Abstract
Background:
Fine needle aspiration cytology (FNAC) is a simple, rapid, inexpensive, and reliable method of diagnosis of breast mass. Cytoprognostic grading in breast cancers is important to identify high-grade tumors. Computer-assisted image morphometric analysis has been developed to quantitate as well as standardize various grading systems.
Aims:
To apply nuclear morphometry on cytological aspirates of breast cancer and evaluate its correlation with cytomorphological grading with derivation of suitable cutoff values between various grades.
Settings and Designs:
Descriptive cross-sectional hospital-based study.
Materials and Methods:
This study included 64 breast cancer cases (29 of grade 1, 22 of grade 2, and 13 of grade 3). Image analysis was performed on Papanicolaou stained FNAC slides by NIS –Elements Advanced Research software (Ver 4.00). Nuclear morphometric parameters analyzed included 5 nuclear size, 2 shape, 4 texture, and 2 density parameters.
Results:
Nuclear size parameters showed an increase in values with increasing cytological grades of carcinoma. Nuclear shape parameters were not found to be significantly different between the three grades. Among nuclear texture parameters, sum intensity, and sum brightness were found to be different between the three grades.
Conclusion:
Nuclear morphometry can be applied to augment the cytology grading of breast cancer and thus help in classifying patients into low and high-risk groups.
Keywords: Breast, carcinoma, nuclear, Papanicolaou
INTRODUCTION
Well-established prognostic factors of breast cancer include stage of the tumors, histological grade, lymphnode status, and estrogen receptor (ER) and progesterone receptor (PR) status. Grading of breast carcinoma before surgery would be an ideal method in selecting the most appropriate therapy for patients.[1] Increasing abnormalities of nuclear morphology correlates with the increasing grade of tumor as well as worsening of prognosis in many cancers, including breast cancer, renal cell carcinoma, and thyroid tumors. However, cytological grading is not perfect and has an element of subjectivity. Standard morphologic analysis is a subjective method compared to morphometric analysis, which is a quantitative method with objective and reproducible results.
Morphometry has a definite role in several areas of diagnostic histopathology. Till now, majority of image analysis studies have been performed on histological sections.[2,3,4,5,6,7] Its application to cytology would be useful for accurate diagnosis and classification of breast cancer.
In the present study, nuclear morphometry was performed on breast FNAC slides. The present study aims at using nuclear morphometry on aspirates of histologically confirmed breast cancer cases and comparing them between different cytological grades of breast cancer with derivation of suitable cut-off values between the three grades.
MATERIALS AND METHODS
The present study was conducted in our institution from 2011 to 2013. Sixty-four cases of breast cancer including carcinoma were selected for the study. FNAC was performed as prediagnosis procedure on 720 patients who presented with a breast lump during this period. Patients on therapy for breast cancer were excluded from the study. Out of these 720 cases, 670 (93.1%) were satisfactory and 50 (6.9%) unsatisfactory due to inadequate material, poor cellularity, nuclear overlapping, degenerated nuclei, or poor staining. Out of 670 cases, trucut/excision biopsy/mastectomy specimens were available only for 122 cases (18.2%). Out of these 122 cases, 64 cases were malignant. These 64 cases were included in the present study. Cytological and histopathological examinations were performed separately by single experienced pathologists.
FNAC slides were stained with routine Papanicolaou method. Cases were graded cytologically by Robinson's grading system into grade I, grade II, and Grade III. Scores of 1–3 were given for each of the six parameters – cell dissociation, cell size, cell uniformity, nucleoli, nuclear margin, and chromatin, and they were added to classify lesions into Grade 1, score 6–11; Grade 2, score 12–14; Grade 3, score 15–18. There were 29 cases of grade I, 22 cases of grade II, and 13 cases of grade III.
Nuclear morphometry was performed on Papanicolaou-stained FNAC slides, and 50 nonoverlapping nuclei were evaluated per case by a trained pathologist who was blinded of cytology and histopathology diagnosis. Images were captured at 100 × magnification under oil immersion. Nuclear morphometry was performed on Papanicolaou-stained FNAC slides, and 50 nonoverlapping nuclei were evaluated using Nikon digital camera (Nikon DS-Ri, Nikon Instruments Inc., America) at the same settings of microscopic light intensity, iris diaphragm, and condenser position. Images were saved in the attached computer in JPEG 2000 format. The saved images were opened in Nikon Imaging Software-AR (ver 4.00), and a background correction was done for uniformity of image intensity. Calibration was performed before each measuring session. Images of selected nuclei were outlined using the mouse of the computer at the same zoom settings. These outlines were refined automatically and automatic measurements were made by the software NIS Element ver. 4.00. A distance was assigned to 1 pixel (by calibration) and automatic measurement was done by comparing objects of different images. For intensity, brightness, and density measurement, image color layer was used by the software and measurement was done in pixels. After measurement, the data were transferred to MS-Excel sheet for further analysis. Nuclear morphometric parameters analyzed included nuclear size, nuclear shape, nuclear texture, and nuclear density parameters [Table 1]. These parameters were compared between the three grades of breast cancer, and cut-off values for nuclear size parameters derived between the three grades.
Table 1.
Nuclear morphometric parameters used in study
Statistical analysis was performed using the SPSS (Statistical Package for the Social Sciences) program for Windows, version 17.0. Chicago. The mean values of each sample with standard deviation (SD) were calculated for all cases. Data were checked for normality before statistical analysis using Shapiro–Wilk test. Normally distributed continuous variables were compared using analysis of variance test (ANOVA). If the P value was significant and variance was homogeneous, Bonferroni multiple comparison test was used to assess the differences between the individual groups; otherwise, Tamhane's T2 test was used. The Kruskal–Wallis test was used for variables that were not normally distributed, and further comparisons were done using Mann–Whitney U test. A receiver operating characteristics (ROC) curve was evaluated for nuclear size parameters to determine the optimal cut-off values between the three grades of breast cancer. For all statistical tests, a P value of less than 0.05 was considered to indicate a significant difference.
RESULTS
Out of the 720 cases for which FNAC was performed during the study period, 64 histologically confirmed malignant cases with satisfactory cytology smears were included in the study.
Based on Robinson's cytological grading, cases were classified as Grade 1, 29 cases [Figure 1], Grade 2, 22 cases [Figure 2], Grade 3, 13 cases [Figure 3]. Age of patients in the study ranged from 25 to 74 years with a median age of 50.17 years in Grade 1, 46.73 years in Grade 2, and 45.69 years in Grade 3.
Figure 1.
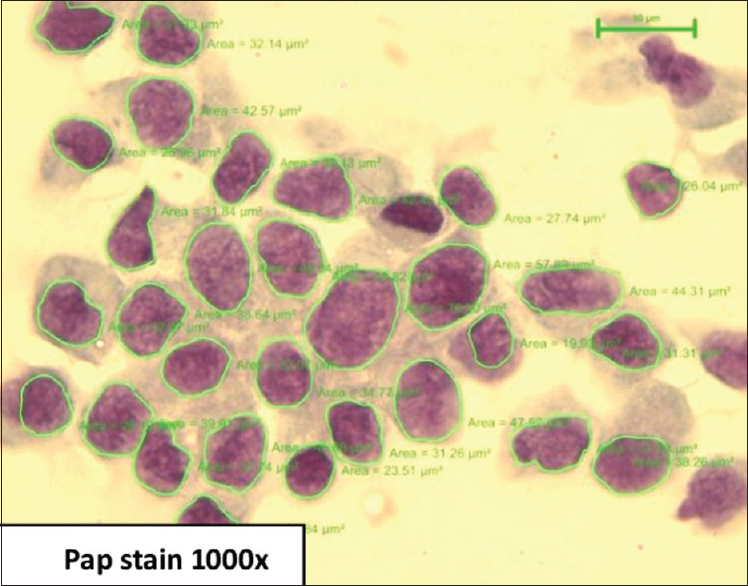
Carcinoma grade 1 – cells with mild pleomorphism, smooth nuclear margin, and inconspicuous nucleoli (Papanicolaou stain, x1000)
Figure 2.
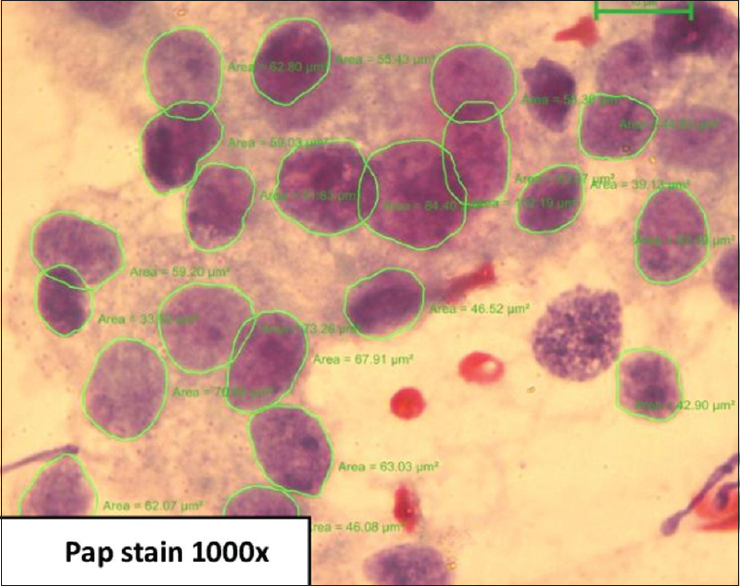
Carcinoma grade 2 – cells with nuclei which are three to four times the erythrocytes, with granular nuclear chromatin, smooth contour, and single prominent nucleoli (Papanicolaou stain, x1000)
Figure 3.
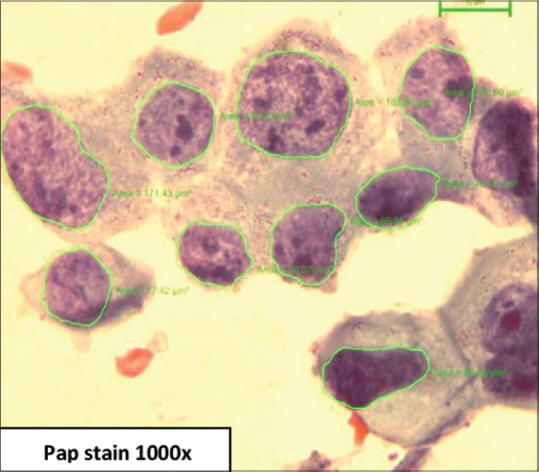
Carcinoma grade 3 – cells with coarsely granular chromatin, irregular nuclear margin, and prominent nucleoli (Pap stain, 1000×)
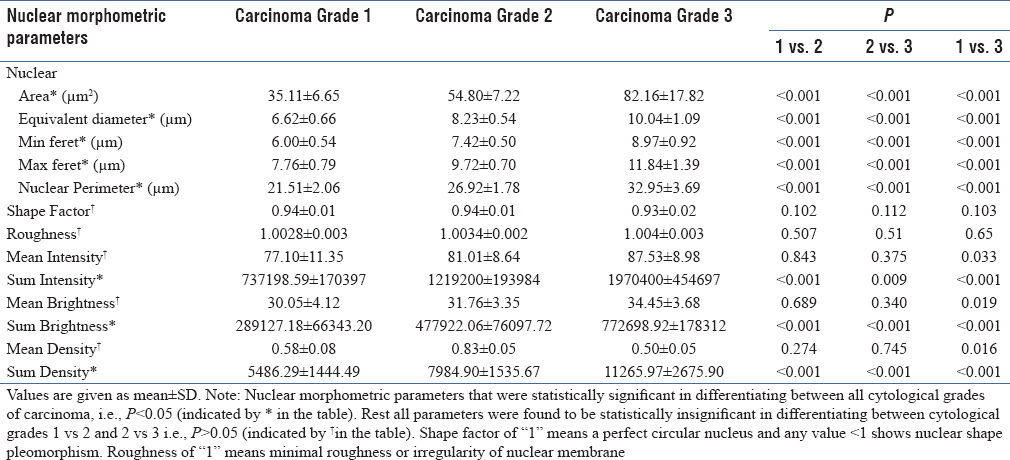
Nuclear morphometry parameters were analyzed and compared between the three cytological grades of breast cancer [Table 2]. On comparing the three cytological grades of breast cancer, all nuclear size parameters showed increasing value with increasing grades of carcinoma. Nuclear size, sum intensity, sum brightness, and sum density parameters were highly significant in differentiating between the three malignant grades. No significant difference was observed for nuclear shape between the three grades. Mean intensity, mean brightness, and mean density parameters were significantly different between grades 1 and 3, but not between grades 1 and 2 or grade 2 and 3.
Table 2.
Comparison of nuclear morphometric parameters among cytological grades of carcinoma
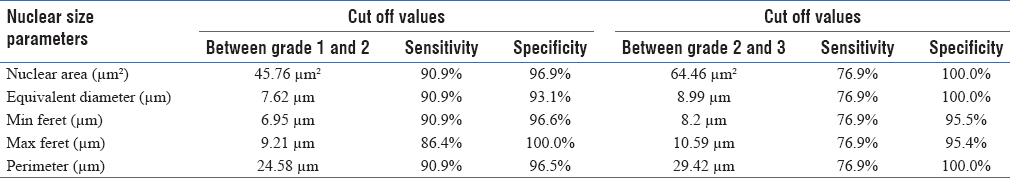
ROC analysis was done to determine the optimal cut-off values for various nuclear size parameters between the three grades. The area under the curve and its sensitivity and specificity were calculated using statistical methods to analyze the diagnostic value of all these variables. Our cut-off values for mean nuclear area, equivalent diameter, minimum feret, maximum ferret, and perimeter between the three grades showed high sensitivity and specificity [Table 3].
Table 3.
Cut-off values of nuclear size parameters between various cytologic grades
DISCUSSION
Cytoprognostic grading in breast cancers is important to identify high-grade tumors, which are more responsive to chemotherapy than the low-grade tumors, in which pretreatment with tamoxifen may be more suitable. Increasing abnormalities of nuclear morphology correlates with the increasing grade of tumor as well as worsening of prognosis in many cancers, including breast cancer, renal cell carcinoma, and thyroid tumors. However, cytological grading is not perfect and has an element of subjectivity. This study emphasizes on morphometry as an objective tool in the diagnosis and grading of breast cancer.
Increasing nuclear morphology abnormalities correlates with tumor progression and grade of breast cancer. Nuclear morphometry can measure these morphological abnormalities in nuclear size, shape, and texture and correlate with tumor grades. Cytological grading is comparable to histological grading and can determine the aggressiveness of breast cancer. Many morphometric studies have been done on breast histology and their results can be compared with our findings on breast cytology.[8,9,10,11,12,13,14,15,16,17]
In our study, all nuclear size parameters, 2 nuclear texture, and 1 nuclear density parameter were significant in differentiating between the three cytological grades of malignant cases. Mean intensity, mean brightness, and mean density were significant in differentiating between grades 1 and 3 but not between grades 1 and 2 or grades 2 and 3. This might be due to the overlap between nuclear texture and density of grades 1 and 2 and grades 2 and 3.
Similarly, Kalhan et al.[18] in their study on cytological aspirates and Ikpatt et al.,[14] Al-Obaidi et al.,[17] and Radwan et al.[15] in their study on histopathological sections of breast found a significant association between nuclear size parameters and grades of carcinoma. Kalhan et al.[18] found that 2 out of 3 textural parameters correlated significantly with cytological grading.
In our study, no significant correlation was found between nuclear shape parameters [Shape factor (P = 0.102) and roughness (P = 0.507)] and different cytological grades of breast carcinoma. This might be due to minor nuclear membrane irregularities being ignored during the process of outlining and digitization of nuclear images.
Similar results were shown by Moroz et al.[2] in their study on 54 FNACs and Radwan et al.[15] in their study on histopathology sections of breast cancer cases, where nuclear shape was not statistically significant in differentiating between different nuclear grades. However, Kalhan et al.[18] found a significant correlation between shape and cytological grading with a significant difference between grades 1 and 2 as well as grades 1 and 3 but not between grades 2 and 3.
Cut-off values of nuclear area, equivalent diameter, minimum feret, maximum feret, and perimeter between carcinoma grades 1 and 2 were 45.76 μm2, 7.62 μm, 6.95 μm, 9.21 μm, and 24.58 μm, respectively, with a good sensitivity and specificity. However, cut-off values of 64.46 μm2, 8.99 μm, 8.2 μm, 10.59 μm, and 29.42 μm, respectively, between carcinoma grades 2 and 3 showed a low sensitivity but good specificity. This low sensitivity could be due to wide variability in the nuclear size parameter values in carcinoma grades 2 and 3 cases in our study.
This study emphasizes on morphometry as an objective tool to augment the cytological grading of breast cancer. However, errors can occur due to technical problems in nuclear morphometry, which can lead to false positive and false negative results. This can be overcome by training, internal calibration, and standardization by an expert observer.
CONCLUSION
Nuclear morphometry correlates with tumor size, lymphnode involvement, and mitotic activity. Nuclear morphometry can be coupled with these clinicopathological features and used to prognosticate and classify breast cancer patients into low and high-risk groups.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Khan MZ, Haleem A, Hassani HA, Kfoury H. Cytopathological grading, as a predictor of histopathological grade, in ductal carcinoma (NOS) of breast, on air-dried Diff-Quick smears. Diagn Cytopathol. 2003;29:185–93. doi: 10.1002/dc.10285. [DOI] [PubMed] [Google Scholar]
- 2.Moroz K, Lipscomb J, Vial LJ, Jr, Dhurandhar N. Cytologic nuclear grade of malignant breast aspirates as a predictor of histologic grade. Light microscopy and image analysis characteristics. Acta Cytol. 1997;41:1107–11. doi: 10.1159/000332796. [DOI] [PubMed] [Google Scholar]
- 3.Pienta KJ, Coffey DS. Correlation of nuclear morphometry with progression of breast cancer. Cancer. 1991;68:2012–6. doi: 10.1002/1097-0142(19911101)68:9<2012::aid-cncr2820680928>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Abdalla F, Boder J, Markus R, Hashmi H, Buhmeida A, Collan Y. Correlation of nuclear morphometry of breast cancer in histological sections with clinicopathological features and prognosis. Anticancer Res. 2009;29:1771–6. [PubMed] [Google Scholar]
- 5.Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, et al. Tumor size, nuclear morphometry, mitotic indices as prognostic factors in axillary-lymph-node-positive breast cancer. Eur Surg Res. 1992;24:160–8. doi: 10.1159/000129203. [DOI] [PubMed] [Google Scholar]
- 6.Baak JP, Kurver PH, de Snoo-Niewlaat AJ, De Graef S, Makkink B, Boon ME. Prognostic indicators in breast cancer--Morphometric methods. Histopathology. 1982;6:327–39. doi: 10.1111/j.1365-2559.1982.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz A, Almenar S, Callaghan RC, Llombart-Bosch A. Benign, preinvasive and invasive ductal breast lesions: A comparative study with quantitative techniques: Morphometry, image and flow cytometry. Pathol Res Pract. 1999;195:741–6. doi: 10.1016/S0344-0338(99)80115-0. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal G, Singh S, Marwah S, Duhan D, Mathur S, Marwah N, et al. Morphometric Analysis in Breast Lesions. A Rapid Conjunct to Intraoperative Imprint Smears. Middle East J Cancer. 2012;3:1–8. [Google Scholar]
- 9.Stenkvist B, Westman-Naeser S, Holmquist J, Nordin B, Benqtsson E, Veqelins J, et al. Computerized Nuclear Morphometry as an Objective Method for Characterizing Human Cancer Cell Populations. Cancer Res. 1978;38:4688–97. [PubMed] [Google Scholar]
- 10.Van der Linden HC, Baak JP, Lindeman J, Hermans J, Meyer CJ. Morphometry and breast cancer. II. Characterisation of breast cancer cells with high malignant potential in patients with spread to lymph nodes: Preliminary results. J Clin Pathol. 1986;39:603–9. doi: 10.1136/jcp.39.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kronqvist P, Kuopio T, Jalava P, Collan Y. Morphometrical malignancy grading is a valuable prognostic factor in invasive ductal breast cancer. Br J Cancer. 2002;87:1275–80. doi: 10.1038/sj.bjc.6600617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan PH, Goh BB, Chiang G, Bay BH. Correlation of nuclear morphometry with pathologic parameters in ductal carcinoma in situ of the breast. Mod Pathol. 2001;14:937–41. doi: 10.1038/modpathol.3880415. [DOI] [PubMed] [Google Scholar]
- 13.Hoque A, Lippman SM, Boiko IV, Atkinson EN, Sneige N, Sahin A, et al. Quantitative nuclear morphometry by image analysis for prediction of recurrence of ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2001;10:249–59. [PubMed] [Google Scholar]
- 14.Ikpatt OF, Kuopio T, Collan Y. Nuclear Morphometry in African Breast Cancer. Image Anal Stereol. 2002;21:145–50. [Google Scholar]
- 15.Radwan MM, Amer KA, Mokhtar NM, Kandil MA, El-Barbary AM, Aiad HA. Nuclear Morphometry in Ductal Breast Carcinoma with Correlation to Cell Proliferative Activity and Prognosis. J Egyptian Nat Cancer Inst. 2003;15:169–82. [Google Scholar]
- 16.Cui Y, Koop EA, Van Diest PJ, Kandel RA, Rohan TE. Nuclear morphometric features in benign breast tissue and risk of subsequent breast cancer. Breast Cancer Res Treat. 2007;104:103–7. doi: 10.1007/s10549-006-9396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Obaidi Z, Fawzi F, Al-Obaidi H. The Value of Nuclear Morphometry in Breast Carcinoma. Iraqi J Med Sci. 2007;5:13–7. [Google Scholar]
- 18.Kalhan S, Dubey S, Sharma S, Duhani S, Preeti, Dixit M. Significance of nuclear morphometry in cytological aspirates of breast masses. J Cytol. 2010;27:16–21. doi: 10.4103/0970-9371.66694. [DOI] [PMC free article] [PubMed] [Google Scholar]


