ABSTRACT
Background: Previous studies have suggested the quorum sensing signal AI-2 as a potential target to prevent the biofilm formation by Streptococcus mutans, a pathogen involved in tooth decay.
Objective: To obtain inhibition of biofilm formation by S. mutans by extracts obtained from the marine bacterium Tenacibaculum sp. 20J interfering with the AI-2 quorum sensing system.
Design: The AI-2 inhibitory activity was tested with the biosensors Vibrio harveyi BB170 and JMH597. S. mutans ATCC25175 biofilm formation was monitored using impedance real-time measurements with the xCELLigence system®, confocal laser microscopy, and the crystal violet quantification method.
Results: The addition of the cell extract from Tenacibaculum sp. 20J reduced biofilm formation in S. mutans ATCC25175 by 40–50% compared to the control without significantly affecting growth. A decrease of almost 40% was also observed in S. oralis DSM20627 and S. dentisani 7747 biofilms.
Conclusions: The ability of Tenacibaculum sp. 20J to interfere with AI-2 and inhibit biofilm formation in S. mutans was demonstrated. The results indicate that the inhibition of quorum sensing processes may constitute a suitable strategy for inhibiting dental plaque formation, although additional experiments using mixed biofilm models would be required.
KEYWORDS: Quorum sensing, quorum quenching, AI-2, biofilms, dental plaque, streptococci
Introduction
Biofilms are complex microbial communities which usually have a key role in virulence and antibiotic resistance in pathogenic bacteria [1,2]. In numerous bacterial species, biofilm formation and maturation are regulated by quorum sensing (QS), a chemical communication system mediated by extracellular signals that are thought to enable bacteria to assess population size and coordinate virulence factor expression [3]. Therefore, the inhibition of QS, a process known as quorum quenching (QQ), constitutes an interesting alternative for controlling pathogenic bacterial behaviours [4]. Oral biofilms comprise about 500–1,000 species, almost half of them still uncultured [5]. Among them, Streptococcus species are the initial colonizers of clean teeth surfaces and the main components of early dental plaque. Although Streptococcus mutans was considered one of the major cariogenic bacteria in humans in the past [6,7], recent DNA- and RNA-based studies from carious lesions indicate that this bacterium represents only a small fraction of the bacterial community present in these lesions [8]. Biofilm formation by S. mutans is regulated by two QS systems, although the hierarchy of their organization is unknown. The QS system typical of streptococci is regulated by a peptide pheromone signal called competence stimulating peptide (CSP) [9], which is species-specific and detected by a histidine-kinase [10]. The inactivation of any of the genes related to the CSP QS system results in the alteration of biofilm architecture [11]. The other QS signal related to biofilm formation in S. mutans is AI-2 [7,12,13], which has been proposed as a universal signalling molecule due to its wide distribution among prokaryotes [14,15], including important oral pathogens [16]. The mutation of the gene of luxS which codifies the enzyme required for AI-2 production affects biofilm formation in S. mutans [12,17]. The QS signal AI-2 seems to be relevant also in other oral pathogens such as Porphyromonas gingivalis, Aggregatibacteractinomycetencomitans Y4, Treponema denticola, and Tannerella forsythia [7,18], which indicates complex signalling networks in the oral biofilm. According to the hypothesis proposed by Kolenbrander et al. [19], high levels of AI-2 would accelerate the growth of pathogens while reducing growth of commensal bacteria, contributing to subgingival plaque formation and maturation and leading to periodontal diseases. However, LuxS is also a central metabolic enzyme which plays a crucial role in methionine metabolism [20] and participates in protein, RNA, and DNA synthesis [17], and therefore, the effects observed when its gene is mutated have to be interpreted carefully. Furanones, which are known to inhibit communication based on acyl homoserine lactone (AHL) and AI-2 signals [21], have been reported to affect biofilm formation in S. mutans. A previous study indicated that the biofilm inhibition observed in S. mutans supplemented with the furanone C52 is linked with the interference with AI-2-controlled processes [22]. However, another study reported the inhibition of biofilm formation in S. mutans by the synthetic furanone C30 through an unknown mechanism not dependent of QS-signal AI-2, since the luxS mutant was also affected [23]. In S. pneumoniae, the use of the natural compound Sinefungin, which interferes with AI-2 synthesis, also caused the alteration of the biofilm structure and a concentration-dependent inhibition in biofilm formation [24].
Preliminary observations of anti-biofilm activity by Tenacibaculum sp. 20J against several Gram-positive and marine bacteria prompted us to study the capacity of cell extracts from 20J to interfere with communication mediated by the QS signal AI-2 and biofilm formation in the oral pathogen S. mutans. Surface-associated growth by S. mutans was monitored in real-time using the xCELLigence® technology that measures total biofilm biomass by combining both cellular growth and matrix production [25–28]. Moreover, confocal laser scanning microscopy (CLSM) as well as crystal violet biofilm staining were used to confirm xCELLigence® results.
Materials and methods
Bacterial strains and growth conditions
The Gram-positive biofilm forming bacteria S. mutans ATCC25175, S. oralis DSM20627, and S. dentisani 7747 were routinely cultured in brain heart infusion (BHI) broth or on BHI agar supplemented with 0.1% sucrose at 37°C. Biofilm formation by S. mutans was also assessed in BHI supplemented with both sucrose and glucose at 0.1% and 0.2%. The strain Tenacibaculum sp.20J CECT7426, isolated in our laboratory from a marine sample [29], and T. maritimum NCIMB2154T were routinely cultured at 22°C on Marine Agar/Broth pH 7.0 (MA/MB, Difco).
The strains used for the AI-2 bioassay, Vibrio harveyi BB170 (HAI-1 negative; AI-2 positive; CAI-1 positive), V. harveyi JMH597 (HAI-1 negative; AI-2 positive; CAI-1 negative) [30], and V. harveyi JAF548, in which bioluminescence is not under the control of QS systems due to the permanent activation of the QS-dependent bioluminescence repressor LuxO [31,32], were cultured in LB supplemented with 30 μg ml−1 of the antibiotic kanamycin (LB+Km) and in autoinducer bioassay medium (AB) [33].
Cell extracts preparation
Purified cell extracts (PCEs) were obtained from 50 ml stationary-phase MB cultures of Tenacibaculum sp. strain 20J and T. maritimum NCIMB2154T. The cultures were centrifuged for 10 min at 5000 × g in order to separate the biomass from the culture media. The pellet was washed with 15 ml of phosphate buffered saline (PBS) pH 6.5, resuspended in 1.5 ml of the same buffer, sonicated for 5 min in ice, and centrifuged at 16,000 × g for 30 min at 4°C. The PCEs obtained in this way were filtered through 0.22 μm and stored at 4°C [34]. Total protein concentration in the PCEs was estimated by Lowry’s method [35]. For the PCE of strain 20J, one aliquot was fractioned using Centricon® Centrifugal Filter Devices (Millipore Corporation, Bedford, MA) of 100 kDa (YM-100), 50 kDa (YM-50), and 10 kDa (YM-10). Filters were pre-washed with NaOH 0.1 N and deionized water. Aliquots of PCEs were also ultracentrifuged (20 min 300,000 g) or dialysed using D-tubeTM Dyalizer Mega (EMD Millipore Corp. Billerica, MA). The PCE (500 μl) was also extracted twice with an equal volume of ethyl acetate (EA-PCE 20J). For obtaining the methanolic cell extract (MCE), the biomass from a 50 ml culture of Tenacibaculum sp. 20J was resuspended in methanol (1.5 ml) and treated in the same way as the PCE. After centrifugation, an aliquot of the MCE was further extracted twice with an equal volume of dichloromethane (DM-MCE 20J). Solvents were evaporated to dryness in a rotary evaporator system at 40°C and resuspended in PBS pH 6.5.
The PCE of Tenacibaculum sp. 20J was also treated with proteinase K (100 μg ml−1; Sigma-Aldrich, MO) at 30°C for 1 h [36]. Thermal stability of the bioactive compound was tested by incubating the PCE of Tenacibaculum sp. 20J at 100°C for 10 min [36]. The residual anti-biofilm activity was measured by using the xCELLigence® System RTCA (ACEA, Biosciences Inc., CA).
Bioluminescence assay
V. harveyi BB170 (HAI-1 negative; AI-2 positive; CAI-1 positive), V. harveyi JMH597 (HAI-1 negative; AI-2 positive; CAI-1 negative), and the QS-independent bioluminescent strain V. harveyi JAF548 were used as biosensor strains [30–32]. They were grown overnight in MB at 30°C with shaking. Aliquots of these overnight cultures were diluted 1:5,000 into fresh AB medium (modified from [30]). In each well of a 96-well black microtiter plate, 180 µl of V. harveyi dilution and 20 µl of tested compounds were added. Photographs were taken every hour using the Gel DocTM XR+ system Bio Rad equipment. Experiments were performed in triplicate. The effect of the cell extracts on growth of the biosensor strains was monitored measuring the optical density at 600 nm under same conditions as the bioluminescence assay.
Biofilm measurement and analysis
The biofilm quantification was done using the xCELLigence® System RTCA (ACEA, Biosciences Inc.) [25–28], in which the increase in the cell adherence on the electrodes causes an increase in the electrode impedance, which is expressed into a dimensionless parameter called cell index. E-plates 16 or E-plate 96 (ACEA, Biosciences Inc.) were inoculated with 180 μl of a 24-h culture of S. mutans in BHI broth supplemented with 0.1% sucrose (OD600 adjusted to 0.05) and 20 μl of extracts or inhibitors. Furanone C30 (0.02 µg ml−1, Sigma-Aldrich) and the AHL-lactonase Aii20J (20 µg ml−1 [36]) were also tested. Twenty microlitres of PBS pH 6.5 were added to the control wells. The system was incubated at 37°C for 24 h.
Biofilms of S. mutans were allowed to form on glass coverslips (18 × 18 mm) using 12-well cell-culture plates containing 3 mL of BHI broth supplemented with 0.1% sucrose (modified from [37]). After incubation, the supernatant was removed, wells were rinsed with PBS to remove the planktonic cells, and the biofilms were stained with either crystal violet or with Syto9 (Thermofisher Scientific) for CLSM observation. For crystal violet quantification [modified from [38]], ethanol was added for the fixation of the biofilms, and after 15 min the supernatant was removed again. Then, 0.1% crystal violet (Gram-Hucker, Panreac) solution was added to all wells, and after 20 min, the excess of dye was removed washing several times. Bound crystal violet was released by adding 33% acetic acid. The absorbance was measured at 590 nm. The results are expressed in terms of percentage of biofilm formed in the presence of extracts in comparison with untreated wells. All experiments were performed in triplicate.
Microscopic observation was performed using a Leica TSC SP2 laser scanning spectral confocal microscope (Leica Microsystem Heidelberg GmbH, Mannheim, Germany) with an HCX PL APO 63x/1.3 Gly. Eight randomly selected fields of each sample were evaluated. The optical sections were scanned in 1-µm sections from the surface of the biofilm to its base, measuring the maximum thickness of the field and subsequently the mean thickness of the biofilm of the corresponding sample. The quantification of the percentage of coverage surface (µm2) was obtained comparing the total surface of the field with the bacterial biofilm. A Nikon Eclipse E400 microscope equipped with a Leica MC170 HD camera was used to image the biofilms stained with crystal violet.
Bacterial growth inhibition
The effect of the cell extracts (100 μg ml−1) and furanone (0.02 μg ml−1) on planktonic growth was investigated by comparing bacterial growth measured at 600 nm with control cultures after 24 h of incubation. Cultures were mixed vigorously with a pipette in order to detach the bacteria. BHI medium supplemented with 0.1% sucrose and the inoculum of S. mutans without PCE were used as controls. All experiments were performed in triplicate. Antimicrobial activity of the cell extracts was also assayed by the disc diffusion test which was performed on BHI agar. Sterile discs (Liofilchem®) with a diameter 6 mm were loaded with 20 µl of the cell extracts and the discs placed over plates inoculated with S. mutans. Plates were then incubated at 37°C for 24 h and checked for the presence of inhibitory halos.
Ahl-degradation activity bioassay
The AHL degradation activity of the different extracts was tested by incubating 500 μl of each extract with N-hexanoyl-L-homoserine lactone (C6-HSL) 10 μM for 24 h at 22°C 200 rpm and the remaining AHL was detected using the biosensor strain Chromobacterium violaceum CV026 [39,40]. After 24 h of degradation, 100 μl of the mixture were placed in wells made on an LB plate overlaid with a 1/100 dilution of an overnight culture of C. violaceum CV026 in soft LB (8% agar). The plates were incubated for 24 h at 30°C and the production of violacein was observed. As control, wells were filled with 100 μl of sterile PBS plus C6-HSL treated as samples.
Statistical analyses
The student t-test was performed to determine the statistical significance of the differences in cell index and bacterial growth by S. mutans between the control and the treated wells. Significant differences were determined at P < 0.05.
Results
Cell extracts of Tenacibaculum sp. 20J interfere with AI-2 signalling
To assess the interference with AI-2-mediated communication, a cell extract from Tenacibaculum sp. 20J was obtained and tested with the reporter strains V. harveyi BB170, in which bioluminescence is under the control of the AI-2 and CAI-1 QS systems, and V. harveyi JMH597 that only senses the AI-2 signal [30]. To exclude that 20J-PCE affects bioluminescence in V. harveyi through factors nondependent on QS, the mutant V. harveyi JAF548 [31] in which bioluminescence is expressed independently of QS control was used. Moreover, the Furanone C30, which was suggested to prevent biofilm formation in S. mutans previously [22,23], and extracts obtained from the closely related species T. maritimum NCBI2154T, a strain with no AHL-lactonase activity [36], were also tested. Results showed that after 8 h, the 20J extract completely eliminates light production by the reporters BB170 and JMH597 compared to control wells (Figure 1(a)). This delay was not caused by a delay in growth since the growth curves of the cultures were identical (data not shown). As expected, the AHL-lactonase Aii20J, that is present in the cell extract, had no effect on the luminescence produced by the reporter BB170. Surprisingly, the addition of the lactonase accelerated the production of bioluminescence in V. harveyi JMH597 (Figure 1(a)). Neither the Furanone C30 nor the extracts of T. maritimum NCBI2154T showed bioluminescence inhibitory activity on BB170 at the concentrations tested. Since the addition of 20J extracts did not cause any change in the production of bioluminescence in the reporter JAF548, these results suggest the presence of an AI-2 specific inhibitory activity in Tenacibaculum sp. 20J. The inhibition is not present in the methanolic biomass extract (MCE) of the same species (Figure 1(b)).
Figure 1.
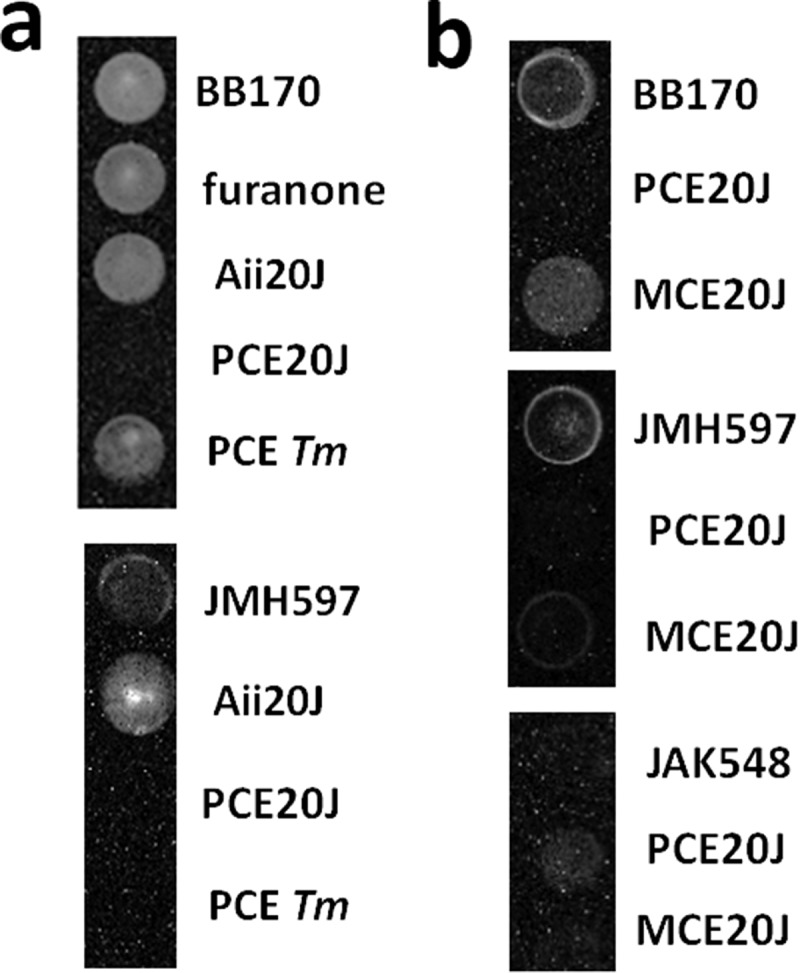
Effect of QS inhibitors and extracts on bioluminescence production by different V. harveyi reporter strains in 96-well cell culture plates. (A) Bioluminescence assays using V. harveyi BB170 (AHL−, AI2+, CAI+) and V. harveyi JMH597 (AHL−, AI2+, CAI−) with furanone (0.02 µg ml−1), the AHL-lactonase Aii20J (20 μg ml−1), and the purified cell extracts from Tenacibaculum sp. 20J (PCE20J) and T. maritimum (PCETm) (100 μg ml−1). (B) Effect of purified cell extract (PCE) and methanolic cell extract on bioluminescence production by V. harvery BB170, JMH597, and the QS independent strain JAF548. Assays were carried out in triplicate. Only one well is shown for each condition.
Tenacibaculum sp. 20J extracts affect biofilm formation in streptococcus mutans
In order to study whether the AI-2 inhibitory activity found in Tenacibaculum sp. 20J extracts could prevent biofilm formation in S. mutans, extracts of strain 20J and T. maritimum were added to cultures of the pathogen and biofilm formation was assessed using the xCELLigence® system. A reduction of 50% in the cell index, the parameter that indicates biofilm formation, was observed when 20J extract was added to S. mutans cultures (Figure 2). On the other hand, although some reduction of cell index was recorded in cultures supplemented with T. maritimum extracts, this effect was not significant (P > 0.05) (Figure 2). Furanone C30 did not cause any reduction in attachment by S. mutans at the concentration tested (0.02 μg ml−1) as measured with the xCELLigence® system (Figure 2). As expected, the AHL-lactonase Aii20J did not display anti-biofilm activity against S. mutans. No effect on Staphylococcus aureus biofilm formation could be observed in treated cultures, indicating the presence of a specific biofilm inhibitory activity against S. mutants in 20J extracts (data not shown).
Figure 2.

Biofilm formation by Streptococcus mutans as measured by xCELLigence® system (■). The effect of purified cell extracts from Tenacibaculum sp. 20J (∆), T. maritimum NCIMB2154T (◊) (100 μg ml−1); furanone C30 (○)(0.02 μg ml−1) and the AHL lactonase Aii20J (˟) (20 μg ml−1) were tested (n = 3).
Optical densities of shaken cultures of S. mutans were measured to evaluate whether the reduction in biofilm formation in cultures supplemented with 20J extracts was derived from growth inhibition. The addition of 20J extracts in S. mutans cultures showed a biofilm inhibition of 50% in the cell index (Figure 3(a)), but it had no significant effect (P > 0.05) on bacterial growth (Figure 3(b)). To further verify that the inhibitory activity on biofilm formation does not result from reducing S. mutans growth, disc diffusion assays were performed with 20J extracts. As a result, no growth inhibition halos around the discs could be observed (data not shown) confirming the absence of growth inhibitory activity against S. mutans in the cell extract of strain 20J.
Figure 3.
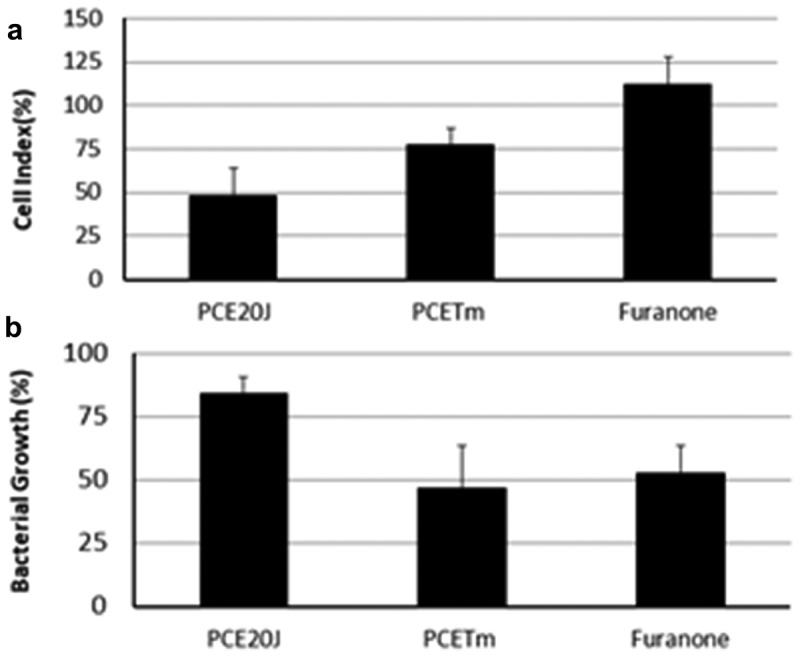
Effect of cell extracts of Tenacibaculum sp. 20J, T. maritimum (100 μg ml−1) and furanone (0.02 μg ml−1) on biofilm formation (A) and cell growth (B). Data were measured as cell index (biofilm growth) and OD600 (cell growth) of S. mutans in comparison to control cultures (100%). Experiments were performed in triplicate.
The biofilm inhibition is not dependent of the culture media
The culture media BHI was supplemented with two concentrations of sucrose and glucose (0.1% and 0.2%). Biofilm formation without sugar supplementation was too low to be reliably detected by the xCELLigence® system (cell index below 0.05). The increase in the concentration of both sugars enhanced the adhesion of S. mutans cells, with the highest cell index being achieved with sucrose 0.2% (Figure 4). The 20J extract inhibited biofilm formation in all conditions, with a reduction of 50% of cell index in both sucrose concentrations and with 0.2% glucose (Figure 4).
Figure 4.
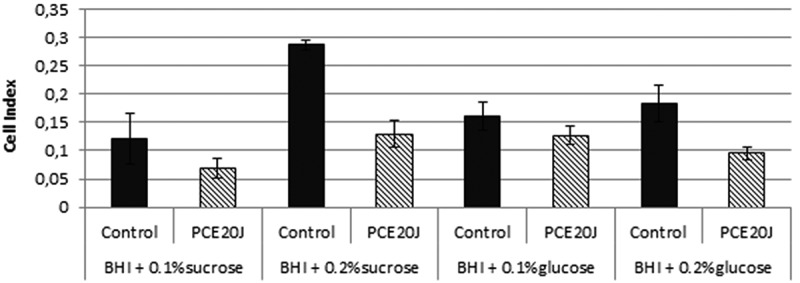
Effect of the cell extract of Tenacibaculum sp. 20J (100 μg ml−1) on S. mutans cultures inoculated with different sucrose (0.1% and 0.2%) and glucose (0.1% and 0.2%) concentrations. Data were measured as cell index (biofilm growth) of S. mutans in comparison to control cultures (100%). Experiments were performed in triplicate.
Validation of xCELLigence® system results
To validate the biofilm formation results obtained with the xCELLigence® system, biofilm formation by S. mutans cultures supplemented with the 20J extract was studied using epifluorescence and CLSM as well as crystal violet staining assays. The CLSM observation showed lower coverage and smaller microcolonies in 20J extract-treated cultures of S. mutans confirming the data obtained with the xCELLigence® system. The addition of the AHL-QQ enzyme Aii20J did not cause significant changes in the structure of the biofilm (data not shown). An average reduction of more than 50% in S. mutans biofilm formation could also be recorded in the culture supplemented with 20J extract used for CLSM analysis, although estimation of the surface coverage using this technique is difficult due to the non-uniform surface coverage (Figure 5). A significant decrease in the biofilm thickness was also observed in the plates (51.38%). The observation of slides stained with crystal violet also revealed important differences in the area coverage and the shape of the cell aggregates between control and 20J-treated biofilms cultured under standard conditions but the quantification showed no differences in biofilm formation (data not shown). However, a decrease of 25% could be observed in S. mutans supplemented with PCE20J using the crystal violet staining assay (Figure 6) when the biofilm formation was promoted by changing the culture media twice a day.
Figure 5.
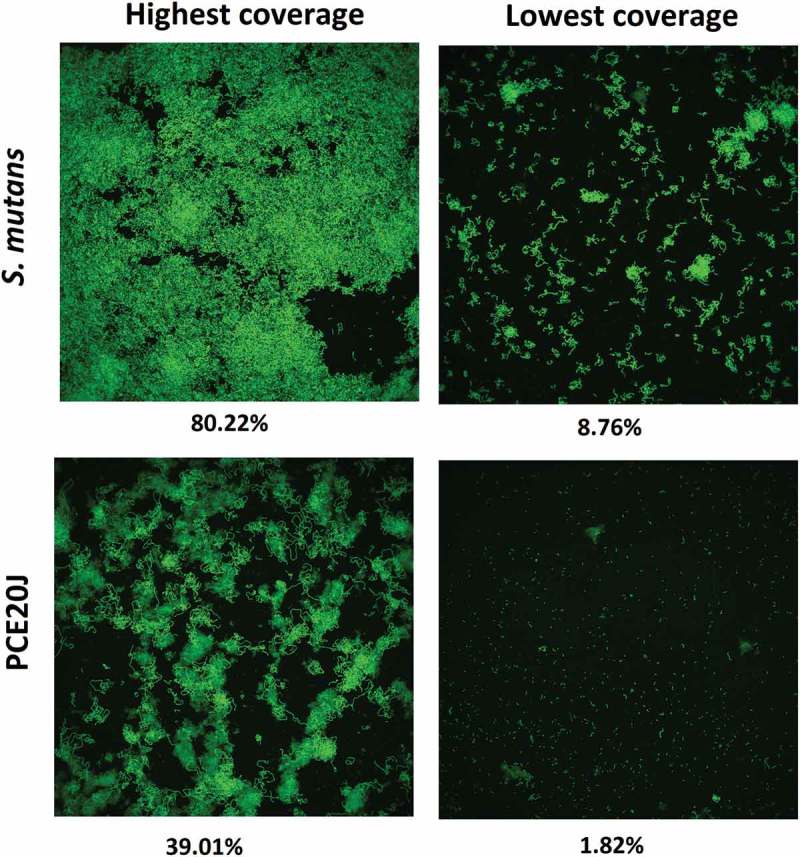
Confocal microscope observation (63 ×) of the effect of the addition of the cell extract if strain 20J (PCE20J) on biofilm formation by S. mutans. The highest and the lowest covered surface fields among the eight fields analysed per sample are shown. A reduction of at least 50% of covered surface is observed in the presence of PCE20J.
Figure 6.
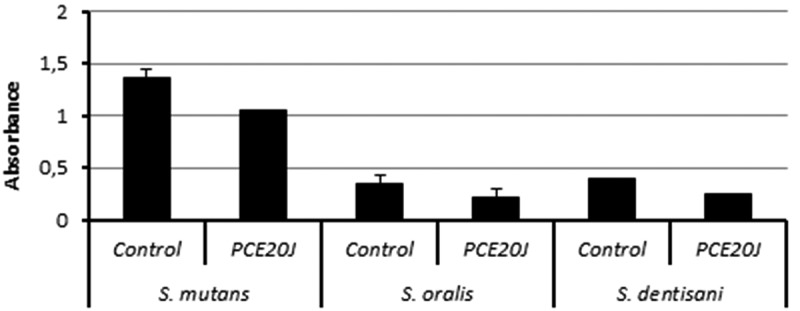
Biofilm formation quantified by crystal violet staining by S. mutans, S. oralis, and S. dentisani in absence or presence of the cell extract of strain 20J (PCE20J). Experiments were performed in triplicate.
Biofilm formation is also affected in other oral Streptococcus species
In order to know whether the activity of PCE20J observed in S. mutans is specific, the anti-biofilm properties of the cell extract were also tested in other oral streptococci. Biofilm formation in S. oralis and S. dentisani was too low to be accurately measured with the xCELLigence® system. The treatment with PCE20J caused a decrease of almost 40% in S. oralis DSM20627 and S. dentisani 7747 (Figure 6).
Characterization of the bioactive component of purified cell extract from Tenacibaculum sp. 20J
To perform a preliminary characterization of the anti-biofilm compound(s) present in the biomass of strain 20J, different 20J extracts were obtained using methanol, ethyl-acetate, and dichloromethane and tested for anti-biofilm activity against S. mutans. No effect on biofilm formation or bacterial growth was recorded for any of the extracts obtained with organic solvents, suggesting that a polar molecule is responsible for the anti-biofilm activity (Figure 7). Moreover, 20J extract fractions with molecular weights below 100 kDa showed no effect on S. mutans attachment (Figure 8(a)), hence either a large molecule should be responsible for the inhibition of biofilm formation in this bacterium or the activity is membrane-bound. To confirm these results, extracts of 20J were either submitted to ultracentrifugation (20 min at 300,000 g) to remove large-size particles or dialyzed to remove molecules smaller than 8 kDa from the extracts. The anti-biofilm activity in dialyzed extracts was similar to that of whole 20J extracts, confirming the high molecular weight of the bioactive compound (Figure 7(a)). While retaining the anti-biofilm activity, the dialyzed extract had no growth-inhibitory activity (Figure 7(b)). Interestingly, a significant loss of anti-biofilm activity was recorded in supernatants from ultracentrifuged extracts (P < 0.05), suggesting that the bioactive compound could be membrane-bound. The same extracts were assayed against the AHL C6-HSL in order to verify the dialysis and ultracentrifugation steps. Expectedly, due to the soluble nature of the AHL-lactonase enzyme present in 20J, dialyzed and ultracentrifuged extracts retained the AHL degradation activity. A 20J extract was then heat-inactivated at 100°C for 10 min or subjected to a proteinase K digestion at 30°C for 1 h. None of the treatments could eliminate the anti-biofilm activity present in the extract (Figure 8(b)), suggesting that either a compound of non-protein nature or a highly resistant enzyme could be responsible for the anti-biofilm activity.
Figure 7.
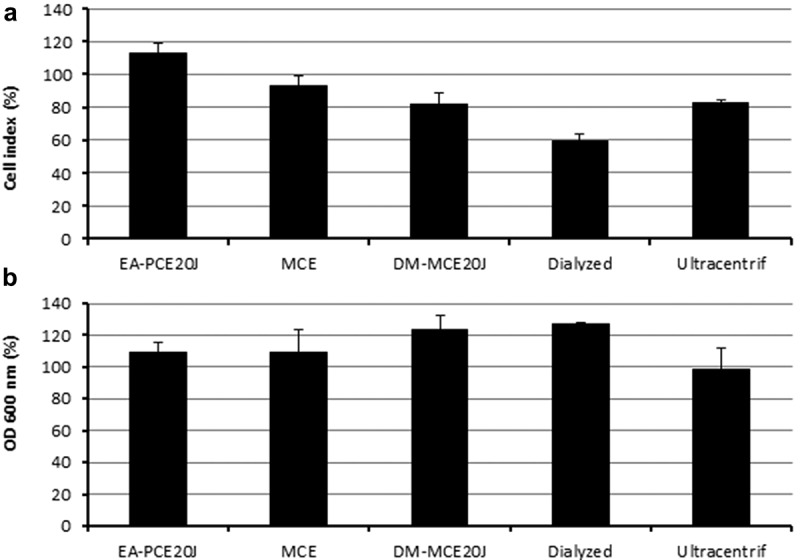
Biofilm formation measured by xCELLigence® (A) and cell growth (B). Measures were done as cell index (biofilm growth) and OD600 (cell growth) of S. mutans in comparison to control cultures (100%). Different types of extracts from Tenacibaculum sp. 20J obtained using different solvents such as methanol (MCE), ethylacetate (EA-PCE) and dichlorometane (DM-MCE20J), and dialyzed PCE and ultracentrifuged PCE were tested as anti-biofilm compounds (n = 3).
Figure 8.
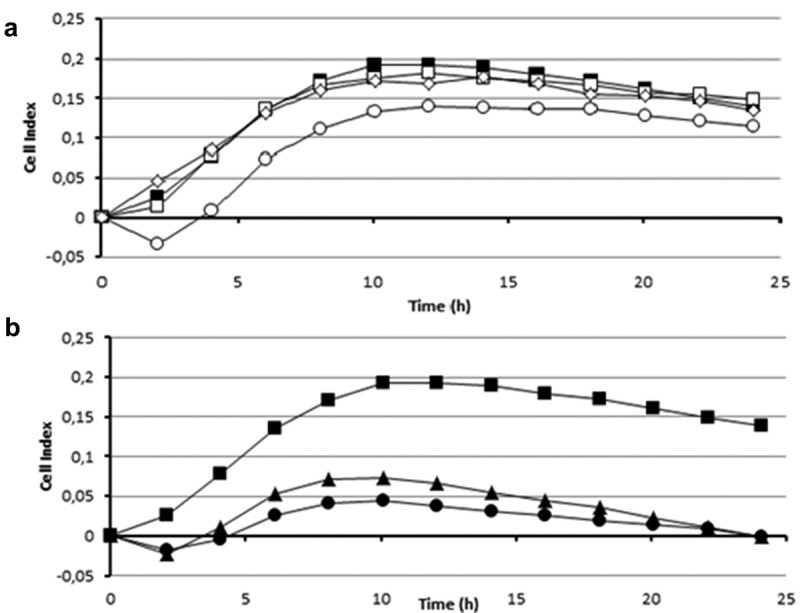
Effect of different treatments on the anti-biofilm activity of the cell extract of strain 20J (100 μg ml−1) against S. mutans measured by xCELLigence® system (■). (A) Activity of extracts fractionated by molecular weight <100 kDa (□), the <50 kDa (○), and the <10 kDa (◊). (B) Effect of temperature (●) (100°C, 10 min) and treatment with proteinase K (▲)(1 h at 30°C) on the activity of the PCE. All cultures were carried out in triplicate.
Discussion
Tenacibaculum sp. 20J was selected as a candidate with possible anti-AI-2 activity due to preliminary observations of anti-biofilm activity in Gram-positive and marine biofilm-forming bacteria not sensitive to the action of the AHL-QQ enzyme Aii20J. The results indicated that the aqueous PCE of Tenacibaculum sp. 20J is able to interfere specifically with the QS system AI-2, in a manner that is independent of the AHL-degradation capacity. The concentration of AI-2 in the culture media of V. harveyi can achieve up to 22.4 μM after 6.5 h of culture [41], therefore the delay in bioluminescence production observed in the first 10 h of growth of cultures supplemented with Tenacibaculum sp. 20J extracts without affecting growth kinetics strongly suggests the presence of AI-2-inhibitory activity in strain 20J extracts. Importantly, 20J extracts did not affect bioluminescence in V. harveyi JAF548, in which bioluminescence is independent of the QS circuits [31], indicating that bioluminescence production was not influenced by unspecific environmental factors [42,43].
Given that biofilm formation is regulated by AI-2 signalling in S. mutans [7,12,13], we decided to assess whether 20J extracts could interfere with biofilm formation of this early dental plaque colonizer. Indeed, the biofilm-inhibitory activity recorded in extracts of strain 20J suggested that it could be derived from the interference with the AI-2 QS system in S. mutans. The reduction of S. mutans biofilm formation is not resulting from growth inhibition since dialyzed extracts retained the anti-biofilm activity and favoured planktonic growth in comparison to untreated and control cultures. A preliminary analysis, including dialysis and ultracentrifugation, indicated that the active compound in 20J extracts is a large, possibly membrane-associated molecule. Despite the bioactive molecule is not affected by heat treatment and proteinase K digestion, it cannot be disregarded that the anti-biofilm activity is enzymatic since similar treatments could not neutralize enzymatic AHL degradation in 20J extracts [36].
In this work, we corroborate previous results that indicate the suitability of the xCELLigence® system RTCA to monitor biofilm formation in S. mutans and other Gram-positive bacteria [25–28]. The xCELLigence® system constitutes a low time-consuming method for real-time quantification and continuous scrutiny of bacterial cell adhesion, in contrast to methods based on staining, microscopic visualization, quantification of biofilm-associated cells in situ, or after detachment from the substrate, and indirect methods such as DNA quantification, XTT reduction assay, or dry cell weight assay which are usually end-point techniques. The inhibitory effect of 20J extracts against S. mutans biofilm formation recorded with the xCELLigence® system was confirmed by microscope observation of crystal violet and fluorescence-stained biofilms indicating the suitability of this technology for monitoring bacterial biofilm. However, the crystal violet quantification method could only detect differences between 20J extract-treated and untreated S. mutans cultures when a thick biofilm formation is promoted by culture medium renewal, being therefore less sensitive than the xCELLigence® system.
Furanone C30 at a concentration of 0.02 μg ml−1 had no effect on biofilm formation by S. mutans as assessed by the xCELLigence® system. Furanone has been reported as a QS inhibitor that affects biofilm formation in S. mutans using the safranine staining and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assays as quantification methods [22,23], although the active concentrations (0.15–4 μg ml−1) were significantly higher than those assayed in the present study (0.02 μg ml−1) and close to the toxicity threshold for this molecule that has been established at concentrations ≥1 µM (0.2 μg ml−1) [44]. He et al. [23] indicate that the furanone acts through an unknown mechanism not dependent of the production of the QS-signal AI-2 since it affected not only S. mutans but also the luxS mutant strain. In any case, the toxicity described for these molecules, causing the rapid death of fish [44], and the development of resistance to furanone-based QS inhibitors [45] exclude the use of these molecules as antimicrobial agents.
Several reports describe the inhibition of biofilm formation by S. mutans through the interference with the AI-2 QS system reducing significantly the biomass, the average depth, and the maximum depth of the biofilm [18]. However, the structure of the AI-2 produced by streptococci is unknown [22], more studies being needed to understand the interference of 20J extracts and other inhibitors with the AI-2 QS system in this species. Although additional experiments using mixed species models would be required in order to evaluate the potential of 20J extracts to prevent dental plaque formation and to control the progress of oral chronic infections such as periodontitis, the inhibition of the biofilm formation observed in another two oral streptococci, S. oralis and S. dentisani, indicates a wide spectrum activity.
Recent studies indicate that the QS networks in S. mutans are complex and should be explored in mixed biofilms in which other QS signals could be present [46–48]. The potential use of QQ strategies like 20J extracts to prevent biofilm formation by S. mutans and other oral pathogens is an attractive alternative since these approaches do not interfere with bacterial growth and hence, the probabilities of inducing resistance or tolerance against these mechanisms are lower. Also the broad spectrum QQ activity present in the cell extracts of Tenacibaculum sp. 20J, interfering with both AI-2 and AHL QS signals, could be applied to prevent mono and multi-species biofilms.
Acknowledgments
We would like to thank Prof. Bonnie Bassler from Princeton University and Prof. Paul Williams from University of Nottingham for kindly providing us with Vibrio harveyi BB170, V. harveyi JMH597, V. harveyi JAF548, and Chromobacterium violaceum CV026 biosensor strains, respectively.
Biographies
Andrea Muras is a PhD fellow in the Department of Microbiology and Parasitology, at the Universidade de Santiago de Compostela. She received her MSc degree in Biotechnology at the Universidade de Santiago de Compostela in 2014. Her research focuses on the identification of novel Quorum Quenching enzymes and the study of Quorum sensing in dental plaque.
Celia Mayer is a PhD fellow in the Department of Microbiology and Parasitology, at the Universidade de Santiago de Compostela. She received her MSc degree in Biotechnology at the Universidade de Santiago de Compostela in 2012. Her research focuses on the Quorum Sensing and Quorum Quenching in Acinetobacter baumannii.
Manuel Romero is post-doctoral fellow in the Microbes group in the University of Nottingham. He obtained his PhD in Microbiology at the Universidade de Santiago de Compostela in 2010, working on the discovery of novel antipathogenic compounds through the inhibition of bacterial communication (quorum sensing). Since 2015 his research focuses in the design and development of new biomaterials with anti-attachment properties against pathogenic bacteria in the project “Next Generation Biomaterials Discovery” (http://www.nottingham.ac.uk/research/groups/biomaterials-discovery/).He has authored 12 research papers (350 citations), 2 international patents and a book chapter.
Tamara Camino is a PhD student in the Obesidomics Group at the Health Research Institute of Santiago de Compostela (IDIS, XXIS/SERGAS). She finished her master's degree in Biomedical Research at the Universidade de Santiago de Compostela in 2017. Her research focuses on the study of exosomes of adipose tissue.
Maria D. Ferrer is a post-doctoral researcher in the oral microbiome group of the Genomics and Health Department at the Foundation for Health and Biomedical Research Promotion of the Valencian Region (FISABIO). She obtained her PhD in Molecular Microbiology at the University CEU Cardenal Herrera in Valencia in 2010, working on the molecular bases of biofilm formation in Staphylococcus aureus. Then she did her first postdoctoral work at the Spanish National Research Council (CSIC) at the Institute of Agrochemistry and Food Technology (IATA) focusing on the development of probiotics against several human diseases. Afterwards, she moved to the industrial field working as a Research and Development Project Manager in the biotechnological company Bioinicia S.L., which is specialized in a novel nanoencapsulation technology of bioactive food ingredients, mainly probiotics. Since 2014, she has been working in the oral biofilms at FISABIO, focusing on the development of an anticaries probiotic and new peptides active against oral pathogens, coordinating various clinical trials. She has published at least 10 scientific articles, half of them as first author.
Alex Mira obtained his PhD at Oxford on microbiology in 1999 and carried out post-doctoral research in the USA and Sweden working on microarray technology, genomics and bioinformatics. In 2003 he initiated his own research group studying the oral microbiota by ''omics'' techniques. In 2009, he was awarded the “Jaime Ferran” Spanish Award for Research in Microbiology. He is currently the principal investigator of the Oral Microbiome Laboratory at the CSISP, in Spain, where he has applied metagenomics and next-generation sequencing technology to the study of oral diseases, as well as to the study of bacteria and fungi from breast milk, saliva, stomach and the respiratory tract.
Ana Otero is Associate Professor of Microbiology at the University of Santiago de Compostela since 2004 and has received the accreditation for Full Professor in 2014. She received her PhD in Microbiology from the Universidade de Santiago in 1995 in the field of Microalgal Biotechnology. She was a Post-doctoral fellow of the European Union in Japan (National Institute for Basic Biology) and Italy (Universitá degli Studi di Firenze). Since 2006 her group is working on the development of novel antipathogenic compounds through the inhibition of bacterial communication (quorum sensing), with special interest in the applications in the field of oral health. A. Otero has co-authored more than 70 papers in international journals, 10 of them in the field of Quorum sensing and Quorum Quenching, and 2 international patents.
Funding Statement
This work was supported by the non-profit organization Fundación Ramón Areces [CIVP16A1814] and by the grant ‘Axudas do Programa de Consolidación e Estructuración de Unidades de Investigación Competitivas (GPC)’ from the Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia (ED431B2017/53). A.M. was supported by a predoctoral fellowship from the Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia (ED481A-2015/311).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–11. [DOI] [PubMed] [Google Scholar]
- [2]. Moreau-Marquis S, Stanton BA, O´Toole GA. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther. 2008;21:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Romero M, Mayer C, Muras A, et al. Silencing bacterial communication through enzymatic quorum sensing inhibition In: Kalia VC, editor. Quorum sensing vs quorum quenching: a battle with no end in sight. India: Springer; 2015. p. 219–236. [Google Scholar]
- [5]. Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacteria flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans . Microbiol Rev. 1980;44:331–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Yoshida A, Toshihiro A, Takehara T, et al. LuxS-based signalling affects Streptococcus mutans biofilm formation. Appl Environ Microbiol. 2005;71:2372–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Simón-Soro A, Guillen-Navarro M, Mira A. Metatranscriptomics reveals overall active bacterial composition in caries lesions. J Microbiol. 2014;6:25443–25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Li YH, Hanna MN, Svensäter G, et al. Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. J Bacteriol. 2001;183:6875–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Petersen FC, Pecharki D, Scheie AA. Biofilm mode of growth of Streptococcus intermedius favored by a competence-stimulating signaling peptide. J Bacteriol. 2004;168:6327–6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Li YH, Tang N, Aspiras MB, et al. A quorum-sensing signalling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J Bacteriol. 2002;184:2699–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Merritt J, Qi F, Goodman SD, et al. Mutation of luxS affects biofilm formation in Streptococcus mutans . Infect Immun. 2003;71:1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Huang Z, Meric G, Liu Z, et al. LuxS-based quorum-sensing signaling affects biofilm formation in Streptococcus mutans . J Mol Microbiol Biotechnol. 2009;17:12–19. [DOI] [PubMed] [Google Scholar]
- [14]. Schauder S, Shokat K, Surrette MG, et al. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing molecule. Mol Microbiol. 2001;41:463–476. [DOI] [PubMed] [Google Scholar]
- [15]. Federle MJ, Bassler BL. Interspecies communication in bacteria. J Clin Invest. 2003;112:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69:3431–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Sztajer H, Lemme A, Viñchez R, et al. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J Bacteriol. 2008;190:401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Jang YJ, Choi YJ, Lee SH, et al. Autoinducer 2 of Fusobacterium nucleatumas a target molecule to inhibit biofilm formation of periodontopathogens. Arch Oral Biol. 2013;58:17–27. [DOI] [PubMed] [Google Scholar]
- [19]. Kolenbrander PE, Palmer RJ, Rickard AH, et al. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42:47–49. [DOI] [PubMed] [Google Scholar]
- [20]. Pereira CS, Thompson JA, Xavier KB. AI-2-mediated signalling in bacteria. FEMS Microbiol Rev. 2013;37:156–181. [DOI] [PubMed] [Google Scholar]
- [21]. Zang T, Lee BW, Cannon LM, et al. A naturally ocurring brominated furanone covalently modifies and inactivates LuxS. Bioorg Med Chem Lett. 2009;19:6200–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Lönn-Stensrud J, Petersen FC, Benneche T, et al. Synthetic bromated furanone inhibits autoinducer-2-mediated communication and biofilm formation in oral streptococci. Oral Microbiol Immunol. 2007;22:340–346. [DOI] [PubMed] [Google Scholar]
- [23]. He Z, Wang Q, Hu Y, et al. Use of the quorum sensing inhibitor furanone C-30 to interfere with biofilm formation by Streptococcus mutans and its luxS mutant strain. Int J Antimicrob Agents. 2012;40:30–35. [DOI] [PubMed] [Google Scholar]
- [24]. Yadav MK, Park SW, Chae SW, et al. Sinefungin, a natural nucleoside analogue of S-Adenosylmethionine, inhibits Streptococcus pneumoniae biofilm growth. Biomed Res Int. 2014;2014:156987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Junka AF, Janczura A, Smutnicka D, et al. Use of the Real Time xCELLigence System for purposes of medical microbiology. Pol J Microbiol. 2012;61:191–197. [PubMed] [Google Scholar]
- [26]. Cihalova K, Chudobova D, Michalek P, et al. Staphylococcus aureus and MRSA growth and biofilm formation after treatment with antibiotics and SeNPs. Int J Mol Sci. 2015;16:24656–24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Ferrer MD, Rodríguez JC, Álvarez L, et al. Effect of antibiotics on biofilm inhibition and indution measured by real-time cell analysis. J Appl Microbiol. 2016. DOI: 10.1111/jam.13368. [DOI] [PubMed] [Google Scholar]
- [28]. Gutiérrez D, Hidalgo-Cantabrana C, Rodríguez A, et al. Monitoring in real time the formation and removal of biofilms from clinical related pathogens using an impedance-based technology. PloS One. 2016;11:e0163966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Romero M, Martín-Cuadrado AM, Roca-Rivada A, et al. Quorum quenching in cultivable bacteria from dense marine coastal microbial communities. FEMS Microbiol Ecol. 2011;75:205–217. [DOI] [PubMed] [Google Scholar]
- [30]. Henke JM, Bassler BL. Three parallel Quorum-sensing systems regulate gene expression in Vibrio harveyi . J Bacteriol. 2004;186:6902–6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi . Mol Microbiol. 1999;31:665–677. [DOI] [PubMed] [Google Scholar]
- [32]. Defoirdt T, Sorgeloos P. Monitoring of Vibrio harveyi quorum sensing activity in real time during infection of brine shrimp larvae. Imse J. 2012;6:2314–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Greenberg EP, Hastings JW, Ulitzur S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol. 1979;120:87–91. [Google Scholar]
- [34]. Romero M, Diggle SP, Heeb S, et al. Quorum quenching activity in Anabaena sp. PCC7120: identification of AiiC, a novel AHL-acylase. FEMS Microbiol Ecol. 2008;75:205–217. [DOI] [PubMed] [Google Scholar]
- [35]. Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- [36]. Mayer C, Romero M, Muras A, et al. Aii20J, a wide spectrum thermo-stable N-acylhomoserine lactonase from the marine bacterium Tenacibaculum sp. 20J can quench AHL-mediated acid resistance in Escherichia coli . Appl Microbiol Biotechnol. 2015;99:9523–9539. [DOI] [PubMed] [Google Scholar]
- [37]. Exterkate RA, Crielaard W, Ten Cate JM. Different response to amine fluoride by Streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res. 2010;44:372–379. [DOI] [PubMed] [Google Scholar]
- [38]. Kerekes EB, É D, Takó M, et al. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J Appl Microbiol. 2013;115:933–942. [DOI] [PubMed] [Google Scholar]
- [39]. McClean KH, Winson MK, Fish L, et al. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiol. 1997;143:3703–3711. [DOI] [PubMed] [Google Scholar]
- [40]. Romero M, Avendaño-Herrera R, Magariños B, et al. Acylhomoserine-lactone production and degradation by the fish pathogen Tenacibaculum maritimum, a member of the Cytophaga–flavobacterium–bacteroides (CFB) group. FEMS Microbiol Lett. 2010;304:131–139. [DOI] [PubMed] [Google Scholar]
- [41]. Campagna SR, Gooding RJ, May AL. Direct quantification of the quorum sensing signal, Autoinducer-2, in clinically relevant samples by liquid chromatography-tandem mass spectometry. Anal Chem. 2009;81:6374–6381. [DOI] [PubMed] [Google Scholar]
- [42]. DeKeersmaecker SC, Vanderleyden J. Constraints on detection of autoinducer-2 (AI-2) signalling molecules using Vibrio harveyi as a reporter. Microbiol. 2003;149:1953–1956. [DOI] [PubMed] [Google Scholar]
- [43]. Turovsky Y, Chikindas ML. Autoinducer-2 bioassay is a qualitative, not quantitative method influenced by glucose. J Microbiol Methods. 2006;66:497–503. [DOI] [PubMed] [Google Scholar]
- [44]. Rasch M, Buch C, Austin B, et al. An inhibitor of bacterial quorum sensing reduces mortalities caused by vibriosis in rainbow trout (Oncorhynchusmykiss, Walbaum). System Appl Microbiol. 2004;27:350–359. [DOI] [PubMed] [Google Scholar]
- [45]. Maeda T, García-Contreras R, Pu M, et al. Quorum quenching quandary: resistance to antivirulence compounds. Isme J. 2012;6:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Murugan K, Sekar K, Sangeetha S, et al. Antibiofilm and quorum sensing inhibitory activity of Achryranthes aspera on cariogenic Streptococcus mutans: an in vitro and in silico study. Pharm Biol. 2013;51:728–736. [DOI] [PubMed] [Google Scholar]
- [47]. Patankar AV, González JE. Orphan LuxR regulators of quorum sensing. FEMS Microbiol Rev. 2009;33:739–756. [DOI] [PubMed] [Google Scholar]
- [48]. Wen ZT, Nguyen AH, Bitoun JP, et al. Transcriptome analysis of LuxS-deficient Streptococcus mutans grown in biofilms. Mol Oral Microbiol. 2011;26:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


