Abstract
Background
Recent outbreaks of swine-origin influenza A(H3N2) variant (H3N2v) viruses have raised public health concerns. Previous studies indicated that older children and young adults had the highest levels of hemagglutination-inhibition (HI) antibodies to 2010–2011 H3N2v viruses. However, newly emerging 2013 H3N2v have acquired antigenic mutations in the hemagglutinin at amino acid position 145 (N145K/R). We estimated the levels of serologic cross-reactivity among humans primed with seasonal influenza A(H3N2) (sH3N2), using postinfection ferret antisera. We also explored age-related HI antibody responses to 2012–2013 H3N2v viruses.
Methods
Human and ferret antisera were tested in HI assays against 1 representative 2012 H3N2v (145N) and 2 2013 H3N2v (145K/R) viruses, together with 9 sH3N2 viruses circulating since 1968.
Results
Low levels of cross-reactivity between the H3N2v and sH3N2 viruses from the 1970s–1990s were observed using postinfection ferret antisera. The overall seroprevalence among the sH3N2-primed population against 2012–2013 H3N2v viruses was >50%, and age-related seroprevalence was observed. Seroprevalence was significantly higher to 2013 H3N2v than to 2012 H3N2v viruses among some children likely to have been primed with A/Sydney/5/97-like (145K) or A/Wuhan/359/95-like viruses (145K).
Conclusions
A single substitution (N145K/R) was sufficient to affect seropositivity to H3N2v viruses in some individuals. Insight into age-related antibody responses to newly emerging H3N2v viruses is critical for risk assessment and pandemic preparedness.
Keywords: Seroprevalence study, human seasonal Influenza A(H3N2) viruses, swine-origin Influenza A(H3N2) variant viruses, antigenic analysis, serum HI antibody response
Triple-reassortant swine influenza A(H3N2) viruses (tr-H3N2 SIVs), which contain genes from human, classic swine, and avian influenza A viruses, have been enzootic among swine herds in the United States since 1998 [1, 2]. The genes encoding the principal surface glycoprotein of the tr-H3N2 SIVs, hemagglutinin (HA), are genetically similar to the 1995, 1997, or 2010–2011 human seasonal influenza A(H3N2) viruses (sH3N2) [1–3]. The first human case in the United States was identified in 2009 [4], and 2009–2010, 7 additional tr-H3N2–infected cases were reported in the United States, primarily among children [5].
In 2010, a novel reassortant H3N2 SIV containing 7 genes from the tr-H3N2 and the matrix gene from influenza A(H1N1)pdm09 virus was identified in US pigs [6, 7]. From August 2011 to October 2016, a total of 364 human infections with the novel reassortant H3N2 variant (H3N2v) viruses were reported from 13 states [8, 9] (http://www.cdc.gov/flu/swineflu). Twenty-one patients were hospitalized, and 1 fatal case was identified. The number of H3N2v-infected cases was probably underestimated, and limited person-to-person transmission was likely [10]. However, no sustained or community transmission of this virus had been identified [10]. The majority of patients with H3N2v virus were children aged <12 years and had reported swine exposure at agricultural fairs [8, 9].
Antibodies against HA are a primary determinant of protection to influenza infection [11]. Serum hemagglutination-inhibition (HI) antibodies that bind or block the receptor-binding site of HA are widely accepted immune correlates of protection [12]. An HI titer of 40 is generally considered a 50% protective titer for sH3N2 viruses in adult populations [12]. Previous seroprevalence studies conducted by 6 countries indicated that approximately 25%–50% of the human populations were seropositive (with HI titers ≥40) to 2010–2011 H3N2v viruses [13–18]. Age-related seroprevalence, based on the percentage of HI titers ≥40, was noted. The older children and young adults had the highest levels of seropositivity to H3N2v viruses, but most young children (aged <10 years) and older adults (aged 40–60 years) had few or no cross-reactive HI antibodies to these viruses. The reasons for this apparent age-related seroprevalence were not discussed in detail [13–18]. Immunization with the 2010–2011 or 2011–2012 trivalent inactivated seasonal influenza vaccine did not result in HI antibody response to H3N2v viruses in children aged <3 years [19], but it slightly boosted the levels of cross-reactive HI antibodies among some older children, adults, and the elderly [15, 19]. On the other hand, vaccination with inactivated A/Beijing/32/1992 vaccine or 2011–2012 trivalent inactivated seasonal influenza vaccine failed to induce HI antibody response to 2011 H3N2v virus and did not provide any significant cross-protection against the H3N2v virus infection in ferrets [20].
The sH3N2 HA genes introduced into the US SIV gene pool in the 1990s followed an evolutionary path in the swine population separate from their human counterparts [5, 21]. This resulted in 2009–2012 H3N2 SIVs, including H3N2v virus, being antigenically distinct from 1990s tr-H3N2 SIVs and sH3N2 viruses circulating since 1979, observed with HI tests using postinfection ferret antisera [15, 22]. Contemporary H3N2 SIVs demonstrated substantial antigenic diversity [21], and continuous genetic and antigenic evolutions of H3N2 SIVs could generate new SIVs with greater antigenic distance from historical and contemporary sH3N2 viruses. Of particular note are the 2013 H3N2v strains, which possess substitutions at position HA-145 (N145K/R). These H3N2v viruses caused 18 human infections in 2013, mainly among children ≤10 years old (http://www.cdc.gov/flu/swineflu).
Amino acid position 145, located in antigenic site A of H3 HA near the tip of the globular head and the receptor-binding site [23], has been under continual antibody-driven selection since the 1968 H3N2 pandemic [24, 25]. The amino acid substitutions at residue 145 of sH3N2 viruses (S145N, N145K, K145N, and N145S) have been identified in multiple antigenic drift events in the past [26–29]. A single N145K substitution was shown to have a large antigenic effect responsible for the A/Sichuan/1987 to A/Beijing/1989 and A/Beijing/1992 to A/ Wuhan/1995 cluster transitions detected by ferret antisera [26, 27]. Convalescent serum samples collected in 1991 from children (aged 12–14 years) infected with sH3N2 viruses containing 145K either lost or reduced their binding capacity to a mutant virus as well as to the 1993–1994 field strains containing 145N [30]. Moreover, the S145N/R or N145K substitution was associated with an emergence of a new antigenic cluster or outliers in European and the US pigs [21, 31].
The human infections with H3N2v viruses possessing the HA N145K/R mutations in 2013 are of public health concern. In the current study, we evaluated the levels of HI antibodies to 1 2012 H3N2v (145N) virus, 2 2013 H3N2v (145K/R) viruses, and 9 1968–2007 sH3N2 viruses in a large set of human serum samples (from subjects aged 6 to ≥80 years) and postinfection ferret antisera. We also explored whether previous exposures to different sH3N2 antigenic variants is associated with age-related cross-reactivity in US children. Analysis of the antigenic relationships among H3N2v and sH3N2 viruses and continuous monitoring of age-based seroprevalence to newly emerging H3N2v drift strains are important for risk assessment and development of medical countermeasures.
MATERIAL AND METHODS
Viruses
Influenza viruses were propagated either in the allantoic cavity of 10-day-old embryonated eggs or in Madin-Darby Canine Kidney cells, following procedures published elsewhere [32] under BSL2-enhanced conditions for H3N2v or BSL2 conditions for sH3N2 viruses.
Serum Samples
Human serum samples collected in 2010 (N = 1007; subjects aged 6 to ≥80 years) were randomly selected from a large set of serum samples provided by the National Health and Nutrition Examination Survey. The protocol for testing of these serum samples at the Centers for Disease Control and Prevention (CDC) was reviewed and approved by the Institutional Review Board of the CDC’s National Center for Immunization and Respiratory Diseases and the Research Ethics Review Board of CDC’s National Center for Health Statistics. Postinfection ferret antisera were prepared by intranasal inoculation with a single dose of wild-type virus.
HI Assay
Serum samples were treated with receptor destroying enzyme (Denke-Seiken) to remove nonspecific inhibitors and adsorbed with packed turkey red blood cells to remove nonspecific agglutinins before testing with 4 HA units of virus and 0.5% turkey red blood cells by means of HI assays [33].
Statistical Analyses
Fisher exact tests and t tests were used to analyze seroprevalence and geometric mean titers (GMTs), respectively. Differences were considered significant at P < .05. SAS software (version 9.3; SAS Institute) was used for statistical analyses.
RESULTS
Antigenic Analysis
The antigenic relationship among 3 2012–2013 H3N2v and 9 sH3N2 viruses associated with the majority of severe epidemics since 1968 was determined by HI assays using primary-infection ferret antisera. Three H3N2v viruses possessing 145N (A/Ohio/13/2012 [OH/13/12]), 145K (A/Indiana/06/2013 [IN/6/13]), or 145R (A/Indiana/17/2013 [IN/17/13]) were tested with HI assays, together with sH3N2 viruses representative of 9 antigenic clusters since 1968. Nine sH3N2 viruses, their prevalent eras, prominent seasons, and vaccine-use eras in the United States are summarized in Table 1, based on previous publications [34, 35] and the annual US influenza surveillance data.
Table 1.
Periods of Prevalence for sH3N2 Viruses Used in Current Study
| Reference Strain (Abbreviation) | Eras of Prevalencea | Prominent Seasonsb | Vaccine-Use Erasa |
|---|---|---|---|
| A/Aichi/2/1968 (AI/68) | 1968–1972 | 1968–1969; 1969–1970; 1971–1972 | 1968–1969; 1969–1970; 1970–1971; 1971–1972; 1972–1973 |
| A/Victoria/3/1975(VIC/75) | 1975–1977 | 1975–1976 | 1976–1977; 1977–1978 |
| A/Bangkok/1/1979(BK/79) | 1979–1983 | 1980–1981; 1982–1983 | 1980–1981; 1981–1982; 1982–1983; |
| A/Shanghai/11/1987(SH/87) | 1987–1990 | 1987–1988; 1989–1990 | 1989–1990 |
| A/Beijing/32/1992(BJ/92) | 1992–1996 | 1992–1993; 1993–1994 | 1993–1994 |
| A/Wuhan/359/1995(WH/95) | 1995–1998 | 1996–1997 | 1996–1997; 1997–1998 |
| A/Sydney/5/1997(SY/97) | 1997–2000 | 1997–1998; 1998–1999; 1999–2000 | 1998–1999; 1999–2000 |
| A/Fujian/411/2002(FJ/02) | 2002–2005 | 2003–2004 | 2004–2005 |
| A/Brisbane/10/2007(BR/07) | 2007–2009 | 2007–2008 | 2008–2009; 2009–2010 |
As shown in Table 2, the 3 H3N2v viruses were well inhibited by ferret antisera raised against these viruses, although somewhat reduced cross-reactivity (2–4 fold) was noticed between 2012 H3N2v (145N) and 2013 H3N2v (145K/R) viruses. However, these viruses were not inhibited or were poorly inhibited by antisera raised against all 9 sH3N2 viruses. The 3 antisera raised by H3N2v viruses also demonstrated low levels of cross-reactivity to the sH3N2 viruses from the1970s–1990s, and 2 antisera raised to WH/95 or BJ/92 showed low levels of cross-reactivity with the 2012 and/or 2013 H3N2v viruses. These data indicate that the 2012–2013 H3N2v viruses were antigenically distinct from the 1968–2007 sH3N2 viruses but antigenically related to the sH3N2 viruses from the1970s–1990s.
Table 2.
HI Reactions of H3N2v and Human sH3N2 Viruses Using Ferret Antisera
| Viruses | HI Antibody Titers With Primary-Infection Ferret Antiseraa | Passage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H3N2v Viruses | Human sH3N2 | ||||||||||||
| OH/13/12 | IN/5/13b | IN/17/13 | HK/68 | VIC/75 | BK/79 | SH/87 | BJ/92 | WH/95 | SY/97 | FJ/02 | BR/07 | ||
| OH/13/12 | 1280c | 160 | 320 | < | 10 | < | < | 160 | < | < | < | < | C3 |
| IN/6/13b | 320 | 640c | 640 | < | 10 | < | 10 | 40 | 20 | < | < | < | C2 |
| IN/17/13 | 640 | 320 | 1280c | < | < | < | < | 80 | 20 | < | < | < | C2 |
| AI/68 | < | < | < | 1280c | 20 | < | 40 | < | < | < | < | < | E6 |
| VIC/75 | 40 | < | 10 | < | 640c | < | 10 | < | < | < | < | < | E6 |
| BK/79 | 40 | < | < | < | 80 | 320c | 20 | < | < | < | < | < | E9 |
| SH/87 | 20 | < | < | < | 10 | < | 640c | 40 | < | < | < | < | EX/E6 |
| BJ/92 | 40 | < | < | < | < | < | 10 | 1280c | < | < | < | < | E2/E4 |
| WH/95 | < | 40 | 40 | < | < | < | < | 80 | 320c | 40 | < | < | E2/E5 |
| SY/97 | < | 20 | 20 | < | 10 | < | < | 10 | 40 | 1280c | 80 | 20 | C2/E3 |
| FJ/02 | < | < | < | < | < | < | < | < | < | 80 | 2560c | 160 | CX/C5 |
| BR/07 | < | < | < | < | < | < | < | < | < | < | 160 | 1280c | E2/E3 |
Abbreviations: H3N2v, influenza A(H3N2) variant; HI, hemagglutination-inhibition; IN/5/13, A/Indiana/5/2013; IN/6/13, A/Indiana/06/2013; IN/17/13, A/Indiana/17/2013; OH/13/12, A/Ohio/13/2012; sH3N2, human seasonal influenza A(H3N2).
Serum samples from ferrets infected with a single dose of H3N2 wild-type virus. Less than symbols (<) denote HI titers <10.
IN/6/13 and A/Indiana/5/2013 (IN/5/13) have identical hemagglutinin sequences.
Reactions to the homologous virus or antigenically closely related virus.
Age-Related Seroprevalence
To determine the seroprevalence of HI antibodies to OH/13/12, IN/6/13, and IN/17/13, a large set of human serum samples (N = 1007) collected from consecutive ages (6 to ≥80 years) in 2010 were examined in HI assays. Most samples yielded similar HI titers against IN/6/13 and IN/17/13 (data not shown). Therefore, seroprevalence, determined on the basis of an HI titer ≥40, and HI GMTs were evaluated for OH/13/12 and IN/6/13 across 9 separate age group categories (Table 3). We also evaluated seroprevalence and HI GMT differences between OH/13/12 (145N) and IN/6/13 (145K).
Table 3.
Cross-Reactive HI Antibodies to H3N2v Viruses, by Age Group, in US Human Serum Samples Collected in 2010
| Age Group in 2010, y |
Birth Years | Subjects, No. | HI Titer ≥40 (95% CI), % | HI GMT (95% CI) | ||
|---|---|---|---|---|---|---|
| OH/13/12 | IN/6/13 | OH/13/12 | IN/6/13 | |||
| 6–9 | 2001–2004 | 48 | 17 (6–27) | 13 (3–22) | 9 (7–11)a | 10 (8–13)a |
| 10–19 | 1991–2000 | 188 | 62 (55–69)b | 82 (77–88)b | 51 (41–64)b | 90 (76–108)b |
| 20–29 | 1981–1990 | 121 | 88 (83–94) | 90 (85–95) | 105 (84–131) | 97 (81–117) |
| 30–39 | 1971–1980 | 121 | 74 (66–81)b | 55 (47–64)b | 49 (41–58)b | 32 (27–39)b |
| 40–49 | 1961–1970 | 125 | 34 (25–42) | 30 (22–38) | 18 (15–22) | 18 (15–22) |
| 50–59 | 1951–1960 | 99 | 35 (26–45) | 35 (26–45) | 19 (15–24) | 20 (15–25) |
| 60–69 | 1941–1950 | 118 | 44 (35–53) | 48 (39–57) | 25 (20–32) | 27 (21–35) |
| 70–79 | 1931–1940 | 108 | 52 (42–61) | 50 (41–59) | 32 (25–41) | 28 (22–36) |
| ≥80 | 1908–1930 | 79 | 46 (35–57) | 39 (28–50) | 23 (17–31) | 20 (15–27) |
| All ages | 1908–2004 | 1007 | 54 (51–57) | 55 (52–58) | 33 (31–36) | 35 (32–38) |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; H3N2v, influenza A(H3N2) variant; HI, hemagglutination-inhibition; IN/6/13, A/Indiana/06/2013; OH/13/12, A/Ohio/13/2012.
P = .04 for difference in HI GMT between OH/13/12 and IN/6/13.
P < .01 for difference in seroprevalence or HI GMT between OH/13/12 and IN/6/13.
The overall seroprevalence was similar against OH/13/12 (54%) and IN/6/13 (55%), although the 2 viruses showed distinctive age-related patterns. Low seropositive rates for both H3N2v viruses were observed among children 6–9 years old. The proportion of seropositivity increased with age, peaked among those aged 20–29 years, then dropped to approximately one-third among those aged 40–49 or 50–59 years, and remained between 39% and 52% among those aged 60–69, 70–79, or ≥80 years. The GMTs had the same age-related trend as the seroprevalence. However, significant differences (P < .01) in seroprevalence and HI GMTs between IN/6/13 and OH/13/12 were observed in those aged 10–19 or 30–39 years (Table 3).
Interestingly, 8% of individuals (83 of 1007; median age, 17 years) showed the pattern of higher seroprevalence to IN/6/13 than to OH/13/12 (HI titers ≥40 to IN/6/13 and ≥4-fold higher for IN/6/13 than for OH/13/12). In contrast, 4% (38 of 1007; median age. 47 years) showed the pattern of higher seroprevalence to OH/13/12 than to IN/6/13 (HI titers ≥40 to OH/13/12 and ≥4-fold higher for OH/13/12 than for IN/6/13). These 2 patterns were also age related: 61% with higher seroprevalence to IN/6/13 were aged 8–20 years, and 95% with higher seroprevalence to OH/13/12 were aged ≥21 years.
Likely Association Between Seroprevalence in Older Children and First Exposure to sH3N2 Viruses in the 1990s
To determine whether the HI antibodies cross-reactive to 2012–2013 H3N2v viruses were associated with exposures to historic sH3N2 viruses, all serum samples from children (n = 211; aged 6–18 years) from a set of 1007 samples were tested against 2 H3N2v and 8 sH3N2 viruses. These serum samples showed unmeasurable HI antibody response (titer, <10) against AI/68, VIC/75, and BK/79 (data not shown). The age distributions for HI antibodies to the 2 H3N2v and 5 1987–2007 sH3N2 viruses are presented in Figure 1.
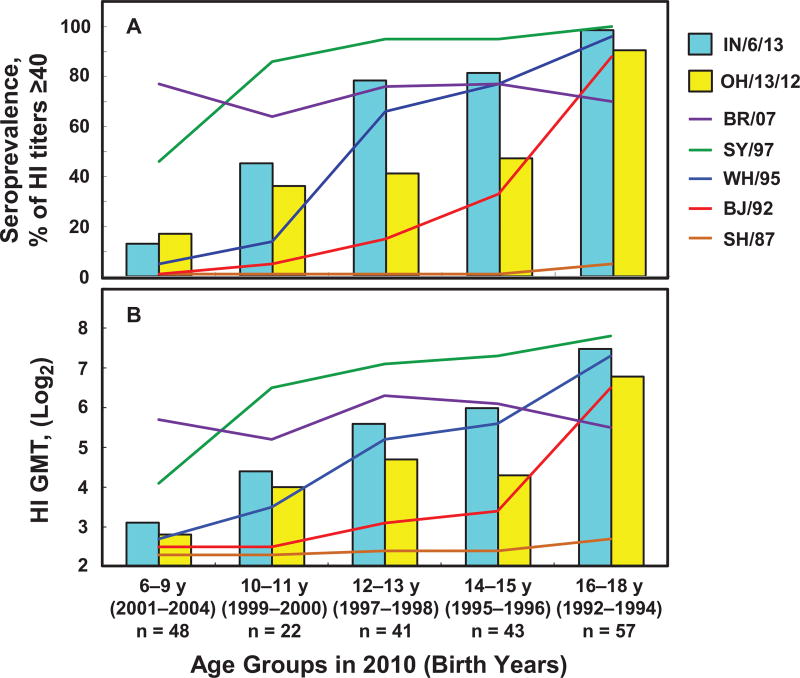
Figure 1.
Prevalence of hemagglutination-inhibition (HI) antibodies cross-reactive to A(H3N2) variant (H3N2v) swine influenza viruses in US children is likely to be associated with exposures to 1990s human seasonal influenza A(H3N2) (sH3N2) viruses. Serum samples from children aged 6 to 18 years (n = 211) were collected in 2010. Seroprevalence (A) and HI geometric mean titers (GMTs) (B) for 2 H3N2v viruses are depicted in bars for A/Indiana/06/2013 (IN/6/13) and A/Ohio/13/2012 (OH/13/12). The seroprevalence and GMTs for the 5 human sH3N2 viruses isolated between 1987 and 2007 are shown as lines. Age groups at the time of serum sample collection (2010) and years of birth are shown on the x-axis. The seroprevalence and HI GMTs against IN/6/13 compared with OH/13/12 were significantly higher in 12–13 and 14–15 age groups.
The majority of children (79%) aged 6–9 years (born 2001–2004) showed seropositivity to BR/07, but ≤17% showed seropositivity to the 2 H3N2v viruses. Increased seroprevalence to IN/6/13 (46%) and OH/13/12 (36%) were observed among 10–11-year-olds (born 1999–2000), along with increasing seroprevalence (86%) to SY/97, which circulated between 1997 and 2000. Further increased seroprevalence to IN/6/13 (80%) was observed in the 12–13- and 14–15-year age groups (born 1995–1998), accompanied by increased seroprevalence (77%) to WH/95, which circulated between 1995 and 1998. Among 16–18-year-olds (born 1992–1994), seroprevalence (~90%) to IN/6/13 and OH/13/12 was the highest, with increasing seroprevalence (88%) to BJ/92, which circulated between 1992 and 1996. Analysis using GMTs demonstrated the same age-related trend as seroprevalence. These data suggested that HI antibodies cross-reactive to 2012 and/or 2013 H3N2v viruses among children (aged 10–18 years) were probably associated with their first exposure to different sH3N2 viruses in the 1990s.
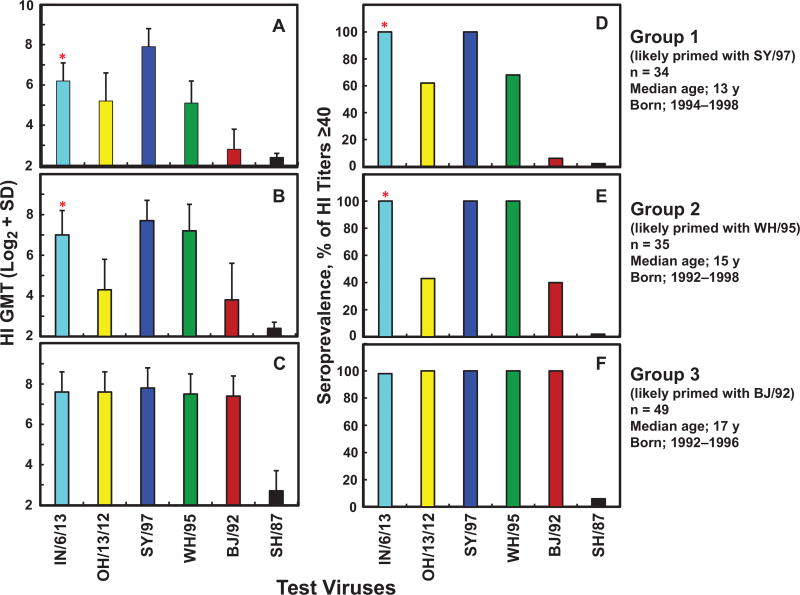
To further clarify the age-related seroprevalence in the older children, the HI data from 141 children (aged 12–18 years) were selected and further analyzed, and 124 children (88%) were seropositive to either IN/6/13 or OH/13/12, or both. Finally, we selected the 118 H3N2v-seropositive children and grouped them into 3 groups based on the 3 unique HI antibody patterns against the 4 1987–1997 sH3N2 viruses (Figure 2 and Supplementary Tables 1–3). Group 1 (median age, 13 years in 2010) included children showing the highest HI titers to SY/97, which were significantly higher (≥4-fold) than titers to WH/95, BJ/92, and SH/87 (Figure 2A and Supplementary Table 1). Group 2 (median age, 15 years in 2010) included children exhibiting similar levels (within 2-fold) of HI antibody titers against SY/97 and WH/95, with titers to WH/95 virus significantly higher than those to BJ/92 and SH/87 (Figure 2B and Supplementary Table 2). Group 3 (median age, 17 years in 2010) included children with similar levels of HI titers to SY/97, WH/95, and BJ/92, with titers to BJ/92 significantly higher than those to SH/87 (Figure 2C and Supplementary Table 3).
Figure 2.
Priming with different 1990s human seasonal influenza A(H3N2) (sH3N2) viruses in US children influences seroprevalence of hemagglutination-inhibition (HI) antibodies to 2012–2013 influenza A(H3N2) variant (H3N2v) virus. Serum samples from children aged 12–18 years (n = 118) were collected in 2010; median ages are for this year. HI geometric mean titers (GMTs) and seroprevalence against the 2 H3N2v and 4 sH3N2 viruses are shown for 3 groups of children likely to have been primed with SY/97 (A, D), WH/95 (B, E), or BJ/92 cluster (C, F). *Significantly higher seroprevalence and HI GMTs against A/Indiana/06/2013 (IN/6/13) compared with A/Ohio/13/2012 (OH/13/12) in groups 1 and 2 (P < .01). Abbreviation: SD, standard deviation.
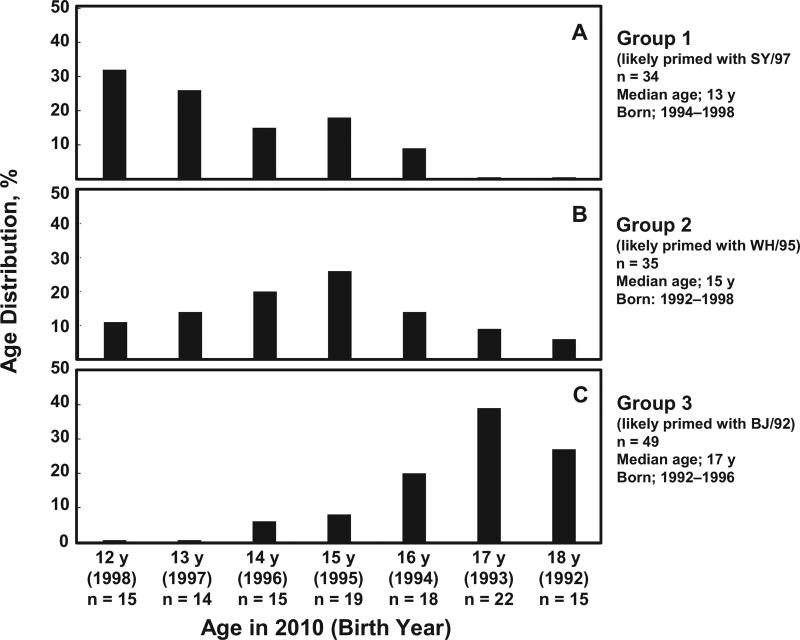
Consistent with recent publications [36–38], our data also showed varied levels of cross-reactive HI antibodies to SH/87, which circulated before the birth of these children (Table 1 and Supplementary Tables 1–3). Considering that the first sH3N2 virus infection can occur among children aged 2 months to 7 years old [39, 40], the age distribution (Figure 3) correlated well with what is known as the “period of prevalence” of the SY/97, WH/95, and BJ/92 antigenic clusters (Supplementary Table 4). Thus, it is reasonable to suggest that most children, if not all, in groups 1, 2, and 3 were highly likely to be primed with the SY/97, WH/95, and BJ/92 clusters, respectively. It is noteworthy that 6 H3N2v-seropositive children were excluded from the analysis (data not shown), including 1 child with the highest HI titers to sH3N2 viruses circulated after 2000, 1 with low HI titers to the sH3N2 viruses tested, and 4 with HI titers to WH/95 2-fold higher than those to BJ/92 but titers to IN/6/13 ≥4-fold higher than those to OH/13/12. The antibody population of these 4 serum samples was verified by antibody adsorption assays; WH/95-specific antibody, but not BJ/92-specific antibody, was detected, suggesting that these children were probably primed with WH/95-like viruses (data not shown). Overall, the 118 samples analyzed in Figure 2 represented 95% of H3N2v-seropositive children available in this age group.
Figure 3.
Age distribution in 3 groups of children (aged 12–18 years, n = 118) likely to have been primed with SY/97, WH/95, or BJ/92 cluster. Median ages shown are for 2010.
All children in group 1, likely to have been primed with SY/97-like virus, showed seropositivity to IN/6/13 and SY/97; 62% also showed seropositivity to OH/13/12 (Figure 2D). Although only a small portion of the children (21%) showed significantly higher HI titers (≥4 fold) against IN/6/13 than against OH/13/12, the data suggested that a certain portion of antibodies in these children could recognize an epitope containing 145K on IN/6/13 HA. All children in group 2, likely to have been primed with WH/95-like virus, showed seropositivity to IN/6/13, WH/95, and SY/97, but only 43% showed seropositivity to OH/13/12 (Figure 2E). Most children (91%) in group 2 had significantly higher HI titers ((≥4 fold) against IN/6/13 than against OH/13/12, thus suggesting that dominant HI antibodies cross-reacting with IN/6/13 in most WH/95-primed children bind to an epitope containing 145K. Most children (98%) in group 3, likely to have been primed with BJ/92-like virus, showed seropositivity and similar levels of HI antibody responses to the 2 H3N2v and the 3 sH3N2 viruses (Figure 2F). Taken together, these data suggested that primary infections with the SY/97, WH/95, or BJ/92 cluster were highly likely to be associated with the age-related seroprevalence to 2012–2013 H3N2v viruses in older children.
Comparison of Amino Acid Sequence in Antigenic Site A Among H3N2 Viruses
To better understand the molecular basis of the different seroprevalence patterns to OH/13/12 and IN/6/13 in children, we compared HA1 amino acid sequences of the H3N2v and the sH3N2 viruses. Because there was only a single amino acid difference at position 145 (antigenic site A) in the entire HA1 domain between the OH/13/12 and IN/6/13, but the 2 viruses demonstrated a moderate antigenic variation (2–4-fold HI titer differences; Table 2), our analysis focused on antigenic Cross-Reactive Antibody to H3N2v Viruses • JID 2017:216 (Suppl 4) • S545 site A. An amino acid difference in antigenic site A between the sH3N2 and the H3N2v viruses is presented in Table 4. The IN/6/13 and WH/95 shared several amino acids in antigenic site A, particularly the 145K, which might provide an explanation of the high seroprevalence to IN/6/13 among possible WH/95-primed children [30]. The data also suggested that the majority of HI antibodies cross-reactive to IN/6/13 among WH/95-primed children bound to an epitope possessing 145K. A single N145K substitution in H3N2v virus HA is sufficient to change an individual’s seropositivity and significantly influence seroprevalence in some age groups.
Table 4.
Antigenic Site A Variation Among Human sH3N2 Viruses and 2012–2013 H3N2v Viruses
| Amino Acid Residues in Antigenic Site Aa
|
Glycosylation Sites, No.b |
Accession No.c |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | 122 | 124 | 126 | 131 | 133 | 135 | 137 | 138 | 140 | 142 | 143 | 144 | 145 | 146 | ||
| sH3N2 viruses | ||||||||||||||||
| SH/87 | N | D | Nd | T | S | G | Y | A | K | G | S | V | N | S | 1 | AF008886 |
| BJ/92 | … | … | … | A | D | … | … | … | … | … | … | … | … | … | 1 | CY113677 |
| WH/95 | … | G | … | A | D | T | … | … | … | … | … | … | K | … | 1 | EU501120 |
| SY/97 | Nd | S | … | A | Nd | T | … | … | … | S | … | I | K | … | 3 | CY112885 |
| BR/07 | Nd | S | … | … | Nd | T | S | … | I | R | … | Nd | … | … | 4 | EU199366 |
|
| ||||||||||||||||
| H3N2v viruses | ||||||||||||||||
| OH/13/12 | Q | S | … | A | D | Y | … | … | R | … | … | … | … | … | 1 | EPI381857 |
| IN/6/13 | Q | S | … | A | D | Y | … | … | R | … | … | … | K | … | 1 | EPI461874 |
| IN/17/13 | Q | S | … | A | D | Y | … | … | R | … | … | … | R | … | 1 | EPI462212 |
Abbreviations: H3N2v, influenza A(H3N2) variant; IN/6/13, A/Indiana/06/2013; IN/17/13, A/Indiana/17/2013; OH/13/12, A/Ohio/13/2012; sH3N2, human seasonal influenza A(H3N2).
The aa sequence in antigenic site A; ellipses denote sequence identical to that for SH/87 virus.
Numbers of potential glycosylation sites in antigenic site A.
Hemagglutinin sequences were accessed from the National Center for Biotechnology Information or the GISAID database.
Potential glycosylation sites (N-X-S/T).
DISCUSSION
Using human and postinfection ferret antisera, we evaluated the levels of HI antibodies against 2012–2013 H3N2v viruses, together with 9 epidemiologically important sH3N2 viruses circulating since 1968. Children born after 2000 had lowest level of cross-reactive HI antibodies to 2012–2013 H3N2v viruses tested. This is consistent with results of previous population serosurveys with 2010–2011 H3N2v viruses [13–18], suggesting that young children are at high risk of infection with the H3N2 SIVs. In agreement with the serological findings, the majority of confirmed H3N2v virus infections in 2011–2015 occurred in children ≤10 years old [9] (http://www.cdc.gov/flu/swineflu). However, more than half of individuals born before 2000 showed seropositivity to 2012–2013 H3N2v viruses.
Low degrees of serologic cross-reactivity between 1975–1997 sH3N2 and the 2012–2013 H3N2v viruses were observed in ferrets after primary infection. However, a high degree of cross-reactivity was observed in some individuals after multiple exposures. Moreover, age-related seroprevalence was observed in humans, and most older children and young adults showed seropositivity to the H3N2v viruses. A 2016 study indicated that HI antibody patterns in ferrets and young children after primary infection with sH3N2 viruses is generally similar [38]. An earlier study found that primary infection with sH3N2 virus could occur in 2-month-old infants, and the second infection in those 12 months old [40].
Our cross-HI data demonstrated that most individuals tested in this study had experienced secondary or even >2 exposures to sH3N2 viruses. Secondary exposures with antigenic drifted strains could shape HI antibody patterns by producing antibodies targeting different subdominant epitopes on the HA [41–43], which may be responsible for the difference observed between the ferrets and humans in this study. It would be interesting to study how the age-related HI antibodies change as an individual experiences first, second, or multiple exposures to sH3N2 antigenic variants. Consistent with previous findings [36, 37, 44], our data indicated that primary exposure with sH3N2 viruses influenced HI antibody response thereafter. Because HI antibodies target the HA globular head and neutralizing antibodies target both globular head and stalk domain [45], it would also be interesting to investigate to what extent such primary exposure with sH3N2 virus affects the neutralizing antibody response.
A single substitution (N145K) was sufficient to affect individuals’ seropositivity to 2012–2013 H3N2v viruses. Significantly higher seropositivity (80%) to IN/6/13 (145K) compared with OH/13/12 (145N; 44%) was observed in children born between 1995 and 1998. The seropositivity to IN/6/13 among older children was also remarkably higher than that to 2011 H3N2v viruses possessing 145N [13, 14, 16]. BJ/92, WH/95, and SY/97 are the representative sH3N2 viruses circulated between 1992 and 2000. To better understand the age-related antibody patterns, we have, for the first time, determined the priming sH3N2 virus in older children with unknown exposure histories. The priming sH3N2 viruses, determined based on each child’s HI antibody pattern, were consistent with surveillance data reflecting the period during which the 3 sH3N2 antigenic cluster circulated in the US population. Grouping the children into 3 sH3N2 primed groups allowed us to understand how sH3N2 priming histories influence cross-reactivity for 2012–2013 H3N2v viruses. For example, most children who were highly likely to have been primed with WH/95-like viruses showed similar levels of HI antibody responses to the WH/95, SY/97, and IN/6/13 viruses, all 3 containing 145K, but their HI antibody levels against OH/13/12 (145N) and BJ/92 (145N) were significantly lower or even undetectable.
Previous studies showed that convalescent serum samples from children infected with BJ/89-like viruses (145K) and antisera from ferrets infected with WH/95-like virus (145K) had significantly decreased HI activity to H3N2 viruses possessing 145N [30, 46]. The addition of glycans at Asn133 on SY/97 HA should not mask the 140 loop [47]. Moreover, a human 145K-specific monoclonal antibody could cross-react with SY/97-like viruses [48]. The HI pattern in the WH/95-primed children, together with previous findings [30, 38, 46–48] suggests that reinfection with SY/97-like viruses probably occurred in these children. The B memory cells targeting an epitope possessing 145K might be restimulated by reinfection with SY/97-like viruses, which might resulted in dominance of the antibodies directed to the epitope possessing 145K in WH/95-primed children [41]. These antibodies are likely to recognize WH/95 (145K), SY/97 (145K), and IN/6/13 (145K) but not OH/13/12 (145N).
Children who were likely to have been primed with SY/97-like virus showed significantly higher levels of HI antibodies to SY/97, compared with 2012–2013 H3N2v viruses, indicating that the majority of these antibodies could not cross-react with 2012–2013 H3N2v viruses. Some SY/97-primed children showed the pattern of higher seroprevalence to IN/6/13 than to OH/13/12, suggesting that some antibodies binding to an epitope possessing 145K have, in part, contributed to the cross-reactivity to IN/6/13 virus. Children primed with BJ/92-like virus showed equally high levels of HI antibody activity to 2012–2013 H3N2v and 1992–1997 sH3N2 viruses, regardless of 145N or 145K, suggesting that there are alternate common epitope(s) between the sH3N2 and 2012–2013 H3N2v viruses. The pattern of higher HI antibody activity to OH/13/12 than to IN/6/13 was observed mainly in individuals born before 1989. It is unknown whether this pattern was associated with exposures to sH3N2 viruses possessing 145N, which circulated in 1970s and 1980s [26]. Further studies are warranted to clarify the influence of priming histories in the cross-reactivity to H3N2v viruses among adults and elderly. The nature of age-related cross-reactive antibodies in different age populations should also be further addressed.
H3N2v viruses can efficiently replicate and transmit in mammals [49]. The continued evolution of H3N2 SIVs among pigs may generate a novel virus, to which the majority of humans, particularly older children and younger adults, may have little or no preexisting immunity. Improved surveillance in pigs for newly emerging H3N2 SIVs remains important. Insight into age-related cross-reactive antibody patterns against circulating SIVs among different age groups is critical in identifying the risks of viruses in causing human infection and interspecies transmission with these viruses, and their pandemic potentials.
Supplementary Material
Acknowledgments
We thank Heather Tatum and Leilani Thomas in the Influenza Division of the CDC for their excellent assistance with this study, the CDC’s National Health and Nutrition Examination Survey for providing human serum samples, and The Influenza Division’s Influenza Sequencing Activity Team for assistance with sequencing.
Financial support. This work was supported by the CDC.
Footnotes
Presented in part: “Options IX for the Control of Influenza,” Chicago, Illinois, 24–28 August 2016.
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Supplement sponsorship. This work is part of a supplement sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zhou NN, Senne DA, Landgraf JS, et al. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J Virol. 1999;73:8851–6. doi: 10.1128/jvi.73.10.8851-8856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webby RJ, Swenson SL, Krauss SL, Gerrish PJ, Goyal SM, Webster RG. Evolution of swine H3N2 influenza viruses in the United States. J Virol. 2000;74:8243–51. doi: 10.1128/jvi.74.18.8243-8251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schicker RS, Rossow J, Eckel S, et al. Outbreak of influenza A(H3N2) variant virus infections among persons attending agricultural fairs housing infected swine—Michigan and Ohio, July–August 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1157–60. doi: 10.15585/mmwr.mm6542a1. [DOI] [PubMed] [Google Scholar]
- 4.Cox CM, Neises D, Garten RJ, et al. Swine influenza virus A (H3N2) infection in human, Kansas, USA, 2009. Emerging Infect Dis. 2011;17:1143–4. doi: 10.3201/eid1706.101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology. 2012;422:151–60. doi: 10.1016/j.virol.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerging Infect Dis. 2012;18:834–7. doi: 10.3201/eid1805.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitikoon P, Nelson MI, Killian ML, et al. Genotype patterns of contemporary reassorted H3N2 virus in US swine. J Gen Virol. 2013;94:1236–41. doi: 10.1099/vir.0.51839-0. [DOI] [PubMed] [Google Scholar]
- 8.Epperson S, Jhung M, Richards S, et al. Human infections with influenza A(H3N2) variant virus in the United States, 2011–2012. Clin Infect Dis. 2013;57:S4–11. doi: 10.1093/cid/cit272. [DOI] [PubMed] [Google Scholar]
- 9.Jhung MA, Epperson S, Biggerstaff M, et al. Outbreak of variant influenza A(H3N2) virus in the United States. Clin Infect Dis. 2013;57:1703–12. doi: 10.1093/cid/cit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biggerstaff M, Reed C, Epperson S, et al. Estimates of the number of human infections with influenza A(H3N2) variant virus, United States, August 2011–April 2012. Clin Infect Dis. 2013;57:S12–5. doi: 10.1093/cid/cit273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu Rev Immunol. 1990;8:737–71. doi: 10.1146/annurev.iy.08.040190.003513. [DOI] [PubMed] [Google Scholar]
- 12.Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther. 2011;9:669–83. doi: 10.1586/eri.11.51. [DOI] [PubMed] [Google Scholar]
- 13.Skowronski DM, Janjua NZ, De Serres G, et al. Cross-reactive and vaccine-induced antibody to an emerging swine-origin variant of influenza A virus subtype H3N2 (H3N2v) J Infect Dis. 2012;206:1852–61. doi: 10.1093/infdis/jis500. [DOI] [PubMed] [Google Scholar]
- 14.Waalen K, Kilander A, Dudman SG, Ramos-Ocao R, Hungnes O. Age-dependent prevalence of antibodies cross-reactive to the influenza A(H3N2) variant virus in sera collected in Norway in 2011. Euro Surveill. 2012;17:pii. 20170. [PubMed] [Google Scholar]
- 15.Hoschler K, Thompson C, Casas I, et al. Population susceptibility to North American and Eurasian swine influenza viruses in England, at three time points between 2004 and 2011. Euro Surveill. 2013;18:pii. doi: 10.2807/1560-7917.es2013.18.36.20578. 20578. [DOI] [PubMed] [Google Scholar]
- 16.Kishida N, Imai M, Xu H, et al. Seroprevalence of a novel influenza A (H3N2) variant virus in the Japanese population. Jpn J Infect Dis. 2013;66:549–51. doi: 10.7883/yoken.66.549. [DOI] [PubMed] [Google Scholar]
- 17.Qiu Y, Muller CP, Van Reeth K. Lower seroreactivity to European than to North American H3N2 swine influenza viruses in humans, Luxembourg, 2010. Euro Surveill. 2015;20:25–33. doi: 10.2807/1560-7917.es2015.20.13.21078. [DOI] [PubMed] [Google Scholar]
- 18.Blumel B, Schweiger B, Dehnert M, et al. Age-related prevalence of cross-reactive antibodies against influenza A(H3N2) variant virus, Germany, 2003 to 2010. Euro Surveill. 2015;20:16–24. [PubMed] [Google Scholar]
- 19.Centers for Disease C, Prevention. Antibodies cross-reactive to influenza A (H3N2) variant virus and impact of 2010–11 seasonal influenza vaccine on cross-reactive antibodies—United States. MMWR Morb Mortal Wkly Rep. 2012;61:237–41. [PubMed] [Google Scholar]
- 20.Houser KV, Pearce MB, Katz JM, Tumpey TM. Impact of prior seasonal H3N2 influenza vaccination or infection on protection and transmission of emerging variants of influenza A(H3N2)v virus in ferrets. J Virol. 2013;87:13480–9. doi: 10.1128/JVI.02434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis NS, Anderson TK, Kitikoon P, Skepner E, Burke DF, Vincent AL. Substitutions near the hemagglutinin receptor-binding site determine the antigenic evolution of influenza A H3N2 viruses in U.S. swine. J Virol. 2014;88:4752–63. doi: 10.1128/JVI.03805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng Z, Gomez J, Bowman AS, et al. Antigenic characterization of H3N2 influenza A viruses from Ohio agricultural fairs. J Virol. 2013;87:7655–67. doi: 10.1128/JVI.00804-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981;289:373–8. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- 24.Bush RM, Bender CA, Subbarao K, Cox NJ, Fitch WM. Predicting the evolution of human influenza A. Science. 1999;286:1921–5. doi: 10.1126/science.286.5446.1921. [DOI] [PubMed] [Google Scholar]
- 25.Shih AC, Hsiao TC, Ho MS, Li WH. Simultaneous amino acid substitutions at antigenic sites drive influenza A hemagglutinin evolution. Proc Natl Acad Sci U S A. 2007;104:6283–8. doi: 10.1073/pnas.0701396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DJ, Lapedes AS, de Jong JC, et al. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305:371–6. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- 27.Koel BF, Burke DF, Bestebroer TM, et al. Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science. 2013;342:976–9. doi: 10.1126/science.1244730. [DOI] [PubMed] [Google Scholar]
- 28.Daum LT, Shaw MW, Klimov AI, et al. Influenza A (H3N2) outbreak, Nepal. Emerg Infect Dis. 2005;11:1186–91. doi: 10.3201/eid1108.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skowronski DM, Chambers C, Sabaiduc S, et al. Interim estimates of 2014/15 vaccine effectiveness against influenza A(H3N2) from Canada’s Sentinel Physician Surveillance Network, January 2015. Euro Surveill. 2015;20:pii. doi: 10.2807/1560-7917.es2015.20.4.21022. 21022. [DOI] [PubMed] [Google Scholar]
- 30.Nobusawa E, Omagari K, Nakajima S, Nakajima K. Reactivity of human convalescent sera with influenza virus hemagglutinin protein mutants at antigenic site A. Microbiol Immunol. 2012;56:99–106. doi: 10.1111/j.1348-0421.2012.00412.x. [DOI] [PubMed] [Google Scholar]
- 31.de Jong JC, Smith DJ, Lapedes AS, et al. Antigenic and genetic evolution of swine influenza A (H3N2) viruses in Europe. J Virol. 2007;81:4315–22. doi: 10.1128/JVI.02458-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 33.WHO. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011 http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf.
- 34.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–50. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057–62. [PubMed] [Google Scholar]
- 36.Lessler J, Riley S, Read JM, et al. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog. 2012;8:e1002802. doi: 10.1371/journal.ppat.1002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonville JM, Wilks SH, James SL, et al. Antibody landscapes after influenza virus infection or vaccination. Science. 2014;346:996–1000. doi: 10.1126/science.1256427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonville JM, Fraaij PL, de Mutsert G, et al. Antigenic maps of influenza A(H3N2) produced with human antisera obtained after primary infection. J Infect Dis. 2016;213:31–8. doi: 10.1093/infdis/jiv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tetsuo Kase SM, Okunoa Y, Maedab A, Babac K. Reinfection with antigenically similar influenza virus observed at a pediatric clinic in Osaka from December 1998 to April 2002. Int Congr Ser. 2004;1263:304–7. [Google Scholar]
- 40.Frank AL, Taber LH, Glezen WP, Paredes A, Couch RB. Reinfection with influenza A (H3N2) virus in young children and their families. J Infect Dis. 1979;140:829–36. doi: 10.1093/infdis/140.6.829. [DOI] [PubMed] [Google Scholar]
- 41.Sato K, Morishita T, Nobusawa E, et al. Amino-acid change on the antigenic region B1 of H3 haemagglutinin may be a trigger for the emergence of drift strain of influenza A virus. Epidemiol Infect. 2004;132:399–406. doi: 10.1017/s0950268803001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linderman SL, Chambers BS, Zost SJ, et al. Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013–2014 influenza season. Proc Natl Acad Sci U S A. 2014;111:15798–803. doi: 10.1073/pnas.1409171111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Myers JL, Bostick DL, et al. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J Exp Med. 2013;210:1493–500. doi: 10.1084/jem.20130212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francis T., Jr On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104:572–8. [Google Scholar]
- 45.Neu KE, Henry Dunand CJ, Wilson PC. Heads, stalks and everything else: how can antibodies eradicate influenza as a human disease? Curr Opin Immunol. 2016;42:48–55. doi: 10.1016/j.coi.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Bostick DL, Sullivan CB, et al. Single hemagglutinin mutations that alter both antigenicity and receptor binding avidity influence influenza virus antigenic clustering. J Virol. 2013;87:9904–10. doi: 10.1128/JVI.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee PS, Ohshima N, Stanfield RL, et al. Receptor mimicry by antibody F045-092 facilitates universal binding to the H3 subtype of influenza virus. Nat Commun. 2014;5:3614. doi: 10.1038/ncomms4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okada J, Ohshima N, Kubota-Koketsu R, et al. Monoclonal antibodies in man that neutralized H3N2 influenza viruses were classified into three groups with distinct strain specificity: 1968–1973, 1977–1993 and 1997–2003. Virology. 2010;397:322–30. doi: 10.1016/j.virol.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Pearce MB, Jayaraman A, Pappas C, et al. Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets. Proc Natl Acad Sci U S A. 2012;109:3944–9. doi: 10.1073/pnas.1119945109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.