Abstract
Purpose
To determine whether abagovomab maintenance therapy prolongs recurrence-free (RFS) and overall survival (OS) in patients with ovarian cancer in first clinical remission.
Patients and Methods
Patients with International Federation of Gynecology and Obstetrics stage III to IV ovarian cancer in complete clinical remission after primary surgery and platinum- and taxane-based chemotherapy were randomly assigned at a ratio of 2:1 in a phase III, double-blind, placebo-controlled, multicenter study. Abagovomab 2 mg or placebo was administered as 1-mL suspension once every 2 weeks for 6 weeks (induction phase) and then once every 4 weeks (maintenance phase) until recurrence or up to 21 months after random assignment of the last patient. The primary end point was RFS; secondary end points were OS and immunologic response.
Results
Characteristics of the 888 patients included: mean age, 56.3 years; Eastern Cooperative Oncology Group performance status, ≤ 1 in > 99% of patients; serous papillary subtype, 81.5%; stage III, 85.9%; and cancer antigen 125 ≤ 35U/mL after third cycle, 80.9%. Mean exposure to study treatment (± standard deviation) was 449.7 ± 333.08 days. Hazard ratio (HR) of RFS for the treatment group using tumor size categorization (≤ 1 cm, > 1 cm) was 1.099 (95% CI, 0.919 to 1.315; P = .301). HR of OS using tumor size categorization (≤ 1 cm, > 1 cm) was 1.150 (95% CI, 0.872 to 1.518; P = .322). The most frequently reported type of adverse event was an injection site reaction in 445 patients (50.2%), followed by injection site erythema and fatigue in 227 (25.6%) and 212 patients (23.9%), respectively. By the final visit, median anti–anti-idiotypic antibody level was 493,000.0 ng/mL, indicating a robust response.
Conclusion
Abagovomab administered as repeated monthly injections is safe and induces a measurable immune response. Administration as maintenance therapy for patients with ovarian cancer in first remission does not prolong RFS or OS.
INTRODUCTION
Ovarian cancer remains the leading cause of mortality among women with gynecologic malignancies. Many patients have achieved complete clinical remission at the conclusion of primary treatment with surgical debulking and platinum- and taxane-based chemotherapy. Recurrence is common and characterized by subsequently shorter intervals of response until uniform chemotherapy resistance develops.1 To improve the clinical outcome of patients with advanced ovarian cancer, maintenance therapy for patients in remission might be beneficial.
No randomized phase III maintenance or consolidation study has shown a statistically significant improvement in overall survival (OS) for those with ovarian cancer in first remission. Examples of negative randomized approaches applied in remission include both subcutaneous and intraperitoneal (IP) interferon alfa,2,3 high-dose chemotherapy,4 continued intravenous carboplatin versus whole-abdominal radiation therapy,5 chemotherapy versus observation versus whole-abdominal radiation therapy,6 IP radioactive phosphorus (phosphorus-32),7 non–cross-resistant chemotherapy,8,9 IP therapy with an yttrium-90–labeled HMFG1 murine monoclonal antibody,10 and oregovomab, a monoclonal antibody that targets cancer antigen 125 (CA-125).11 Extended paclitaxel use prolonged progression-free survival but not OS.12
There is evidence supporting a role for the immune system in ovarian cancer surveillance. With regard to potential targets, CA-125 is a cell-surface, high–molecular weight mucin (MUC16) expressed by > 80% of nonmucinous epithelial ovarian cancers, and changes in its value are closely associated with disease recurrence and progression.13–16 MUC16 expression has been directly correlated with platinum resistance and tumor invasiveness.17,18 Two major obstacles have hampered the development of CA-125–directed immunotherapy. First, these peptides are self-antigens, which are tolerated by the host, and attempts at vaccination with irradiated autologous or allogeneic tumor cells or tumor lysates have not produced meaningful immune responses.19,20 Second, the successful cloning of CA-125, categorizing it as a complex mucin (MUC16), occurred only recently, leading to its recognition as a massive transmembrane glycoprotein with > 60 repeat domains and an amino terminus.21 Although understanding the structure has made the development of directly targeted synthetic immunogenic constructs possible, work is necessary to understand which parts of the larger structure need to be included in a vaccine approach, because size prohibits immunization with the entire construct.22
Abagovomab is an anti-idiotypic antibody produced by a mouse hybridoma and generated against OC125. The murine monoclonal antibody recognizes the tumor-associated antigen CA-125. The induction of a specific immune response (both humoral and cellular) was confirmed in preclinical studies, and a phase I/II trial with 119 patients showed an association between prolonged survival in patients with ovarian cancer who demonstrated an anti–anti-idiotypic antibody (Ab3) response to vaccination (68%) versus those who did not (23.4 v 4.9 months). No significant adverse events were noted.16,23 Subsequent phase I studies confirmed safety and efficacy of the subcutaneous route and suggested that longer vaccination sequences produced more robust immune responses.16,24 These data provided the rationale for the phase III randomized trial reported here.
PATIENTS AND METHODS
Eligibility Criteria
Patients were accrued at multiple institutions from December 2006 to February 2009. Eligible patients had a history of histologically and serologically CA-125–confirmed diagnosis (CA-125 > 35 U/mL) of stage III to IV epithelial ovarian, fallopian tube, or primary peritoneal cancer. Patients underwent debulking surgery and six to eight cycles of standard taxane- and platinum-based treatment, resulting in a complete clinical response. Complete clinical response was defined as normal physical examination, computed tomography scan and chest radiograph without definite evidence of disease, and serum CA-125 within normal laboratory range. Patients were enrolled within 12 weeks of last chemotherapy treatment. Adequate hematologic, renal, and hepatic function were required to include absolute neutrophil count ≥ 1.5 × 109/L; platelets ≥ 75 × 109/L; hemoglobin ≥ 9.9 g/dL; serum creatinine ≤ 1.5 × upper limit of normal (ULN); bilirubin ≤ 1.5 × ULN; and AST, ALT, and alkaline phosphatase ≤ 2.5 × ULN. Patients with known autoimmune disease requiring treatment with immunosuppressive agents were excluded. Prior vaccine or monoclonal antibody treatment was not allowed.
Study Design
The study was a randomized, double-blind, placebo-controlled, multicenter trial of abagovomab maintenance therapy versus placebo in patients with epithelial ovarian, primary peritoneal, or fallopian tube cancer in first complete clinical remission. Registration and random assignment in a 2:1 fashion favoring abagovomab was centralized. Predetermined strata included International Federation of Gynecology and Obstetrics (FIGO) stage (III v IV), tumor size after debulking surgery (residual tumor ≤ 1 or > 1 cm), and serum CA-125 after first three cycles of chemotherapy (≤ 35 or > 35 U/mL). The primary study end point was recurrence-free survival (RFS) at the end of double-blind observation. Secondary end points included OS; safety; and immunologic parameters, including human antimouse antibody (HAMA), Ab3, Ab1′, and serum CA-125 (blinded during study). The double-blind observation period extended from random assignment of the first patient to 24 months after random assignment of the last patient. The open survival follow-up period started at the end of the double-blind observation period for a planned additional 5 years. Abagovomab was administered subcutaneously in a 1-mL suspension once every 2 weeks for three injections (induction phase) and then once every 4 weeks for up to 21 months after random assignment of the last patient (maintenance phase). A steering committee, independent radiology review panel, and data and safety monitoring board were established for study management.
Dose Modifications
Dose modification was not permitted. Patients were to be removed for dose-limiting toxicity using Common Terminology Criteria for Adverse Events version 3.0 criteria defined by grade 2 allergic reaction, grade ≥ 2 autoimmune reaction, grade ≥ 3 hematologic or nonhematologic toxicity including fever, or grade 3 injection site reaction.
Baseline and Treatment Assessments
Radiologic tests were performed within 28 days of study entry. Medical history, laboratory tests, urinalysis, and ECG were performed within 14 days. Interval assessments included physical examination, concomitant medication assessment, hematologic and serum chemistries, and immune assessments at weeks 4 and 10 and then every 12 weeks. Serum CA-125 (kept blinded) and computed tomography imaging were obtained at week 10 and then every 12 weeks in both arms. RECIST version 1.0 was used to assess for disease progression. Central radiology review was provided. Adverse events and survival status were monitored throughout the observation period.
Statistical Methods
The planned study population was 870 patients (580 to receive abagovomab, 290 to receive placebo). The expected RFS for the placebo arm was 18 months, and the expected number of recurrence events was 535 (338 in abagovomab arm, 197 in placebo arm). The study was powered to detect a hazard ratio (HR) between abagovomab and placebo of 1.33 (leading to an approximate benefit of abagovomab over placebo of 6 months). Significance level (α) = 5% (two sided), and the expected dropout rate was 10%. The primary analysis for RFS was run on progression-free survival as assessed by the central radiology review committee. A Cox proportional hazards model was used for the primary analysis, which had treatment as a major covariate, adjusted only for the predefined prognostic stratification factors (ie, FIGO stage, tumor size after debulking, and CA-125 level after first three cycles of chemotherapy). Kaplan-Meier estimation analysis was used to support the results seen in the Cox regression model. Safety parameters were descriptively analyzed using Common Terminology Criteria for Adverse Events version 3.0 descriptors. The following subgroups were also analyzed for effect on the primary end point: tumor size after debulking (residual tumor ≤ 1 or > 1 cm), FIGO stage (III v IV), and serum CA-125 level after the first three cycles of chemotherapy (≤ 35 or > 35 U/mL). For the immunologic parameters Ab3 and HAMA, descriptive statistics by time point (ie, baseline, week 10, and week 22) were performed for FIGO stage, tumor size after debulking, and CA-125 level after the first three cycles of chemotherapy.
RESULTS
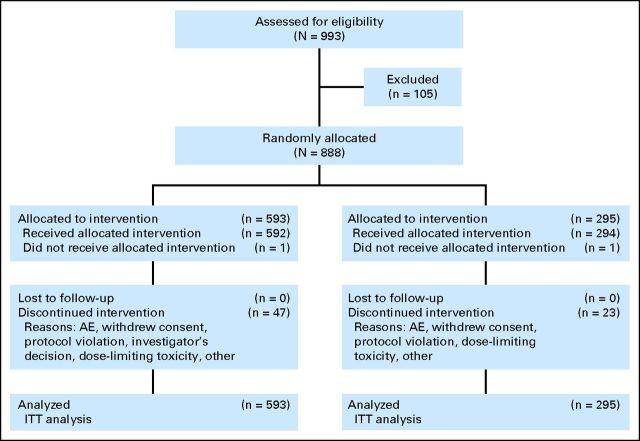
Patient Characteristics
Patient flow is outlined in the CONSORT diagram (Fig 1). Patient characteristics, which were similar for both groups, are listed in Table 1. Overall mean age (± standard deviation [SD]) was 56.3 ± 10.53 years. At week 0, 78.8% of patients had Eastern Cooperative Oncology Group performance status of 0, and 20.9% had performance status of 1. Of the total population, the majority (82%) had stage III disease and had serous histology (81.5%), followed by endometrioid histology (6.7%). All patients received surgical debulking and platinum- and taxane-based primary therapy for six to eight cycles. The mean time from primary surgery to random assignment (± SD) was 192 ± 43 days. Most patients (80.1%) were debulked to < 1 cm, and 47.7% had no visible residual disease at the conclusion of primary surgery. There were no observed differences between treatment groups regarding tumor size after debulking surgery. The majority of patients (80.9%) experienced reduction in CA-125 to ≤ 35 U/mL after three cycles of primary therapy. There were no observed differences between treatment groups regarding serum CA-125 level after the first three cycles of chemotherapy.
Fig 1.
CONSORT diagram. AE, adverse event; ITT, intention to treat.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Abagovomab (n = 593) |
Placebo (n = 295) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Mean | 56.3 | 56.0 | ||
| SD | 10.57 | 10.47 | ||
| Ethnicity | ||||
| Hispanic or Latino | 121 | 20.4 | 66 | 22.4 |
| Non-Hispanic or non-Latino | 471 | 79.6 | 229 | 77.6 |
| Race | ||||
| White | 582 | 98.1 | 291 | 98.6 |
| Black or African American | 3 | 0.5 | 3 | 1.0 |
| Hawaiian or Pacific Islander | 1 | 0.2 | 0 | 0 |
| Asian | 2 | 0.3 | 1 | 0.3 |
| Other | 5 | 0.8 | 0 | 0 |
| FIGO stage | ||||
| III | 513 | 86.6 | 252 | 85.7 |
| IV | 80 | 13.5 | 42 | 14.3 |
| Tumor size after debulking, cm | ||||
| ≤ 1 | 479 | 80.8 | 232 | 78.6 |
| 0 | 285 | 48.1 | 139 | 47.1 |
| > 0 to ≤ 1 | 194 | 32.7 | 93 | 31.5 |
| > 1 | 114 | 19.2 | 63 | 21.4 |
| Serum CA-125 after three cycles, U/mL | ||||
| ≤ 35 | 479 | 80.8 | 239 | 81.3 |
| > 35 | 114 | 19.2 | 55 | 18.7 |
| Histology | ||||
| Serous | 481 | 81.5 | 245 | 83.1 |
| Endometrioid | 38 | 6.4 | 21 | 7.1 |
| Mucinous | 6 | 1.0 | 3 | 1.0 |
| Other | 65 | 11.1 | 26 | 8.9 |
Abbreviation: CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; SD, standard deviation.
Dose Administration
All patients (100%) completed the screening visit. At week 10 (end of induction and start of maintenance phase), 825 patients (92.9%) had completed the visit. Patients received all doses in the induction phase, and compliance was approximately 70% during the maintenance phase. Mean exposure to study treatment (± SD) was 449.7 ± 333.08 days for the overall study population, with no differences observed between treatment groups (450.6 ± 335.49 days in abagovomab group; 447.9 ± 328.73 days in placebo group). For the abagovomab arm, median (quartile one to quartile three) exposure was 351.0 days (range, 160.5 to 702.5 days), whereas for the placebo arm, it was 377 days (range, 157.0 to 686.0 days).
Mean exposure (± SD) was longer among patients with residual tumor size ≤ 1 cm (468.7 ± 336.27 days) versus patients with residual tumor size > 1 cm (373.2 ± 309.16 days), among patients with baseline FIGO stage III (470.4 ± 338.0 days) versus baseline FIGO stage IV (319.8 ± 266.91 days), and among patients with CA-125 ≤ 35 U/mL after the first three cycles of chemotherapy (489.8 ± 336.18 days) versus CA-125 > 35 U/mL (280.7 ± 260.13 days).
Efficacy
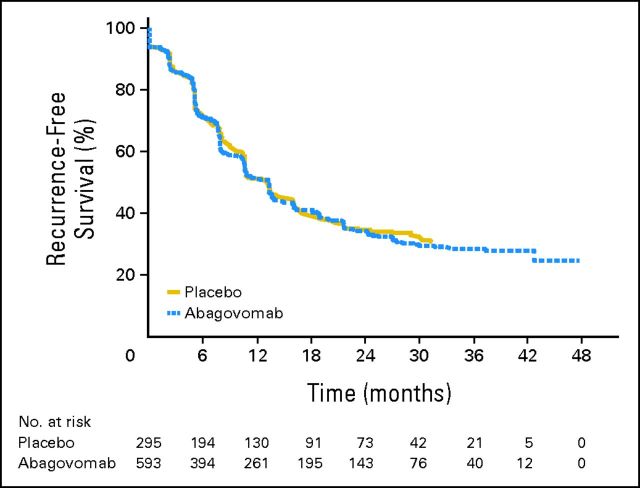
At the end of double-blind observation period, 554 recurrence events had been observed in total (374 in abagovomab arm; 180 in placebo arm). HR of RFS was 1.099 (95% CI, 0.919 to 1.315; P = .301). This showed no statistical difference in risk of recurrence between the abagovomab-treated and placebo groups.
Figure 2 shows the survival distribution against time for RFS. The results of this confirmatory analysis support those seen in the primary Cox regression model. The median estimated time to recurrence was similar between both treatment groups (abagovomab group: 403 days; 95% CI, 323 to 414; placebo group: 402 days; 95% CI, 323 to 487).
Fig 2.
Primary end point: recurrence-free survival distribution against time.
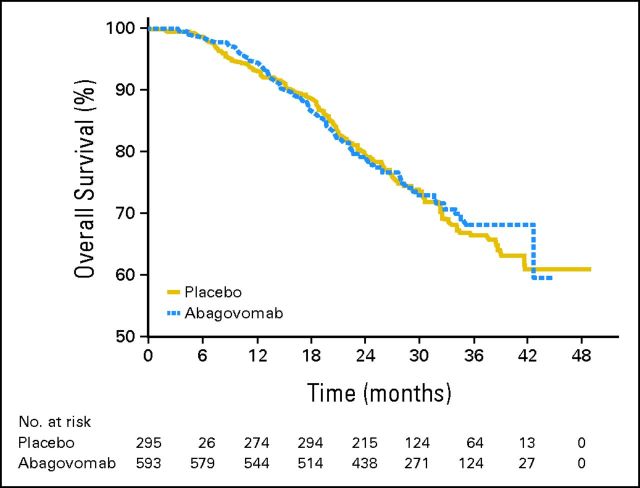
At the end of the double-blind observation period, 251 patients had died (171 in abagovomab arm; 80 in placebo arm). HR of OS for the treatment group was 1.150 (95% CI, 0.872 to 1.518; P = .322). This showed no statistical difference in the risk of death between patients receiving abagovomab versus placebo. OS rate at 2 years was 80% in both arms, with SE equal to 1.71 and 2.43 for abagovomab and placebo, respectively. Figure 3 shows the survival distribution against time for OS. No statistically significant difference was observed in the survival curves (P = .607).
Fig 3.
Secondary end point: overall survival distribution against time.
Primary Immune Response Parameters
Response parameters are listed in Table 2. Median Ab3 level was set to 0 (ie, below predefined limit of quantification) at baseline in both treatment groups. In the placebo group, median Ab3 level remained at 0 ng/mL, whereas in the abagovomab-treated group, it increased during the course of study. At week 10 (end of induction/start of maintenance phase), median Ab3 level was 63,550.0 ng/mL, and at week 22, median Ab3 level was 335,500.0 ng/mL. By the final study visit, median Ab3 level was 493,000.0 ng/mL.
Table 2.
Immune Response Parameters (Ab3, HAMA) at Week 10, Week 22, and Final Study Visit in Overall ITT Population
| Parameter | Abagovomab (n = 593) |
Placebo (n = 295) |
Total (N = 888) |
|||
|---|---|---|---|---|---|---|
| Actual Value | Change From Baseline | Actual Value | Change From Baseline | Actual Value | Change From Baseline | |
| Ab3, ng/mL | ||||||
| Baseline | ||||||
| No. | 576 | 288 | 864 | |||
| Mean | 893.5 | 985.0 | 924.0 | |||
| SD | 7,585.82 | 5,613.77 | 6,987.36 | |||
| Median | 0.0 | 0.0 | 0.0 | |||
| Q1 to Q3 | 0 to 0 | 0 to 0 | 0 to 0 | |||
| Week 10 | ||||||
| No. | 538 | 532 | 269 | 263 | 807 | 795 |
| Mean | 89,952.1 | 89,230.7 | 1,128.9 | 500.4 | 60,344.4 | 59,877.1 |
| SD | 91,321.64 | 92,190.47 | 6,588.91 | 4,141.14 | 85,592.98 | 86,224.51 |
| Median | 63,550.0 | 63,150.0 | 0.0 | 0.0 | 31,400.0 | 30,700.0 |
| Q1 to Q3 | 31,400.0 to 115,500.0 | 30,100.0 to 115,500.0 | 0 to 0 | 0 to 0 | 0 to 88,000.0 | 0 to 88,000.0 |
| Week 22 | ||||||
| No. | 472 | 460 | 234 | 228 | 706 | 688 |
| Mean | 404,625.5 | 407,134.7 | 1,245.3 | 533.3 | 270,927.2 | 272,388.9 |
| SD | 271,569.40 | 273,703.11 | 6,433.85 | 4,396.22 | 292,219.18 | 294,520.64 |
| Median | 335,500.0 | 339,000.0 | 0.0 | 0.0 | 225,500.0 | 227,000.0 |
| Q1 to Q3 | 224,000.0 to 536,500.0 | 225,500.0 to 540,000.0 | 0 to 0 | 0 to 0 | 0 to 455,000.0 | 0 to 457,500.0 |
| Final study visit | ||||||
| No. | 449 | 441 | 230 | 224 | 679 | 665 |
| Mean | 595,209.8 | 596,580.7 | 3,950.5 | 2,936.4 | 394,930.5 | 396,616.3 |
| SD | 469,647.94 | 470,366.17 | 33,697.04 | 33,040.60 | 473,866.07 | 475,199.78 |
| Median | 493,000.0 | 493,000.0 | 0.0 | 0.0 | 256,000.0 | 258,000.0 |
| Q1 to Q3 | 258,000.0 to 794,000.0 | 258,000.0 to 798,000.0 | 0.0 | 0.0 | 0 to 627,000.0 | 0 to 625,400.0 |
| HAMA, ng/mL | ||||||
| Baseline | ||||||
| No. | 576 | 288 | 864 | |||
| Mean | 13.730 | 4.712 | 10.724 | |||
| SD | 117.7044 | 28.4410 | 97.5600 | |||
| Median | 0.000 | 0.000 | 0.000 | |||
| Q1 to Q3 | 0 to 0 | 0 to 0 | 0 to 0 | |||
| Week 10 | ||||||
| No. | 538 | 532 | 269 | 263 | 807 | 795 |
| Mean | 832.904 | 822.022 | 6.800 | 1.602 | 557.536 | 550.613 |
| SD | 1,523.2716 | 1,522.1293 | 35.2562 | 38.6689 | 1,303.1505 | 1,303.5092 |
| Median | 326.000 | 322.500 | 0.000 | 0.000 | 110.000 | 106.000 |
| Q1 to Q3 | 101.0 to 824.0 | 95.9 to 818.0 | 0 to 0 | 0 to 0 | 0 to 548.0 | 0 to 537.0 |
| Week 22 | ||||||
| No. | 472 | 460 | 234 | 228 | 706 | 688 |
| Mean | 9,844.807 | 9,833.055 | 13.795 | 8.922 | 6,586.370 | 6,577.383 |
| SD | 13,721.0703 | 13,779.9604 | 95.4973 | 100.9670 | 12,133.7797 | 12,177.3228 |
| Median | 6,380.000 | 6,415.000 | 0.000 | 0.000 | 2,795.000 | 2,760.000 |
| Q1 to Q3 | 2,760.0 to 11,850.0 | 2,745.0 to 11,850.0 | 0 to 0 | 0 to 0 | 0 to 8,370.0 | 0 to 8,465.0 |
| Final study visit | ||||||
| No. | 449 | 441 | 230 | 224 | 679 | 665 |
| Mean | 21,990.183 | 22,028.887 | 633.039 | 645.449 | 14,755.804 | 14,826.045 |
| SD | 40,079.0892 | 40,272.9965 | 7,038.0481 | 7,131.7401 | 34,357.8623 | 34,556.3083 |
| Median | 11,300.000 | 11,300.000 | 0.000 | 0.000 | 2,840.000 | 2,950.000 |
| Q1 to Q3 | 295.0 to 26,100.0 | 295.0 to 26,100.0 | 0 to 0 | 0 to 0 | 0 to 16,500.0 | 0 to 16,600.0 |
NOTE. Baseline value is defined as last measurement taken before first administration of study drug. Values specified as below limit of quantification were set to 0 in summary tables.
Abbreviations: Ab3, anti–anti-idiotypic antibody; HAMA, human antimouse antibody; Q, quartile; SD, standard deviation.
Median HAMA level was set to 0 (ie, below predefined limit of quantification) at baseline in both treatment groups. In the placebo group, median HAMA level remained at 0 ng/mL, whereas in the abagovomab-treated group, it increased during the course of study. At week 10 (end of induction/start of maintenance phase), median HAMA level was 326.0 ng/mL, and at week 22, median HAMA level was 6380.0 ng/mL. By the final study visit, median HAMA level was 11,300.0 ng/mL.
Analyses by FIGO stage, tumor debulking status, and CA-125 level after the first three cycles of chemotherapy did not reveal any differences in the subgroups regarding the time course of median Ab3 level (data not shown).
Secondary Immune Response Parameters
The secondary immune response parameters will be reported separately.
Adverse Events
In the total study population, 564 patients (95.3%) in the abagovomab group and 278 patients (94.6%) in the placebo group experienced an adverse event (Tables 3 and 4). The proportions of patients experiencing treatment-related adverse events, serious adverse events, treatment-related serious adverse events, and adverse events leading to permanent withdrawal from study medication were similar between the abagovomab- and placebo-treated groups. The most frequently reported adverse event was an injection site reaction, reported in 443 patients (50%), characterized overall by localized erythema. Fatigue was reported in 170 patients (19.1%). For the majority of patients, the most severe adverse events were grade 1 (n = 212; 23.9%) or 2 (n = 423; 47.7%). A total of 182 patients (20.5%) experienced grade 3 adverse events, with 115 (19.4%) in the abagovomab group and 67 (22.8%) in the placebo group. The number of grade 4 adverse events was small, with 12 (2.0%) in the abagovomab group and five (1.7%) in the placebo group. Overall, 213 patients (24.0%) experienced a serious adverse event, the most commonly attributed cause of which was recurrent ovarian cancer (11.5% of patients).
Table 3.
Summary of Treatment-Emergent AEs
| AE | Abagovomab |
Placebo |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Treatment-emergent AE | 564 | 95.3 | 278 | 94.6 |
| Grade 3 AE | 115 | 19.4 | 67 | 22.8 |
| Grade 4 AE | 12 | 2.0 | 5 | 1.7 |
| Treatment-emergent related AE | 507 | 85.6 | 246 | 83.7 |
| SAE | 141 | 23.8 | 72 | 24.5 |
| Treatment-related SAE | 12 | 2.0 | 3 | 1.0 |
| SAE leading to withdrawal of study drug | 93 | 15.7 | 57 | 19.4 |
Abbreviations: AE, adverse event; SAE, serious adverse event.
Table 4.
AEs by Maximum Relationship Related to Study Medication Occurring in ≥ 5% Patients
| System Organ Class | Abagovomab (n = 592) |
Placebo (n = 294) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Possible |
Probable |
Certain |
Possible |
Probable |
Certain |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Abdomen pain | 38 | 6.4 | 3 | 0.5 | 4 | 0.7 | 6 | 2.0 | 4 | 1.4 | 3 | 1.0 |
| Diarrhea | 38 | 6.4 | 5 | 0.8 | 9 | 1.5 | 8 | 2.7 | 4 | 1.4 | 2 | 0.7 |
| Nausea | 43 | 7.3 | 12 | 2.0 | 4 | 0.7 | 14 | 4.8 | 3 | 1.0 | 11 | 3.7 |
| Fatigue | 89 | 15.0 | 22 | 3.7 | 12 | 2.0 | 33 | 11.2 | 10 | 3.4 | 4 | 1.4 |
| Injection site reaction | 13 | 2.2 | 48 | 8.1 | 246 | 41.6 | 5 | 1.7 | 25 | 8.5 | 106 | 36.1 |
| Arthralgia | 52 | 8.8 | 17 | 2.9 | 13 | 2.2 | 25 | 8.5 | 8 | 2.7 | 8 | 2.7 |
| Myalgia | 25 | 4.2 | 8 | 1.4 | 5 | 0.8 | 18 | 6.1 | 5 | 1.7 | 7 | 2.4 |
| Headache | 37 | 6.3 | 3 | 0.5 | 7 | 1.2 | 10 | 3.4 | 9 | 3.1 | 2 | 0.7 |
Abbreviation: AE, adverse event.
DISCUSSION
Despite the association between Ab3 production and OS seen in the previous phase I/II trial evaluating abagovomab in patients with advanced ovarian cancer, no benefit with regard to RFS or OS was seen in this large international randomized phase III study evaluating abagovomab for patients in first clinical remission. The patient characteristics evaluated here were typical for patients with ovarian cancer. A majority were optimally debulked, and the requirement for complete clinical remission provided a good patient group for the evaluation of an immunotherapeutic approach. The study used central randomization, and both patients and investigators were blinded to treatment arms and serum CA-125 values. The time to RFS was adjudicated by a central radiology review committee and was as expected for the patient groups. Study compliance was good overall, and the treatment was well tolerated. In addition to the lack of benefit in the overall intention-to-treat population, no benefit was seen in those characterized by the planned subgroups based on FIGO stage, size of residual tumor, or normalization of CA-125 after three cycles of primary chemotherapy.
Vaccination with abagovomab resulted in a robust Ab3 response. The lack of benefit seen in this study despite its immunogenicity is in contrast to that seen in the phase II study, in which a strong association was seen between antibody response and OS (23.5 v 4.9 months; P > .001).25 This illustrates the importance of phase III randomized trials in drawing any conclusions regarding efficacy for maintenance approaches. This finding may indicate that in the phase II study, antibody production was a biomarker for improved outcome in that patients who were able to generate such a response despite disease status had longer survival. The proactive induction of the antibody response using abagovomab did not show similar results. The high percentage of immune responders with regard to Ab3 in this study does not permit a comparison between those who produced antibodies and those who did not.
The lack of a RFS or OS benefit with abagovomab parallels the data recently reported with oregovomab, which is a murine monoclonal antibody specific for CA-125. It similarly had strong phase II supporting data, but no benefit was seen in a randomized phase III trial.26 Although neither antibody-directed approach showed a survival improvement, much interest remains in considering CA-125 (MUC16) as one viable target for future studies with other effectors. MUC16 is overexpressed on most epithelial ovarian cancer cells with its cleaved and released domain (CA-125) as well as a retained domain (MUC-CD). Because it is otherwise expressed at low levels in other tissue sites, and preclinical data support its modulation of ovarian tumor growth and invasiveness, it is ideally suited for targeting. Recent studies have shown that T cells, for example, modified to express a chimeric antigen receptor (4H11) specific to the MUC-CD of MUC16 can lyse human ovarian cancer cells in vitro and have shown tumor kill in orthotopic xenotransplant tumor models.27 Ovarian cancer is a markedly immunogenic tumor, and there is significant evidence that the presence of both antibody and T-cell effectors correlate with outcome.28,29 Recent data in renal cell cancer suggest that multiple tumor-associated peptide targets have a positive clinical effect.30 Exploring multiple targets simultaneously in addition to CA-125 (MUC16) for immunotherapy, exploiting other effectors, and combining these approaches with immunomodulatory efforts directed toward CTLA4 or PDL1 remain reasonable approaches to try and improve outcome for patients with ovarian cancer.
Acknowledgment
We thank the following investigators in this international effort: L. Dirix, J.C. Goeminne, J. Van Erps, H. Denys, J.F. Baurain (Belgium); J. Chovanec, M. Bláha, Z. Novotny, P. Sák, J. Spacek, J. Vaňásek, M. Kohoutek, M. Kubecová, L. Sevcik (Czech Republic); A. Lortholary, H. Bourgeois (France); W. Bauer, K. Baumann, M. Beckmann, A. Belau, J. Bischoff, A. Burges, U. Canzler, G. Emons, A. Feldmann, K. Freese, M. Valter, B. Gerber, L. Hanker, A. Hasenburg, F. Hilpert, G. Hoffmann, C. Höß, T. Fehm, J.P. Scharf, C. Kahl, P. Krabisch, R. Kreienberg, R. Kullmer, C. Liebrich, S. Mahner, W. Meinerz, A. Ober, H.M. Enzinger, M. Pölcher, B. Richter, M. Schmidt, M. Aydogdu, B. Rack, O. Tomàe, H.G. Strauß, G. Feisel-Schwickardi, H. Wolf, T.W. Park-Simon, I. Runnebaum, R.S. Baum, J. Kosse (Germany); F. Bánhidy, K. Boer, Z. Hernadi, K. Pap, A. Szánthó, P. Gocze (Hungary); C. Boni, P.F. Conte, F. Petruzzelli, L. Frati, M. Nardi, D. Bilancia, S. Pignata, F. Artioli, S. Tamberi, C. Benedetto, A. Sobrero, G. Amunni, F. Raspagliesi (Italy); A. Deptała, W. Rogowski, A. Kalmuk, P. Koralewski, J. Markowska, J. Pikiel, M. Ziobro, Z. Rusinowska, P. Sawrycki, G. Slomian, C. Szczylik (Poland); L. Alonso Carrión, M. Amenedo Gancedo, M. Beltrán, A.B. Sanchez, P. Gascon, E. Ortega, J. Rífá, M.J. Rubio Pérez, E. Calvo, A. Gonzalez Martin, S. Menjon, I. Bover, C. Caballero (Spain); and A. Alvarez Secord, J. Burke II, P. Konstantinopoulos, S. Davidson, D. Dizon, R. Edwards, D. Matei, R. Morris, K. Odunsi, C. Brewer, D. Smith, N. Spirtos, T. Tillmanns, K. Easley, W. McGuire (United States)
Footnotes
Written on behalf of the MIMOSA (Monoclonal Antibody Immunotherapy for Malignancies of the Ovary by Subcutaneous Abagovomab) investigators.
Supported by Menarini Ricerche.
Presented at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00418574.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Simona Scartoni, Menarini Ricerche (C); Monica Bertolotti, Menarini Ricerche (C); Cecilia Simonelli, Menarini Ricerche (C); Angela Capriati, Menarini Ricerche (C); Carlo Alberto Maggi, Menarini Ricerche (C) Consultant or Advisory Role: Karel Cwiertka, Menarini Ricerche (C); Jan B. Vermorken, Menarini Ricerche (C); Eric Pujade-Lauraine, Menarini Ricerche (C) Stock Ownership: None Honoraria: Jacobus Pfisterer, Menarini Recerche Research Funding: Paul Sabbatini, Menarini Ricerche; Karel Cwiertka, Menarini Ricerche; Jan B. Vermorken, Menarini Ricerche; Jonathan S. Berek, Menarini Ricerche; Jacobus Pfisterer, Menarini Recerche Expert Testimony: None Other Remuneration: Christian Kurzeder, Menarini Recerche
AUTHOR CONTRIBUTIONS
Conception and design: Paul Sabbatini, Angela Capriati, Carlo Alberto Maggi, Jonathan S. Berek, Jacobus Pfisterer
Financial support: Monica Bertolotti, Cecilia Simonelli
Administrative support: Monica Bertolotti, Cecilia Simonelli
Provision of study materials or patients: Paul Sabbatini, Philipp Harter, Giovanni Scambia, Jalid Sehouli, Werner Meier, Pauline Wimberger, Klaus H. Baumann, Christian Kurzeder, Barbara Schmalfeldt, David Cibula, Mariusz Bidzinski, Antonio Casado, Andrea Martoni, Nicoletta Colombo, Robert W. Holloway, Luigi Selvaggi, Andrew Li, Jose del Campo, Karel Cwiertka, Tamas Pinter, Eric Pujade-Lauraine, Simona Scartoni, Jonathan S. Berek, Jacobus Pfisterer
Collection and assembly of data: Paul Sabbatini, Philipp Harter, Andrew Li, Monica Bertolotti, Cecilia Simonelli, Jonathan S. Berek, Jacobus Pfisterer
Data analysis and interpretation: Paul Sabbatini, Philipp Harter, Giovanni Scambia, Jalid Sehouli, Werner Meier, Pauline Wimberger, Klaus H. Baumann, Christian Kurzeder, Barbara Schmalfeldt, David Cibula, Mariusz Bidzinski, Antonio Casado, Andrea Martoni, Nicoletta Colombo, Robert W. Holloway, Luigi Selvaggi, Andrew Li, Jose del Campo, Karel Cwiertka, Tamas Pinter, Jan B. Vermorken, Eric Pujade-Lauraine, Simona Scartoni, Jonathan S. Berek, Jacobus Pfisterer
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Markman M Markman J Webster K , etal : Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: Implications for patient management and clinical trial design J Clin Oncol 22: 3120– 3125,2004. [DOI] [PubMed] [Google Scholar]
- 2.Hall GD Brown JM Coleman RE , etal : Maintenance treatment with interferon for advanced ovarian cancer: Results of the Northern and Yorkshire Gynaecology Group randomised phase III study Br J Cancer 91: 621– 626,2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberts DS Hannigan EV Liu PY , etal : Randomized trial of adjuvant intraperitoneal alpha-interferon in stage III ovarian cancer patients who have no evidence of disease after primary surgery and chemotherapy: An Intergroup study Gynecol Oncol 100: 133– 138,2006. [DOI] [PubMed] [Google Scholar]
- 4.Schilder RJ Brady MF Spriggs D , etal : Pilot evaluation of high-dose carboplatin and paclitaxel followed by high-dose melphalan supported by peripheral blood stem cells in previously untreated advanced ovarian cancer: A Gynecologic Oncology Group study Gynecol Oncol 88: 3– 8,2003. [DOI] [PubMed] [Google Scholar]
- 5.Lambert HE Rustin GJ Gregory WM , etal : A randomized trial comparing single-agent carboplatin with carboplatin followed by radiotherapy for advanced ovarian cancer: A North Thames Ovary Group study J Clin Oncol 11: 440– 448,1993. [DOI] [PubMed] [Google Scholar]
- 6.Sorbe B: Consolidation treatment of advanced ovarian carcinoma with radiotherapy after induction chemotherapy Int J Gynecol Cancer 13: 192– 195,2003. suppl 2 [DOI] [PubMed] [Google Scholar]
- 7.Varia MA Stehman FB Bundy BN , etal : Gynecologic Oncology Group: Intraperitoneal radioactive phosphorus (32P) versus observation after negative second-look laparotomy for stage III ovarian carcinoma: A randomized trial of the Gynecologic Oncology Group J Clin Oncol 21: 2849– 2855,2003. [DOI] [PubMed] [Google Scholar]
- 8.Nicoletto MO Tumolo S Falci C , etal : A randomized study of epithelial ovarian cancer: Is chemotherapy useful after complete remission? Int J Med Sci 1: 116– 125,2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Placido S Scambia G Di Vagno G , etal : Topotecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer: Multicenter Italian Trials in Ovarian Cancer (MITO-1) randomized study J Clin Oncol 22: 2635– 2642,2004. [DOI] [PubMed] [Google Scholar]
- 10.Verheijen RH Massuger LF Benigno BB , etal : Phase III trial of intraperitoneal therapy with yttrium-90–labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission J Clin Oncol 24: 571– 578,2006. [DOI] [PubMed] [Google Scholar]
- 11.Berek J Taylor P McGuire W , etal : Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer J Clin Oncol 27: 418– 425,2009. [DOI] [PubMed] [Google Scholar]
- 12.Markman M Liu PY Wilczynski S , etal : Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group trial J Clin Oncol 21: 2460– 2465,2003. [DOI] [PubMed] [Google Scholar]
- 13.Levine D Park K Juretzka M , etal : A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission Cancer 110: 2448– 2456,2007. [DOI] [PubMed] [Google Scholar]
- 14.Berek JS, Taylor PT, Nicodemus CF: CA125 velocity at relapse is a highly significant predictor of survival post relapse: Results of a 5-year follow-up survey to a randomized placebo-controlled study of maintenance oregovomab immunotherapy in advanced ovarian cancer J Immunother 31: 207– 214,2008. [DOI] [PubMed] [Google Scholar]
- 15.Duffy MJ Bonfrer JM Kulpa J , etal : CA125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use Int J Gynecol Cancer 15: 679– 691,2005. [DOI] [PubMed] [Google Scholar]
- 16.Sabbatini P Dupont J Aghajanian C , etal : Phase I study of abagovomab in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer Clin Cancer Res 12: 5503– 5510,2006. [DOI] [PubMed] [Google Scholar]
- 17.Bafna S, Kaur S, Batra SK: Membrane-bound mucins: The mechanistic basis for alterations in the growth and survival of cancer cells Oncogene 29: 2893– 2904,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gubbels JA Belisle J Onda M , etal : Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors Mol Cancer 5: 50,2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya-Chatterjee M, Chatterjee SK, Foon KA: Anti-idiotype antibody vaccine therapy for cancer Expert Opin Biol Ther 2: 869– 881,2002. [DOI] [PubMed] [Google Scholar]
- 20.Leffers N Fehrmann RS Gooden MJ , etal : Identification of genes and pathways associated with cytotoxic T lymphocyte infiltration of serous ovarian cancer Br J Cancer 103: 685– 692,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien TJ Beard JB Underwood LJ , etal : The CA 125 gene: An extracellular superstructure dominated by repeat sequences Tumour Biol 22: 348– 366,2001. [DOI] [PubMed] [Google Scholar]
- 22.Yin BW, Lloyd KO: Molecular cloning of the ca125 ovarian cancer antigen: Identification as a new mucin, muc 16 J Biol Chem 276: 27371– 27375,2001. [DOI] [PubMed] [Google Scholar]
- 23.Reinartz S Wagner U Giffels P , etal : Immunological properties of a single-chain fragment of the anti-idiotypic antibody ACA125 Cancer Immunol Immunother 49: 186– 192,2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfisterer J du Bois A Sehouli J , etal : The anti-idiotypic antibody abagovomab in patients with recurrent ovarian cancer: A phase I trial of the AGO-OVAR Ann Oncol 17: 1568– 1577,2006. [DOI] [PubMed] [Google Scholar]
- 25.Reinartz S Köhler S Schlebusch H , etal : Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: Immunological response and survival (phase Ib/II) Clin Cancer Res 10: 1580– 1587,2004. [DOI] [PubMed] [Google Scholar]
- 26.Berek JS Taylor PT Gordon A , etal : Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer J Clin Oncol 22: 3507– 3516,2004. [DOI] [PubMed] [Google Scholar]
- 27.Chekmasova AA, Brentjens RJ: Adoptive T cell immunotherapy strategies for the treatment of patients with ovarian cancer Discov Med 9: 62– 70,2010. [PubMed] [Google Scholar]
- 28.Anderson KS Wong J Vitonis A , etal : p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer Cancer Epidemiol Biomarkers Prev 19: 859– 868,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato E Olson SH Ahn J , etal : Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer Proc Natl Acad Sci U S A 102: 18538– 18543,2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walter S Weinschenk T Stenzl A , etal : Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival Nat Med [epub ahead of print on July 29, 2012] [DOI] [PubMed] [Google Scholar]