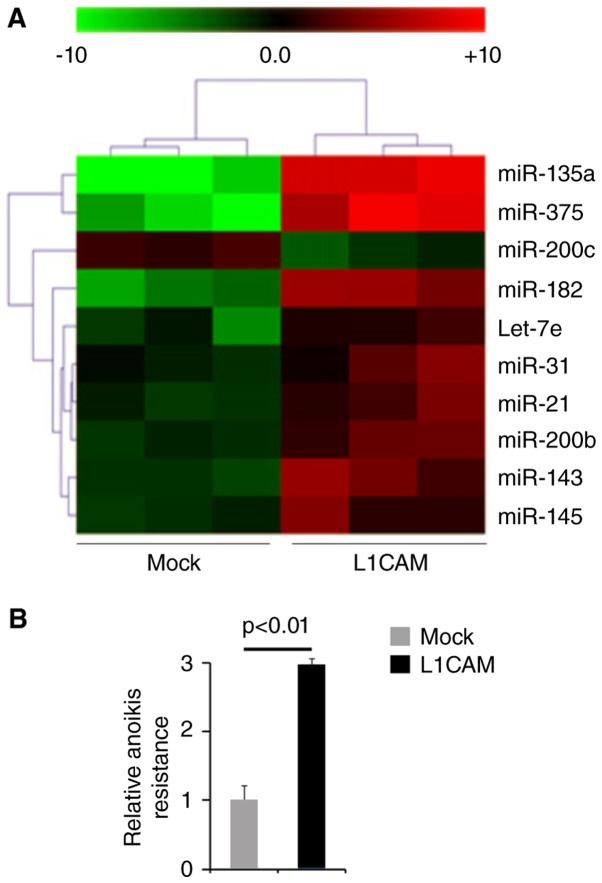
Oncogenes can promote endometrial cancer progression by regulating microRNA expression and microRNA involved in endometrial cancer pathogenesis can function as oncogene or tumor suppressor gene (38–40). Thus, we reasoned that L1CAM could function as an oncogene by regulating miRNAs expression. We performed microarrays to detect miRNA expression. RNAs isolated from L1CAM expressing plasmids or empty vectors transfected HEC-1A cells were hybridized to a custom miRNA microarray platform. We found that miR-135a, miR-375, miR-200c, miR-182, let-7e, miR-31, miR-21, miR-200b, miR-143 and miR-145 were changed more than 10 folds in the cells (Fig. 4A).
Figure 4.
L1CAM regulates paclitaxel resistance-associated microRNAs expression and promotes anoikis resistance in endometrial cancer HEC-1A cells. (A) miRNA microarray analysis for L1CAM expressing plasmids or empty vectors (mock) transfected HEC-1A cells. n=3. (B) Anoikis assays for L1CAM expressing plasmids or empty vectors (mock) transfected HEC-1A cells. n=3.
L1CAM promotes anoikis resistance in human endometrial cancer HEC-1A cells
To study the roles of L1CAM on metastasis, we used anoikis assays to detect its role regulating anoikis resistance. Cells transfected with L1CAM expressing plasmids showed about 200% increased resistance to anoikis-mediated cell death (Fig. 4B).
Discussion
The expression of L1CAM is a strong predictor of poor outcome in endometrial cancer (41). EMT plays an important role in invasion and metastasis of endometrial cancer and enables cancer cells to obtain malignant characters and traits of CICs (42). Critical molecular features of this process are the deregulation of E-cadherin and vimentin expression (43). Consistent with previous report that L1CAM was inversely associated with E-cadherin expression (44), we found that overexpressing L1CAM induced EMT and inhibited E-cadherin expression in endometrial cancer HEC-1A cells.
CICs have been proposed as the major power of EMT and responsible for poor survival (45). Musashi-1 and CD133 have been proposed as markers of CICs for endometrial cancer (46). In line with previous report that L1CAM is required for maintaining CICs and targeting L1CAM may represent a novel therapeutic strategy (47), we showed that its overexpression evidently promoted formation of CICs traits and upregulated Musashi-1 and CD133 protein. All the results indicated that L1CAM might be a therapeutic target for eradicating CICs in endometrial cancer.
Chemotherapy is a common therapeutic strategy for cancer, but it fails to eradicate cancer cells, because of primary resistance or acquired drug resistance. Elucidating the mechanisms of drug resistance for cancer will yield vital information about how to improve cancer chemotherapy and circumvent the resistance. In line with previous report that L1CAM can confer chemoresistance in malignant tumor (48,49), we showed that over-expressing L1CAM could promote paclitaxel resistance in endometrial cancer HEC-1A cells. Cyclophilin A expression was increased in paclitaxel-resistant endometrial cancer cells, as well as silencing Cyclophilin A reversed paclitaxel resistance (50). We showed that Cyclophilin A was upregulated by L1CAM. microRNAs have recently been identified as key genes implicated in mechanisms of chemoresistance. Upregulation of miR-135a and miR-375 can contribute to paclitaxel resistance. Up-regulating miR-200c in ovarian cancer reduced tumor burden and improved paclitaxel sensitivity. We showed that L1CAM significantly upregulatedmiR-135a and miR-375 expression and downregulated miR-200c expression. The results indicated that L1CAM may induce paclitaxel resistance by regulating microRNAs.
Glossary
Abbreviations
- CICs
cancer initiating cells
- EMT
epithelial to mesenchymal transition
- EC
endometrial cancer
References
- 1.Koval OA, Sakaeva GR, Fomin AS, Nushtaeva AA, Semenov DV, Kuligina EV, Gulyaeva LF, Gerasimov AV, Richter VA. Sensitivity of endometrial cancer cells from primary human tumor samples to new potential anticancer peptide lactaptin. J Cancer Res Ther. 2015;11:345–351. doi: 10.4103/0973-1482.157301. [DOI] [PubMed] [Google Scholar]
- 2.Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annu Rev Pathol. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
- 3.Kadar N, Homesley HD, Malfetano JH. Prognostic factors in surgical stage III and IV carcinoma of the endometrium. Obstet Gynecol. 1994;84:983–986. [PubMed] [Google Scholar]
- 4.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A gynecologic oncology group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-K. [DOI] [PubMed] [Google Scholar]
- 5.Haspel J, Grumet M. The L1CAM extracellular region: A multi-domain protein with modular and cooperative binding modes. Front Biosci. 2003;8:s1210–s1225. doi: 10.2741/1108. [DOI] [PubMed] [Google Scholar]
- 6.Rathjen FG, Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984;3:1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pancook JD, Reisfeld RA, Varki N, Vitiello A, Fox RI, Montgomery AM. Expression and regulation of the neural cell adhesion molecule L1 on human cells of myelomonocytic and lymphoid origin. J Immunol. 1997;158:4413–4421. [PubMed] [Google Scholar]
- 8.Debiec H, Christensen EI, Ronco PM. The cell adhesion molecule L1 is developmentally regulated in the renal epithelium and is involved in kidney branching morphogenesis. J Cell Biol. 1998;143:2067–2079. doi: 10.1083/jcb.143.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thor G, Probstmeier R, Schachner M. Characterization of the cell adhesion molecules L1, N-CAM and J1 in the mouse intestine. EMBO J. 1987;6:2581–2586. doi: 10.1002/j.1460-2075.1987.tb02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebeling O, Duczmal A, Aigner S, Geiger C, Schöllhammer S, Kemshead JT, Möller P, Schwartz-Albiez R, Altevogt P. L1 adhesion molecule on human lymphocytes and monocytes: Expression and involvement in binding to alpha v beta 3 integrin. Eur J Immunol. 1996;26:2508–2516. doi: 10.1002/eji.1830261035. [DOI] [PubMed] [Google Scholar]
- 11.Altevogt P, Doberstein K, Fogel M. L1CAM in human cancer. Int J Cancer. 2016;138:1565–1576. doi: 10.1002/ijc.29658. [DOI] [PubMed] [Google Scholar]
- 12.Colombo F, Meldolesi J. L1-CAM and N-CAM: From adhesion proteins to pharmacological targets. Trends Pharmacol Sci. 2015;36:769–781. doi: 10.1016/j.tips.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Notaro S, Reimer D, Duggan-Peer M, Fiegl H, Wiedermair A, Rössler J, Altevogt P, Marth C, Zeimet AG. Evaluating L1CAM expression in human endometrial cancer using qRT-PCR. Oncotarget. 2016;7:40221–40232. doi: 10.18632/oncotarget.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryffel B, Woerly G, Greiner B, Haendler B, Mihatsch MJ, Foxwell BM. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology. 1991;72:399–404. [PMC free article] [PubMed] [Google Scholar]
- 15.Nigro P, Pompilio G, Capogrossi MC. Cyclophilin A: A key player for human disease. Cell Death Dis. 2013;4:e888. doi: 10.1038/cddis.2013.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonfils C, Bec N, Larroque C, Del Rio M, Gongora C, Pugnière M, Martineau P. Cyclophilin A as negative regulator of apoptosis by sequestering cytochrome c. Biochem Biophys Res Commun. 2010;393:325–330. doi: 10.1016/j.bbrc.2010.01.135. [DOI] [PubMed] [Google Scholar]
- 17.Bosco DA, Eisenmesser EZ, Pochapsky S, Sundquist WI, Kern D. Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A; Proc Natl Acad Sci USA; 2002; pp. 5247–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brazin KN, Mallis RJ, Fulton DB, Andreotti AH. Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A; Proc Natl Acad Sci USA; 2002; pp. 1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard BR, Vajdos FF, Li S, Sundquist WI, Hill CP. Structural insights into the catalytic mechanism of cyclophilin A. Nat Struct Biol. 2003;10:475–481. doi: 10.1038/nsb927. [DOI] [PubMed] [Google Scholar]
- 20.Göthel SF, Marahiel MA. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci. 1999;55:423–436. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer G, Tradler T, Zarnt T. The mode of action of peptidyl prolyl cis/trans isomerases in vivo: Binding vs. FEBS Lett. 1998;426:17–20. doi: 10.1016/S0014-5793(98)00242-7. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Wang Q, Yao J, Jiang H, Xiao C, Wu F. MicroRNA let-7g and let-7i inhibit hepatoma cell growth concurrently via downregulation of the anti-apoptotic protein B-cell lymphoma-extra large. Oncol Lett. 2015;9:213–218. doi: 10.3892/ol.2014.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang Y, Lu DL, Li JP, Yu CX, Zheng DL, Huang X, Wang ZY, Hu P, Liao XH, Zhang TC. Myocardin inhibits estrogen receptor alpha-mediated proliferation of human breast cancer MCF-7 cells via regulating MicroRNA expression. IUBMB Life. 2016;68:477–487. doi: 10.1002/iub.1507. [DOI] [PubMed] [Google Scholar]
- 24.Ren ZG, Dong SX, Han P, Qi J. miR-203 promotes proliferation, migration and invasion by degrading SIK1 in pancreatic cancer. Oncol Rep. 2016;35:1365–1374. doi: 10.3892/or.2015.4534. [DOI] [PubMed] [Google Scholar]
- 25.Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li YQ, Yan TB, Sun XG, Hu P, Zhang TC. Estrogen receptor α mediates proliferation of breast cancer MCF-7 cells via a p21/PCNA/E2F1-dependent pathway. FEBS J. 2014;281:927–942. doi: 10.1111/febs.12658. [DOI] [PubMed] [Google Scholar]
- 26.Liao XH, Li YQ, Wang N, Zheng L, Xing WJ, Zhao DW, Yan TB, Wang Y, Liu LY, Sun XG, et al. Re-expression and epigenetic modification of maspin induced apoptosis in MCF-7 cells mediated by myocardin. Cell Signal. 2014;26:1335–1346. doi: 10.1016/j.cellsig.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Liao XH, Wang Y, Wang N, Yan TB, Xing WJ, Zheng L, Zhao DW, Li YQ, Liu LY, Sun XG, et al. Human chorionic gonadotropin decreases human breast cancer cell proliferation and promotes differentiation. IUBMB Life. 2014;66:352–360. doi: 10.1002/iub.1269. [DOI] [PubMed] [Google Scholar]
- 28.Liao XH, Xiang Y, Yu CX, Li JP, Li H, Nie Q, Hu P, Zhou J, Zhang TC. STAT3 is required for MiR-17-5p-mediated sensitization to chemotherapy-induced apoptosis in breast cancer cells. Oncotarget. 2017;8:15763–15774. doi: 10.18632/oncotarget.15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao XH, Li JY, Dong XM, Wang X, Xiang Y, Li H, Yu CX, Li JP, Yuan BY, Zhou J, Zhang TC. ERα inhibited myocardin-induced differentiation in uterine fibroids. Exp Cell Res. 2017;350:73–82. doi: 10.1016/j.yexcr.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Song Z, Liu Z, Sun J, Sun FL, Li CZ, Sun JZ, Xu LY. The MRTF-A/B function as oncogenes in pancreatic cancer. Oncol Rep. 2016;35:127–138. doi: 10.3892/or.2015.4329. [DOI] [PubMed] [Google Scholar]
- 31.Zhang WL, Lv W, Sun SZ, Wu XZ, Zhang JH. miR-206 inhibits metastasis-relevant traits by degrading MRTF-A in anaplastic thyroid cancer. Int J Oncol. 2015;47:133–142. doi: 10.3892/ijo.2015.2993. [DOI] [PubMed] [Google Scholar]
- 32.Xin J, Zhang XK, Xin DY, Li XF, Sun DK, Ma YY, Tian LQ. FUS1 acts as a tumor-suppressor gene by upregulating miR-197 in human glioblastoma. Oncol Rep. 2015;34:868–876. doi: 10.3892/or.2015.4069. [DOI] [PubMed] [Google Scholar]
- 33.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns; Proc Natl Acad Sci U S A; 1998; p. 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 35.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myatt SS, Wang J, Monteiro LJ, Christian M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S, Lam EW. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res. 2010;70:367–377. doi: 10.1158/0008-5472.CAN-09-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YW, Liu JC, Deatherage DE, Luo J, Mutch DG, Goodfellow PJ, Miller DS, Huang TH. Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer. Cancer Res. 2009;69:9038–9046. doi: 10.1158/0008-5472.CAN-09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong P, Kaneuchi M, Watari H, Hamada J, Sudo S, Ju J, Sakuragi N. MicroRNA-194 inhibits epithelial to mesenchymal transition of endometrial cancer cells by targeting oncogene BMI-1. Mol Cancer. 2011;10:99. doi: 10.1186/1476-4598-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dellinger TH, Smith DD, Ouyang C, Warden CD, Williams JC, Han ES. L1CAM is an independent predictor of poor survival in endometrial cancer-An analysis of the cancer genome atlas (TCGA) Gynecol Oncol. 2016;141:336–340. doi: 10.1016/j.ygyno.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friel AM, Sergent PA, Patnaude C, Szotek PP, Oliva E, Scadden DT, Seiden MV, Foster R, Rueda BR. Functional analyses of the cancer stem cell-like properties of human endometrial tumor initiating cells. Cell Cycle. 2008;7:242–249. doi: 10.4161/cc.7.2.5207. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Y, Terai Y, Kawaguchi H, Fujiwara S, Yoo S, Tsunetoh S, Takai M, Kanemura M, Tanabe A, Ohmichi M. Prognostic impact of EMT (epithelial-mesenchymal-transition)-related protein expression in endometrial cancer. Cancer Biol Ther. 2013;14:13–19. doi: 10.4161/cbt.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huszar M, Pfeifer M, Schirmer U, Kiefel H, Konecny GE, Ben-Arie A, Edler L, Münch M, Müller-Holzner E, Jerabek-Klestil S, et al. Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J Pathol. 2010;220:551–561. doi: 10.1002/path.2673. [DOI] [PubMed] [Google Scholar]
- 45.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Götte M, Wolf M, Staebler A, Buchweitz O, Kelsch R, Schüring AN, Kiesel L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol. 2008;215:317–329. doi: 10.1002/path.2364. [DOI] [PubMed] [Google Scholar]
- 47.Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Held-Feindt J, Schmelz S, Hattermann K, Mentlein R, Mehdorn HM, Sebens S. The neural adhesion molecule L1CAM confers chemoresistance in human glioblastomas. Neurochem Int. 2012;61:1183–1191. doi: 10.1016/j.neuint.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Yoon H, Min JK, Lee DG, Kim DG, Koh SS, Hong HJ. L1 cell adhesion molecule and epidermal growth factor receptor activation confer cisplatin resistance in intrahepatic cholangiocarcinoma cells. Cancer Lett. 2012;316:70–76. doi: 10.1016/j.canlet.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Min W, Gou J. Knockdown of cyclophilin A reverses paclitaxel resistance in human endometrial cancer cells via suppression of MAPK kinase pathways. Cancer Chemother Pharmacol. 2013;72:1001–1011. doi: 10.1007/s00280-013-2285-8. [DOI] [PubMed] [Google Scholar]