Abstract
The purpose of this study was to investigate the curative effect of 1α-hydroxyvitamin D3 on the benign paroxysmal positional vertigo (BPPV). Fifty BPPV patients diagnosed in the ENT Department of Anzhen Hospital from October 2015 to December 2016 were randomly selected as the treatment group, and treated with 0.25 µg 1α-hydroxyvitamin D3 once per day, in addition to the routine diagnosis and treatment. Moreover, 50 BPPV patients in the same period were selected as the control group, and received the routine diagnosis and treatment. The detection results of bone mineral density (BMD) t-value, vitamin D3 and bone metabolic markers before and after treatment were compared, and statistical analysis was performed on the results. There were no differences in the general data between treatment group and control group. There were no statistically significant differences in the BMD and age distribution of males and females between treatment group and control group (P>0.05). The BMD of male BPPV patients in each age group in the treatment group was significantly increased after treatment, and the difference was statistically significant (P<0.05). Although the BMD of male BPPV patients in each age group in control group was somewhat increased after treatment, the difference was not statistically significant (P>0.05). The BMD of female BPPV patients in each age group in treatment group was increased after treatment, and the difference was statistically significant (P<0.05). Similarly, although the BMD of female BPPV patients in each age group in control group was somewhat increased after treatment, the difference was not statistically significant (P>0.05). The average BMD of female BPPV patients in each age group was significantly lower than that of male patients, and the difference was statistically significant (P<0.05) (Table II). The BMD t-value of patients in treatment group showed a decreasing trend with the increase of age (Fig. 1). The levels of 25-hydroxyvitamin D3 and bone metabolic markers in treatment group were significantly improved compared with those before treatment (P<0.05). Multivariate Logistic regression analysis was performed to identify whether the treatment of BPPV was effective or not as a dependent variable, and six items, including the sex (female), hypertension, diabetes mellitus, age (>50 years), 25-hydroxyvitamin D3 and osteopenia/osteoporosis, as the independent variables, and the results suggested that the level of 25-hydroxyvitamin D3 and osteopenia/osteoporosis are the clinical features of whether the BPPV treatment is effective (P<0.05). The results showed that the treatment of BPPV with 1α-hydroxyvitamin D3 can effectively improve the symptoms of patients, and the level of vitamin D3 and the occurrence of osteopenia/osteoporosis are the clinical indexes of whether the BPPV treatment is effective.
Keywords: benign paroxysmal positional vertigo, 1α-hydroxyvitamin D3, curative effect, bone metabolism
Introduction
Benign paroxysmal positional vertigo (BPPV) is a kind of clinically common peripheral vestibular disease, which has the highest incidence rate in vertigo derived from the inner ear (1). Its main manifestation is that the transient paroxysmal vertigo can be caused when the head moves to a specific position, accompanied by nystagmus and autonomic neural symptoms (2). BPPV can occur in all age groups and is common in middle-aged and elderly patients. The disease is self-limited, and the vertical semicircular canal is involved most easily (approximately 80–90%), followed by the horizontal semicircular canal (approximately 10%), and the superior semicircular canal is the least involved (approximately 2%). BPPV is manifested as transient vertigo accompanied with visual rotation and nystagmus, when it occurs, so patients are initially diagnosed in the Neurology Department (3). Jeong et al reported the levels of 25-hydroxyvitamin D in serum in 100 idiopathic BPPV patients (63 females and 37 males) in 2013 and found that the level of 25-hydroxyvitamin D in serum in BPPV patients was lower than that in control group, and it was also found that the probability of vitamin D deficiency (<20 ng/ml) in BPPV patients was significantly higher than that in control group; moreover, the Multivariate Logistic regression analysis of the age, sex, body mass index (BMI), hypertension, diabetes mellitus, proteinuria, conventional exercise and decreased bone mineral density (BMD) proved that the vitamin D insufficiency (10–20 ng/ml) and vitamin D deficiency (<10 ng/ml) are associated with BPPV, and the decreased serum vitamin D is a risk factor of BPPV (4). 1α-Hydroxyvitamin D3 is an important active metabolite of vitamin D3, which can regulate the calcium-phosphorus balance in the body, improve various symptoms caused by vitamin D metabolic disturbance, increase the intestinal absorption of calcium and phosphorus, reduce the level of parathyroid hormone in plasma and improve osteoporosis due to women's menopause and application of hormone drugs (5). At present, there has been no report on the effectiveness of 1α-hydroxyvitamin D3 in the treatment of BPPV in China. In this study, 50 BPPV patients and 50 BBPV controls were selected, and the changes in bone metabolic markers, such as bone alkaline phosphatase (BALP), osteocalcin N-terminal middle molecular fragment (N-MID) and β-collagen degradation product (β-CTX), and BMD value in patients and control group before and after treatment with 1α-hydroxyvitamin D3 were detected, so as to provide a reference for the evaluation of curative effect of BPPV.
Patients and methods
Patients of study
Fifty BPPV patients diagnosed in the ENT Department of Anzhen Hospital from October 2015 to December 2016 were randomly selected by the research group. According to the following criteria, 50 BPPV patients were set as the treatment group. In addition to the routine diagnosis and treatment, the treatment group was given 0.25 µg 1α-hydroxyvitamin D3 (Kunming Baker Norton) once per day for 14 consecutive days. Moreover, another 50 BPPV patients in the same period were selected as the control group and received the routine diagnosis and treatment. This study was approved by the Ethics Committee of Anzhen Hospital. Signed written informed consents were obtained from the patients and/or guardians.
Inclusion criteria
Patients aged between 40 and 75 years and diagnosed as BPPV. The diagnosis of BPPV was according to the Diagnostic Criteria of Benign Paroxysmal Positional Vertigo issued by Barany Institute in 2015 (6): a) Posterior semicircular canal BPPV: 1) typical and recurrent positional vertigo or positional dizziness; 2) the duration of vertigo <1 min; 3) Dix-Hallpike (DH) test or side-lying test could induce the positional nystagmus, lasting for less than 1 min; 4) BPPV caused by other central nervous system diseases should be eliminated by senior doctors in the Neurology Department. b) Lateral semicircular canal BPPV: 1) typical and recurrent positional vertigo or positional dizziness; 2) the duration of vertigo <1 min; 3) The supine-position test could induce the positional nystagmus with very short or no latency, lasting for less than 1 min; 4) BPPV caused by other central nervous system diseases should be eliminated by senior doctors in the Neurology Department. c) Anterior semicircular canal BPPV: 1) typical and recurrent positional vertigo or positional dizziness; 2) the duration of vertigo <1 min; 3) DH test (unilateral or bilateral) or supine-position center head hanging test could induce the positional nystagmus, lasting for less than 1 min; 4) BPPV caused by other central nervous system diseases should be eliminated by senior doctors in the Neurology Department.
Exclusion criteria
1) Patients with a history of typical and recurrent positional vertigo or positional dizziness, but without a history of positional nystagmus; 2) patients with a history of inner ear diseases (including Meniere's disease, vestibular neuritis, positional vertigo, zoster oticus, sudden deafness, noise-induced hearing loss, presbycusis, drug-induced deafness, temporal bone fracture and acoustic neuroma); 3) patients with a history of noise exposure; 4) patients with a history of head trauma; 5) patients with organic diseases in major organs, such as cerebral infarction, cerebral hemorrhage, myocardial infarction, liver and kidney dysfunction.
Reasons for loss to follow-up and solutions
1) Expected reasons: the subjects dropped out of the test and were transferred to another hospital for treatment; 2) solutions: the subjects were supplemented following the inclusion and exclusion criteria by 1:1 ratio.
Medical ethics
1) The subjects and their family members signed the informed consent about participating in the study. 2) The safety of subjects was fully protected according to the relevant principles of clinical guidelines. 3) The diagnosis and treatment records of patients were kept secret, and privacy right of patients was protected.
Double-blind principal in clinical trials
1) The researchers were divided into 4 groups. Group 1 screened and distributed the subjects; group 2 performed the treatment; group 3 observed and collected the index data and group 4 summarized the data and wrote the study. 2) The grouping of subjects was kept strictly confidential. 3) The operations of researchers in 4 groups were kept confidential.
Detection of BMD
XR-46 digital dual energy X-ray bone densitometer (Norland Corp., Fort Atkinson, WI, USA) was used and the detection site was lumbar spine. Detection results are presented as t-value: t-value = (measured value - peak BMD)/standard deviation of BMD in normal adults. Patients with space occupying lesion in lumbar spine found in the detection were excluded, and then the appropriate cases were selected in accordance with the standard. According to the diagnosis and treatment guideline of primary osteoporosis (2011), BMD t-value > −1 was deemed as normal, t-value between −1 to −2.5 was deemed as low BMD and t-value < −2.5 was deemed as osteoporosis.
Serum vitamin D
Fasting elbow vein blood was drawn from patients in the two groups at 8:00–9:00 in the morning at 1 day before treatment and 1 day after treatment. The serum 25-hydroxyvitamin D3 was detected using the Cobas Modular E170 full-automatic electrochemical luminescence immunoassay system (Roche, Mannheim, Germany) via the immunochemiluminometric assay. The detection kits were purchased from Roche. Judgment criteria of detection results: The serum 25-hydroxyvitamin D3 >20 ng/ml was deemed as normal, serum 25-hydroxyvitamin D3 between 10 and 20 ng/ml was deemed as vitamin D3 insufficiency, and serum 25-hydroxyvitamin D3 <10 ng/ml was deemed as vitamin D3 deficiency.
Detection of serum bone metabolic markers
Bone alkaline phosphatase (BALP), osteocalcin N-terminal middle molecular fragment (N-MID) were selected as the markers of bone formation level, while β-collagen degradation product (β-CTX) was selected as the marker of bone resorption level. BALP was detected via enzyme-linked immunosorbent assay (ELISA), and N-MID and β-CTX were detected using the Cobas Modular E170 full-automatic electrochemical luminescence immunoassay system (Roche) and the corresponding kits.
Statistical analysis
SPSS 19.0 software (IBM, Armonk, New York, USA) was used for the statistical analysis and processing of research data. Measurement data are presented as mean ± standard deviation, and t-test was used for the intergroup comparison. Enumeration data are presented as %, and Chi-square test was used for the intergroup comparison. The paired t-test was used for the comparison before and after treatment, and analysis of variance was used for the comparison among groups. Multivariate Logistic regression analysis was performed with 6 items of patients, such as sex and age, as the independent variables. P<0.05 was considered to indicate a statistically significant difference.
Results
General results
There were 50 BPPV patients in treatment group, including 20 males and 30 females with an average age of 51.36±9.42 years. There were 16 cases of hyperlipidemia, 23 cases of diabetes mellitus, 37 cases of hypertension, 8 cases of drinking history and 21 cases of smoking history. BMI was 22.46±2.48 kg/m2.
There were 50 BPPV patients in control group, including 22 males and 28 females with an average age of 52.79±10.58 years. There were 17 cases of hyperlipidemia, 22 cases of diabetes mellitus, 35 cases of hypertension, 7 cases of drinking history and 19 cases of smoking history. BMI was 23.43±2.38 kg/m2. The Chi-square test and t-test showed that the general data of patients in the two groups had no statistically significant differences (P>0.05) (Table I).
Table I.
Comparisons of general data between BPPV treatment group and control group (n).
| Item | Treatment group (n=50) | Control group (n=50) | χ2/t-value | P-value |
|---|---|---|---|---|
| Male/female | 20/30 | 22/28 | 0.164 | 0.653 |
| Age | 0.053 | 0.908 | ||
| ~40 years | 15 | 16 | ||
| ~50 years | 19 | 18 | ||
| ~60 years | 16 | 16 | ||
| Hyperlipidemia | 16 | 17 | 0.042 | 0.816 |
| Diabetes mellitus | 23 | 22 | 0.044 | 0.807 |
| Hypertension | 37 | 35 | 0.194 | 0.650 |
| Drinking | 8 | 7 | 0.074 | 0.794 |
| Smoking | 21 | 19 | 0.167 | 0.631 |
| BMI (kg/m2) | 22.46±2.48 | 23.43±2.38 | 2.121 | 0.793 |
Clinical characteristics of patients in the two groups
BPPV patients in treatment group suffered from unilateral BPPV, including 27 cases involving the right ear and 23 cases involving the left ear. There were 32 cases (64.0%) involving the posterior semicircular canal, 11 cases (22.0%) involving the horizontal semicircular canal and only 7 cases (14.0%) involving the superior semicircular canal. In BPPV patients involving the horizontal semicircular canal, there were 7 cases of pipe stone disease and 4 cases of ridge stone disease. The above-mentioned detections of patients were conducted at an interval of 1–14 days with an average of 4.9±2.4 days. BPPV patients in control group suffered from unilateral BPPV, including 25 cases involving the right ear and 25 cases involving the left ear. There were 30 cases (60.0%) involving the posterior semicircular canal, 12 cases (24.0%) involving the horizontal semicircular canal and only 8 cases (16.0%) involving the superior semicircular canal. In BPPV patients involving the horizontal semicircular canal, there were 8 cases of pipe stone disease and 4 cases of ridge stone disease. The above-mentioned detections of patients were conducted at an interval of 1–14 days with an average of 5.6±2.5 days. There were no statistically significant differences in the clinical characteristics between the two groups (P>0.05).
Comparison of correlations of BMD with sex and age between the two groups
Comparison of BMD in treatment group before and after treatment: the BMD of males was generally increased by 1.39±1.17, while that of females in each age group was significantly increased after treatment, and the difference was statistically significant (P<0.05). Comparison of BMD in control group before and after treatment: the BMD of males was generally increased by 0.48±0.77, and the difference was not statistically significant (P>0.05). The differences in BMD between treatment group and control group before treatment and between male group and female group were not statistically significant (P>0.05). Comparison of BMD between treatment group and control group after treatment: The BMD in each male group was 0.41±1.18, −1.25±1.27 and −1.42±0.94 in turn, and the BMD in treatment group was significantly higher than that in control group (P<0.05). The BMD in each female group was −0.112±0.84, −1.77±0.68 and −1.91±0.72 in turn, which was significantly higher than that in the corresponding age group in control group, and the differences were statistically significant (P<0.05). The results showed that the average BMD in female BPPV patients in each age group was significantly lower than that in male patients (Table II). The BMD t-value in treatment group showed a decreasing trend with the increase of age (Fig. 1). There were no statistically significant differences in the BMD between different sides of BPPV (Table III) and different semicircular canals of BPPV (Table IV) in treatment group before and after treatment (P>0.05).
Table II.
Comparisons of BMD of BPPV patients in different sexes in each age group before and after treatment.
| Treatment group | Control group | |||||
|---|---|---|---|---|---|---|
| Item | n | Before treatment | After treatment | n | Before treatment | After treatment |
| Male | ||||||
| ~40 years | 7 | −0.34±1.57 | 0.41±1.18a | 7 | −0.29±1.37b | −0.07±1.51c |
| ~50 years | 7 | −2.05±1.42 | −1.25±1.27a | 8 | −2.17±1.26b | −1.61±1.35c |
| ~60 years | 6 | −2.61±1.34 | −1.42±0.94a | 7 | −2.63±1.42b | −2.03±1.52c |
| Female | ||||||
| ~40 years | 8 | −0.71±1.33 | −0.12±0.84a | 9 | −0.82±1.24b | −0.52±0.84c |
| ~50 years | 12 | −2.61±1.21 | −1.77±0.68a | 10 | −2.71±1.29b | −2.21±1.49c |
| ~60 years | 10 | −2.72±1.34 | −1.91±0.72a | 9 | −2.78±1.27b | −2.28±1.43c |
P<0.05, the difference is statistically significant in the comparison of BMD in treatment group before and after treatment.
P>0.05, the difference is not statistically significant in the comparison of BMD between treatment group and control group before treatment.
P<0.05, the difference is statistically significant in the comparison of BMD between treatment group and control group after treatment.
Figure 1.
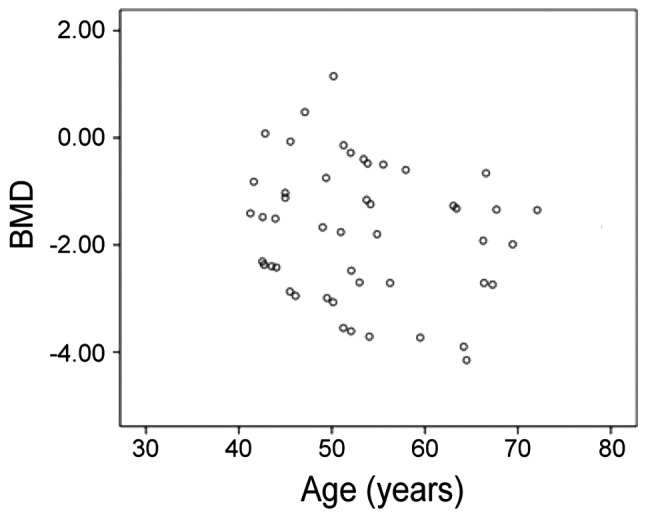
Scatter diagram about the relationship between age and BMD of BPPV patients in treatment group before treatment: the BMD t-value of patients shows a gradually decreasing trend with the increase of age.
Table III.
Comparisons of BMD in patients in treatment group in different sides before and after treatment.
| Item | Left-ear BPPV (n=23) | Right-ear BPPV (n=27) | P-value |
|---|---|---|---|
| Before treatment | −1.77±1.54 | −1.72±1.62 | >0.05 |
| After treatment | −1.11±0.84 | −1.23±0.64 | >0.05 |
| t-value | 1.957 | 2.359 | |
| P-value | <0.05 | <0.05 |
Table IV.
Comparisons of BMD in patients in treatment group in different semicircular canals before and after treatment.
| Item | Posterior semicircular canal (32) | Horizontal semicircular canal (11) | Superior semicircular canal (7) | P-value |
|---|---|---|---|---|
| Before treatment | −1.73±1.35 | −2.54±1.46 | −0.97±0.82 | >0.05 |
| After treatment | −1.14±0.75 | −1.68±0.85 | −0.37±0.22 | >0.05 |
| t-value | 2.385 | 3.152 | 2.559 | |
| P-value | <0.05 | <0.05 | <0.05 |
Comparisons of 25-hydroxyvitamin D3 and bone metabolic markers in the treatment group before and after treatment
Before treatment, there were 26 cases (52.0%) of vitamin D insufficiency and 8 cases (16.0%) of vitamin D deficiency in treatment group, while there were 25 cases (50.0%) of vitamin D insufficiency and 9 cases (18.0%) of vitamin D deficiency in control group, and the differences were not statistically significant between the two groups (P>0.05). In treatment group, the level of 25-hydroxyvitamin D3 was 17.62±9.03 µg/l before treatment and increased to 36.71±11.93 µg/l after treatment; the level of BALD was 14.61±5.21 µg/l before treatment and increased to 23.77±11.33 µg/l after treatment; the level of N-MID was 18.71±6.33 µg/l before treatment and increased to 27.12±7.84 µg/l after treatment; the level of β-CTX was 0.52±0.31 µg/l before treatment and increased to 1.49±0.72 µg/l after treatment; and each index was significantly improved compared with that before treatment (P<0.05) (Table V).
Table V.
Comparisons of 25-hydroxyvitamin D3 and bone metabolic markers in BPPV treatment group before and after treatment (µg/l).
| Item | Before treatment | After treatment | t-value | P-value |
|---|---|---|---|---|
| 25-Hydroxyvitamin D3 | 17.62±9.03 | 36.71±11.93 | 2.564 | <0.05 |
| BALD | 14.61±5.21 | 23.77±8.68 | 4.059 | <0.05 |
| N-MID | 18.71±6.33 | 27.12±7.84 | 6.112 | <0.05 |
| β-CTX | 0.52±0.31 | 1.49±0.72 | 5.101 | <0.05 |
Influencing factors of 1α-hydroxyvitamin D3 for the curative effect on BPPV
Multivariate Logistic regression analysis was performed with whether the treatment of BPPV with 1α-hydroxyvitamin D3 was effective or not as a dependent variable, and six items, including the sex (female), hypertension, diabetes mellitus, age (>50 years), 25-hydroxyvitamin D3 and osteopenia/osteoporosis, as the independent variables, and the results suggested that the level of 25-hydroxyvitamin D3 is the clinical feature of whether the treatment of BPPV is effective (P<0.05, OR=0.673, 95% CI=0.259–0.675), and osteopenia/osteoporosis is also the clinical feature of whether the treatment of BPPV is effective (P<0.05, OR=4.637, 95% CI=1.212–20.242). Female (P=0.089, OR=6.923), hypertension (P=0.096, OR=0.664), diabetes mellitus (P=0.096, OR=0.256) and age above 50 years (P=0.087, OR=0.358) are related to the BPPV treatment and prognosis (Table VI).
Table VI.
Logistic regression analysis of influencing factors of 1α-hydroxyvitamin D3 for the curative effect on BPPV.
| 95%CI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable name | B | SE | Wald | df | P-value | OR | Lower limit | Upper limit |
| Sex (female) | 2.103 | 1.288 | 3.614 | 1 | 0.089 | 6.923 | 0.944 | 66.345 |
| Hypertension | −2.113 | 1.316 | 3.711 | 1 | 0.096 | 0.664 | 0.025 | 2.115 |
| Diabetes mellitus | −2.005 | 1.228 | 3.687 | 1 | 0.084 | 0.256 | 0.053 | 2.253 |
| Age (>50 years) | −1.952 | 1.185 | 3.752 | 1 | 0.087 | 0.358 | 0.056 | 1.686 |
| 25-Hydroxyvitamin D3 | −0.537 | 0.342 | 1.437 | 1 | 0.012 | 0.673 | 0.259 | 0.865 |
| Osteopenia/osteoporosis | 2.354 | 1.159 | 4.438 | 1 | 0.032 | 4.637 | 1.212 | 20.242 |
Discussion
BPPV is a kind of common labyrinth disorder caused by the mechanical stimulation against the vestibular receptors in semicircular canal, whose typical features are positional vertigo and positional nystagmus, caused by changes in head position relative to the gravity (7,8). In most patients, it is easy to be diagnosed, and the treatment effect is very satisfactory for patients and physicians. However, some cases are challenging in differential diagnosis, and the treatment effect on these cases is often poor. BPPV is the most common cause of vertigo in adults, and its incidence rate in normal population can be as high as approximately 10% (9). BPPV accounts for approximately 15% in all balanced diseases, and the average onset age is close to 60 years. After the age of 60 years, the incidence rate is increased, and it is lower in population aged below 40 years. Moreover, it is rare in children, and women are more likely to suffer form it than men with a ratio of approximately 2:1 (10). BPPV is a kind of self-limited disease that can be alleviated spontaneously within several days to weeks after onset. Approximately half of patients are prone to recurrence. Since the posterior semicircular canal is most susceptible to the gravity, it is most likely to be involved by BPPV. In fact, approximately 70% BPPV patients are diagnosed as unilateral posterior semicircular canal BPPV. In particular, the right posterior semicircular canal is 1.5 times of the left posterior semicircular canal, which is associated with the right-side sleeping of most people (11).
BPPV is also known as ‘otolithiasis’. Otolith is an important organ to control the body's balance, and the normal otoliths are in the three semicircular canals. Since the otoliths are composed of calcium carbonate, the formation of otolith requires the increase in the concentration of local calcium ions (Ca2+) and carbonate (CO3−), forming the crystals on the protein core. It is important to maintain a low calcium ion concentration in the vestibule of inner ear, because it can prevent the production of abnormal crystals in the endolymph (12,13). The calcium uptake will be increased when the free calcium concentration in the endolymph increase, reducing the ability of endolymph of dissolving the fallen otoliths (14,15). In addition, 1,25-hydroxyvitamin D3 in epithelial cells of rat semicircular canal can upregulate the expression of some calcium channel-binding proteins (16). Thus, we can assume that vitamin D deficiency promotes the calcium deposition in vestibular organs, thus producing BPPV (17). Therefore, vitamin D supplement has a preventive effect on BPPV recurrence for patients with BPPV and low serum vitamin D. Vitamin D receptor (VDR) exists in cells of the whole body (18). The function of vitamin D is mediated by nuclear VDR. Previous studies found VDR in epithelial cell of crista ampullaris, membranous semicircular canals and surrounding bone cells in mice (19). In addition, the accelerated rotation, inclined platform, rotation and swimming tests show that the equilibrium function of mice with mutant VDR is decreased, suggesting that vitamin D insufficiency may cause the vestibular dysfunction, such as BPPV (20,21).
It was found in this study that the proportion of osteopenia/osteoporosis in female BPPV patients aged above 50 years was significantly higher than that in female patients aged below 50 years, and the BMD of patients could be effectively improved after treatment with 1α-hydroxyvitamin D3. The BMD t-value in treatment group showed a decreasing trend with the increase of age. The levels of 25-hydroxyvitamin D3 and bone metabolic markers in treatment group were significantly improved compared with those before treatment (P<0.05). Logistic regression analysis suggested that the level of 25-hydroxyvitamin D3 in patients is the clinical features indicating whether the treatment of BPPV is effective (P<0.05), and osteopenia/osteoporosis is also the clinical feature indicating whether the treatment of BPPV is effective (P<0.05). This is basically consistent with the research results in literature of others is.
In conclusion, the treatment of BPPV with 1α-hydroxyvitamin D3 can effectively improve the symptoms of patients, and the level of vitamin D3 and the occurrence of osteopenia/osteoporosis are the clinical indexes indicating whether the BPPV treatment is effective.
References
- 1.Parham K, Leonard G, Feinn RS, Lafreniere D, Kenny AM. Prospective clinical investigation of the relationship between idiopathic benign paroxysmal positional vertigo and bone turnover: A pilot study. Laryngoscope. 2013;123:2834–2839. doi: 10.1002/lary.24162. [DOI] [PubMed] [Google Scholar]
- 2.Whitman GT, Baloh RW. Seasonality of benign paroxysmal positional vertigo. JAMA Otolaryngol Head Neck Surg. 2015;141:188–189. doi: 10.1001/jamaoto.2014.2941. [DOI] [PubMed] [Google Scholar]
- 3.Nuti D, Masini M, Mandalà M. Benign paroxysmal positional vertigo and its variants. Handb Clin Neurol. 2016;137:241–256. doi: 10.1016/B978-0-444-63437-5.00018-2. [DOI] [PubMed] [Google Scholar]
- 4.Jeong SH, Kim JS, Shin JW, Kim S, Lee H, Lee AY, Kim JM, Jo H, Song J, Ghim Y. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J Neurol. 2013;260:832–838. doi: 10.1007/s00415-012-6712-2. [DOI] [PubMed] [Google Scholar]
- 5.Sheikhzadeh M, Lotfi Y, Mousavi A, Heidari B, Bakhshi E. The effect of serum vitamin D normalization in preventing recurrences of benign paroxysmal positional vertigo: A case-control study. Caspian J Intern Med. 2016;7:173–177. [PMC free article] [PubMed] [Google Scholar]
- 6.von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, Newman-Toker D. Benign paroxysmal positional vertigo: Diagnostic criteria. J Vestib Res. 2015;25:105–117. doi: 10.3233/VES-150553. [DOI] [PubMed] [Google Scholar]
- 7.Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370:1138–1147. doi: 10.1056/NEJMcp1309481. [DOI] [PubMed] [Google Scholar]
- 8.Taneja MK, Taneja V. Vitamin D deficiency in e.N.T. patients. Indian J Otolaryngol Head Neck Surg. 2013;65:57–60. doi: 10.1007/s12070-012-0603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhim GI, PhD GIRM Serum vitamin D and recurrent benign paroxysmal positional vertigo. Laryngoscope Investig Otolaryngol. 2016;1:150–153. doi: 10.1002/lio2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Büki B, Ecker M, Jünger H, Lundberg YW. Vitamin D deficiency and benign paroxysmal positioning vertigo. Med Hypotheses. 2013;80:201–204. doi: 10.1016/j.mehy.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talaat HS, Abuhadied G, Talaat AS, Abdelaal MS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur Arch Otorhinolaryngol. 2015;272:2249–2253. doi: 10.1007/s00405-014-3175-3. [DOI] [PubMed] [Google Scholar]
- 12.Pérez P, Franco V, Cuesta P, Aldama P, Alvarez MJ, Méndez JC. Recurrence of benign paroxysmal positional vertigo. Otol Neurotol. 2012;33:437–443. doi: 10.1097/MAO.0b013e3182487f78. [DOI] [PubMed] [Google Scholar]
- 13.Talaat HS, Kabel AM, Khaliel LH, Abuhadied G, El-Naga HA, Talaat AS. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx. 2016;43:237–241. doi: 10.1016/j.anl.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Sanyelbhaa H, Sanyelbhaa A. Vestibular-evoked myogenic potentials and subjective visual vertical testing in patients with vitamin D deficiency/insufficiency. Eur Arch Otorhinolaryngol. 2015;272:3233–3239. doi: 10.1007/s00405-014-3395-6. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi D, Raveendran NN, Pondugula SR, Kampalli SB, Sanneman JD, Harbidge DG, Marcus DC. Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem Biophys Res Commun. 2005;331:1353–1357. doi: 10.1016/j.bbrc.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 16.Kasapoğlu Aksoy M, Altan L, Ökmen Metin B. The relationship between balance and vitamin 25(OH)D in fibromyalgia patients. Mod Rheumatol. 2017;27:868–874. doi: 10.1080/14397595.2016.1259603. [DOI] [PubMed] [Google Scholar]
- 17.Kahraman SS, Ozcan O, Arli C, Ustun I, Erduran R, Akoglu E, Gokce C. Calcium homeostasis during attack and remission in patients with idiopathic benign paroxysmal positional vertigo. Otol Neurotol. 2016;37:1388–1392. doi: 10.1097/MAO.0000000000001167. [DOI] [PubMed] [Google Scholar]
- 18.Giannini S, Signorini L, Bonanome L, Severino M, Corpaci F, Cielo A. Benign paroxysmal positional vertigo (BPPV): It may occur after dental implantology. A mini topical review. Eur Rev Med Pharmacol Sci. 2015;19:3543–3547. [PubMed] [Google Scholar]
- 19.Sheikhzadeh M, Lotfi Y, Mousavi A, Heidari B, Monadi M, Bakhshi E. Influence of supplemental vitamin D on intensity of benign paroxysmal positional vertigo: A longitudinal clinical study. Caspian J Intern Med. 2016;7:93–98. [PMC free article] [PubMed] [Google Scholar]
- 20.Minasyan A, Keisala T, Zou J, Zhang Y, Toppila E, Syvälä H, Lou YR, Kalueff AV, Pyykkö I, Tuohimaa P. Vestibular dysfunction in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009;114:161–166. doi: 10.1016/j.jsbmb.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Lips P, Binkley N, Pfeifer M, Recker R, Samanta S, Cohn DA, Chandler J, Rosenberg E, Papanicolaou DA. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: Effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am J Clin Nutr. 2010;91:985–991. doi: 10.3945/ajcn.2009.28113. [DOI] [PubMed] [Google Scholar]


