Abstract
Purpose
Although neoadjuvant chemoradiotherapy achieves low local recurrence rates in clinical stages II to III rectal cancer, it delays administration of optimal chemotherapy. We evaluated preoperative infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX)/bevacizumab with selective rather than consistent use of chemoradiotherapy.
Patients and Methods
Thirty-two patients with clinical stages II to III rectal cancer participated in this single-center phase II trial. All were candidates for low anterior resection with total mesorectal excision (TME). Patients were to receive six cycles of FOLFOX, with bevacizumab included for cycles 1 to 4. Patients with stable/progressive disease were to have radiation before TME, whereas responders were to have immediate TME. Postoperative radiation was planned if R0 resection was not achieved. Postoperative FOLFOX × 6 was recommended, but adjuvant regimens were left to clinician discretion. The primary outcome was R0 resection rate.
Results
Between April 2007 and December 2008, 32 (100%) of 32 study participants had R0 resections. Two did not complete preoperative chemotherapy secondary to cardiovascular toxicity. Both had preoperative chemoradiotherapy and then R0 resections. Of 30 patients completing preoperative chemotherapy, all had tumor regression and TME without preoperative chemoradiotherapy. The pathologic complete response rate to chemotherapy alone was 8 of 32 (25%; 95% CI, 11% to 43%). The 4-year local recurrence rate was 0% (95% CI, 0% to 11%); the 4-year disease-free survival was 84% (95% CI, 67% to 94%).
Conclusion
For selected patients with clinical stages II to III rectal cancer, neoadjuvant chemotherapy and selective radiation does not seem to compromise outcomes. Preoperative Radiation or Selective Preoperative Radiation and Evaluation Before Chemotherapy and TME (PROSPECT), a randomized phase III trial to validate this experience, is now open in the US cooperative group network.
INTRODUCTION
The advantage of combined-modality therapy (CMT) in rectal cancer is that it has reduced local pelvic recurrence—a dreaded and morbid event—to rates of < 10%.1,2 In 2004, a German randomized trial established the superiority of preoperative administration of fluorouracil-based chemoradiotherapy (FUCMT).3,4 Subsequently, neoadjuvant FUCMT followed by a total mesorectal excision (TME) and postoperative systemic therapy has been standard practice in North America.5 Most trials comparing CMT regimens demonstrated that oral capecitabine and parenteral FU have equal efficacy and that intensification with oxaliplatin has no incremental benefit.6 As a result, the current trimodality paradigm of fluoropyrimidine-containing CMT, then TME, and finally adjuvant systemic therapy, prevails.
Although local recurrence (LR) has been relegated to a rare complication, distant recurrence rates for stages II to III rectal cancers are still consistently > 25% and patients therefore more commonly succumb to rectal cancer as a consequence of metastatic disease. One strategy to reduce the distant recurrence rate, and thereby increase the cure rate, would be to introduce systemic treatment earlier to prevent dissemination of micrometastases. Trials of neoadjuvant infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) or capecitabine plus oxaliplatin before chemoradiotherapy reported by British and Spanish investigators have demonstrated increased chemotherapy treatment exposure, high response rates, and favorable outcomes.7,8
On the basis of the results of these trials, as well as our own experiences treating stage IV rectal cancers with FOLFOX,9 we hypothesized that radiotherapy could be selectively omitted for patients who respond to neoadjuvant FOLFOX and bevacizumab (FOLFOX/bevacizumab). We were motivated to investigate this strategy both by the possibility of avoiding the toxicities of radiation without compromising local control, and the possibility that earlier introduction of full dose chemotherapy might improve distant control. In 2007, bevacizumab plus chemotherapy was the preferred first-line regimen for metastatic colorectal cancer and was undergoing phase III testing in adjuvant colon cancer10; therefore, we included bevacizumab in our neoadjuvant chemotherapy regimen. We designed an investigator-initiated, pharmaceutical-sponsored single institution pilot study to determine if neoadjuvant systemic therapy, with selective use of radiation reserved for nonresponders, was a safe and effective treatment strategy for patients with clinical stages II to III rectal cancer amenable to low anterior resection.
PATIENTS AND METHODS
The study was an open label, single arm, single-center phase II study conducted at Memorial Sloan-Kettering Cancer Center and was approved by the institution's review board. All study participants provided written informed consent.
Patient Selection
Eligibility criteria required adults with pathologically confirmed rectal adenocarcinoma and no previous treatment. All participants had baseline staging that included a contrast-enhanced computed tomography (CT) scan of the chest, abdomen, and pelvis to rule out metastatic disease. The colorectal surgeon performed a baseline rigid proctoscopy and identified that the tumor was amenable to sphincter-preserving TME and had a distal edge located between 5 and 12 cm of the anal verge. All patients had radiologic staging with endorectal ultrasound (ERUS), as well as contrast enhanced pelvic magnetic resonance imaging (MRI) to estimate tumor size and the extent of nodal involvement. Patients were included if imaging suggested clinical cT3N− or cT3N+ disease. Clinical T stage was estimated based on both MRI and ERUS with review of discrepant estimates by the surgeon and the study radiologist. Patients were ineligible if the primary tumor was T4, encroaching on the mesorectal fascia, fixed or deemed unresectable before administration of any preoperative therapy on the basis of the surgeon's clinical assessment and image review. Patients with clinical obstruction requiring a temporary diverting ostomy or endorectal stent to maintain bowel patency were excluded. Given the inability to precisely determine nodal status on the basis of either MRI or ERUS, clinical nodal status was estimated as node-negative (N−) if there were no perirectal lymph nodes > 5 mm, and node-positive (N+) if there were one or more perirectal lymph nodes larger than 5 mm. Patients with four or more pelvic lymph nodes > 2 cm manifest on MRI or ERUS were deemed to have bulky nodal disease and were excluded.
Patients with treatment for another primary cancer within 5 years or a thrombotic episode within 6 months of enrollment were excluded. Patients on stable doses of anticoagulant therapy were eligible as were those with controlled hypertension. Patients had to be candidates for systemic chemotherapy with FOLFOX/bevacizumab and therefore needed to have an Eastern Cooperative Oncology Group performance status of 0 to 2, adequate hematologic, liver, and renal function (ie, neutrophils ≥ 1.5 × 109/L; platelet count ≥ 100 × 109/L; creatinine clearance ≥ 30 mL/min; total bilirubin concentration not ≥ two times the upper limit of normal; and liver transaminase or alkaline phosphatase concentrations not ≥ three times the upper limit of normal).
Treatment
Neoadjuvant treatment.
The treatment schema (Figs 1 and 2) included four cycles of modified FOLFOX6 (mFOLFOX6)10 with bevacizumab, followed by two cycles of mFOLFOX6 alone. Patients who were unable to tolerate neoadjuvant FOLFOX/bevacizumab were treated with combined modality fluorouracil and pelvic radiation (FUCMT). Patients who had any clinical evidence of progression during chemotherapy were to proceed to FUCMT. Toxicity was assessed before each 2-week cycle according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Standard dose modifications were used. Pegfilgrastim (Neulasta; Amgen, Thousand Oaks, CA) was not administered prophylactically with the first cycle, but use was permitted during later cycles of therapy. Medical therapy to maintain blood pressure within normal limits and antiemetic support were prescribed according to institutional guidelines.
Fig 1.
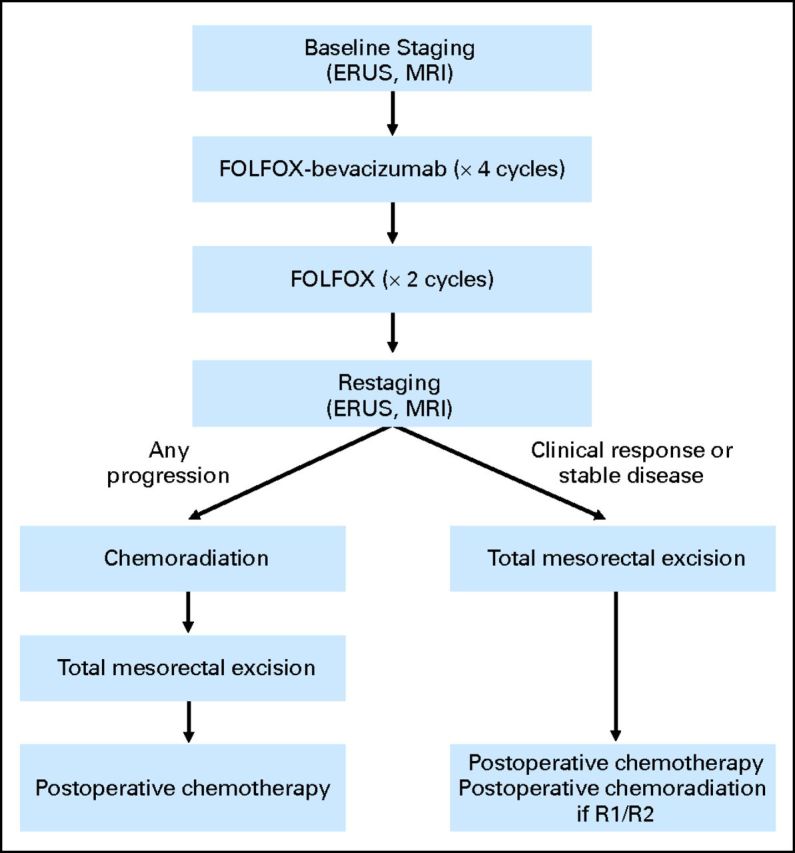
Patient flow diagram. ERUS, endorectal ultrasound; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; MRI, magnetic resonance imaging.
Fig 2.
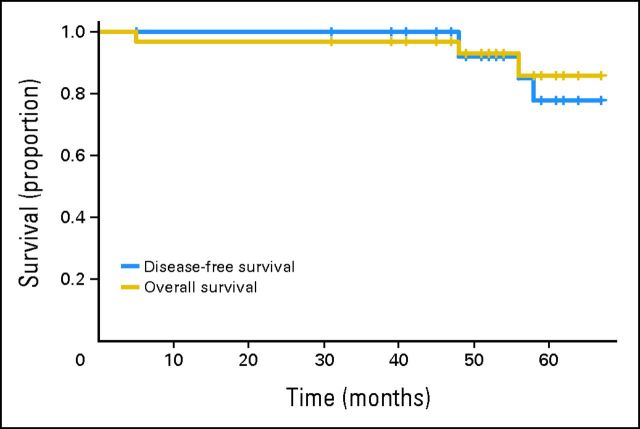
Disease-free and overall survival for the 32 study participants. A single patient who died as a result of postoperative complications but without disease is not censored, but is considered to have had an event.
Surgery with TME was performed 3 to 6 weeks from completion of FUCMT or the final cycle of FOLFOX/bevacizumab. Creation of a temporary diverting ostomy was at the discretion of the primary surgeon. Delaying ostomy takedown until the completion of all systemic therapy was recommended.
Synchronous chemoradiotherapy.
Patients underwent either 3D conformal radiotherapy or intensity modulated radiotherapy treatment planning. The treatment volume included the primary tumor and the mesorectal, presacral, and internal iliac lymph nodes up to the level of the bottom part of the fifth lumbar vertebra. For 3D conformal radiotherapy plans, the regional nodes were treated to 45 Gy in 1.8 Gy fractions followed by a 5.4 Gy boost to primary tumor. Patients treated with intensity-modulated radiotherapy received 45 Gy in 1.8 Gy fractions to regional nodes and 50 Gy in 2 Gy fractions to the primary or, in the postoperative setting, the tumor bed and anastomosis, as an integrated boost. During radiotherapy, continuous infusion fluorouracil was to be delivered at 225 mg/m2 with standard dose adjustments. The criteria for receiving preoperative FUCMT included patients who had either intolerance of bevacizumab; no response to FOLFOX/bevacizumab; or progression during treatment. The criteria for administration of postoperative FUCMT were microscopic (R1) or macroscopic (R2) evidence of tumor in the surgical specimen. Consideration for chemoradiotherapy was advised for patients found by pathology to have T4 or N2 disease.
Restaging after neoadjuvant chemotherapy.
Proctoscopy, ERUS, and pelvic MRI were repeated within 2 to 3 weeks for all patients completing induction FOLFOX/bevacizumab. Any evidence of progression was an indication for FUCMT. In addition, patients showing no evidence of any tumor shrinkage on either clinical examination or ERUS were also to be referred for FUCMT. Final determination of clinical response was based on the composite of each restaging modality and was adjudicated by the primary colorectal surgeon who directly visualized the tumor before and after induction treatment. Surgery with TME was performed 3 to 6 weeks from completion of FUCMT or the final cycle of FOLFOX/bevacizumab. Creation of a temporary diverting ostomy was at the discretion of the primary surgeon; ostomy takedown was advised after completion of all systemic therapy.
Postoperative adjuvant chemotherapy.
For patients who did not receive FUCMT, six cycles of postoperative FOLFOX were recommended. However, a patient's tolerance of neoadjuvant FOLFOX was incorporated into the choice of postoperative regimen. The specific dosing for the postoperative chemotherapy regimen was at the discretion of the primary oncologist.
Post-treatment surveillance.
Patients without evidence of disease recurrence had symptom assessment and physical examination every 3 months in year 1 and at least every 6 months thereafter. Proctoscopy and contrast enhanced CT scans of the chest/abdomen and pelvis were performed at least annually.
Evaluation of Study End Points
Pathologic response.
The primary study outcome was the R0 resection rate. An R0 resection was defined as no evidence of tumor within 1 mm of the distal, proximal, or radial margins on the basis of the review by the study pathologist (J.S.). An R1 resection was defined as microscopic and R2 as macroscopic evidence of residual disease. A pathologic complete response (CR) was defined based on the absence of viable tumor cells in both the primary tumor and in the lymph nodes (ypT0N0). The extent of tumor response to neoadjuvant treatment was categorized based on the amount of viable carcinoma cells within the tumor as described previously.11–13 The extent of residual tumor in the resected TME specimen was classified according to the TNM staging system of the American Joint Committee on Cancer version 6.
LR was identified on the basis of physical examination or imaging demonstrating disease in the pelvis. Metastatic recurrence was identified based on physical examination or imaging. Isolated elevation of the carcinoembryonic antigen absent other evidence of disease was not considered to be recurrence. Disease-free survival (DFS) was measured based on the lack of any evidence of tumor recurrence including development of second primary colorectal cancer. The proportion of trial participants who received preoperative, postoperative, or any radiation was also a specified end point.
Statistical Considerations
A two-stage design, as proposed by Simon,14 was used to allow early termination if the ability to maintain an R0 resection rate > 90% was threatened. With 32 patients, we had 90% power to distinguish between rates of 80% and 95% with a type 1 error probability of 10%. The statistical plan required initial enrollment of seven patients with termination if five or fewer achieved R0 resections. If six or more achieved an R0 resection, an additional 24 patients were to be enrolled. If the R0 resection rate exceeded 90% (28 of the total 32 patients), then we would consider the treatment approach to warrant validation in a multicenter study in comparison to standard neoadjuvant FUCMT. Because of the importance of pathologic CR in rectal cancer, we also required that at least six (18.8%) of the 32 patients achieve a CR. The probability of observing at least six CRs is > 80% if the true CR rate is 25%. This probability reduces to < 10% if the true CR rate is 10%.
LR was measured from the date of enrollment until the date of pelvic recurrence on the basis of imaging or proctoscopy. DFS was measured from the date of enrollment until disease progression, any rectal relapse, or death from any cause and is illustrated using the Kaplan-Meier method. Patient records were reviewed in April 2013 to assess recurrence and were censored at the date of last follow-up. Statistical calculations were performed using SAS 9.1 for Windows (SAS Institute, Cary, NC).
RESULTS
Between March 2007 and October 2009, 32 patients were enrolled. The cutoff date for this report was April 15, 2013, by which time participants had been followed for a mean of 53 and a median of 54 months (range, 43 to 73 months). The 32 participants had median age of 52 (range, 26 to 81), and 17 of 32 (53%) were women. The majority (23 of 32 [72%]) had clinically node-positive tumors. There were 20 participants with T3N+, nine with T3N−, and three with T2N+ rectal tumors.
Summary study results are displayed in Table 1. All protocol participants were able to undergo an R0 resection, and 8 of 32 (25%) had a pathologic CR. To date, no participant has had an LR. Only two participants, both intolerant of FOLFOX/bevacizumab, received preoperative chemoradiotherapy. Four of 32 (12.5%) have developed metastatic disease, all first appearing in the lung. A total of three participants have died, two as a result of metastatic rectal cancer and one as a result of postoperative complications. Of the two living participants with metastatic disease, one had surgical resection of an isolated lung metastasis and is alive and free of disease. The 4-year DFS rate is 92.0% (95% CI, 82.1% to 100%), and the overall survival rate is 91.6% (95% CI, 84.0% to 100%).
Table 1.
Summary of Study Outcomes With Mean of 53 Months of Follow-Up Since Enrollment
| Study Outcome | No. | % | 95% CI |
|---|---|---|---|
| R0 resection rate | 32 | 100 | 89 to 100 |
| Pathologic complete response rate | 8 | 25 | 11 to 43 |
| Completion of neoadjuvant FOLFOX/bevacizumab | 30 | 93.8 | 79 to 99 |
| Preoperative chemoradiation | 2 | 6.3 | 1 to 21 |
| Postoperative radiation | 1 | 3.1 | 1 to 16 |
| 4-year local recurrence rate | 0 | 0 | 0 to 11 |
| 4-year disease-free survival | 27 | 84 | 67 to 94 |
| 4-year overall survival rate | 29 | 91 | 75 to 98 |
Abbreviation: FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin.
Of 32 patients, two (6.3%) did not complete the prescribed neoadjuvant treatment regimen, both secondary to cardiovascular toxicity of chemotherapy. One patient, a 63-year-old woman with cT3N+ tumor, developed an arrhythmia after her first cycle of FOLFOX/bevacizumab. Therapy was discontinued, and she was treated with standard chemoradiotherapy and went on to have an R0 resection of a pathologic T3N1 tumor and received postoperative FOLFOX without difficulty. She remains rectal cancer free as of April 2013. A 69-year-old man with a cT3N+ tumor developed angina after his second cycle of FOLFOX/bevacizumab. He was triaged to a local hospital with S-T changes on his ECG and had emergent angiography with stent placement. The study regimen was discontinued. He went on to receive neoadjuvant radiation without chemotherapy and had an R0 resection with yT3N1 disease. He developed metastatic lung disease and succumbed to his rectal cancer 57 months later.
The remaining 30 of 32 (93.8%) patients completed four cycles of FOLFOX/bevacizumab and two cycles of FOLFOX alone. At restaging, all patients had evidence of clinical response to therapy and therefore went directly to surgery for TME, almost always with a temporary diverting ostomy. At surgery, all of these 30 participants had R0 resections. One patient, an 81-year-old woman with cT3N1 tumor, had yT3N2 with 14 of 16 lymph nodes containing tumor. She also had a close radial margin with residual tumor detected within 3 mm of the surgical specimen. In the setting of a close surgical margin, postoperative radiotherapy was recommended and delivered. She developed pulmonary metastases within 1 year of her operation and ultimately died as a result of metastatic disease without local recurrence.
The correlation between participants' clinical, pathologic stage and outcomes is listed in Table 2. No participants have experienced an LR but four have developed systemic recurrence, all with pulmonary metastases. In total, three participants have died, one postoperatively and two as a result of metastatic disease. The postoperative death was a 64-year-old man with a cT3N0, yT1N1 tumor who presented to the emergency department 17 days postoperatively with syncope and new renal failure attributed to dehydration from high volume ileostomy output. Despite triage to the intensive care unit, he succumbed to renal failure and did not have autopsy. No evidence of infection, thromboembolism, or precipitating event was manifest.
Table 2.
Demographic Characteristics and Clinical and Pathologic Stage of Participants, in Order of Study Accrual Date
| Patient No. | Age (years) | Sex | Clinical Stage | Pathologic Stage | Tumor Regression (%) | Radiation | First Site of Recurrence | Vital/Disease Status |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 | F | T3N+ | T0N0 | CR | No | Lung | Alive, metCA, NED |
| 2 | 70 | M | T3N+ | T3N1 | 50 | Preop | Lung | Dead as result of CA |
| 3 | 48 | F | T3N− | T2N0 | 80 | No | — | Alive, NED |
| 4 | 72 | M | T3N+ | T0N0 | CR | No | — | Alive, NED |
| 5 | 63 | M | T3N+ | T0N0 | CR | No | — | Alive, NED |
| 6 | 64 | M | T3N− | T2N0 | 80 | No | — | Dead, postop, NED |
| 7 | 54 | M | T3N+ | T2N0 | 90 | No | — | Alive, NED |
| 8 | 81 | F | T3N+ | T3N2 | 40 | Postop | Lung | Dead as result of CA |
| 9 | 64 | F | T3N+ | T2N0 | 70 | Preop | — | Alive, NED |
| 10 | 56 | M | T3N+ | T3N0 | 90 | No | — | Alive, NED |
| 11 | 49 | M | T3N+ | T2N1 | 90 | No | — | Alive, NED |
| 12 | 67 | F | T3N+ | T0N0 | CR | No | — | Alive, NED |
| 13 | 32 | F | T2N+ | T3N1 | 40 | No | — | Alive, NED |
| 14 | 28 | F | T3N+ | T3N0 | 90 | No | — | Alive, NED |
| 15 | 46 | M | T3N+ | T0N0 | CR | No | — | Alive, NED |
| 16 | 46 | F | T3N+ | T2N0 | 80 | No | — | Alive, NED |
| 17 | 56 | F | T3N+ | T2N0 | 80 | No | — | Alive, NED |
| 18 | 37 | M | T3N− | T2N1 | 70 | No | — | Alive, NED |
| 19 | 26 | F | T3N+ | T3N2 | 30 | Postop | — | Alive, NED |
| 20 | 58 | M | T2N+ | T0N0 | CR | No | — | Alive, NED |
| 21 | 53 | M | T3N+ | T2N1 | 50 | No | — | Alive, NED |
| 22 | 49 | M | T2N+ | T1N0 | 90 | No | — | Alive, NED |
| 23 | 46 | F | T3N− | T3N0 | 10 | No | Lung | Alive, metCA |
| 24 | 42 | F | T3N+ | T3N1 | 60 | No | — | Alive, NED |
| 25 | 54 | M | T3N− | T1N0 | 90 | No | — | Alive, NED |
| 26 | 64 | F | T3N− | T3N1 | 60 | No | — | Alive, NED |
| 27 | 36 | M | T3N− | T0N0 | CR | No | — | Alive, NED |
| 28 | 42 | F | T3N+ | T2N0 | 20 | No | — | Alive, NED |
| 29 | 51 | F | T3N− | T3N0 | 90 | No | — | Alive, NED |
| 30 | 61 | M | T3N− | T0N0 | CR | No | — | Alive, NED |
| 31 | 41 | F | T3N+ | T2N1 | 90 | No | — | Alive, NED |
| 32 | 57 | F | T3N+ | T2N0 | 90 | No | — | Alive, NED |
Abbreviations: CR, complete response; metCA, metastatic cancer; NED, no evidence of disease; postop, postoperative; preop, preoperative.
DISCUSSION
Before integration of pelvic radiation into curative rectal cancer treatment 25 years ago, pelvic recurrence was a commonplace occurrence that resulted in debilitating complications such as abscess, fistula, and chronic pain. Since then, other major strides in rectal cancer therapeutics have been made including better surgical technique with TME and better imaging with CT, MRI, and ERUS, better chemotherapy regimens,15 and optimal ways to deliver chemoradiotherapy.3,4 Notwithstanding these advances, in recent trials, > 25% of patients with locally advanced rectal cancer have still developed distant metastases. In this context, the neoadjuvant paradigm, which delays delivery of systemic therapy until 3 to 4 months after diagnosis, seemed disadvantageous and encouraged us and others to investigate earlier integration of systemic chemotherapy into neoadjuvant treatment. Indeed, both randomized trials and observational studies indicate low adherence to postoperative systemic therapy after chemoradiotherapy and resection.16,17
Our pilot study findings demonstrate that indeed, a neoadjuvant systemic therapy approach that eliminates routine use of pelvic radiation can be delivered without apparent compromise of either short- or long-term outcomes in carefully staged patients with rectal cancer. With more than 4 years of follow-up, there have been no pelvic recurrences. Notwithstanding the small number of participants and the drawback that the study was performed in a single center where colorectal surgeons are skilled at performing TME, the strength and consistency of these results warrant corroboration in a multicenter study. Our treatment strategy is distinct from neoadjuvant paradigms that include pelvic radiotherapy as has been reported by European investigators7 and recently from Brown University18 because these protocols have not omitted pelvic radiation. In 2010, Fernandez-Martos et al19 reported favorable results of a trial with similar design to our pilot, and these final results will help to substantiate or negate our findings.
Several features of our study design warrant special mention. First, longstanding controversy about whether pelvic radiation is indicated for proximal rectal tumors (> 12 cm from the anal verge) persists. To avoid results that could be dismissed as attributable to favorable selection for proximal tumors, we restricted eligibility to patients whose primary rectal tumors were 5 to 12 cm from the anal verge on the basis of the surgeon's proctoscopic evaluation. Second, 23 of 32 (72%) study participants had clinical evidence of nodal involvement. With these eligibility criteria and the clinical stage distribution listed in Table 2, the low recurrence rate we observed cannot simply be attributed to selection of node-negative patients.
At the time we designed our study in 2006, bevacizumab had recently demonstrated superiority in the metastatic setting and was also expected to prove beneficial for adjuvant colon cancer treatment.10,20 As a result, we included bevacizumab in our neoadjuvant regimen. Two patients in our study developed cardiac complications during neoadjuvant FOLFOX/bevacizumab. There was also one postoperative death that seemed attributable to dehydration. We cannot exclude the possibility that bevacizumab contributed to these treatment complications. Given the negative results of two large-scale trials of bevacizumab in the adjuvant setting in colon cancer, we do not believe that our favorable results are attributable to bevacizumab and do not plan to incorporate it into future studies.
Pelvic radiation can have long-term impact on bowel, bladder, and sexual function and can impair bone marrow reserve, diminishing future tolerance of chemotherapy.21,22 For these reasons, a rectal cancer treatment paradigm that incorporates radiation selectively, as opposed to reflexively, would be advantageous. To confirm this auspicious pilot experience in the multicenter context, the PROSPECT trial (N1048, available at www.ctsu.org) has recently opened in North America with participation of all US and Canadian cooperative groups. It will compare our pilot study regimen (minus the bevacizumab) to the prevailing standard with neoadjuvant chemoradiotherapy strategy.
Footnotes
Supported by Genentech, who provided funding as well as the study drug bevacizumab.
Bevacizumab was provided free of charge to study participants, who were not otherwise compensated.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00462501.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Diane L. Reidy-Lagunes, Novartis (C); Leonard B. Saltz, Roche (C), Genentech (C), Bristol-Myers Squibb (C), ImClone Systems (C), Pfizer (C), Bayer HealthCare Pharmaceuticals (C), sanofi-aventis (U), Boehringer Ingleheim (C), Taiho Pharmaceutical (U), EMD Serono (U) Stock Ownership: None Honoraria: None Research Funding: Andrea Cercek, Bayer; Diane L. Reidy-Lagunes, Novartis; Leonard B. Saltz, Roche, Genentech, Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals, Taiho Pharmaceutical, Synta Pharmaceuticals Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Deborah Schrag, Martin R. Weiser, Karyn A. Goodman, Mithat Gon̈en, Diane L. Reidy-Lagunes, Jose G. Guillem, Larissa K.F. Temple, Philip B. Paty, Leonard B. Saltz
Provision of study materials or patients: Deborah Schrag, Martin R. Weiser, Karyn A. Goodman, Jose G. Guillem, Larissa K.F. Temple, Philip B. Paty, Leonard B. Saltz
Collection and assembly of data: Deborah Schrag, Martin R. Weiser, Karyn A. Goodman, Ellen Hollywood, Andrea Cercek, Marc J. Gollub, Jinru Shia, Jose G. Guillem, Larissa K.F. Temple, Philip B. Paty, Leonard B. Saltz
Data analysis and interpretation: Deborah Schrag, Martin R. Weiser, Karyn A. Goodman, Mithat Gon̈en, Andrea Cercek, Diane L. Reidy-Lagunes, Marc J. Gollub, Jinru Shia, Jose G. Guillem, Larissa K.F. Temple, Philip B. Paty, Leonard B. Saltz
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1. Adjuvant therapy for patients with colon and rectal cancer JAMA 264: 1444– 1450,1990. NIH Consensus Conference [PubMed] [Google Scholar]
- 2. Kapiteijn E Marijnen CA Nagtegaal ID , etal: Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer N Engl J Med 345: 638– 646,2001. [DOI] [PubMed] [Google Scholar]
- 3. Sauer R Liersch T Merkel S , etal: Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years J Clin Oncol 30: 1926– 1933,2012. [DOI] [PubMed] [Google Scholar]
- 4. Sauer R Becker H Hohenberger W , etal: Preoperative versus postoperative chemoradiotherapy for rectal cancer N Engl J Med 351: 1731– 1740,2004. [DOI] [PubMed] [Google Scholar]
- 5. Benson AB Bekaii-Saab T Chan E , etal: NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer 2013. Version 4 [DOI] [PubMed] [Google Scholar]
- 6. Roh MS Yothers GA O'Connell MJ , etal: The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04 J Clin Oncol 29: 221s,2011. suppl abstr 3503 [Google Scholar]
- 7. Chau I Brown G Cunningham D , etal: Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer J Clin Oncol 24: 668– 674,2006. [DOI] [PubMed] [Google Scholar]
- 8. Fernandez-Martos C Pericay C Aparicio J , etal: Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study J Clin Oncol 28: 859– 865,2010. [DOI] [PubMed] [Google Scholar]
- 9. Cercek A Weiser MR Goodman KA , etal: Complete pathologic response in the primary of rectal or colon cancer treated with FOLFOX without radiation J Clin Oncol 28,2010. suppl abstr 481 [Google Scholar]
- 10. Allegra CJ Yothers G O'Connell MJ , etal: Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08 J Clin Oncol 29: 11– 16,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dworak O, Keilholz L, Hoffmann A: Pathological features of rectal cancer after preoperative radiochemotherapy Int J Colorectal Dis 12: 19– 23,1997. [DOI] [PubMed] [Google Scholar]
- 12. Quah HM Chou JF Gonen M , etal: Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation Cancer 113: 57– 64,2008. [DOI] [PubMed] [Google Scholar]
- 13. Shia J Guillem JG Moore HG , etal: Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome Am J Surg Pathol 28: 215– 223,2004. [DOI] [PubMed] [Google Scholar]
- 14. Simon R: Optimal two-stage designs for phase II clinical trials Control Clin Trials 10: 1– 10,1989. [DOI] [PubMed] [Google Scholar]
- 15. Andre T Boni C Navarro M , etal: Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial J Clin Oncol 27: 3109– 3116,2009. [DOI] [PubMed] [Google Scholar]
- 16. Bosset JF Collette L Calais G , etal: Chemotherapy with preoperative radiotherapy in rectal cancer N Engl J Med 355: 1114– 1123,2006. [DOI] [PubMed] [Google Scholar]
- 17. Khrizman P Niland JC ter Veer A , etal: Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: A national comprehensive cancer network analysis J Clin Oncol 31: 30– 38,2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perez K Pricolo V Vrees M , etal: A phase II study of complete neoadjuvant therapy in rectal cancer (CONTRE): The Brown University Oncology Group J Clin Oncol 30,2013. suppl 4 abstr 335 [DOI] [PubMed] [Google Scholar]
- 19. Fernandez-Martos C Safont M Feliu J , etal: Induction chemotherapy with or without chemoradiation in intermediate-risk rectal cancer patients defined by magnetic resonance imaging (MRI): A GEMCAD study J Clin Oncol 29: 30s,2010. suppl abstr TPS196 [Google Scholar]
- 20. Hurwitz H Fehrenbacher L Novotny W , etal: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer N Engl J Med 350: 2335– 2342,2004. [DOI] [PubMed] [Google Scholar]
- 21. Bruheim K Guren MG Skovlund E , etal: Late side effects and quality of life after radiotherapy for rectal cancer Int J Radiat Oncol Biol Phys 76: 1005– 1011,2010. [DOI] [PubMed] [Google Scholar]
- 22. Temple LK, Wong WD, Minsky B: The impact of radiation on functional outcomes in patients with rectal cancer and sphincter preservation Semin Radiat Oncol 13: 469– 477,2003. [DOI] [PubMed] [Google Scholar]