Abstract
Purpose
A clinical study to characterize renal masses with positron emission tomography/computed tomography (PET/CT) was undertaken.
Patients and Methods
This was an open-label multicenter study of iodine-124 (124I) -girentuximab PET/CT in patients with renal masses who were scheduled for resection. PET/CT and contrast-enhanced CT (CECT) of the abdomen were performed 2 to 6 days after intravenous 124I-girentuximab administration and before resection of the renal mass(es). Images were interpreted centrally by three blinded readers for each imaging modality. Tumor histology was determined by a blinded central pathologist. The primary end points—average sensitivity and specificity for clear cell renal cell carcinoma (ccRCC)—were compared between the two modalities. Agreement between and within readers was assessed.
Results
124I-girentuximab was well tolerated. In all, 195 patients had complete data sets (histopathologic diagnosis and PET/CT and CECT results) available. The average sensitivity was 86.2% (95% CI, 75.3% to 97.1%) for PET/CT and 75.5% (95% CI, 62.6% to 88.4%) for CECT (P = .023). The average specificity was 85.9% (95% CI, 69.4% to 99.9%) for PET/CT and 46.8% (95% CI, 18.8% to 74.7%) for CECT (P = .005). Inter-reader agreement was high (κ range, 0.87 to 0.92 for PET/CT; 0.67 to 0.76 for CECT), as was intrareader agreement (range, 87% to 100% for PET/CT; 73.7% to 91.3% for CECT).
Conclusion
This study represents (to the best of our knowledge) the first clinical validation of a molecular imaging biomarker for malignancy. 124I-girentuximab PET/CT can accurately and noninvasively identify ccRCC, with potential utility for designing best management approaches for patients with renal masses.
INTRODUCTION
There were an estimated 60,920 new cases of renal carcinoma in the United States in 2011, with an associated mortality of 13,120.1 Renal cortical tumors are diverse, with variable metastatic potential, from benign (20%, including oncocytoma, angiomyolipoma) to indolent (papillary and chromophobe carcinoma) with limited metastatic potential to the more potentially metastatic conventional clear cell renal cell carcinoma (ccRCC).
Approximately 70% of renal cortical tumors are confined to the kidney at presentation; 30% of patients either present with or later develop metastatic disease.2–4 ccRCC has a poor prognosis, largely because of its higher metastatic potential.5–10 Thus, a priori identification of this phenotype is important in clinical decision making.
For large renal tumors that have replaced the entire kidney, radical nephrectomy (RN) remains the surgical treatment of choice. However, for small renal masses (SRMs), 70% of which are detected incidentally at a median size of 4 cm or less,11 nephron-sparing surgical approaches are increasingly performed. There is emerging evidence that RN for SRM can cause or worsen preexisting chronic kidney disease and increase cardiovascular morbidity and mortality.12–14 In appropriately selected vulnerable patients—those who have a limited life expectancy, have competing comorbidities, or are surgically fragile for other reasons—the use of active surveillance may be an acceptable option.15 The creation of an individualized treatment plan is thus increasingly warranted.
The standard for definitive characterization of a renal mass remains surgical histopathology. Presurgical renal mass biopsy has limitations. A recent analysis of community practice suggests that less than 10% of patients with suspected RCC undergo renal mass sampling before nephrectomy, and the current rate of nondiagnostic biopsies ranges from 10% to 20% (inversely correlated to tumor size), even in the most experienced hands.16–18
Positron emission tomography/computed tomography (PET/CT) offers the ability to noninvasively characterize, in vivo, numerous pathophysiologic characteristics. Iodine-124 (124I) is a positron-emitting radionuclide with favorable physical properties for PET/CT imaging.19 The chimeric antibody cG250 (girentuximab) binds with carbonic anhydrase IX, a cell-surface antigen highly and homogeneously expressed in more than 95% of ccRCC.20 A PET/CT imaging study that used 124I-labeled girentuximab (124I-girentuximab) PET/CT in 26 presurgical patients with renal masses demonstrated a sensitivity of 94% and a specificity of 100%, with a negative predictive value (NPV) of 90% and a positive predictive value (PPV) of 100%.21 On the basis of these promising preliminary results, a phase III multicenter, open-label trial (REnal Masses: Pivotal Study to DETECT Clear Cell Renal Cell Carcinoma With Pre-Surgical PET/CT [REDECT]) was conducted by using presurgical 124I-girentuximab PET/CT in a contemporary cohort of patients with renal cortical tumors.
PATIENTS AND METHODS
This trial was designed to compare the sensitivity and specificity of 124I-girentuximab PET/CT to that of multiphasic contrast-enhanced CT (CECT). Patients scheduled for surgical resection of a renal mass underwent PET/CT after an infusion of 124I-girentuximab 5 mCi/13.7 mg and CECT. PET/CT was obtained 2 to 6 days after study drug infusion and before surgery. This range was feasible, given the 4.2-day half-life of 124I. CECT of the kidneys/abdomen was performed within 48 hours of PET/CT. CECT was acquired with contrast injection to scan delays of 30 seconds for the corticomedullary phase and 80 to 120 seconds for the parenchymal/excretory phase. Central blinded evaluation of all PET/CT and CECT scans was performed at a single imaging core laboratory (ICON Medical Imaging, Warrington, PA) by a panel of three independent reviewers per imaging modality, in accordance with predefined criteria and after training on image interpretation.
PET/CT was evaluated for evidence of radioactive uptake in the tumor and dichotomously designated positive or negative on qualitative assessment. A lesion was classified as positive for ccRCC if tumor radioactivity was visible and greater than that in normal kidney, normal liver, and blood. If any of these qualitative criteria were not met, the lesion was classified as negative. A positive 124I-girentuximab PET/CT scan was defined as a scan with at least one positive renal lesion. This dichotomous assessment was used in the analysis of the diagnostic efficacy variables.
A tumor was described as ccRCC on CECT if predefined enhancement properties were met22 in regions of interest drawn by the individual reader. Tumors that did not meet these criteria were recorded as non-ccRCC. Reviewers identified those instances when their own interpretation differed with the assessment based on enhancement cutoffs.
A central pathologist blinded to all imaging and local clinical site pathology results similarly categorized tumor specimens as positive (ccRCC present) or negative (ccRCC absent) by using the WHO 2004 classification system.23
The overall sample size was selected to provide at least 80% power to compare sensitivity and specificity of PET/CT to CECT by using two-sided McNemar's tests at a significance level of 0.05. We postulated values of sensitivity and specificity as 0.90 for PET/CT and 0.75 for CECT. Sample size considerations were driven by the analysis required for regulatory purposes, which included comparing each PET/CT reader separately to a consensus CECT interpretation. We projected a sample size of 166 patients for the study, with the expectation that this would ensure the availability of complete data on 158 patients. On the basis of clinical information, we initially projected that 40% of patients would not have histopathologically diagnosed ccRCC. However, because the actual proportion of patients without ccRCC was smaller than projected (52 of 195; 26.7%) and the proportion of evaluable patients was also lower than projected (195 of 226; 86.3%), we recruited a total of 226 patients in the study over an 18-month period.
Sensitivity and specificity were estimated for each of the three PET/CT and the three CECT readers separately, along with 95% CIs computed according to the Agresti-Coull method. The main analysis was based on data for those patients with complete data sets.
Primary efficacy variables for each imaging modality consisted of sensitivity and specificity. PPV, NPV, and accuracy were secondary efficacy variables. Only the independent central review of image results was considered in the analysis of diagnostic efficacy.
The average sensitivity of PET/CT readers was compared with that of CECT readers by using a mixed model approach24 that accounts for correlation due to the fact that readers of each modality interpreted scans from the same set of patients; two-sided P values were calculated. For descriptive purposes, we also derived averages of the PPV, NPV, and the overall accuracy for the two modalities. For descriptive purposes, a tabulation of imaging results by histologic subtype was developed. In this tabulation, imaging results were defined by the majority rule between the three readers in each imaging modality. We computed κ statistics25 as descriptive measures of agreement among readers; intrareader variability was assessed by percentage agreement on rereading a set of scans from a randomly selected 10% of patients.
RESULTS
A total of 226 patients were accrued at 14 centers in the United States (Appendix Table A1, online only) between May 2008 and November 2009. PET/CT was performed in all of the 204 patients who received the study drug; 203 patients also underwent CECT, and 202 of these had surgery. All but one of the PET/CT scans from the 204 patients were considered adequate for evaluation by all three PET/CT readers, and 198 of the 203 CECT images were considered adequate by all three CECT readers; 195 patients (96%) had all image sets and surgical histopathology available, and they formed the cohort analyzed in this study. Figure 1 illustrates the study schema in detail.
Fig 1.

Patient flow diagram. ccRCC, clear cell renal cell carcinoma; CECT, contrast-enhanced computed tomography; IP, investigational product; PET/CT, positron emission tomography/computed tomography.
Of the 202 patients who underwent surgery, 42 (20.8%) had multiple lesions. Lesion size ranged from 0.2 to 22 cm; the mean number of lesions per kidney was similar in patients with multiple lesions (2.5 lesions in the right kidney and 2.7 lesions in the left kidney). Only one patient had surgery for bilateral kidney tumors. The primary renal mass was ≤ 7 cm in diameter (T1b) in 158 (81.0%) of the 195 patients, and 101 of those (51.8%) were ≤ 4 cm (T1a). Demographic characteristics are presented in Table 1.
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Characteristic | Safety Population (n = 226) |
Patients With Complete Data Sets (n = 195)* |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years (mean ± SD) | 56.1 ± 12.0 | 55.8 ± 11.8 | ||
| Male | 142 | 62.8 | 126 | 64.6 |
| Race/ethnicity | ||||
| White | 200 | 88.5 | 177 | 90.8 |
| Black | 8 | 3.5 | 6 | 3.1 |
| Hispanic | 9 | 4.0 | 5 | 2.6 |
| Asian | 5 | 2.2 | 4 | 2.4 |
| Other | 4 | 1.8 | 3 | 1.5 |
| No. of patients who underwent surgery | 202 | 89.4 | 195 | 100.0 |
| With readable PET/CT and readable CECT | 195 | 86.3 | 195 | 100.0 |
| With multiple renal masses (n = 202) | 42 | 20.8 | 41 | 21.0 |
| Size of smallest/largest mass surgically resected, cm | ||||
| Range | 0.2-22 | 0.2-22 | ||
| T1a (≤ 4) | 101 | 51.8 | ||
| T1b (> 4 to ≤ 7) | 57 | 29.2 | ||
Abbreviations: CECT, contrast-enhanced computed tomography; PET/CT, positron emission tomography/computed tomography; SD, standard deviation.
Histopathologic diagnosis, PET/CT, and CECT results available for all image readers.
124I-girentuximab was well tolerated. There was no evidence of allergic reaction or drug intolerance. Adverse events judged by the investigators to be related to treatment were reported in 30 (13.3%) of the 226 patients. Of these treatment-related adverse events, 64% were grade 1 and 33% were grade 2. The most common treatment-related adverse event was headache, reported in 10 patients (4.4%), followed by nausea in three patients (1.3%), and diarrhea, dizziness, and hot flashes in two patients each (0.9%). One patient had a grade 3 agent-related adverse event—transient liver enzyme increase 3 weeks after administration of the study drug. This was reported by the investigator as study-drug–related, although the patient had also received ciprofloxacin before the transaminase increase. Serum human antichimeric antibodies were detected in 28% (56 of 198) of evaluable patients, with no difference in the frequency/severity of adverse events or diagnostic product performance compared with those without human antichimeric antibodies.
Imaging
Table 2 lists results for each imaging modality. The average sensitivity was 86.2% for 124I-girentuximab PET/CT readers and 75.5% for CECT readers (95% CI for difference, 2.4% to 19%; P = .023). The average specificity was 85.9% for 124I-girentuximab PET/CT readers and 46.8% for CECT readers (95% CI for difference, 19.5% to 58.7%; P = .005). Appendix Table A2 (online only) provides individual and average reader values for each primary efficacy variable.
Table 2.
Average Diagnostic Performance Data of PET/CT and CECT
| Imaging Modality | Primary Efficacy Variables |
Secondary Efficacy Variables |
|||||
|---|---|---|---|---|---|---|---|
| Sensitivity* | 95% CI† | Specificity* | 95% CI† | PPV* | NPV* | Accuracy* | |
| PET/CT | 0.862 | 0.753 to 0.971 | 0.859 | 0.694 to 0.999 | 0.944 | 0.694 | 0.862 |
| CECT | 0.755 | 0.626 to 0.884 | 0.468 | 0.188 to 0.747 | 0.796 | 0.410 | 0.679 |
| Difference (PET/CT-CECT) | 0.107 | 0.024 to 0.190 | 0.391 | 0.195 to 0.587 | 0.148 | 0.284 | 0.183 |
| P‡ | .023 | .005 | N/D | N/D | N/D | ||
Abbreviations: CECT, contrast-enhanced computed tomography; N/D, not done; NPV, negative predictive value; PET/CT, positron emission tomography/computed tomography; PPV, positive predictive value.
Average estimate of three independent, blinded central readers per imaging modality.
95% CIs for averages and differences of averages were derived via a mixed model approach to account for correlations in the data; 95% CIs for differences of averages not calculated for secondary efficacy variables.
Two-sided P values; calculated only for primary efficacy variables.
Table 2 tabulates individual accuracy values for each imaging modality. The secondary efficacy variables of overall accuracy, PPV, and NPV were also estimated for each reader and modality (Appendix Table A3, online only). PET/CT estimates were consistently higher than those for CECT. In particular, PET/CT accuracy ranged from a minimum of 85.6% (95% CI, 80.0% to 89.9%) to a maximum of 86.7% (95% CI, 81.1% to 90.8%), and CECT accuracy ranged from a minimum of 66.2% (95% CI, 59.3% to 72.4%) to a maximum of 69.2% (95% CI, 62.4% to 75.3%). PPV estimates for PET/CT ranged from 93.9% (95% CI, 88.2% to 97.0%) to 94.7% (95% CI, 89.2% to 97.6%); CECT ranged from 78.1% (95% CI, 70.4% to 84.2%) to 80.5% (95% CI, 72.8% to 86.3%); NPV estimates for PET/CT ranged from 68.8% (95% CI, 56.6% to 78.8%) to 70.3% (95% CI, 58.2% to 80.1%); CECT ranged from 37.9% (95% CI, 26.6% to 50.8%) to 43.1% (95% CI, 31.2% to 55.9%). The detection of ccRCC was independent of Fuhrman grade, as detailed in Table 3, which also provides detail on histologic subtype. The sensitivity of PET/CT for T1a and T1b ccRCC lesions was 82.8% and 95.7%, respectively. Four lesions ≤ 1 cm were detected and correctly diagnosed. Sensitivity of PET/CT was 70.8% for lesions ≤ 2 cm and 89.4% for lesions more than 2 cm and ≤ 4 cm.
Table 3.
PET/CT and CECT Results for Each Histologic Subtype of Renal Mass
| Tumor Category-Diagnosis | Central Pathologist* |
Positive on PET/CT† |
Positive on CECT† |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| ccRCC | 143 | 73.3 | 124 | 86.7 | 109 | 76.2 |
| Grade 1 to 2 | 90 | 62.9 | 78 | 86.7 | 67 | 74.4 |
| Grade 3 to 4 | 53 | 37.1 | 46 | 86.8 | 42 | 79.2 |
| Papillary type 1 RCC | 11 | 5.6 | 0 | 2 | ||
| Papillary type 2 RCC | 5 | 2.6 | 1 | 0 | ||
| Chromophobe RCC | 7 | 3.6 | 0 | 3 | ||
| Other RCCs‡ | 2 | 1.0 | 0 | 2 | ||
| Total other RCCs | 25 | 12.8 | 1 | 4.0 | 7 | 28.0 |
| Oncocytomas | 16 | 8.2 | 5 | 16 | ||
| Angiomyolipoma | 4 | 2.1 | 0 | 3 | ||
| Other tumors§ | 7 | 3.6 | 1 | 2 | ||
| Total non-RCCs | 27 | 13.8 | 6 | 22.2 | 21 | 77.8 |
| Total non-ccRCCs | 52 | 26.7 | 7 | 13.5 | 28 | 53.8 |
Abbreviations: ccRCC, clear cell renal cell carcinoma; CECT, contrast-enhanced computed tomography; PET/CT, positron emission tomography/computed tomography; RCC, renal cell carcinoma.
Including all patients with complete data sets (n = 195).
Readout using majority rule for the three readers per imaging modality.
One RCC unclassified, grade 3; one RCC oncocytic papillary.
One each: metanephric adenoma, simple cyst, cystic nephroma, multiloculated benign cyst, organizing hematoma, poorly differentiated malignant neoplasm, low-grade leiomyosarcoma.
For inter-reader variability, the κ statistics for pairs of readers of the 124I-girentuximab PET/CT scans ranged from 0.87 to 0.92, indicating excellent, robust agreement between readers. The κ statistics for pairs of CECT readers ranged from 0.67 to 0.76. Intrareader variability was relatively similar between the two imaging modalities. The percentage of agreement between two image evaluations on a randomly selected set of 10% of patients for 124I-girentuximab PET/CT ranged from 87.0% to 100% and for CECT, from 73.7% to 91.3%.
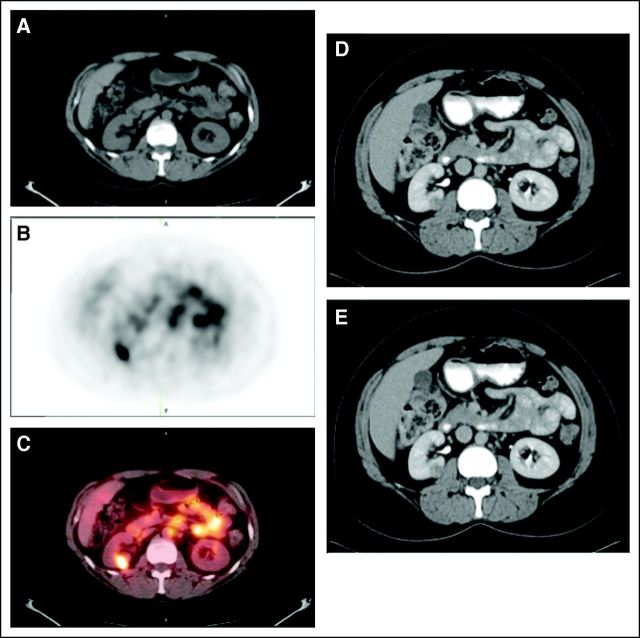
Figure 2 shows CECT and PET/CT images from a patient with a 1.0-cm lesion that was positive on both CECT and PET/CT. Figure 3 shows a 1.8-cm oncocytoma that was negative for ccRCC on 124I-girentuximab PET/CT and (false) positive on CECT, with agreement between reader interpretation and enhancement assessment.
Fig 2.
Patient with a 1.0-cm right renal clear cell carcinoma. (A) The mass is evident in the noncontrast computed tomography (CT) component of the positron emission tomography/computed tomography (PET/CT) scans, (B) is positive on the iodine-124 (124I) -girentuximab PET component, and (C) is clearly evident on the fused image. The mass was deemed to be positive on the contrast-enhanced CT scan of the (D) parenchymal component and (E) excretory component by Hounsfield criteria and qualitatively.
Fig 3.

Patient with a 1.8-cm right renal oncocytoma. The mass was deemed to be positive for clear cell renal cell carcinoma on the contrast-enhanced computed tomography scan of the (A) parenchymal component and (B) excretory component by Hounsfield criteria and qualitatively. (C) The iodine-124 (124I) -girentuximab positron emission tomography/computed tomography scan is negative.
DISCUSSION
This study confirms the high accuracy of 124I-girentuximab PET/CT in the preoperative, noninvasive identification of the ccRCC phenotype. Centralized production and distribution of 124I-girentuximab was demonstrated to be feasible. The trial was conducted at 14 centers in the United States. The average estimated sensitivity and specificity for detection of ccRCC in this multicenter trial were 86.2% and 85.9%, respectively, using a qualitative dichotomous classification based on our earlier results and for ease of and confidence in interpretation. Although sensitivity was lower for tumors ≤ 2 cm and higher for tumors more than 2 cm, all lesions below 1 cm were visualized. The study was not designed to assess lesion size detection limits. Secondary efficacy variables of PPV and NPV for PET/CT were 94.4% and 69.4%, respectively. Both inter- and intraobserver agreement were high, underscoring the potential for broad applicability of this imaging technique for the noninvasive identification of ccRCC.
A possible limitation of this study in assessment of 124I-girentuximab PET/CT utility was the requirement of presurgical patients. Every patient was eligible for surgery, and surgical biopsy was considered the standard of reference against which the imaging modalities were considered. The study design was considered essential by us, as well as by the US Food and Drug Administration, for validation of PET/CT performance. However, 124I-girentuximab PET/CT will probably be most useful in patients in whom surgical resection of their renal mass cannot (due to comorbidities) or need not (if benign without associated symptoms) be carried out. Therefore, its role in influencing outcome would perhaps be best assessed in a clinical trial carried out in patients with SRM tumors and associated comorbidities.
Currently, a renal mass enhanced on CT is considered malignant until proven otherwise, although there is increasing awareness that smaller masses are more likely to be benign. Retrospective and prospective data sets have confirmed postoperative benign disease in 15% to 30% of clinical T1a lesions in patients with a presurgical diagnosis of suspected renal cancer.3,26–29 The therapeutic role of tumor resection in this setting is likely multifaceted; however, it is conceivable that a proportion of patients might have been managed effectively with active surveillance if more precise preoperative histologic information had been available. Importantly, and clearly illustrative of the impact that presurgical knowledge of clear cell tumor histology has on clinical decision making, retrospective analyses show that if a sufficiently diagnostic renal mass biopsy is obtained, the results lead to a change in clinical management 40% to 60% of the time.30–32 Diagnostic RN for SRMs should no longer be considered automatic or acceptable surgical care without preoperative tissue characterization.
In renal neoplasms, current preoperative histologic characterization can be achieved only by biopsy. Although the accuracy of renal biopsy can approach more than 90% at established centers,33,34 rates vary depending on tumor size, tumor location, and the physician's technique.16,35,36 Needle biopsy is invasive and is associated with inherent risks, and its usefulness is particularly limited in patients with comorbidities.36,37 Furthermore, biopsy is problematic in patients with multiple renal masses, which may confound resection choice and surgical management. The role of preoperative renal mass biopsy thus remains controversial because of issues relating to diagnostic accuracy, dependence on an adequate biopsy sample for analysis, inability to distinguish tumor histologic subtypes and histologic grade, and concerns by clinicians of how negative or equivocal results would alter the ultimate treatment plan. As a consequence, its actual clinical use is estimated at less than 10%.17
There is thus a need for better methods for preoperative characterization of tumor histology. This multicenter trial demonstrated that 124I-girentuximab PET/CT could provide information on the presence or absence of ccRCC with accuracy at least comparable to that of biopsy, while obviating the need for this procedure with its inherent risks. Moreover, chromophobe and most papillary (type 1) cancers (which account for up to 15% to 20% of all RCCs) are largely indolent, and thus a negative 124I-girentuximab PET/CT scan may allow risk-stratified management for this group of patients. A recent pooled analysis of 936 localized renal masses concluded that active surveillance was an acceptable approach in patients with competing health risks.38,39 With the additional information provided by 124I-girentuximab PET/CT, a broader population of vulnerable patients may benefit from active surveillance. In addition, 124I-girentuximab PET/CT can be carried out in patients with renal dysfunction or other comorbidities. Beyond the applications already mentioned, this specific PET/CT scan may play an important role in surgical planning by improving tumor characterization in patients with unilateral multicentric or bilateral lesions. Although not specifically addressed in this study, 124I-girentuximab PET/CT has been shown to identify anatomically occult regional nodal metastases, which may guide extent of resection and optimize renal surgery.40,41
These data suggest that patients presenting with incidentally identified T1 renal masses may benefit from the incorporation of 124I-girentuximab PET/CT to optimally inform a clinical management decision and add confidence and clarity to rational therapeutic recommendations for the surgically fragile, elderly, or comorbidly ill patient. Other studies have evaluated molecular imaging agents in stratification of patients with malignancy. Fluorodeoxyglucose PET/CT has assumed a role as a pharmacodynamic biomarker in many malignancies and is used clinically to evaluate early response in a variety of cancers,42,43 although it has been shown to be of limited utility in evaluating renal neoplasms.44,45 Similarly, molecular imaging is used to direct therapy in neuroendocrine tumors46 and has shown promise in selecting patients with breast cancer for endocrine therapy.47 Most of these studies, however, have been retrospective, and there has been no systematic, prospective evaluation of the relevant agent as a molecular imaging biomarker. 124I-girentuximab PET/CT represents the first molecular imaging modality that identifies an immunohistologically specific prognostic marker for a solid human tumor, and the observations in the initial, single-center, verification study21 have been validated in this prospective, multicenter validation clinical trial.
FDA has, as part of its Critical Path Initiative, provided guidance for the development of biomarkers48 that would enable the rapid qualification of biomarkers as drug development tools. In that guidance, a prognostic biomarker is defined as a “baseline patient or disease characteristic that categorizes patients by degree of risk for disease occurrence or progression.” PET/CT with 124I-girentuximab may fulfill these characteristics for identification of a malignant phenotype well established as conferring a poor prognosis. A negative 124I-girentuximab PET/CT could lead to an active surveillance schedule in an elderly or comorbidly ill individual or to an extended partial nephrectomy, ablation, or active surveillance in a medically fit individual, in which previously radical nephrectomy would have been planned on the basis of tumor size or difficult tumor location alone. A positive PET/CT, although it is unable to differentiate low- and high-grade ccRCC, would signal the conventional clear cell phenotype and could prompt surgical resection in the form of partial or radical nephrectomy or ablative intervention in the elderly or medically unfit patient.
In conclusion, PET/CT with 124I-girentuximab can accurately and noninvasively identify ccRCC. PET/CT with 124I-girentuximab may be of value in risk stratification of patients with renal masses, and it fulfills an unmet medical need to improve appropriate patient care while minimizing the risks of invasive diagnostics and potentially unnecessary surgery.
Acknowledgment
This research was funded in part through the National Cancer Institute Cancer Center Support Grant P30 CA008748.
We acknowledge Lloyd J. Old, MD, for his vision in developing radiolabeled antibodies for cancer imaging. We also thank Aviva Asnis-Alibozek for editorial assistance.
Appendix
The following were principal investigators in the REDECT trial: Chaitanya R. Divgi (coordinating investigator), Columbia University Medical Center, New York, NY; David Chen, Fox Chase Cancer Center, Philadelphia, PA; Steven Larson, Memorial Sloan-Kettering Cancer Center, New York, NY; Robert Bahnson, Ohio State University, Columbus, OH; John Libertino, Lahey Clinic, Burlington, MA; Wade Sexton, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; Nicholas Vogelzang and Wolfram Samlowski, Nevada Cancer Institute, Las Vegas, NV; Allan Pantuck, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA; Thomas Polascik, Duke University Medical Center, Durham, NC; Khaled Hafez, University of Michigan, Ann Arbor, MI; Benjamin Chung, Stanford University, Palo Alto, CA; Daniel Pryma, University of Pennsylvania, Philadelphia, PA; Shyam Srinivas, Cleveland Clinic Foundation, Cleveland, OH; Christopher Wood, MD Anderson Cancer Center, Houston, TX; Arif Sheikh, University of North Carolina School of Medicine at Chapel Hill, Chapel Hill, NC.
Table A1.
List of Participating Centers With Number of Patients Accrued Per Site
| Participating Site | Site Location | No. of Patients Accrued |
|---|---|---|
| Fox Chase Cancer Center | Philadelphia, PA | 54 |
| Memorial Sloan-Kettering Cancer Center | New York, NY | 42 |
| Ohio State University | Columbus, OH | 35 |
| Lahey Clinic | Burlington, MA | 24 |
| H. Lee Moffitt Cancer Center and Research Institute | Tampa, FL | 18 |
| Nevada Cancer Institute | Las Vegas, NV | 17 |
| David Geffen School of Medicine, UCLA | Los Angeles, CA | 11 |
| Duke University Medical Center | Durham, NC | 6 |
| University of Michigan | Ann Arbor, MI | 5 |
| Stanford University | Palo Alto, CA | 4 |
| Hospital of the University of Pennsylvania | Philadelphia, PA | 3 |
| Cleveland Clinic Foundation | Cleveland, OH | 3 |
| MD Anderson Cancer Center | Houston, TX | 3 |
| University of North Carolina School of Medicine at Chapel Hill | Chapel Hill, NC | 1 |
| Total | 226 |
Table A2.
Sensitivity and Specificity Estimates Per Individual Reader (primary efficacy variables)*
| Reader | Sensitivity |
Specificity |
||||
|---|---|---|---|---|---|---|
| Estimate | Patients (true positive/all positive) | 95% CI† | Estimate | Patients (true negative/all negative) | 95% CI† | |
| PET-D | 0.860 | 123/143 | 0.793 to 0.908 | 0.846 | 44/52 | 0.722 to 0.923 |
| PET-E | 0.860 | 123/143 | 0.793 to 0.908 | 0.865 | 45/52 | 0.744 to 0.936 |
| PET-F | 0.867 | 124/143 | 0.801 to 0.914 | 0.865 | 45/52 | 0.744 to 0.936 |
| Average | 0.862 | 0.753 to 0.971 | 0.859 | 0.694 to 0.999 | ||
| CT-A | 0.748 | 107/143 | 0.671 to 0.813 | 0.423 | 22/52 | 0.299 to 0.557 |
| CT-B | 0.748 | 107/143 | 0.671 to 0.813 | 0.50 | 26/52 | 0.369 to 0.631 |
| CT-C | 0.769 | 110/143 | 0.693 to 0.831 | 0.481 | 25/52 | 0.351 to 0.613 |
| Average‡ | 0.755 | 0.626 to 0.884 | 0.468 | 0.188 to 0.747 | ||
| Difference (PET-CT) | 0.107 | 0.024 to 0.190 | 0.391 | 0.195 to 0.587 | ||
| P§ | .023 | .005 | ||||
Abbreviations: CT, computed tomography; PET, positron emission tomography.
Estimates were computed for each reader separately by using data on patients with complete data sets.
For individual readers, 95% CIs were computed by using the Agresti-Coull method.
For averages and differences of averages, 95% CIs were derived via a mixed model approach to account for correlations in the data; 95% CIs for differences of averages were not calculated for secondary efficacy variables.
Two-sided P values.
Table A3.
PPV, NPV, and Accuracy Estimates per Individual Reader (secondary efficacy variables)*
| Reader | PPV |
NPV |
Accuracy |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Patients (true positive/true + false positive) | 95% CI† | Estimate | Patients (true negative/true + false negative) | 95% CI† | Estimate | True Positive/All Patients | 95% CI† | |
| PET-D | 0.939 | 123/131 | 0.882 to 0.970 | 0.688 | 44/64 | 0.566 to 0.788 | 0.856 | 167/195 | 0.800 to 0.899 |
| PET-E | 0.946 | 123/130 | 0.891 to 0.976 | 0.692 | 45/65 | 0.572 to 0.792 | 0.861 | 168/195 | 0.806 to 0.910 |
| PET-F | 0.947 | 124/131 | 0.892 to 0.976 | 0.703 | 45/64 | 0.582 to 0.801 | 0.867 | 169/195 | 0.811 to 0.908 |
| Average | 0.944 | 0.694 | 0.862 | ||||||
| CT-A | 0.781 | 107/131 | 0.704 to 0.842 | 0.379 | 22/58 | 0.266 to 0.508 | 0.662 | 129/195 | 0.593 to 0.724 |
| CT-B | 0.805 | 107/133 | 0.728 to 0.863 | 0.419 | 26/62 | 0.305 to 0.543 | 0.682 | 133/195 | 0.614 to 0.743 |
| CT-C | 0.803 | 110/137 | 0.728 to 0.861 | 0.431 | 25/68 | 0.312 to 0.559 | 0.692 | 135/195 | 0.624 to 0.753 |
| Average‡ | 0.796 | 0.410 | 0.679 | ||||||
| Difference (PET-CT)‡ | 0.148 | 0.284 | 0.183 | ||||||
Abbreviations: CT, computed tomography; NPV, negative predictive value; PET, positron emission tomography; PPV, positive predictive value.
Estimates were computed for each reader separately by using data on patients with complete datasets.
95% CIs for individual readers were computed by using the Agresti-Coull method.
95% CIs for averages and differences of averages were not calculated for secondary efficacy variables.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00606632.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Constantine Gatsonis, WILEX (U); Roman Bartz, WILEX (C); Silke Treutner, WILEX (C); Paul Bevan, WILEX (C) Consultant or Advisory Role: Chaitanya R. Divgi, WILEX (C); Robert G. Uzzo, WILEX (C); Constantine Gatsonis, WILEX (C); Paul Russo, WILEX (C) Stock Ownership: Roman Bartz, WILEX Honoraria: None Research Funding: Chaitanya R. Divgi, WILEX; Robert G. Uzzo, WILEX; Jian Qin Yu, WILEX; David Chen, WILEX; Jorge A. Carrasquillo, WILEX; Steven Larson, WILEX; Paul Russo, WILEX Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Chaitanya R. Divgi, Robert G. Uzzo, Constantine Gatsonis, Roman Bartz, Silke Treutner, Paul Bevan, Paul Russo
Collection and assembly of data: Chaitanya R. Divgi, Constantine Gatsonis, Roman Bartz, Silke Treutner, Jian Qin Yu, David Chen, Jorge A. Carrasquillo, Steven Larson, Paul Russo
Data analysis and interpretation: Chaitanya R. Divgi, Robert G. Uzzo, Constantine Gatsonis, Silke Treutner, Jian Qin Yu, Paul Bevan, Paul Russo
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1. Siegel R Ward E Brawley O , etal: Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths CA Cancer J Clin 61: 212– 236,2011 [DOI] [PubMed] [Google Scholar]
- 2. Linehan WM, Walther MM, Zbar B: The genetic basis of cancer of the kidney J Urol 170: 2163– 2172,2003 [DOI] [PubMed] [Google Scholar]
- 3. Gill IS Aron M Gervais DA , etal: Small renal mass N Engl J Med 362: 624– 634,2010 [DOI] [PubMed] [Google Scholar]
- 4. Klatte T Pantuck AJ Kleid MD , etal: Understanding the natural biology of kidney cancer: Implications for targeted cancer therapy Rev Urol 9: 47– 56,2007 [PMC free article] [PubMed] [Google Scholar]
- 5. Kattan MW Reuter V Motzer RJ , etal: A postoperative prognostic nomogram for renal cell carcinoma J Urol 166: 63– 67,2001 [PubMed] [Google Scholar]
- 6. Sorbellini M Kattan MW Snyder ME , etal: A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma J Urol 173: 48– 51,2005 [DOI] [PubMed] [Google Scholar]
- 7. Motzer RJ Bacik J Mariani T , etal: Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology J Clin Oncol 20: 2376– 2381,2002 [DOI] [PubMed] [Google Scholar]
- 8. Cheville JC Lohse CM Zincke H , etal: Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma Am J Surg Pathol 27: 612– 624,2003 [DOI] [PubMed] [Google Scholar]
- 9. Gudbjartsson T Hardarson S Petursdottir V , etal: Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: A retrospective nation-wide study of 629 patients Eur Urol 48: 593– 600,2005 [DOI] [PubMed] [Google Scholar]
- 10. Leibovich BC Lohse CM Crispen PL , etal: Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma J Urol 183: 1309– 1315,2010 [DOI] [PubMed] [Google Scholar]
- 11. Berland LL Silverman SG Gore RM , etal: Managing incidental findings on abdominal CT: White paper of the ACR incidental findings committee J Am Coll Radiol 7: 754– 773,2010 [DOI] [PubMed] [Google Scholar]
- 12. Huang WC Levey AS Serio AM , etal: Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study Lancet Oncol 7: 735– 740,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang WC Elkin EB Levey AS , etal: Partial nephrectomy versus radical nephrectomy in patients with small renal tumors: Is there a difference in mortality and cardiovascular outcomes? J Urol 181: 55– 61,2009. discussion 61-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zini L Perrotte P Capitanio U , etal: Radical versus partial nephrectomy: Effect on overall and noncancer mortality Cancer 115: 1465– 1471,2009 [DOI] [PubMed] [Google Scholar]
- 15. Novick AC Campbell SC Belldegrun A , etal: Guideline for Management of the Clinical Stage 1 Renal Mass 2009. American Urological Association Education and Research; http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/renalmass09.pdf [Google Scholar]
- 16. Leveridge MJ Finelli A Kachura JR , etal: Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy Eur Urol 60: 578– 584,2011 [DOI] [PubMed] [Google Scholar]
- 17. Larson S Sarnes M McLaughlin T , etal: Real world use of diagnostic procedures in renal cell carcinoma J Nucl Med 52,2011. suppl 1 abstr 1419 [Google Scholar]
- 18. Volpe A Mattar K Finelli A , etal: Contemporary results of percutaneous biopsy of 100 small renal masses: A single center experience J Urol 180: 2333– 2337,2008 [DOI] [PubMed] [Google Scholar]
- 19. Belov VV Bonab AA Fischman AJ , etal: Iodine-124 as a label for pharmacological PET imaging Mol Pharm 8: 736– 747,2011 [DOI] [PubMed] [Google Scholar]
- 20. Stillebroer AB Mulders PF Boerman OC , etal: Carbonic anhydrase IX in renal cell carcinoma: Implications for prognosis, diagnosis, and therapy Eur Urol 58: 75– 83,2010 [DOI] [PubMed] [Google Scholar]
- 21. Divgi CR Pandit-Taskar N Jungbluth AA , etal: Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: A phase I trial Lancet Oncol 8: 304– 310,2007 [DOI] [PubMed] [Google Scholar]
- 22. Kim JK Kim TK Ahn HJ , etal: Differentiation of subtypes of renal cell carcinoma on helical CT scans AJR Am J Roentgenol 178: 1499– 1506,2002 [DOI] [PubMed] [Google Scholar]
- 23. Eble JN Sauter G Epstein JI , etal: World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs 2004. Lyon, France: IARC Press [Google Scholar]
- 24. Obuchowski N, Rockette HE: Hypothesis testing of diagnostic accuracy for multiple readers and multiple tests: An ANOVA approach with dependent observations Commun Stat Simul Comput 24: 285– 308,1995 [Google Scholar]
- 25. Landis JR, Koch GG: The measurement of observer agreement for categorical data Biometrics 33: 159– 174,1977 [PubMed] [Google Scholar]
- 26. Kutikov A Fossett LK Ramchandani P , etal: Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging Urology 68: 737– 740,2006 [DOI] [PubMed] [Google Scholar]
- 27. Schachter LR Cookson MS Chang SS , etal: Second prize: Frequency of benign renal cortical tumors and histologic subtypes based on size in a contemporary series: What to tell our patients J Endourol 21: 819– 823,2007 [DOI] [PubMed] [Google Scholar]
- 28. Snyder ME Bach A Kattan MW , etal: Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in radiological diameter: Influence of sex J Urol 176: 2391– 2395,2006. discussion 2395-2396 [DOI] [PubMed] [Google Scholar]
- 29. Frank I Blute ML Cheville JC , etal: Solid renal tumors: An analysis of pathological features related to tumor size J Urol 170: 2217– 2220,2003 [DOI] [PubMed] [Google Scholar]
- 30. Maturen KE Nghiem HV Caoili EM , etal: Renal mass core biopsy: Accuracy and impact on clinical management AJR Am J Roentgenol 188: 563– 570,2007 [DOI] [PubMed] [Google Scholar]
- 31. Neuzillet Y Lechevallier E Andre M , etal: Accuracy and clinical role of fine needle percutaneous biopsy with computerized tomography guidance of small (less than 4.0 cm) renal masses J Urol 171: 1802– 1805,2004 [DOI] [PubMed] [Google Scholar]
- 32. Wood BJ Khan MA McGovern F , etal: Imaging guided biopsy of renal masses: Indications, accuracy and impact on clinical management J Urol 161: 1470– 1474,1999 [DOI] [PubMed] [Google Scholar]
- 33. Samplaski MK Zhou M Lane BR , etal: Renal mass sampling: An enlightened perspective Int J Urol 18: 5– 19,2011 [DOI] [PubMed] [Google Scholar]
- 34. Lane BR Samplaski MK Herts BR , etal: Renal mass biopsy: A renaissance? J Urol 179: 20– 27,2008 [DOI] [PubMed] [Google Scholar]
- 35. Herts BR, Baker ME: The current role of percutaneous biopsy in the evaluation of renal masses Semin Urol Oncol 13: 254– 261,1995 [PubMed] [Google Scholar]
- 36. Wang R Wolf JS Jr Wood DP Jr , etal: Accuracy of percutaneous core biopsy in management of small renal masses Urology 73: 586– 590,2009. discussion 590-591 [DOI] [PubMed] [Google Scholar]
- 37. Volpe A Kachura JR Geddie WR , etal: Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy J Urol 178: 379– 386,2007 [DOI] [PubMed] [Google Scholar]
- 38. Smaldone MC Kutikov A Egleston BL , etal: Small renal masses progressing to metastases under active surveillance: A systematic review and pooled analysis Cancer 118: 997– 1006,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jewett MA Mattar K Basiuk J , etal: Active surveillance of small renal masses: Progression patterns of early stage kidney cancer Eur Urol 60: 39– 44,2011 [DOI] [PubMed] [Google Scholar]
- 40. Bahnson EE Murrey DA Mojzisik CM , etal: PET/CT imaging of clear cell renal cell carcinoma with I labeled chimeric antibody Ther Adv Urol 1: 67– 70,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Povoski SP Hall NC Murrey DA Jr , etal: Multimodal imaging and detection strategy with 124 I-labeled chimeric monoclonal antibody cG250 for accurate localization and confirmation of extent of disease during laparascopic and open surgical resection of clear cell renal cell carcinoma Surg Innov [epub ahead of print on April 11, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Juweid ME Wiseman GA Vose JM , etal: Response assessment of aggressive non-Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography J Clin Oncol 23: 4652– 4661,2005 [DOI] [PubMed] [Google Scholar]
- 43. Weber WA: Assessing tumor response to therapy J Nucl Med 50: 1S– 10S,2009. suppl 1 [DOI] [PubMed] [Google Scholar]
- 44. Krajewski KM Giardino AA Zukotynski K , etal: Imaging in renal cell carcinoma Hematol Oncol Clin North Am 25: 687– 715,2011 [DOI] [PubMed] [Google Scholar]
- 45. Frangioni JV: The problem is background, not signal Mol Imaging 8: 303– 304,2009 [PubMed] [Google Scholar]
- 46. Kwekkeboom DJ Kam BL van Essen M , etal: Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors Endocr Relat Cancer 17: R53– R73,2010 [DOI] [PubMed] [Google Scholar]
- 47. Dehdashti F Mortimer JE Trinkaus K , etal: PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer Breast Cancer Res Treat 113: 509– 517,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Food and Drug Administration: FDA's Critical Path Initiative; 2011. Jan 20, http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/ucm076689.htm.